Abstract.
Severe flooding has been linked to outbreaks of leptospirosis. Two sequential extreme flood events in Western Fiji caused the largest outbreak of leptospirosis recorded in the South Pacific, with 1,217 total suspected cases, of which 314 were probable and confirmed. Most (83%) cases occurred within 6 weeks of the flood events, displaying a biphasic epidemic curve associated with the floods. Given the temporal proximity of cases to flooding events, most of the transmission appeared to occur during or immediately after the floods; therefore, prevention of exposure to contaminated environments is a priority in the immediate flood and post-flood period. In addition, genotyping studies suggest that multiple animal reservoirs were implicated in the outbreak, reaffirming the importance of integrated human and animal health strategies for leptospirosis control.
Severe flooding has been linked to outbreaks of leptospirosis.1–5 During January and March 2012, successive tropical depressions caused two severe flooding events in Western Fiji, leading to the largest outbreak of leptospirosis reported in the Pacific region. A total of 44 reported deaths and several hundred cases were attributed to the outbreak. Leptospirosis was first reported in Fiji in 1952 and is one of seven priority national notifiable diseases.6,7 The estimated incidence is 20–100 cases per 100,000 per year, considered high when compared with other Asia-Pacific countries.8–10 Transmission is seasonal with peak case-reporting between February and June, largely corresponding to the rainy season (January to April).10 To better understand the epidemiology of the outbreak in 2012, including the spatial and temporal features of transmission in relationship to precipitation, we conducted a retrospective outbreak investigation in the Western division of Fiji (population 340,000),11 the region most severely affected by the flooding events.
We included individuals who 1) sought medical care in the Western Division between December 28, 2011 and May 17, 2012 (21 weeks), 2) the treating clinician suspected leptospirosis, and 3) provided biological samples for testing. Cases were classified as flood associated if they sought medical care 2–6 weeks after the flooding events, or not flood associated if they sought medical care outside this period. The date of health-care visit was based on the date of sample collection at the health-care facilities where patients sought medical care. If the date of health care visit was not reported, we used the date of sample arrival at the Fiji Center for Communicable Diseases Control (FCCDC) minus the average interval in days from sample collection to arrival at the FCCDC (for all samples in the study cohort that included this information). The definition of flood associated was based on prior studies demonstrating an increase in leptospirosis cases approximately 2 weeks after flooding and persisting for several weeks thereafter.1,12–16 We extracted demographic and laboratory data from the FCCDC national leptospirosis database. Rainfall data were obtained from the Fiji Meteorological Service and the National Oceanic and Atmospheric Administration.17,18
We achieved laboratory confirmation of cases using anti-Leptospira immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) (Panbio Diagnostics, Brisbane, Australia), lipL32-based polymerase-chain reaction (PCR) detection assay,19 and microscopic agglutination testing (MAT) using a panel of 28 Leptospira serovars selected for the Pacific region.20 Immunohistochemical detection of Leptospira in lung, kidney, liver, heart, and spleen tissue samples was performed on tissues from eight case patients who died of an acute febrile illness in flood-affected areas in February 2012, during the study period. Seventeen samples were genotyped using methods previously reported.21 Confirmed cases were defined as a positive qPCR result, MAT titer ≥ 1:800, or immunohistochemical detection of Leptospira in tissues.22 Probable cases were defined as unconfirmed cases that had positive anti-Leptospira IgM ELISA or MAT titer 1:200–1:400.
Of 1,217 suspected cases identified during the study period, 31 (3%) were confirmed cases (all by PCR and none by MAT) and 283 (23%) were probable cases, respectively; 1,009 of 1,217 cases (83%) were flood associated. The mean weekly case count was 91.1 and 27.0 during flood-associated (10 weeks) and not flood-associated (11 weeks) periods (risk ratio 3.37 [95% confidence interval: 3.24, 3.51]). Most cases were males (61%) and indigenous Fijians (59%). The median age was 30 years (interquartile range [IQR]: 19–42). Of 1,217 total blood samples tested using ELISA, 181 (15%) and 23 (2%) were also tested by PCR and MAT, respectively. Immunohistochemistry confirmed leptospirosis among the eight fatal cases, with positive results being obtained from the lung, kidney, liver, and heart samples. The qPCR positivity rate was higher among flood-associated cases (21% [29/141] versus 5% [2/40], P = 0.02). Of 17 samples genotyped, 10 were suggestive of a rodent reservoir (serogroups Icterohaemorrhagiae [N = 7] and Ballum [N = 3]) and seven a livestock reservoir (serogroups Australis [N = 4] and Pomona [N = 3]).23
This study describes the largest outbreak of leptospirosis reported in the South Pacific. The number of laboratory-confirmed and probable cases that were identified may underestimate the overall burden attributable to the outbreak. Key findings are as follows: 1) most of the cases were identified within 6 weeks after the flood events, indicating most exposures occurred during or shortly after the floods, with notable implications for public health interventions, and 2) multiple animal reservoirs likely contributed to the outbreak, indicating that a variety of control interventions would be necessary to interrupt transmission. The large number of suspected leptospirosis cases reported, including specimen submission and diagnostic testing, may be in part due to enhanced outbreak response activities. In March 2012, the Fiji Ministry of Health and Medical Services (MHMSs) and the World Health Organization (WHO) conducted training seminars for clinicians in the affected areas on the identification and management of leptospirosis. In addition to recommending presumptive treatment of clinically suspected cases, clinicians were encouraged to submit samples for testing if they suspected leptospirosis. The MHMS also formed the National Taskforce for the Control of Outbreak Prone Diseases to monitor outbreak progression and provide technical guidance for control and prevention of leptospirosis. Health promotion activities such as radio messaging were conducted in affected areas to raise public awareness on the prevention of leptospirosis. A study limitation was the low number of probable and confirmed cases. However, given the poor sensitivity of anti-Leptospira IgM ELISA in the first 5–7 days of illness,24 and given that only a small proportion of samples (18%) were tested by PCR, additional cases would likely have been confirmed if comprehensive testing had been implemented.
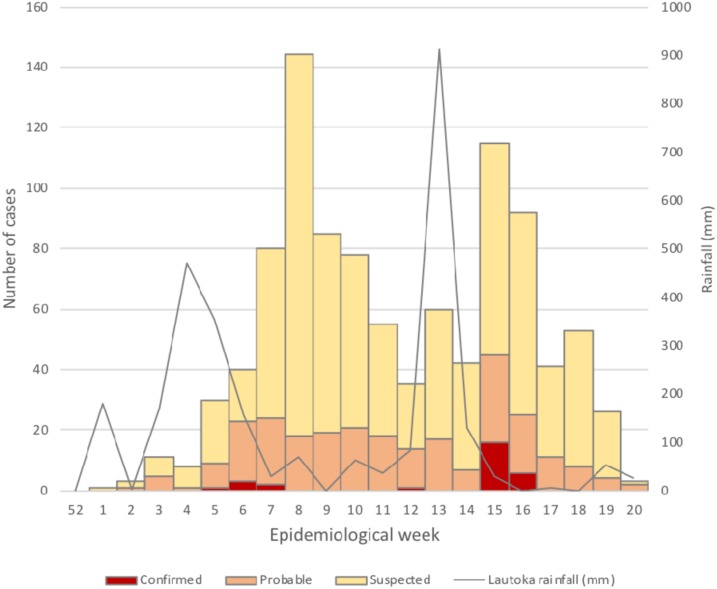
A 2012 study reported that most patients in Fiji with undifferentiated fever seek health care within the first several days of illness (median 3 days [IQR 1–5]) (MHMS/WHO, unpublished), when anti-Leptospira IgM ELISA and MAT are unlikely to be positive. Because of the intermittent availability of leptospirosis testing protocols and reagents before the 2012 outbreak, the reported baseline incidence of leptospirosis may be less reliable than if we had more consistent historical data; however, we are confident that this was a large leptospirosis outbreak, given that 1) the weekly incidence was more than 3-fold higher during the post-flood period, 2) the qPCR positivity rate was more than 4-fold higher post-flood, 3) all eight fatal cases with autopsy tissue available were confirmed as leptospirosis by immunohistochemistry, 4) the biphasic epidemic curve paralleled the successive flood events (Figure 1), and 5) multiple hospitals in the Western Division reported abnormally high rates of severe disease and deaths (mostly due to pulmonary hemorrhage) among patients with clinically suspected leptospirosis during the post-flood periods (M. Kama, E. J. Nilles, unpublished data).
Figure 1.
Rainfall and suspected, probable and confirmed cases of leptospirosis by date of laboratory sample collection—Western Division, Fiji, 2012.*† *Rainfall in Lautoka, the largest city in the Western Division of Fiji. †Includes cases with date of sample collection (n = 1,002). This figure appears in color at www.ajtmh.org.
Given their unique geographic and demographic characteristics, the Pacific island region is among the most vulnerable globally, and the impact of extreme weather events leading to epidemics of infectious diseases has been identified as a high-priority public health threat to the region.25,26 Countries, including those in the Pacific, that are susceptible to extreme weather events such as flooding are encouraged to strengthen emergency surveillance and response capacities—in line with the International Health Regulations (2005)27—to rapidly detect and control infectious disease outbreaks. This study builds on the limited body of literature characterizing the epidemiology of leptospirosis outbreaks in these settings and aims to better inform policy-makers and control programs on prevention and control priorities after flooding events. Given that most transmission occurs during or immediately post-flood, investing limited public health resources in source reduction after flooding may not be effective, and avoidance or barrier protection to prevent exposure to contaminated environments should be a public health priority. The implication that multiple animal reservoirs were likely involved in this outbreak reaffirms the importance of integrated human, animal, and environmental health strategies for leptospirosis control.
Acknowledgments:
This study was supported by a grant of the French Government through the fonds de coopération économique, sociale et culturelle pour le Pacifique (« Fonds Pacifique »), as well as support from Yale Global Health Initiative Field Experience Award 2015, and NIH grants R01 TW009504 and R01 AI121207. We would like to thank the Immunohistochemistry Laboratory of the Infectious Diseases Pathology Branch at the US Centers for Disease Control and Prevention for developing and performing the immunohistochemistry assay.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Amilasan AS, et al. 2012. Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg Infect Dis 18: 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau CL, Smythe LD, Craig SB, Weinstein P, 2010. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Trans R Soc Trop Med Hyg 104: 631–638. [DOI] [PubMed] [Google Scholar]
- 3.Reis RB, et al. 2008. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl Trop Dis 2: e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar U, et al. 2002. Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am J Trop Med Hyg 66: 605–610. [DOI] [PubMed] [Google Scholar]
- 5.McBride AJ, Athanazio DA, Reis MG, Ko AI, 2005. Leptospirosis. Curr Opin Infect Dis 18: 376–386. [DOI] [PubMed] [Google Scholar]
- 6.Fiji Ministry of Health , 2010. Fiji National Communicable Disease Surveillance and Outbreak Response Guidelines. Suva, Fiji: Fiji Ministry of Health and Medical Services.
- 7.Ram P, 1977. History of leptospirosis in Fiji. Fiji Med J 3: 68–69. [Google Scholar]
- 8.Victoriano AF, et al. 2009. Leptospirosis in the Asia Pacific region. BMC Infect Dis 9: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinsstag J, Schelling E, Waltner-Toews D, Whittaker M, Tanner M, 2015. One Health: The Theory and Practice of Integrated Health Approaches. Oxfordshire, United Kingdom: Centre for Agriculture and Bioscience International (CABI), 477.
- 10.Ghosh A, Khan S, Kishore K, 2010. Leptospirosis in Fiji: incidence between 2000 to 2007 and review of literature. Fiji Med J 29: 8–14. [Google Scholar]
- 11.Fiji Bureau of Statistics, 2012. 2007 Census of Population and Housing. Suva, Fiji: Fiji Bureau of Statistics.
- 12.Dechet AM, et al. 2012. Leptospirosis outbreak following severe flooding: a rapid assessment and mass prophylaxis campaign; Guyana, January–February 2005. PLoS One 7: e39672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaki SA, Shanbag P, 2010. Clinical manifestations of dengue and leptospirosis in children in Mumbai: an observational study. Infection 38: 285–291. [DOI] [PubMed] [Google Scholar]
- 14.Barcellos C, Sabroza PC, 2001. The place behind the case: leptospirosis risks and associated environmental conditions in a flood-related outbreak in Rio de Janeiro. Cad Saude Publica 17 (Suppl): 59–67. [DOI] [PubMed] [Google Scholar]
- 15.Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD, Jr, Riley LW, 1999. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet 354: 820–825. [DOI] [PubMed] [Google Scholar]
- 16.Casanovas-Massana A, et al. 2018. Spatial and temporal dynamics of pathogenic Leptospira in surface waters from the urban slum environment. Water Res 130: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiji Meteorological Service, 2015. Fiji Meteorological Service Available at: http://www.met.gov.fj/index.php. Accessed July 24, 2016.
- 18.National Oceanic and Atmospheric Administration, 2015. National Oceanic and Atmospheric Administration Available at: http://www.noaa.gov/. Accessed July 28, 2016.
- 19.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR, 2009. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn Microbiol Infect Dis 64: 247–255. [DOI] [PubMed] [Google Scholar]
- 20.Lau CL, Watson CH, Lowry JH, David MC, Craig SB, Wynwood SJ, Kama M, Nilles EJ, 2016. Human leptospirosis infection in Fiji: an eco-epidemiological approach to identifying risk factors and environmental drivers for transmission. PLoS Negl Trop Dis 10: e0004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez J, Goarant C, 2010. Rapid Leptospira identification by direct sequencing of the diagnostic PCR products in New Caledonia. BMC Microbiol 10: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention , 2013. National Notifiable Diseases Surveillance System, Diseases & Conditions, Leptospirosis Summary, Leptospirosis (Leptospira interrogans) 2013 Case Definition. Atlanta, GA: Centers for Disease Control and Prevention.
- 23.Levett PN, 2001. Leptospirosis. Clin Microbiol Rev 14: 296–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reller ME, Bodinayake C, Nagahawatte A, Devasiri V, Kodikara-Arachichi W, Strouse JJ, Flom JE, Dumler JS, Woods CW, 2011. Leptospirosis as frequent cause of acute febrile illness in southern Sri Lanka. Emerg Infect Dis 17: 1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IPCC , 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri RK and Meyer LA, eds.]. Geneva, Switzerland: IPCC, 151. [Google Scholar]
- 26.McIver L, et al. 2016. Health impacts of climate change in Pacific island countries: a regional assessment of vulnerabilities and adaptation priorities. Environ Health Perspect 124: 1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization , 2008. International Health Regulations (2005), 2nd edition. Geneva, Switzerland: WHO Press. [Google Scholar]