Abstract.
Community prevalence of infection is a widely used, standardized metric for evaluating malaria endemicity. Conventional methods for measuring prevalence include light microscopy and rapid diagnostic tests (RDTs), but their detection thresholds are inadequate for diagnosing low-density infections. The significance of submicroscopic malaria infections is poorly understood in Madagascar, a country of heterogeneous malaria epidemiology. A cross-sectional community survey in the western foothills of Madagascar during the March 2014 transmission season found malaria infection to be predominantly submicroscopic and asymptomatic. Prevalence of Plasmodium infection diagnosed by microscopy, RDT, and molecular diagnosis was 2.4%, 4.1%, and 13.8%, respectively. This diagnostic discordance was greatest for Plasmodium vivax infection, which was 98.5% submicroscopic. Village location, insecticide-treated bednet ownership, and fever were significantly associated with infection outcomes, as was presence of another infected individual in the household. Duffy-negative individuals were diagnosed with P. vivax, but with reduced odds relative to Duffy-positive hosts. The observation of high proportions of submicroscopic infections calls for a wider assessment of the parasite reservoir in other regions of the island, particularly given the country’s current focus on malaria elimination and the poorly documented distribution of the non–P. falciparum parasite species.
INTRODUCTION
Malaria remains a major health problem across most of Madagascar.1–3 The malaria burden is monitored through clinical case reports submitted to the Ministry of Health’s routine health management information system2 and by Malaria Indicator Surveys that measure national malaria prevalence every 2–3 years.4–6 These data sources indicated a sharply increased malaria burden between 2011 and 2015, with a slight subsequent decrease into 2016.2,3 The launch of the 2018–2022 Malaria National Strategic Plan offered an opportunity to review the impact of existing interventions and to formulate new plans to achieve “geographically progressive elimination.”1 Monitoring progress toward achieving these targets requires diagnostic methods with appropriate sensitivity to detect the overall reservoir of parasite infection and documenting infections that result in patients seeking treatment of clinical episodes.7 These two complimentary malariometrics allow a more comprehensive description of the overall status of malaria than a single one in isolation.8 Here, we present a study investigating the parasite reservoir and infection risk factors in an area of central Madagascar known to be co-endemic for both Plasmodium falciparum and Plasmodium vivax clinical malaria.9,10
Four human Plasmodium parasite species have been detected in Madagascar, with P. falciparum being predominant.6,11 Parasite species-specific routine case reporting is not yet established, although it is a stated ambition in the latest strategic plan, making it difficult, at present, to assess species-specific contributions to malaria morbidity and mortality. Plasmodium vivax parasites from Madagascar are of particular interest following the observation of their ability to infect red blood cells and cause clinical illness in patients of the Duffy-negative blood group (absence of the atypical chemokine receptor 1), individuals traditionally considered “resistant” to infection.9,12 These P. vivax infections of Duffy-negative hosts are particularly pertinent to sub-Saharan African countries (excluding the Horn of Africa) where most of the population is Duffy negative13 and P. vivax is considered absent, but growing numbers of infections in Duffy-negative hosts are reported.14,15 Madagascar’s diverse population origins, being from both continental Africa and Indonesia,16 mean that a unique combination of Duffy-positive and Duffy-negative hosts co-exist there, allowing assessment of differential infection risk by P. vivax.
Here, we revisit an area of Madagascar where P. vivax infections were previously described in Duffy-negative patients, and where clinical P. vivax malaria exists alongside P. falciparum.9,10 In this context, the present study addresses two principal issues: 1) the proportions and characteristics of submicroscopic infections missed by conventional point-of-care diagnostic tests to provide a more complete description of the parasite reservoir, and 2) P. vivax infection risks according to Duffy genotype.
MATERIALS AND METHODS
Ethics approval and consent to participate.
This study protocol was approved by the ethical review panels of University Hospitals Case Medical Center, Cleveland, OH (DMID Protocol #13-0067), the National Institutes of Health, United States (1R01AI097366), and the Ministry of Public Health, Madagascar (No. 099-MSANP/CE). Community-based discussions were conducted with area administrators, community leaders and their constituents before initiating this study. Written informed consent was obtained from all subjects (or guardians of participants younger than 18 years) enrolled into the study.
Study site population.
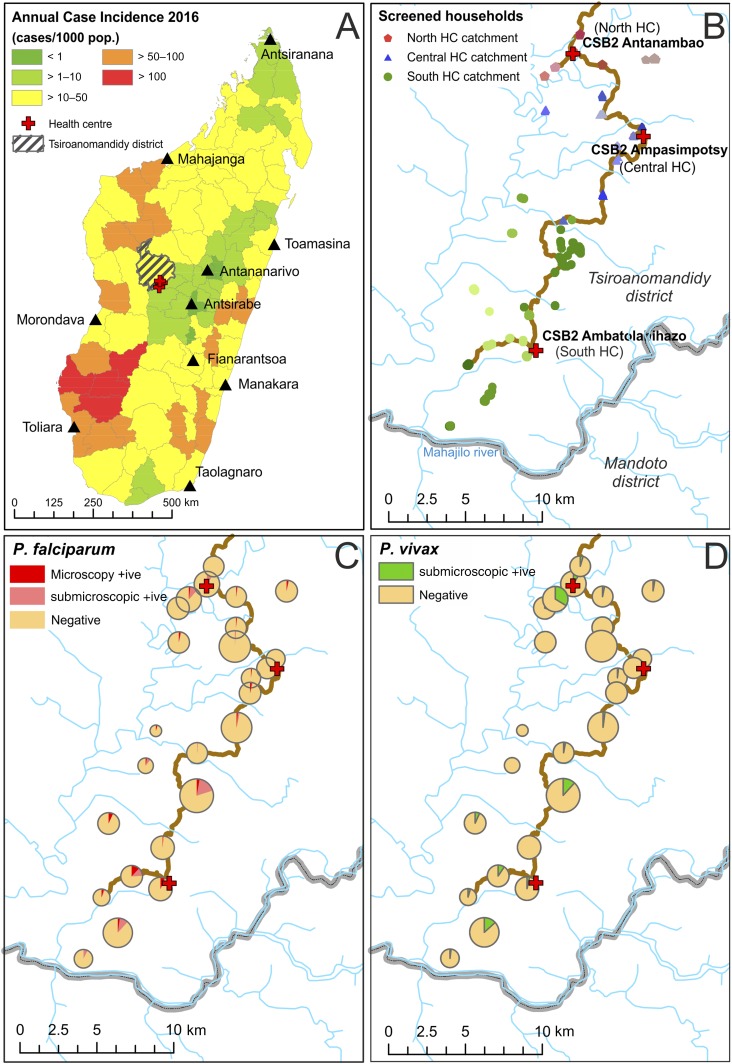
The study was conducted in Tsiroanomandidy district in the region of Bongolava, a rural area in the western foothills of Madagascar which fringes the low transmission central highlands and the high endemicity tropical west coast (Figure 1A). Plasmodium falciparum and P. vivax malaria are endemic in the area, with marked seasonal trends in their transmission intensity2,17 (Supplemental Figure 1). The study site falls into the country’s West Highland Fringe ecozone, an area with rapid diagnostic test (RDT)–confirmed reported annual case incidence of 20.7/1,000 population in 2015, with higher incidence in children younger than 6 years compared with the rest of the population.2 The primary local vector species is Anopheles funestus, although Anopheles arabiensis is also implicated in transmission.17
Figure 1.
Study site map and distribution of diagnosed infections. (A) The study site location (red crosses), with the hatched polygon representing Tsiroanomandidy district. Background districts are colored categorically by annual case incidence estimates for 2016 (source: National Malaria Control Program of Madagascar). (B) A higher resolution map of the study region, with the principal access track marked in brown, health centers denoted by red crosses, and households colored by village and by health center catchment area. (C and D) Observed Plasmodium infection prevalence by village. Pie chart sizes reflect the number of individuals tested by village (range: 21–179). This figure appears in color at www.ajtmh.org.
The study was conducted in collaboration with three private health clinics that provide health care to a migrant population relocated from the generally malaria-free capital city, Antananarivo, to the rural endemic zone approximately 200 km west of the city. The relocation program is run by the nongovernmental organization (NGO) “Ankohonana Sahirana Arenina (ASA)” (http://www.asa-madagascar.org/). The relocated population lives in 25 villages across the ASA territory, with at least one new village established annually since the NGO’s formation. The central health center in Ampasimpotsy (lat. 19.24525, long. 46.18026) provides care to the longest established villages; to the south, the health center in Ambatolahihazo (−19.37066, 46.11724) includes villages with local residents and migrant families, whereas the most recently established villages are in the north, provided for by a health center in Antanambao (−19.19685, 46.13900) (Figure 1B).
Sampling method.
A full census with housing details and relocation dates was available from the ASA administrators. The study’s aim was to achieve universal sampling of the migrant community of 2,783 individuals, the only criterion for inclusion was being > 6 months old. Neighboring long-term resident communities were not included. The study was conducted between March 17 and March 27, 2014, corresponding to the end of the wet season, and close to the transmission peak in April–May2 (Supplemental Figure 1).
Following informed consent, a questionnaire was completed which included self-reported ethnicity, insecticide-treated bednet (ITN) ownership, and clinical details including participant height, weight, and axillary temperature. Finally, capillary blood samples were taken for diagnostic screening.
Field-based diagnostics.
All study participants were screened for malaria parasites from fingertip capillary blood using three diagnostic approaches. First, the SD Bioline Combo Pf/Pan RDT for malaria was used to screen for parasitemia at the point of contact with the study participant. Second, thick and thin blood films were prepared for light microscopy diagnosis following standard World Health Organization (WHO) protocols. The thin smear was examined for Plasmodium species, parasite life stage, and density relative to white blood cell count. A slide was only declared negative after examining all the slide fields. Blood smears were examined by two independent microscopists blinded to prior results, with discrepancies settled by a WHO-certified third reader. Third, blood spots were dried onto filter paper for molecular diagnosis. Finally, 20% of participants were selected (through random number assignment before enrolment) for hemoglobin concentration measurement (HemoCue Diagnostic).
Rapid diagnostic test–positive participants were treated with a weight-adjusted course of artesunate–amodiaquine, in accordance with the Malagasy Ministry of Health guidelines.18
Molecular diagnostics.
Details of the molecular screening procedures used have been previously published for Plasmodium species diagnosis19 and Duffy genotyping.9 The polymerase chain reaction (PCR)-based nucleic acid amplification assay used was the ligase detection reaction-fluorescent microsphere assay.
Statistical analysis.
Spatial mapping of households was performed using a handheld Garmin global positioning system (GPS) (projection Datum WGS 84). Distances (in meters) to the river network were calculated for each household in relation to the HydroSHEDS river network dataset (the 15-second version).20,21 Univariable and stepwise multivariable logistic regression was used to evaluate association between different levels of infection status (microscopy versus molecular versus submicroscopic) for P. falciparum and P. vivax infection. All explanatory variables (health center catchment area, gender, age, years lived in the malaria-endemic area [“exposure time”], ethnicity, fever, and ITN ownership) and outcomes were categorical except age, exposure time, and distance to rivers. Categorical groupings for age and exposure time were investigated but did not provide further analytical resolution (Supplemental Figure 2). Variable selection for the multivariable regression models was based on Akaike Information Criterion (AIC) values, with a stepwise algorithm optimizing the combination of variables to achieve the lowest possible AIC. The resulting models were assessed for goodness-of-fit by area under the curve (AUC) measures on a 10% subset of datapoints. Analyses were conducted in R statistical software (R Core Team, The R Foundation for Statistical Computing 2017, version 3.4.3).
RESULTS
Population.
Of the 2,783 eligible individuals resident in the ASA study region, 2,143 participated in the screening (77%), with others either absent from their homes at the time of screening or refusing consent. Participants came from 25 villages in the catchment areas of the three health centers (Figure 1B), and self-reported to be from two predominant highland ethnic groups: Merina (73%) and Betsileo (22%); the remaining 5% self-reported to a variety of minority ethnic groups. The study population included slightly more females (53%) than males, and the mean participant age was 19.6 years (median: 14 years; standard deviation: 16.5 years) (Supplemental Figure 2).
Duration of residency in the endemic region had a low but significant correlation with age (Spearman test = 0.49, P < 0.0001), consistent with 67% of the population having been born elsewhere and then relocated to the endemic study area. The other 33% had been born to migrant parents in the study zone.
Hemoglobin (Hb) concentration was assessed in 406 randomly selected participants and ranged from 6.5 g/dL to 17.9 g/dL (mean: 13.6 g/dL; 95% confidence interval [CI]: 13.5–13.8). When adjusting for gender and age as per the WHO guidelines,22 89.9% of individuals were non-anemic, 7.8% had mild anemia, and 2.1% had moderate anemia. A single individual had severe anemia (Hb: 6.5 g/dL), and four individuals had Hb < 10g/dL; all were free of malaria infection. Anemia was therefore not found to be common in the population.
Prevalence of infection.
The full trio of diagnostic results – microscopy, RDT and molecular analysis – were available from 2,063 individuals (Tables 1 and 2). Symptomatic Plasmodium infections were exceedingly rare in this cross-sectional survey, with only 11 individuals (0.5% prevalence) meeting the criteria for symptomatic infection (RDT positive with a temperature ≥ 37.5°C). Plasmodium infections were overwhelmingly submicroscopic, with 82.8% being undetected by microscopy (overall prevalence of microscopy-positive participants: 2.4% [N = 49] versus PCR-positive hosts: 13.8% [N = 285]) (Table 2). All four human Plasmodium species were identified although Plasmodium ovale and Plasmodium malariae were not detected by microscopy. Plasmodium falciparum prevalence by microscopy was 2.3%, whereas 7.0% by molecular diagnosis (combining mono- and mixed-species infections), corresponding to 67.6% of infections being submicroscopic, in contrast to P. vivax which was 98.5% submicroscopic, with only two of 137 infections detected by microscopy. The prevalence of P. falciparum and P. vivax infections (mono or mixed) by molecular diagnosis was therefore 7.0% and 6.6%, respectively. Infection densities of the two microscopy-positive P. vivax infections were 387 and 219 parasites/μL, with both detected as positive by the RDT Plasmodium lactate dehydrogenase band. The 47 P. falciparum microscopy-positive cases had an overall mean parasitemia of 3,717 parasites/μL (range: 54–63, 105). Consistent with the high proportions of submicroscopic infections, the sensitivity of microscopy to detect infections was low, being at 25% (95% CI: 18–33) for P. falciparum and 1% (0–5) for P. vivax. Specificity meanwhile was ≥ 99% (99–100%) for all species (Supplemental Table 1).
Table 1.
Diagnostic results by species and detection method
| Molecular | Microscopy | RDT | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Pf | Pv | Neg | HRP2 | pLDH | HRP2/pLDH | Neg | |
| Negative | 1,778 | 10 | 1,768 | 23 | 2 | 6 | 1,747 | |
| Pf | 113 | 35 | 78 | 8 | 1 | 35 | 69 | |
| Pv | 100 | 1 | 2 | 97 | 1 | 4 | 95 | |
| Pm | 25 | 25 | 25 | |||||
| Po | 5 | 5 | 5 | |||||
| Pf, Pv | 25 | 1 | 24 | 3 | 1 | 21 | ||
| Pf, Pm | 5 | 5 | 5 | |||||
| Pv, Pm | 9 | 9 | 1 | 8 | ||||
| Pv, Po | 1 | 1 | 1 | |||||
| Pf, Pv, Pm | 1 | 1 | 1 | |||||
| Pf, Pv, Pm, Po | 1 | 1 | 1 | |||||
| Sub-total | 2,063 | 47 | 2 | 2,014 | 36 | 7 | 42 | 1,978 |
| TOTAL | 2,063 | 2,063 | 2,063 | |||||
HRP2 = histidine-rich protein 2 (a P. falciparum-specific band); Pf = Plasmodium falciparum; pLDH = Plasmodium lactate dehydrogenase (indicator of Plasmodium infection, but not species specific); Pm = Plasmodium malariae; Po = Plasmodium ovale; Pv = Plasmodium vivax; RDT = rapid diagnostic test. The RDT categories correspond to specific bands on the RDT cassette.
Table 2.
Sub-microscopic infection (SMI) prevalence and proportion by Plasmodium species
| Species | Microscopy | PCR | Prop SMI* | ||
|---|---|---|---|---|---|
| Mono or mixed infections | |||||
| Plasmodium falciparum | 47 | (2.3%) | 145 | (7.0%) | 67.6% |
| Plasmodium vivax | 2 | (< 0.1%) | 137 | (6.6%) | 98.5% |
| Plasmodium malariae | 0 | (0.0%) | 41 | (2.0%) | 100% |
| Plasmodium ovale | 0 | (0.0%) | 7 | (0.3%) | 100% |
| Mixed infections | 0 | (0.0%) | 42 | (2.0%) | 100% |
| N individuals infected by any Plasmodium species | 49 | (2.4%) | 285 | (13.8%) | 82.8% |
Percentages refer to population prevalence of the different infection types.
Proportion of infections which were submicroscopic (SMI): .
No mixed infections were detected by microscopy, despite 42 detected by molecular analysis (15% of all infections). These were mainly double-species infections, although one triple-species (P. falciparum, P. vivax, malariae) and one quadruple-species (P. falciparum, P. vivax, malariae, P. ovale) infections were also diagnosed (Table 1). Mixed infections (P. falciparum/P. vivax) were more common than expected by random chance based on overall population prevalence (observed P. falciparum/P. vivax: 27 [including one triple and one quadruple species infection]; expected: 9.6; χ2 test of independence: 7.14; P = 0.0075).
Infection risk factors: logistic regression analysis.
Heterogeneity in Plasmodium infections across the population was investigated by logistic regression analysis for the four infection outcomes of interest: 1) P. falciparum detectable by microscopy, 2) by molecular diagnosis, 3) as a submicroscopic infection, and 4) P. vivax by molecular diagnosis (this included the two microscopy-positive samples; 98.5% of the P. vivax infections were at submicroscopic densities). Both univariable and multivariable analyses were performed (Tables 3 and 4). Although all models presented in Tables 3 and 4 were significantly more informative relative to the null model (P < 0.001), and variables in the multivariable analyses were individually significant by sequential analysis of variance (ANOVA), overall model predictive capacity varied. Weakest model predictive capacity was for microscopically diagnosed P. falciparum infections (AUC: 0.652). The best model fit was for PCR-detected P. falciparum (AUC: 0.839), whereas the model for P. falciparum submicroscopic infections had an AUC of 0.758. The multivariable model predictions for molecular P. vivax diagnosis had an AUC of 0.752.
Table 3.
Logistic regression analysis of Plasmodium falciparum infection explanatory variables
| P. falciparum (microscopy-detectable) | P. falciparum (molecularly diagnosed) | P. falciparum (submicroscopic) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable model | Multivariable model | Univariable model | Multivariable model | Univariable model | Multivariable model | ||||||||||||||||||
| N* | PfPR† | OR | 95% CI‡ | P | AOR | 95% CI‡ | P | PfPR† | OR | 95% CI‡ | P | AOR | 95% CI‡ | P | PfPR† | OR | 95% CI‡ | P | AOR | 95% CI‡ | P | ||
| Gender | Female | 1,086 | 2.6% | Reference | 7.0% | Reference | 5.2% | Reference | |||||||||||||||
| Male | 977 | 1.9% | 0.75 | (0.41–1.34) | 0.3400 | 7.1% | 1.01 | (0.72–1.42) | 0.95 | 5.4% | 1.06 | (0.72–1.55) | 0.790 | ||||||||||
| Age (year) | 2,061 | 2.3% | 0.99 | (0.97–1.01) | 0.3800 | 7.0% | 1.00 | (0.99–1.01) | 0.79 | 4.8% | 1.00 | (0.99–1.01) | 0.600 | ||||||||||
| Exposure (year)§ | 2,050 | 2.3% | 0.99 | (0.93–1.05) | 0.7600 | Not selected | 7.0% | 0.98 | (0.95–1.02) | 0.35 | 4.8% | 0.98 | (0.94–1.02) | 0.400 | |||||||||
| Not selected | Not selected | ||||||||||||||||||||||
| Other | 103 | 3.9% | Reference | 10.7% | Reference | 6.8% | Reference | ||||||||||||||||
| Ethnicity‖ | Betsileo | 455 | 1.8% | 0.45 | (0.13–2.10) | 0.5100 | 7.0% | 0.67 | (0.31–1.62) | 0.53 | 5.5% | 0.86 | (0.34–2.61) | 0.860 | |||||||||
| Merina | 1,505 | 2.3% | 0.60 | (0.21–2.54) | 6.8% | 0.65 | (0.32–1.49) | 5.1% | 0.80 | (0.34–2.32) | |||||||||||||
| Water distance (m)¶ | 2,063 | 2.3% | 1.00 | (1.00–1.00) | 0.0005 | 1.00 | (1.00–1.00) | 0.012 | 7.0% | 1.00 | (1.00–1.00) | 0.043 | 4.8% | 1.00 | (1.00–1.00) | 0.480 | |||||||
| Insecticide-treated bednet ownership | No | 242 | 5.0% | Reference | Reference | 17.4% | Reference | Reference | 12.8% | Reference | Reference | ||||||||||||
| Yes | 1,810 | 1.9% | 0.37 | (0.19–0.75) | 0.0035 | 0.31 | (0.14–0.70) | 0.004 | 5.6% | 0.28 | (0.19–0.42) | < 0.0001 | 0.39 | (0.26–0.59) | < 0.0001 | 4.3% | 0.31 | (0.20–0.48) | < 0.0001 | 0.43 | (0.28–0.68) | 0.0002 | |
| Center | 775 | 1.2% | Reference | Reference | 1.9% | Reference | Reference | 1.0% | Reference | Reference | |||||||||||||
| Health center | North | 492 | 1.2% | 1.05 | (0.35–2.93) | 0.0004 | 0.49 | (0.14–1.50) | 0.043 | 7.3% | 4.00 | (2.21–7.60) | < 0.0001 | 3.02 | (1.61–5.96) | < 0.0001 | 6.1% | 6.23 | (2.97–14.68) | < 0.0001 | 5.31 | (2.51–12.60) | < 0.0001 |
| South | 796 | 4.0% | 3.56 | (1.76–7.99) | 1.72 | (0.72–4.33) | 11.8% | 6.78 | (4.02–12.28) | 5.98 | (3.46–11.13) | 8.9% | 9.39 | (4.77–21.29) | 8.22 | (4.14–18.73) | |||||||
| Fever# | No | 1,904 | 2.0% | Reference | Reference | 6.5% | Reference | Reference | 5.0% | Reference | Not selected | ||||||||||||
| Yes | 111 | 6.3% | 3.22 | (1.29–6.95) | 0.0057 | 3.79 | (1.46–8.74) | 0.003 | 16.2% | 2.78 | (1.58–4.65) | 0.0002 | 2.56 | (1.25–3.91) | 0.0051 | 9.9% | 2.07 | (1.02–3.83) | 0.029 | ||||
AOR = adjusted odds ratios; CI = confidence interval; OR = odds ratio; PfPR = prevalence of Plasmodium falciparum. Each infection level was modeled separately, using univariable (OR) and multivariable (AOR) models.
Totals tested by category. Any totals less than 2,063 are because of incomplete information about the different fields.
PfPR infection including both mono- and mixed-species infections.
95% CI of the AOR. P values were derived from the Wald test and represent each categorical factor’s overall statistical significance. Significant P values (P < 0.05) are identified in bold.
Exposure time corresponds to the total years of residency in the malaria-endemic area. Participants born in the study area are considered to have been exposed since birth.
Ethnicity is self-reported and inherited via the female line.
Fever was present if axillary temperature was ≥ 37.5°C.
Table 4.
Logistic regression analysis of Plasmodium vivax infection explanatory variables
| P. vivax (molecularly diagnosed; 98.5% sub-microscopic) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable model | Multivariable model | ||||||||
| N* | PvPR† | OR | 95% CI‡ | P | AOR | 95% CI‡ | P | ||
| Sex | Female | 1,086 | 6.2% | Reference | Not selected | ||||
| Male | 977 | 7.2% | 1.17 | (0.83–1.66) | 0.3700 | ||||
| Age (year) | 2,061 | 6.6% | 0.99 | (0.98–1.00) | 0.2600 | ||||
| Exposure (year)§ | 2,050 | 6.6% | 0.89 | (0.85–0.92) | < 0.0001 | ||||
| Ethnicity‖ | Other | 103 | 4.9% | Reference | Reference | ||||
| Betsileo | 455 | 5.7% | 1.55 | (0.52–6.55) | 0.3600 | 0.98 | (0.32–4.27) | 0.0074 | |
| Merina | 1,505 | 7.0% | 1.92 | (0.70–7.92) | 2.01 | (0.71–8.44) | |||
| Water distance (m)¶ | 2,063 | 6.6% | 1.00 | (1.00–1.00) | 0.4100 | Not selected | |||
| Insecticide-treated bednet ownership | No | 242 | 14.9% | Reference | Reference | ||||
| Yes | 1,810 | 5.6% | 0.34 | (0.23–0.51) | < 0.0001 | 0.46 | (0.30–0.71) | 0.0004 | |
| Health center | Center | 775 | 0.8% | Reference | Reference | ||||
| North | 492 | 15.0% | 22.69 | (10.64–58.85) | < 0.0001 | 22.68 | (10.51–59.26) | < 0.0001 | |
| South | 796 | 7.2% | 9.89 | (4.59–25.77) | 8.41 | (3.88–22.03) | |||
| Fever# | No | 1904 | 6.4% | Reference | Not selected | ||||
| Yes | 111 | 9.9% | 1.62 | (0.80–2.98) | 0.1400 | ||||
Only two P. vivax infections were identified by light microscopy.
–# See notes from Table 3.
Gender and age were nonsignificant explanatory variables and were not selected by the stepwise multivariable regression models as they did not improve AIC values, whereas fever (temperature ≥ 37.5°C) was significantly associated with all levels of P. falciparum infection (P < 0.05), but not P. vivax (P = 0.140). Fever was more strongly associated with microscopy-detectable infection than molecularly diagnosed infections (odds ratio [OR]: 3.22 versus 2.78). Overall, the two most significant explanatory variables across all infection types were the health facility catchment area (Figure 1C and D) and ITN ownership (P < 0.05 for all outcomes). Odds ratios of infection in the north and south were ≥ 4.0 relative to the central region for molecularly diagnosed infections for both species. Insecticide-treated bednet ownership reduced by at least half the odds of infection relative to non-ownership, both for microscopy-detectable and submicroscopy density infections.
Distance to the river network was significantly associated with microscopy-detectable P. falciparum infection (P = 0.0005), but not submicroscopic infections of either species; the ORs and coefficients were indicative of a very small effect size over the range of study distances.
Two explanatory variables were significantly associated with P. vivax infection by univariable analysis, but not P. falciparum: exposure years and ethnicity. Univariable analysis of P. vivax infection indicated decreasing infection risk as exposure time increased (OR: 0.89; P < 0.0001); however, this effect was not significant when considered in the multivariable framework. Although neither of the main ethnic groups (Betsileo and Merina) had an individually significantly different risk of infection in relation to “other” ethnic groups, ethnicity overall was a significant variable (P = 0.0074) in the multivariable model.
Infection risk factors: Duffy blood group.
Duffy blood group genotypes were available from 1,878 study participants. Three Duffy alleles were detected, the most common being the erythrocyte silent FY*BES allele (frequency: 0.678), followed by FY*A (0.233) and FY*B (0.089). Almost half of the participants had the Duffy-negative phenotype (48.7%); among the Duffy-positive individuals, Fy(a+b−) was the most common phenotype at 34.5%, with a minority Fy(a−b+) at 11.6% and Fy(a+b+) at 5.2%.
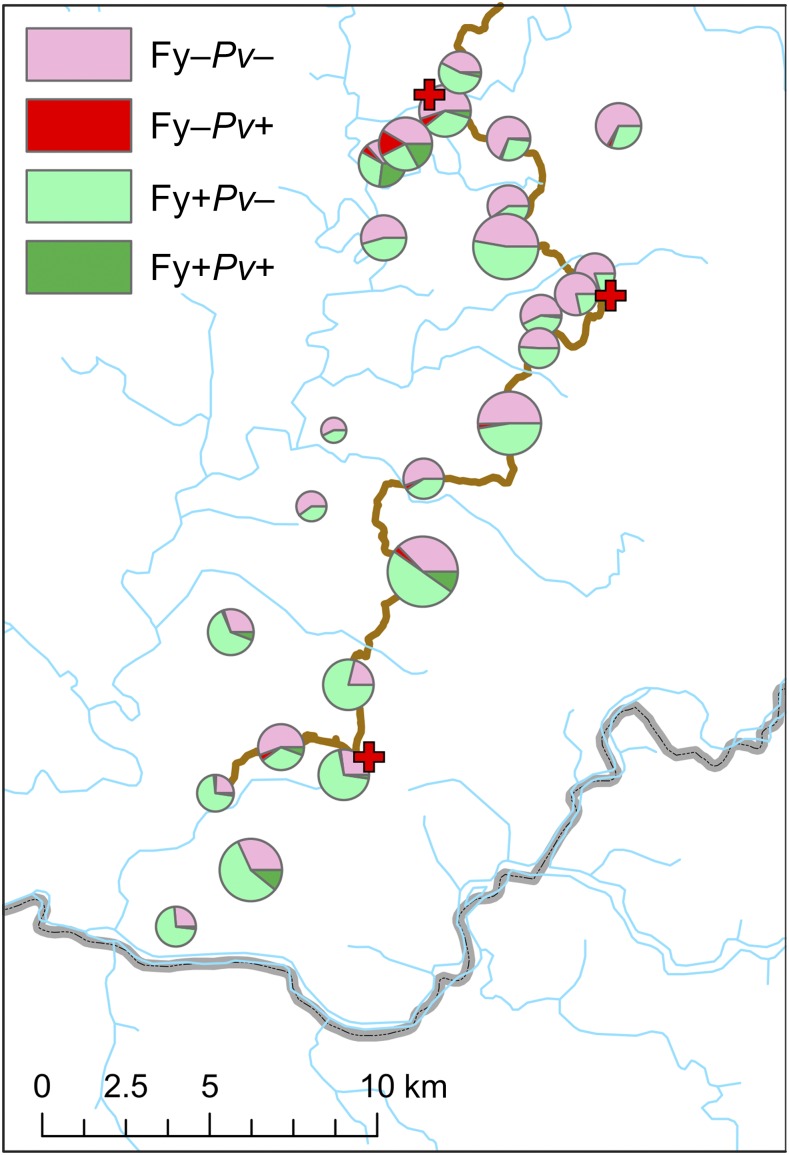
Table 5 shows the association between Duffy blood type and P. vivax infection, with both Duffy-positive and Duffy-negative hosts being diagnosed as infected. Risk of infection for Duffy-negative hosts was half that of Duffy-positives (4.8% versus 8.9% prevalence; OR: 0.52 [95% CI: 0.35–0.75], P < 0.001). The distribution of P. vivax infections among Duffy-positive and Duffy-negative hosts is plotted by village in Figure 2, showing a higher number of infected Duffy-negative hosts in the northern villages, consistent with the greater prevalence of P. vivax in that catchment area (Figure 1D, Table 4). No statistically significant difference was identified between infection odds of Duffy homozygote positive hosts and Duffy heterozygotes (P = 0.429), although heterozygotes had a slightly lower prevalence of infection (8.5% versus 10.2%). No association was found between Duffy blood type and P. falciparum infection (Supplemental Table 3).
Table 5.
Plasmodium vivax infection among Duffy-positive and Duffy-negative hosts, as detected by molecular diagnosis
| N | P. vivax infection | Odds ratio (95% confidence interval) | χ2 P value | ||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | ||||||
| Phenotype | |||||||
| Duffy positive | 964 | 878 | (91.1%) | 86 | (8.9%) | Reference | |
| Duffy negative | 914 | 870 | (95.2%) | 44 | (4.8%) | 0.52 (0.35–0.75) | < 0.001 |
| Genotype | |||||||
| (+/+) | 246 | 221 | (89.8%) | 25 | (10.2%) | Reference | |
| (+/−) | 718 | 657 | (91.5%) | 61 | (8.5%) | 0.82 (0.50–1.34) | 0.429 |
| (−/−) | 914 | 870 | (95.2%) | 44 | (4.8%) | 0.45 (0.27–0.75) | 0.002 |
Odds ratios calculated by unconditional maximum likelihood estimation (Wald).
Figure 2.
Village-level summary of Plasmodium vivax infection status (from molecular diagnosis) according to Duffy phenotype. Pie chart sizes reflect the number of individuals tested by village for whom both the Duffy phenotype and P. vivax infection status data were available (range: 21–164). This figure appears in color at www.ajtmh.org.
Infection risk factors: household level infection risks.
The logistic regression models indicated important spatial heterogeneity across the study area as measured by associations with health center catchment areas (P < 0.05) and relatively low multivariable model predicted performances (AUC ≤ 0.84). The role of household infection status was, therefore, explored further as a potential additional spatial explanatory variable of infection risk. Of the 484 households in the study area, 433 were represented in the screening (89%), with a mean 4.8 individuals screened per household (standard deviation: 2.3; range: 1–23). Two-thirds of households were fully infection free by molecular diagnosis (63%, N = 271). Among the remaining households with at least three individuals screened (N = 152), the prevalence of Plasmodium infection was ≥ 50% in 34 of these.
The odds of infections if living with either RDT-positive or PCR-positive individuals were consistently significantly higher than overall population prevalence rates (OR > 1; Supplemental Table 2). This association was stronger among PCR-level infections, rather than RDT-detectable infections (Supplemental Table 2A). Cohabiting with an RDT-positive individual doubled the odds of PCR-positivity by P. falciparum (OR: 1.98; P = 0.0001), whereas being RDT-positive was only significantly more likely if living with at least two RDT-positive individuals (OR: 2.97; P = 0.0107). The odds of P. vivax infection, however, were unchanged by cohabiting with an RDT-positive individual (P > 0.05). Cohabiting with a molecularly diagnosed infected individual significantly increased the odds of infection by that same species, with a higher effect size for concurrent P. vivax infection (OR: 1.84; P = 0.0004; Supplemental Table 2C) over P. falciparum (OR: 1.48; P = 0.0253; Supplemental Table 2B). There was also evidence of a cross-species association, with odds of P. vivax infection increased by cohabiting with a P. falciparum–positive individual (OR: 1.84; P = 0.0003; Supplemental Table 2B), and to a similar extent vice-versa (OR: 1.77; P = 0.0008; Supplemental Table 2C). The odds of P. falciparum infection were therefore greater if cohabiting with a P. vivax–positive individual than with a fellow P. falciparum–diagnosed individual.
These odds were decreased in households where study participants were exclusively Duffy negative, relative to Duffy-positive households (Supplemental Table 2D), although observed infection numbers were low in some cases, leading to large CIs and nonsignificant effects.
DISCUSSION
The Plasmodium parasite reservoir is increasingly recognized as a hurdle to parasite elimination.7 Regionally specific characteristics of low-density infections and their role toward sustaining transmission are widely reported from endemic settings globally, emphasizing heterogeneity across locations.23–26 The status of, and risk factors associated with, the parasite reservoir in Madagascar were not previously described, despite a national commitment to shift from malaria case control toward parasite pre-elimination by 2022.1 Madagascar’s volatile malaria epidemiology,2,3,27 which has seen important fluctuations likely to be associated with political and financial uncertainties since 2009, includes important epidemic outbreaks,28 the causes of which remain poorly defined.29
The cross-sectional population survey described here identified malaria infection in the western foothills of central Madagascar to be almost exclusively asymptomatic and submicroscopic. These characteristics were more pronounced for P. vivax (100% of infections were asymptomatic and 98.5% submicroscopic) than for P. falciparum. This may help explain the perception of P. vivax being a minority prevalence Plasmodium species in Madagascar. For example, in the 2017 World Malaria report, 100% of clinical cases were attributed to P. falciparum.11 However, molecular diagnostics indicate that P. vivax and P. falciparum are at almost equal prevalence in this population (6.6% versus 7.0%). As previously reported from this region,9,30 Duffy negativity did not confer full protection against P. vivax infection, but did significantly reduce the odds of infection (OR: 0.52; Table 5). Efforts to monitor the local status of P. vivax toward elimination must therefore be based on higher sensitivity diagnostics able to detect the parasite’s low peripheral parasitemias.31
Although the chances of onward transmission decrease as gametocyte densities decrease, mosquito infection can successfully occur even at low parasite densities.32 Model estimates from submicroscopic asexual infection data indicate a ∼20% mosquito infection success rate at gametocyte densities > 600 gametocytes/μL, which drops to ∼4% at densities ∼10 gametocytes/μL33 both from submicroscopic infections. Although this present study did not specifically diagnose gametocyte carriage, it can be inferred from the prevalence of submicroscopic infections that these carriers together represent a significant force of onward vector infection.25,32,34
The positive association of fever with submicroscopic P. falciparum infection (though not P. vivax) by univariable analysis also raises questions in relation to the contribution of malaria infections toward clinical burden which are undetectable by the point-of-care diagnostic tests used in Madagascar (RDTs in field clinics and microscopy in referral hospitals).1 Longitudinal monitoring of infections in this area has been established following this initial cross-sectional descriptive study to assess more comprehensively the local role of different infection densities on clinical burden.
Overall, the multivariable model goodness-of-fit metrics indicated that the available explanatory variables could not fully explain heterogeneity in the responses. Further study is required to fully address the distribution of infections. For example, a limitation of the cross-sectional study design used here includes the lack of associated clinical case incidence data; however, longitudinal surveillance will allow further insight into the relationships between exposure time, clinical burden of disease, prevalence of low-density asymptomatic infections, and ecological variables as possible complementary explanatory drivers of infection dynamics. The broader representativeness of the study population, being largely relocated to the field study zone from the malaria transmission-free capital city, is unclear. Although exposure time was not found to be significant in terms of P. falciparum infection, it was significant for P. vivax (Tables 3 and 4). As carried out here, analyses must be adjusted to account for this potentially influential parameter.
The existence of a silent and invisible parasite reservoir has implications for public health policy and surveillance strategies. The expansion of antimalarial treatment more broadly across apparently healthy and uninfected individuals (by RDT or microscopy) could impact transmission rates beyond simply treating symptomatic cases.35,36 Although the cross-sectional design of the present study did not promote recruitment of symptomatic cases, clustering of infections revealed a strongly increased household-level infection risk (Supplemental Table 2), with implications for active case detection strategies. The interaction between clinical cases and submicroscopic infections at the household level ought to be investigated through longitudinal surveillance and targeted screening at the household and village level to assess the potential benefit of broader access to treatment.
Supplementary Material
Supplemental figures and tables
Acknowledgments:
We are grateful to the communities who generously participated in this study, to the ASA organization for permission to perform the study, to Pact Madagascar for administrative and logistical support, and to the National Malaria Control Program for support with project implementation. Harry Gibson (Malaria Atlas Project, University of Oxford) is thanked for his help with the HydroSHEDS dataset.
Note: Supplemental figures and tables appear at www.ajtmh.org.
REFERENCES
- 1.National Malaria Control Programme of Madagascar , 2017. National Strategic Plan for Malaria Control in Madagascar 2018–2022. Progressive Malaria Elimination from Madagascar. Madagascar: Ministry of Health of Madagascar.
- 2.Howes RE, Mioramalala SA, Ramiranirina B, Franchard T, Rakotorahalahy AJ, Bisanzio D, Gething PW, Zimmerman PA, Ratsimbasoa A, 2016. Contemporary epidemiological overview of malaria in Madagascar: operational utility of reported routine case data for malaria control planning. Malar J 15: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang SY, et al. 2018. Spatio-temporal mapping of Madagascar’s malaria indicator survey results to assess Plasmodium falciparum endemicity trends between 2011 and 2016. BMC Med 16: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institut National de la Statistique (INSTAT), Programme National de Lutte contre le Paludisme (PNLP), and ICF International , 2011. Madagascar Malaria Indicator Survey 2011 [Enquête sur les Indicateurs du Paludisme (EIPM)]. Calverton, MC: INSTAT, PNLP, and ICF International. [Google Scholar]
- 5.Institut National de la Statistique (INSTAT), Programme National de Lutte contre le Paludisme (PNLP), Institut Pasteur de Madagascar (IPM), and ICF International , 2013. Madagascar Malaria Indicator Survey 2013 [Enquête sur les Indicateurs du Paludisme (EIPM)]. Calverton, MC: INSTAT, PNLP, IPM and ICF International. [Google Scholar]
- 6.Institut National de la Statistique (INSTAT), Programme National de Lutte contre le Paludisme (PNLP), Institut Pasteur de Madagascar (IPM), and ICF International , 2016. Madagascar Malaria Indicator Survey 2016 [Enquête sur les Indicateurs du Paludisme (EIPM)]. Calverton, MD: INSTAT, PNLP, IPM and ICF International. [Google Scholar]
- 7.malERA Refresh Consultative Panel on Characterising the Reservoir and Measuring Transmission , 2017. malERA: an updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med 14: e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen JM, Le Menach A, Pothin E, Eisele TP, Gething PW, Eckhoff PA, Moonen B, Schapira A, Smith DL, 2017. Mapping multiple components of malaria risk for improved targeting of elimination interventions. Malar J 16: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menard D, et al. 2010. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci USA 107: 5967–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehlotra RK, et al. 2017. Long-term in vitro culture of Plasmodium vivax isolates from Madagascar maintained in Saimiri boliviensis blood. Malar J 16: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO , 2017. World Malaria Report 2017. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 12.Miller LH, Mason SJ, Clyde DF, McGinniss MH, 1976. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med 295: 302–304. [DOI] [PubMed] [Google Scholar]
- 13.Howes RE, et al. 2011. The global distribution of the Duffy blood group. Nat Commun 2: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howes RE, et al. 2015. Plasmodium vivax transmission in Africa. PLoS Negl Trop Dis 9: e0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman PA, 2017. Plasmodium vivax infection in Duffy-negative people in Africa. Am J Trop Med Hyg 97: 636–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tofanelli S, Bertoncini S, Castri L, Luiselli D, Calafell F, Donati G, Paoli G, 2009. On the origins and admixture of Malagasy: new evidence from high-resolution analyses of paternal and maternal lineages. Mol Biol Evol 26: 2109–2124. [DOI] [PubMed] [Google Scholar]
- 17.Ratovonjato J, et al. 2014. Entomological and parasitological impacts of indoor residual spraying with DDT, alphacypermethrin and deltamethrin in the western foothill area of Madagascar. Malar J 13: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Malaria Control Programme of Madagascar , 2015. National Strategic Plan for Malaria Control in Madagascar 2013–2017: Consolidating the Gains with a View to Elimination of Malaria from Madagascar, 2015–2017 Revision. Madagascar: Ministry of Health of Madagascar.
- 19.McNamara DT, Kasehagen LJ, Grimberg BT, Cole-Tobian J, Collins WE, Zimmerman PA, 2006. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am J Trop Med Hyg 74: 413–421. [PMC free article] [PubMed] [Google Scholar]
- 20.Farr TG, et al. 2007. The shuttle radar topography mission. Rev Geophys 45: RG2004. [Google Scholar]
- 21.World Wildlife Fund (WWF) and U.S. Geological Survey , 2007. HydroSHEDS Database Available at: http://hydrosheds.org/. Accessed January 29, 2018.
- 22.WHO , 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 23.Waltmann A, et al. 2015. High rates of asymptomatic, sub-microscopic Plasmodium vivax infection and disappearing Plasmodium falciparum malaria in an area of low transmission in Solomon Islands. PLoS Negl Trop Dis 9: e0003758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosas-Aguirre A, Ponce OJ, Carrasco-Escobar G, Speybroeck N, Contreras-Mancilla J, Gamboa D, Pozo E, Herrera S, Llanos-Cuentas A, 2015. Plasmodium vivax malaria at households: spatial clustering and risk factors in a low endemicity urban area of the northwestern Peruvian coast. Malar J 14: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ, 2012. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 3: 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Q, Cunningham J, Gatton ML, 2015. Systematic review of sub-microscopic P. vivax infections: prevalence and determining factors. PLoS Negl Trop Dis 9: e3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ihantamalala FA, Rakotoarimanana FMJ, Ramiadantsoa T, Rakotondramanga JM, Pennober G, Rakotomanana F, Cauchemez S, Metcalf CJE, Herbreteau V, Wesolowski A, 2018. Spatial and temporal dynamics of malaria in Madagascar. Malar J 17: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girond F, et al. 2017. Analysing trends and forecasting malaria epidemics in Madagascar using a sentinel surveillance network: a web-based application. Malar J 16: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesteman T, et al. 2016. Multiple causes of an unexpected malaria outbreak in a high-transmission area in Madagascar. Malar J 15: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menard D, et al. 2013. Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis 7: e2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruenberg M, et al. 2018. Plasmodium vivax molecular diagnostics in community surveys: pitfalls and solutions. Malar J 17: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Churcher TS, Trape JF, Cohuet A, 2015. Human-to-mosquito transmission efficiency increases as malaria is controlled. Nat Commun 6: 6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouedraogo AL, Basanez MG, 2013. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife 2: e00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bousema T, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 35.Okell LC, Griffin JT, Kleinschmidt I, Hollingsworth TD, Churcher TS, White MJ, Bousema T, Drakeley CJ, Ghani AC, 2011. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS One 6: e20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjorkman A, Cook J, Sturrock H, Msellem M, Ali A, Xu W, Molteni F, Gosling R, Drakeley C, Mårtensson A, 2017. Spatial distribution of falciparum malaria infections in Zanzibar: implications for focal drug administration strategies targeting asymptomatic parasite carriers. Clin Infect Dis 64: 1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures and tables