Abstract.
An important component of malaria control programs is the ability to assess the effectiveness of the insecticide in insecticide-treated nets (ITNs) during normal usage. The standard technique to measure insecticidal activity is the World Health Organization (WHO) cone test, which in many circumstances, may be difficult to implement. We have evaluated an alternative technique, the colorimetric field test (CFT) on a group of 24-month-old Permanet® 2.0 (Vestergaard-Frandsen, Denmark) nets collected in Colombia. The CFT, which measures surface levels (SL) of deltamethrin is compared with standard high-performance liquid chromatography (HPLC) and the WHO cone test. Effective concentrations of deltamethrin for 80% mortality (EC80) were determined from the CFT and HPLC results. Distribution of insecticide SL after 24 months of use reveal that sampling of the midsection best represents the condition of the entire net. We conclude that the CFT is a practical alternative to the WHO cone test for assessing ITN efficacy.
The addition of an insecticide to a mosquito net increases its effectiveness by repelling and/or killing infective vectors, even after the integrity of the insecticide-treated net (ITN) has been compromised through the accumulation of rips, tears, or holes in the fabric. Two types of insecticide treatments are used to produce ITNs. Either the insecticide is chemically coated onto the net surface, or the insecticide is impregnated throughout the thread (polymer) used to weave the net, and therefore present throughout the netting polymer.1 Regardless of the manufacturing process, the mosquito must come in contact with the insecticide (at present a pyrethroid-class product) at the surface of the net. However, through storage effects, as well as washing and abrasive contact with objects or people, the available insecticide depletes over time in a nonuniform fashion, especially at the surface of the net.
Present methods of monitoring net efficacy include the World Health Organization (WHO) cone test (mosquito bioassay) and chemical analysis using high-performance liquid chromatography (HPLC). The WHO cone test measures mosquito mortality as a function of exposure to available insecticide present on the surface of the net, whereas HPLC measures the entire insecticide content per area of net. The WHO cone test relies on rearing large numbers of insecticide-susceptible vectors in-colony, a technologically challenging activity, especially in resource-poor countries. The assay, as described, requires five portions of the net to be tested (bottom to roof). Nets with an average percentage mortality greater than 80% are considered “optimally effective.”1 Because of difficulty in maintaining standardized reference strains of mosquitoes, insecticide susceptibility is quite varied; making comparisons of net effectiveness difficult. The expense of maintaining mosquito colonies, the inherent variability of biological assay data, and the cost of surveillance by this method make this assay difficult to sustain on a large scale. Although measurements of residual insecticide levels using HPLC methods provide a more objective assessment for net comparisons, this technique destroys the net, is expensive, and requires a significant amount of expertise and resources. Because bioassay results depend on exposure of the mosquito to available insecticide on the surface of the net and chemical analysis measures total levels of insecticide, these two techniques do not always correspond. Therefore, a practical field-adapted technique is needed to measure surface levels (SL) of insecticide.
The cyanopyrethroid field test (CFT) is a simple colorimetric assay that has successfully been used to measure available insecticide present at the net surface.2,3 Briefly, SL of insecticide are collected by rubbing a portion of net material with filter paper using a “magnetic sampling device” or MSD. The MSD is constructed from commonly found materials, i.e., microcentrifuge tubes and magnets. The filter papers are attached to the tubes with adhesive material and placed on both sides of a net. The magnets provide a constant force while the filter papers are systematically rubbed across the net surface. The amount of insecticide adhered to the filter paper is measured using a colorimetric test specific for the cyanopyrethroid class of insecticide, in this case, deltamethrin. The technique does not destroy the net and does not require sophisticated equipment. Samples can be collected in the field while the net is still in use. Once the samples have been collected, they can be stored until the colorimetric assay is ready to be performed. Considering these advantages, the CFT is a practical field method for assessing efficacy while the nets are in use. Therefore, the objective of this report is to validate the CFT by comparing SL (CFT) and total levels (HPLC) of insecticide with results from the WHO cone test and evaluate the technique as a predictor of net bioefficacy. Colorimetric field test data from other published studies were combined with results from this study to construct a contour plot of deltamethrin SL on a typical 24-month-old net. From this plot, a sampling location was determined which reflected the average condition of the entire net.
The WHO cone test was performed on 42 24-month old PermaNet® 2.0 nets collected as part of a separate study conducted in northeast Colombia (manuscript in preparation). Female adult delta strain Anopheles albimanus mosquitoes were used for the cone test.4,5 Based on the range of %Mortality (maximum to minimum), a subset of 16 nets was chosen for insecticide analysis. The CFT was performed on the midsection of each of five panels relative to the orientation of the occupant, that is, the roof (R), head (H), feet (F), next to the wall (W), and the entrance (E). Before HPLC analysis, filter paper disks were collected for the CFT assay. For the HPLC assay, five 10 × 10-cm cut pieces were combined for HPLC processing, resulting in an averaged deltamethrin concentration. The total contents of deltamethrin per unit area (mg/m2) of net material were obtained using the standard extraction Collaborative International Pesticides Analytical Council protocol.5 The concentrations of the sample nets were converted to %whole net levels (%WL) by relative comparison to a new unused “reference” net. Likewise, CFT values (μg/sample) were converted to %SL relative to the “reference” net for direct comparisons.
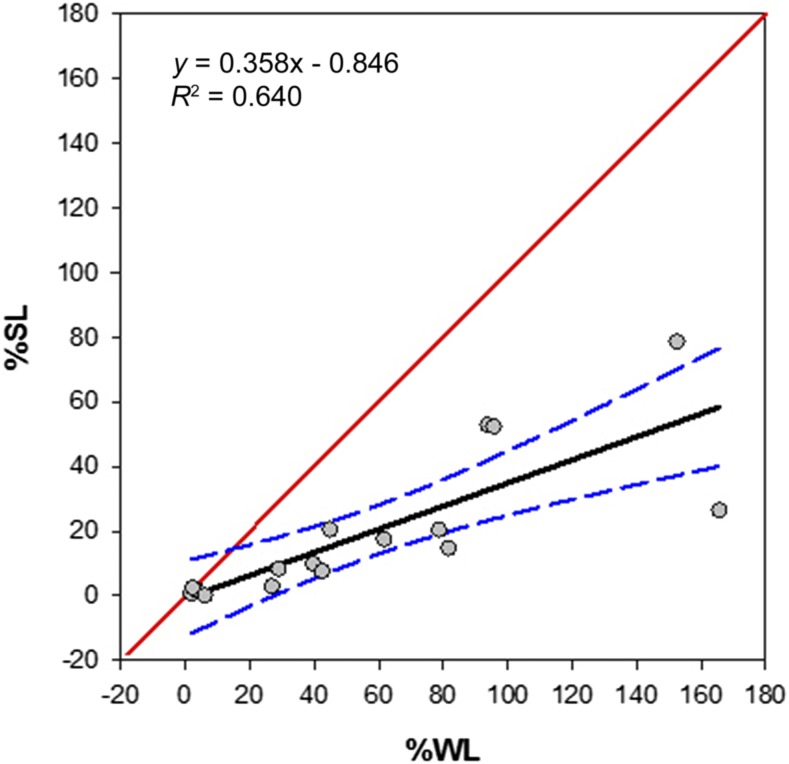
Figure 1 shows the relationship of %SL and %WL values for nets after 24 months of use (y = 0.358x − 0.846), suggesting a preferential loss of deltamethrin at the surface relative to the WL. These nets were sampled laterally at the midsection of each side relative to the orientation of the sleeping occupant, that is, head, entrance, foot, wall, and roof. A Kruskal–Wallis test revealed a statistically significant difference between the lateral %SL values (H[t] = 10.7, P = 0.03) with a post-hoc analysis showing the “roof” (mean rank = 126.6) to be statistically different from the “Entrance” (mean rank = 83.3).
Figure 1.
A comparison of deltamethrin surface levels (%SL) and whole net levels (%WL) on 24-month-old nets as determined by the colorimetric field test and high-performance liquid chromatography methods, respectively. This figure appears in color at www.ajtmh.org.
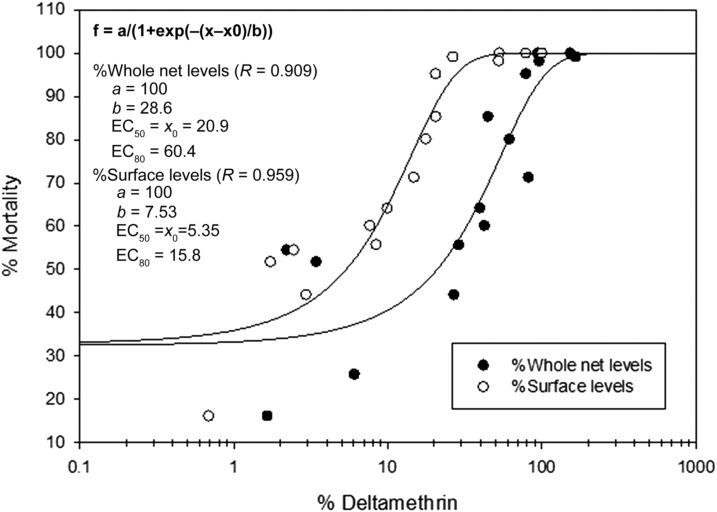
The relationship between %WL, %SL, and %mortality was obtained by plotting a best-fit sigmoidal curve (y = a/[1 + exp(−[x − x0]/b)]) using nonlinear regression software (Sigmaplot 12.3, Systat Software, Inc., San Jose, CA). From these curves shown in Figure 2, the effective concentrations associated with 50% and 80% mortality (EC50 and EC80) were calculated for both %WL and %SL. The ECxx (xx = %Mortality) serve as threshold values to determine a “failed” or “passed” net. EC50 and EC80 values for SL of deltamethrin are 5.4% (0.055 mg/m2) and 15.8% (0.16 mg/m2), respectively. While EC50 and EC80 values of 20.9% (11 mg/m2) and 60.4% (33 mg/m2) were determined for whole net levels (WL), respectively. An improved correlation for %SL (R = 0.959) compared with %WL (R = 0.909) suggests that the measurement of SL of insecticide is more relevant to bioassay results.
Figure 2.
Sigmoid regression curves with parameters “a,” “b,” and “x0” used in calculating the effective concentrations of deltamethrin associated with 50% (EC50) and 80% (EC80) mortality determined from the World Health Organization cone test.
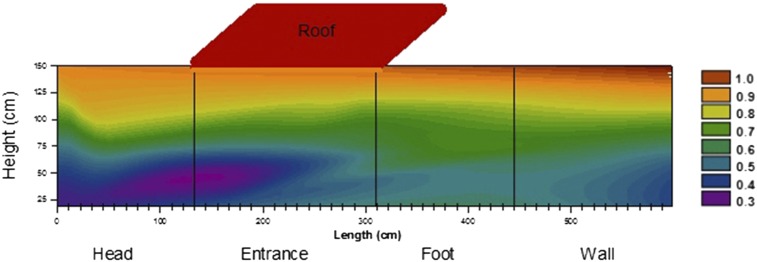
The contour plot in Figure 3 was constructed by estimating the sampling locations on a medium-sized Permanet® 2.0 net (height = 150 cm, length = 180 cm, and width = 130 cm). When splayed out into four panels: head, entrance, foot, and wall, the total length was 620 cm. For example, the estimated sampling locations (length × height) for the WHO cone test are 16 × 10 cm, 153 × 56 cm, 326 × 94 cm, 598 × 131 cm, and the roof. The %SL levels of 24-month-old nets from Benin2 (N = 42) and Lao PDR3 (N = 43) were estimated from published data and normalized to levels found on the roof. These values, along with the Columbian data, were plotted against the dimensions of a medium-sized Permanet® 2.0 net (Sigmaplot 12.3) to form the contour plot. As expected, less deltamethrin remained at the bottom (blue) where the net comes in contact with the bedding material and at the “entrance” toward the “head” (purple) where manipulation of the net material by the occupant is most likely to occur.6 The green portion represents the location of the net where the average %SL for the entire net is found. The average %SL for the midsection-foot of these 24-month old nets is 16.3% (95% confidence interval: 9.4–23.1). If we use the EC50 of 5.4% as the threshold for a failed net, a minimum of 37 nets should be analyzed (α = 0.05, β = 0.20). In conclusion, the SL of insecticide is relevant to the bioefficacy of mosquito nets, thus the CFT can be used to assess ITNs. According to the distribution of deltamethrin SL in Figure 3, we have found that the average deltamethrin levels for the entire net can be found at the midsection (green) with less variability at the “foot.” It is suggested that sampling in this area closely represents the condition for the entire net, thus making the CFT even more cost-effective and convenient than the WHO cone test for malaria control in developing countries.
Figure 3.
Contour plot of deltamethrin surface levels showing insecticide density typically found in nets after 24 months of normal use. Values with the associated colors are proportions relative to levels found in the roof (roof = Red = 1). The green portion represents the average level for the entire net. This figure appears in color at www.ajtmh.org.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.World Health Organization , 2013. World Health Organization Guidance Note for Estimating the Longevity of Long-Lasting Insecticidal Nets in Malaria Control (September):2011–3. Geneva, Switzerland: World Health Organization.
- 2.Green MD, Atieli F, Akogbeto M, 2009. Rapid colorimetric field test to determine levels of deltamethrin on PermaNet surfaces: association with mosquito bioactivity. Trop Med Int Health 14: 1–8. [DOI] [PubMed] [Google Scholar]
- 3.Green MD, Mayxay M, Beach R, Pongvongsa T, Phompida S, Hongvanthong B, Vanisaveth V, Newton PN, Vizcaino L, Swamidoss I, 2013. Evaluation of a rapid colorimetric field test to assess the effective life of long-lasting insecticide-treated mosquito nets in the Lao PDR. Malar J 12: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO , 2011. Guidelines for Laboratory and Field Testing of Long-Lasting Insecticidal Mosquito Nets. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 5.WHO , 2011. Vector Control Technical Expert Group Report to MPAC September 2013. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/malaria/mpac/mpac_sep13_vcteg_llin_survival_report.pdf. Accessed July 2, 2018.
- 6.Sutcliffe JF, Yin S, 2014. Behavioural responses of females of two anopheline mosquito species to human-occupied, insecticide-treated and untreated bed nets. Malar J 13: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]