Abstract.
Pulmonary tuberculosis (PTB) is associated with modulation of levels of adipokines, specifically adiponectin and leptin, but the effect of standard antituberculosis treatment (ATT) on the systemic levels of adiponectin, resistin, and leptin has not been well explored. To identify the association of adipokines with PTB and their relationship with disease severity and bacterial burden, we measured the levels of adiponectin, resistin, and leptin in PTB individuals and compared them with latent tuberculosis (LTB) and healthy control (HC) individuals. Pulmonary tuberculosis was characterized by diminished circulating levels of adiponectin and leptin and heightened circulating levels of resistin in comparison to that in LTB and HC individuals. However, PTB with bilateral or cavitary disease did not exhibit any increased systemic levels of these adipokines in comparison with those with unilateral or non-cavitary disease, respectively. In addition, none of the adipokines exhibited a positive correlation with bacterial burdens, but adiponectin alone exhibited a negative correlation with body mass index in PTB individuals. Finally, on successful completion of ATT, PTB individuals exhibited significantly increased levels of adiponectin and leptin and significantly decreased levels of resistin. Therefore, our data identify an important association of systemic adipokine levels with PTB disease and its alteration following ATT.
INTRODUCTION
Adipokines are cytokines secreted predominantly by the adipose tissue. The most well-studied adipokines are adiponectin, resistin, and leptin. Adipokines can function as hormones to control energy homeostasis and to stabilize endocrine function and also as cytokines to influence immune functions and inflammatory processes.1 Adiponectin, leptin, and resistin are thought to provide an important link between insulin resistance and related inflammatory disorders.2–6 Adiponectin and leptin function in a hormone-like manner and have many of the features of conventional cytokines, and there is escalating evidence that they are involved in many diseases and, under certain circumstances, might cross-regulate each other.1
Adiponectin is an adipokine with both pro- and anti-inflammatory effects on the immune system.7 It can not only inhibit adhesion molecules and the release of certain pro-inflammatory cytokines but can also program pro-inflammatory effects in macrophages and act as an enhancer of inflammation.8 Resistin is known to be induced in various inflammatory disorders and can exert potent pro-inflammatory effects.9 Studies have also revealed that leptin is an immune system regulator in addition to its effect on food intake.10,11 Leptin has a dual role in inflammation. It not only activates monocytes/macrophages to release pro-inflammatory cytokines, but can also exert an anti-inflammatory effect by its ability to induce IL-4 and IL-1Ra.12 Plasma levels of leptin can be altered in disease states associated with malnutrition.13
Low plasma leptin and high adiponectin levels are associated with wasting and inflammation in pulmonary tuberculosis (PTB).14,15 In addition, resistin has been postulated to serve as a surrogate biomarker for PTB.16 Because these adipokines are intimately associated with the disease process in PTB and are known to affect host immunity, we hypothesized that PTB would be associated with changes in systemic levels and that antituberculosis treatment (ATT) would modify the levels. Therefore, we examined circulating levels of adipokines in the plasma of individuals with PTB and compared them with latent tuberculosis (LTB) or healthy control (HC) individuals. We also determined the relationship of adipokines with the extent and severity of PTB. We also examined the changes in adipokine levels following standard ATT in PTB.
MATERIALS AND METHODS
Ethics statement.
All individuals were examined as part of a clinical protocol approved by the Institutional Review Board of the National Institute of Research in Tuberculosis (NCT01154959), and informed written consent was obtained from all participants.
Study population.
We studied a group of 44 individuals with active PTB, 44 individuals with LTB, and 44 HC individuals. The demographics of the study population are shown in Table 1. Individuals with pulmonary tuberculosis (TB) were diagnosed on the basis of positive culture for Mycobacterium tuberculosis (Mtb) on Lowenstein–Jensen medium. They were classified as having unilateral or bilateral lung disease and cavitary or non-cavitary disease based on radiological findings. Sputum smear grades were determined by sputum microscopy and graded as 0, 1+, 2+, and 3+, with zero being no bacteria in microscopy and 3+ the maximum number of bacteria. All PTB individuals were newly diagnosed, drug-sensitive patients and not re-treatment or multi-drug resistant cases, as determined by culture and drug sensitivity tests. Chest X-rays were read by two different radiologists and consenus opinion was obtained. Pretreatment samples were collected before the commencement of therapy. Standard ATT was administered to PTB individuals using the directly observed treatment, short course strategy. At 6 months following ATT initiation, fresh plasma samples were obtained. All PTB individuals were culture negative at the end of ATT. Latent infection was diagnosed on the basis of being positive in the Quantiferon-TB Gold in Tube (Cellestis) assay that measures the release of interferon (IFN) gamma after stimulation in vitro by Mtb antigens such as ESAT-6, CFP-10, and TB7.7. The result is described as quantification of IFNγ in international units (IUs) per mL. An individual is measured positive for Mtb infection if the IFNγ response to TB antigens is greater than the test cut-off (>0.35 IU after subtracting the background IFNγ response in the negative control), but with an absence of pulmonary symptoms coexisting with a normal chest radiograph. Healthy control individuals were Quantiferon-TB Gold in Tube negative, and had no pulmonary symptoms and a normal chest radiograph. All the individuals were HIV negative and nondiabetic and non-prediabetic. All individuals were antituberculous treatment naïve. Anthropometric measurements, including height, weight, and body mass index (BMI), and biochemical parameters, including plasma glucose and HbA1c, were obtained using standardized techniques as detailed elsewhere.17 Sample sizes were based on convenient sampling.
Table 1.
Demographics of the study population
| Study demographics | Pulmonary tuberculosis | Latent tuberculosis | Healthy control |
|---|---|---|---|
| No. of subjects recruited | 44 | 44 | 44 |
| Gender (male/female) | 36/8 | 22/22 | 19/25 |
| Median age (range) | 40 (19–54) | 36 (22–65) | 36 (21–58) |
| Median height, cm (range) | 163 (147–182) | 163 (146–175) | 162 (143–179) |
| Median weight, kg (range) | 45 (33–68) | 59 (37–80) | 61 (41–95) |
| Median body mass index (range) | 17 (13–22) | 23 (17–34) | 24 (16–37) |
Enzyme linked immunosorbent assay (ELISA).
Plasma was collected in heparin sodium tubes and after centrifugation stored at −80°C. Adipokines were measured using ELISA kits from R&D Systems. All assays were run in duplicates. The lower limits of detection were as follows: adiponectin, 3,654 pg/mL; leptin, 1,476 pg/mL; and resistin, 197 pg/mL.
Statistical analysis.
Geometric means (GM) were used for measurements of central tendency. Statistically significant differences between three groups were analyzed by the Kruskal–Wallis with Dunn’s post hoc test and between two groups were analyzed using the nonparametric Mann–Whitney U test with Holm’s correction for multiple comparisons. Correlations were calculated using Spearman rank correlation or using linear trend post hoc analysis. Changes following ATT were calculated using the Wilcoxon signed rank test. Analyses were performed using GraphPad PRISM version 5.01.
RESULTS
Diminished levels of adiponectin and leptin and elevated levels of resistin in PTB.
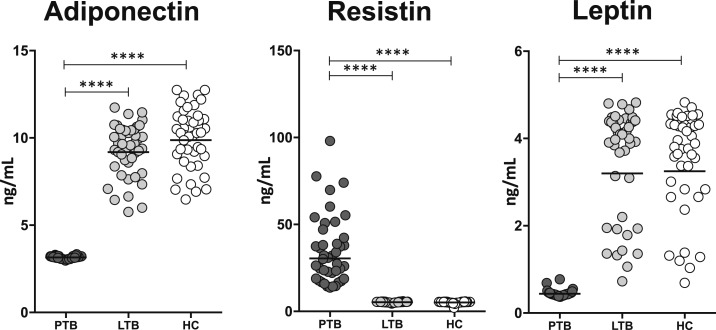
To determine the circulating levels of adipokines in PTB, we measured the circulating levels of adiponectin, resistin, and leptin in PTB and compared them with LTB and HC individuals. As shown in Figure 1, PTB individuals exhibited significantly diminished levels of adiponectin and leptin and significantly elevated levels of resistin in comparison to LTB and HC individuals. Thus, PTB is associated with modulation in the levels of adipokines.
Figure 1.
Altered systemic levels of adipokines in pulmonary tuberculosis (PTB). The plasma levels of adiponectin, resistin, and leptin were measured by using ELISA in PTB (N = 44), latent tuberculosis (LTB) (N = 44), and healthy control (HC) (N = 44) individuals. The data are represented as scatter plots with each circle representing a single individual and the line representing the geometric mean. P values were calculated using the Kruskal–Wallis test (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
Adipokine levels in PTB do not reflect disease extent or severity.
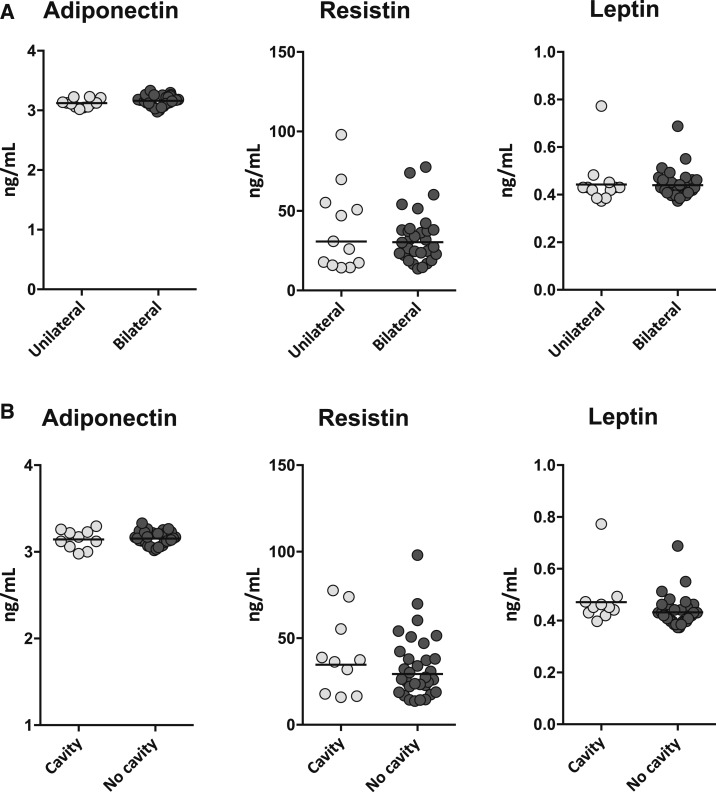
To determine the relationship of adipokines with disease extent in PTB, we measured the levels of adiponectin, resistin, and leptin in PTB individuals with unilateral lung disease and compared them with those with bilateral disease. As shown in Figure 2A, there were no significant differences in the levels of adiponectin, resistin, or leptin between the two groups of PTB individuals. To determine the relationship of adipokines with disease severity in PTB, we measured the levels of adiponectin, resistin, and leptin in PTB individuals with cavitary disease and compared them with those without cavitary disease. As shown in Figure 2B, there were no significant differences in the levels of adiponectin, resistin, or leptin between the two groups of PTB individuals. Thus, adipokine levels in PTB do not reflect disease extent or severity.
Figure 2.
Adipokines do not reflect disease extent or severity in pulmonary tuberculosis (PTB). (A) The plasma levels of adiponectin, resistin, and leptin were measured in PTB individuals with unilateral or bilateral lung involvement. (B) The plasma levels of adiponectin, resistin, and leptin were measured in PTB individuals with cavitary or non-cavitary disease. The data are represented as scatter plots with each circle representing a single individual and the line representing the geometric mean. P values were calculated using the Mann–Whitney U test.
Adipokine levels in PTB do not reflect bacterial burdens, but adiponectin exhibits a negative relationship with BMI.
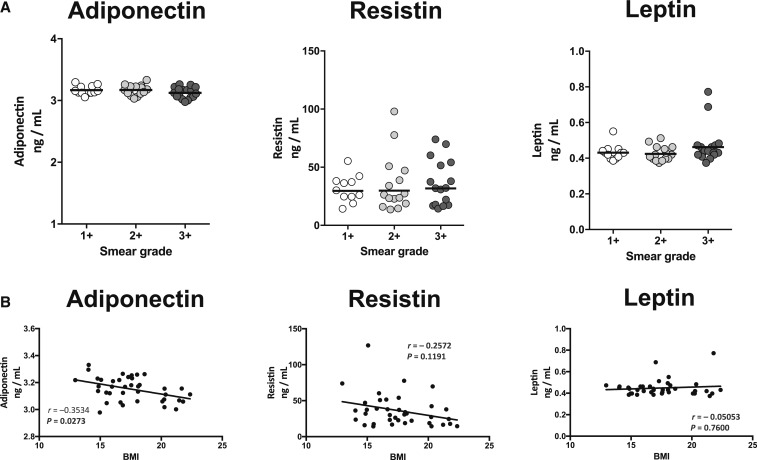
To determine the relationship of adipokines with bacterial burdens in PTB, we examined the correlation of the levels of adiponectin, resistin, and leptin in PTB individuals with smear grades as determined by sputum smear microscopy. As shown in Figure 3A, there were no significant correlations in the levels of adiponectin, resistin, or leptin with smear grades of PTB individuals. Thus, adipokine levels did not exhibit any significant association with bacterial burdens in PTB in our study.
Figure 3.
Relationship between adipokines and bacterial burden or body mass index (BMI). (A) Correlation between adipokines and bacterial burdens in pulmonary tuberculosis (PTB) individuals. (B) Correlation between adipokines and BMI levels in PTB individuals. The relationship between the plasma levels of adiponectin, resistin, and leptin and bacterial burdens or BMI was examined in PTB (N = 44) individuals. The data are represented as scatter plots with each circle representing a single individual and the line represents the linear curve fit. P values were calculated using the linear trend analysis or Spearman rank correlation.
To determine the relationship of adipokines with BMI in PTB, we examined the correlation of the levels of adiponectin, resistin, and leptin with BMI. As shown in Figure 3B, only adiponectin exhibited a significant negative correlation with BMI. Thus, adiponectin is negatively associated with BMI in PTB individuals.
Effect of ATT on adipokine levels in PTB.
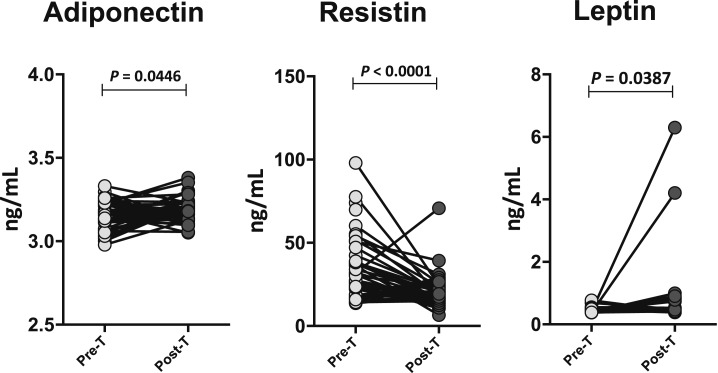
To determine the effect of ATT on adipokine levels in PTB, we measured the circulating levels of adiponectin, resistin, and leptin in PTB before (pre-T) and at the end of ATT (post-T). As shown in Figure 4, PTB individuals exhibited significantly increased levels of adiponectin and leptin and significantly decreased levels of resistin at post-T compared with pre-T values. Thus, ATT reverses (at least partially) the modulation of adipokines in PTB individuals.
Figure 4.
Changes in adipokine levels in pulmonary tuberculosis (PTB) following antituberculosis treatment (ATT). The plasma levels of adiponectin, resistin, and leptin in PTB (N = 44) individuals before (pre-T) and after (post-T) standard ATT. The data are represented as line diagrams with each line representing a single individual. P values were calculated using the Wilcoxon signed rank test.
DISCUSSION
Adiponectin is an adipokine secreted only by the adipose tissue and acts by improving insulin sensitivity and glucose metabolism.18 Adiponectin can suppress the secretion of type 1 cytokines and is a negative regulator of T cells.19 Previous studies have documented increased levels of adiponectin in PTB individuals and its association with low BMI.15,20 In addition, we have previously demonstrated modulation of adiponectin levels in TB—diabetes comorbidity. Our present study followed up on these observations and described the diminished levels of adiponectin in PTB individuals. However, these results are in contrast to previous reports and could potentially reflect the effect of gender or ethnicity of our population or other bacterial factors. Our study also demonstrated a lack of association between disease severity or extent and bacterial burdens with systemic adiponectin levels in these individuals, although larger sample sizes are needed to confirm this finding. In addition, we also corroborated the negative correlation between adiponectin and BMI in PTB individuals. Finally, our study also confirmed previous reports on the increased levels of adiponectin post-TB treatment.15
Resistin is an adipokine that is secreted by adipose tissue in mice and monocytes/macrophages in humans.18 It is implicated in glucose metabolism and gluconeogenesis.18 Resistin has also been found to be expressed at elevated levels in PTB individuals previously,21 and interestingly, the mRNA for resistin was part of a transcriptional signature to discriminate active TB from latent infection.22 Our data showed that resistin levels were indeed significantly increased in PTB individuals compared with the other two groups. However, like the other adipokines studied, systemic resistin levels did not exhibit any association with either disease severity/extent or bacterial burdens. In addition, resistin levels did not correlate with BMI in PTB individuals. Finally, similar to a previous report,21 resistin levels were significantly diminished following treatment of PTB individuals.
Leptin is an adipokine secreted predominantly by white adipose tissue and regulates energy intake, expenditure, and feeding behavior.18 It is also known to regulate storage of fat and insulin signaling.18 Previous studies have shown that leptin concentrations are low in PTB individuals20 and that low leptin levels are associated with wasting and weight loss.23 Our study provided further corroboration of this finding and demonstrated that leptin levels were significantly diminished in PTB individuals in comparison with both LTB and HC individuals. Our study also demonstrated that leptin levels in the circulation did not reflect either disease extent/severity or bacterial burden in PTB. There are conflicting reports on the relationship between leptin and BMI with one study showing a positive correlation20 and another study showing no significant correlation.24 Our study showed no significant relationship between leptin and BMI in our group of PTB individuals. Studies in animal models have shown an important role for leptin in host resistance against Mtb by the induction of Th1 cytokine responses.25 Our data on the diminished levels of leptin, therefore, suggest that decreased leptin levels could be a potential factor in the pathogenesis of TB disease in PTB individuals. Similarly, previous reports have also found that ATT is associated with increased systemic levels of leptin in PTB individuals.26–28 Our data confirmed and extended these results and demonstrated that successful completion of ATT (and consequent cure of TB) was associated with elevated levels of leptin.
We have performed a comprehensive evaluation of three adipokines in PTB individuals from South India. We have not performed multivariate analysis on our data and hence cannot rule out an effect of gender on the levels of adipokines in our study. Our study has several limitations including the small sample size, the lack of multivariate analysis, being descriptive, and not being able to account for all the confounding factors. However, our data reinforced previous findings and suggested an important association of adiponectin, resistin, and leptin with the pathogenesis of TB disease. Our data also clearly delineated an important effect of ATT on the restoration of normal levels of adipokines in PTB. Thus, our data further highlight the recent findings on the important nexus between TB and metabolic function in the host immune response. Future studies evaluating the role of these adipokines in modifying host immune responses could perhaps lead to important insights on the role of these cytokines in immunity to TB.
Acknowledgments:
We thank the staff of the Department of Clinical Research and the Department of Bacteriology, NIRT.
REFERENCES
- 1.Tilg H, Moschen AR, 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783. [DOI] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS, 2005. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calle EE, Kaaks R, 2004. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4: 579–591. [DOI] [PubMed] [Google Scholar]
- 4.La Cava A, Matarese G, 2004. The weight of leptin in immunity. Nat Rev Immunol 4: 371–379. [DOI] [PubMed] [Google Scholar]
- 5.Kusminski CM, McTernan PG, Kumar S, 2005. Role of resistin in obesity, insulin resistance and type II diabetes. Clin Sci (Lond) 109: 243–256. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW, Jr., 2006. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shehzad A, Iqbal W, Shehzad O, Lee YS, 2012. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens) 11: 8–20. [DOI] [PubMed] [Google Scholar]
- 8.Cheng X, Folco EJ, Shimizu K, Libby P, 2012. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem 287: 36896–36904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A, 2005. Resistin, an adipokine with potent proinflammatory properties. J Immunol 174: 5789–5795. [DOI] [PubMed] [Google Scholar]
- 10.Wiedmer P, Nogueiras R, Broglio F, D’Alessio D, Tschop MH, 2007. Ghrelin, obesity and diabetes. Nat Clin Pract Endocrinol Metab 3: 705–712. [DOI] [PubMed] [Google Scholar]
- 11.Leite-Moreira AF, Soares JB, 2007. Physiological, pathological and potential therapeutic roles of ghrelin. Drug Discov Today 12: 276–288. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C, Sanchez-Margalet V, 2010. Role of leptin in the activation of immune cells. Mediators Inflamm 2010: 568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Lee CT, Yoon HI, Song J, Shin WG, Lee JH, 2010. Relation of ghrelin, leptin and inflammatory markers to nutritional status in active pulmonary tuberculosis. Clin Nutr 29: 512–518. [DOI] [PubMed] [Google Scholar]
- 14.van Crevel R, Karyadi E, Netea MG, Verhoef H, Nelwan RH, West CE, van der Meer JW, 2002. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab 87: 758–763. [DOI] [PubMed] [Google Scholar]
- 15.Keicho N, Matsushita I, Tanaka T, Shimbo T, Hang NT, Sakurada S, Kobayashi N, Hijikata M, Thuong PH, Lien LT, 2012. Circulating levels of adiponectin, leptin, fetuin-A and retinol-binding protein in patients with tuberculosis: markers of metabolism and inflammation. PLoS One 7: e38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehtesham NZ, Nasiruddin M, Alvi A, Kumar BK, Ahmed N, Peri S, Murthy KJ, Hasnain SE, 2011. Treatment end point determinants for pulmonary tuberculosis: human resistin as a surrogate biomarker. Tuberculosis (Edinb) 91: 293–299. [DOI] [PubMed] [Google Scholar]
- 17.Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, Mohan V, 2003. The Chennai Urban Rural Epidemiology Study (CURES)–study design and methodology (urban component) (CURES-I). J Assoc Physicians India 51: 863–870. [PubMed] [Google Scholar]
- 18.Makki K, Froguel P, Wolowczuk I, 2013. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013: 139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang JY, Li D, Ho D, Peng J, Xu A, Lamb J, Chen Y, Tam PK, 2011. Novel immunomodulatory effects of adiponectin on dendritic cell functions. Int Immunopharmacol 11: 604–609. [DOI] [PubMed] [Google Scholar]
- 20.Santucci N, D’Attilio L, Kovalevski L, Bozza V, Besedovsky H, del Rey A, Bay ML, Bottasso O, 2011. A multifaceted analysis of immune-endocrine-metabolic alterations in patients with pulmonary tuberculosis. PLoS One 6: e26363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang SW, Pan WS, Lozano Beltran D, Oleyda Baldelomar L, Solano MA, Tuero I, Friedland JS, Torrico F, Gilman RH, 2013. Gut hormones, appetite suppression and cachexia in patients with pulmonary TB. PLoS One 8: e54564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan L, et al. 2017. Genome-wide transcriptional profiling identifies potential signatures in discriminating active tuberculosis from latent infection. Oncotarget 8: 112907–112916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Lettow M, van der Meer JW, West CE, van Crevel R, Semba RD, 2005. Interleukin-6 and human immunodeficiency virus load, but not plasma leptin concentration, predict anorexia and wasting in adults with pulmonary tuberculosis in Malawi. J Clin Endocrinol Metab 90: 4771–4776. [DOI] [PubMed] [Google Scholar]
- 24.Yurt S, Erman H, Korkmaz GG, Kosar AF, Uysal P, Gelisgen R, Simsek G, Uzun H, 2013. The role of feed regulating peptides on weight loss in patients with pulmonary tuberculosis. Clin Biochem 46: 40–44. [DOI] [PubMed] [Google Scholar]
- 25.Wieland CW, Florquin S, Chan ED, Leemans JC, Weijer S, Verbon A, Fantuzzi G, van der Poll T, 2005. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol 17: 1399–1408. [DOI] [PubMed] [Google Scholar]
- 26.Perna V, Perez-Perez A, Fernandez-Riejos P, Polo-Padillo J, Batista N, Dominguez-Castellano A, Sanchez-Margalet V, 2013. Effective treatment of pulmonary tuberculosis restores plasma leptin levels. Eur Cytokine Netw 24: 157–161. [DOI] [PubMed] [Google Scholar]
- 27.Yuksel I, Sencan M, Dokmetas HS, Dokmetas I, Ataseven H, Yonem O, 2003. The relation between serum leptin levels and body fat mass in patients with active lung tuberculosis. Endocr Res 29: 257–264. [DOI] [PubMed] [Google Scholar]
- 28.Mexitalia M, Dewi YO, Pramono A, Anam MS, 2017. Effect of tuberculosis treatment on leptin levels, weight gain, and percentage body fat in Indonesian children. Korean J Pediatr 60: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]