Abstract.
Among pyogenic liver abscesses, melioid etiology is considered in endemic regions in the presence of known health or occupational risk factors. “Honeycomb sign,” used to describe an abscess with multiple internal septations dividing the abscess cavity into multiple loculations of comparable sizes on imaging, is a sensitive sign for melioid liver abscess. This is a retrospective case–control study investigating incidence, sensitivity, and specificity of “honeycomb sign” in melioid liver abscess, in a cohort of patients with culture-proven melioidosis infection. Abscesses ≥ 2 cm were analyzed for the honeycomb sign. P value < 0.05 was taken as statistically significant. Interobserver agreement was calculated between two radiologists for the presence of the sign, sensitivity, and specificity. A total of 40 abscesses were analyzed. Thirty-four abscesses (85%) manifested the honeycomb sign with interobserver agreement (kappa = 0.70 and 0.92). Sensitivity of the sign is 85% (95% confidence interval [CI]: 70–94%), specificity is 75% (95% CI: 59–87%), positive predictive value is 77% (95% CI: 62–88%), and negative predictive value is 83% (95% CI: 67–94%). If abscess size is ≥ 3 cm, the sensitivity is 91% (95% CI: 77–98%), specificity is 75% (95% CI: 59–87%), positive predictive value is 76% (95% CI: 61–88%), and negative predictive value is 91% (95% CI: 76–98%). Honeycomb sign is a novel imaging marker for melioid liver abscess. Increased awareness and recognition of this imaging feature has the potential to affect patient management.
INTRODUCTION
Pyogenic liver abscesses are the most common intra-abdominal visceral abscesses.1,2 The routes of infection are via the portal circulation, biliary tract, or by arterial hematological seeding. The common causative pyogenic organisms are Escherichia coli, Staphylococcus sp., Streptococcus sp., Klebsiella sp., and parasitic Entamoeba hystolytica.2 Burkholderia pseudomallei liver abscess is to be considered in endemic regions and in the presence of known health or occupational risk factors. The known risk factors are diabetes mellitus, male gender, excessive alcohol consumption, chronic diseases such as thalassemia and chronic lung disease, and other debilitating illnesses. Occupational risk factors include those with soil and/or water exposure such as farmers.3 Pyogenic liver abscesses respond to standard antibiotic therapy, whereas melioidosis need specific antibiotics both intravenous and oral for a long duration to completely eliminate the infection from the bloodstream.4–6 Imaging plays a major role in diagnosis, evaluation of extent of infection, and evaluation of complications such as abscess rupture with peritonitis or vascular thrombosis. Ultrasound and contrast-enhanced computed tomography are the appropriate imaging modalities of choice. The role of ultrasound also includes monitoring disease resolution.7 Most microbiological laboratories misidentify or underreport B. pseudomallei by routinely used laboratory facilities. There is a need for alertness to the clinical entity and further, once isolated, specific identification of the organism can take more than 48 hours in the laboratory.3 Numerous advances in ultrasonogram and computed tomography make medical imaging a contender for a diagnostic tool,8 thereby bringing to attention, its potential in early diagnosis and initiation of appropriate and effective antibiotics. In addition, imaging of liver abscess is an easy-to-perform test and does not require sophisticated training to read the images. The limitations of imaging, however, is that no one imaging feature may be specific for a particular infective organism.9 The “honeycomb sign” has been described in melioid liver abscess10–12; the sign describes an abscess in the liver with multiple internal thin septations dividing the abscess cavity into small loculations of comparable sizes. It has also been used in Klebsiella sp. or E. coli liver abscess13,14 as well as in a few of tuberculous liver abscesses15,16 to describe conglomerate of small abscesses in various stages of coalescence. In the following study, we have looked at the incidence of the “honeycomb sign” in a discrete abscess as a diagnostic marker for melioid liver abscess in a cohort of culture-proven cases and the sensitivity and specificity of the sign against a cohort of controls. To our knowledge, this is the largest number of melioid liver abscesses to have been analyzed for the sign.
MATERIALS AND METHODS
After obtaining approval from the institutional review board, we retrospectively analyzed the imaging of 189 consecutive patients, over a period of 6 years, with blood, pus, or tissue culture–proven B. pseudomallei infection, using images stored on the picture archiving and communication system. Informed consent was waived on account of study data being obtained from preexisting clinical, laboratory, and radiology electronic records alone.
Twenty-three of 189 patients with melioidosis had liver abscesses. We went through imaging of each of these patients and recorded the number of abscess, size, and the presence of the sign and other characteristics such as calcification or complications such as rupture or thrombosis of regional vessels. Computed tomography scan had been performed for 17 patients in three different scanners available at our institution (Discovery CT750 HD [GE Healthcare, Milwaukee, WI], in eight patients, Phillips Brilliance 6 [Phillips Healthcare, Eindhoven, Netherlands] in seven patients, and Somatom Emotion 16 [Siemens AG, Erlangen, Germany] in two patients) and ultrasound had been performed for six patients in two different machines available at our institution (Toshiba Xario 100 Platinum series [Toshiba Medical System, Japan] in five patients and Siemens Acuson S2000TM ultrasound system [Siemens AG, Erlangen, Germany] in one patient). Six patients had predominant infection in the lungs and spleen with very small liver abscesses measuring less than 2 cm, so these cases were not further analyzed. Based on observations by Apisarnthanarak et al.,11 abscesses measuring more than or equal to 2 cm, a total number of 40 abscesses, were included in the study for analysis.
The presence of the honeycomb sign was defined as a discrete abscess focus in the liver with or without a definite wall, with obvious subjective and visually assessed, multiple small loculations of comparable sizes giving a heterogeneous appearance of hyperdensity interspersed with hypodense areas.11,17
A year-wise list of patients with liver abscess of different pyogenic etiology, presenting to the hospital at the same period as the study population, was generated. Systematic random sampling was carried out by going through the first 10 cases in the list of each year, starting with the later year, progressing to the earliest year, and repeating in a circular manner at the end of the list for case and control population ratio of 1:1. Forty patients with one liver abscess documented on imaging, microbiological culture–proven pathogen, and age matched for our study population were included in the control group. The presence of multiloculations in the abscesses giving appearance of honeycomb sign was noted.
Two radiologists, blinded to the microbiological diagnosis of the liver abscess, participated to test the interobserver variability. The predefined honeycomb sign definition and a folder consisting of randomly arranged imaging of cases and controls were presented to reader 1 and 2 who then delivered their results independent of one another.
Statistical analysis.
Data are presented as mean with standard deviations where appropriate. Categorical data are presented with number and percentage. Sensitivity and specificity were calculated for the diagnostic performance of the honeycomb sign for melioid liver abscess. Interobserver agreement between two radiologists was assessed using kappa coefficient. The guidelines of Landis and Koch were followed for the interpretation of these values: 0.00–0.20, indicating slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.00, almost perfect agreement.18 A P value of < 0.05 was considered as significant. All analyses were performed using the SAS package (version 9.4; SAS® Institute Inc., Cary, NC) for statistical evaluation.
RESULTS
A total of 23 patients (12%) were found to have liver involvement of melioidosis. Six patients with liver abscess size less than 2 cm were not further analyzed. Seventeen patients had liver abscess measuring more than 2 cm (74%). Of these 17 patients, eight (47%) had multiple abscesses and nine (53%) had solitary abscess in the liver. A total of 40 melioid liver abscesses were analyzed. Summary of demography, clinical characteristics and organs where the infection has disseminated in the cohort of patients with melioid liver abscess (N = 17), and relevant details of non-melioid liver abscess (N = 40) are depicted in Table 1.
Table 1.
Summary of demography and other characteristics of cases (n = 17) and controls (n = 40)
| Characteristics | Case n (%) | Control n (%) | Total n (%) |
|---|---|---|---|
| Gender | |||
| Male | 15 (88) | 32 (80) | 47 (83) |
| Female | 2 (12) | 8 (20) | 10 (17) |
| Age (years) | |||
| Mean | 47 | 47.8 | 47 |
| Standard deviation | 12.1 | 13 | 13 |
| Culture sample positive for Burkholderia pseudomallei in the case cohort: | |||
| Blood culture | 9 (53) | – | 9 (53) |
| Liver abscess pus | 6 (35) | – | 6 (35) |
| Blood culture and liver abscess pus | 1 (6) | – | 1 (6) |
| Pus from other site of infection | 1 (6) | – | 1 (6) |
| Type of disease at presentation: | |||
| Localized disease | 1 (6) | – | – |
| Disseminated disease | 16 (94) | – | – |
| Associated organ involvement in the cases (mutually inclusive): | |||
| Splenic abscess | 16 (94) | – | – |
| Pancreatitis | 1 (6) | – | – |
| Genitourinary tract (renal abscess) | 2 (12) | – | – |
| Musculoskeletal involvement (osteomyelitis, arthritis, and intramuscular abscess) | 7 (41) | – | – |
| Free fluid | 4 (24) | – | – |
| Organism identified from liver pus culture in the control cohort: | |||
| Escherichia sp. | – | 9 (23) | 9 (23) |
| Klebsiella sp. | – | 7 (18) | 7 (18) |
| Staphylococcus sp. | – | 5 (29) | 5 (29) |
| Nonfermenting GNB | – | 4 (24) | 4 (24) |
| Escherichia sp. and Enterococcus sp. | – | 4 (24) | 4 (24) |
| Streptococcus sp. | – | 3 (8) | 3 (8) |
| Escherichia sp., Klebsiella sp., and Enterococcus sp. | – | 2 (5) | 2 (5) |
| Escherichia sp., Enterococcus sp., and Citrobacter sp., and Pseudomonas sp. | – | 1 (3) | 1 (3) |
| Escherichia sp. and Citrobacter sp. | – | 1 (3) | 1 (3) |
| Escherichia sp. and Klebsiella sp. | – | 1 (3) | 1 (3) |
| Klebsiella sp. and Staphylococcus sp. | – | 1 (3) | 1 (3) |
| Nonfermenting gram negative bacteria and Enterococcus sp. | – | 1 (3) | 1 (3) |
| Morganella sp. and Proteus sp. | – | 1 (3) | 1 (3) |
| Imaging modality: | |||
| Contrast-enhanced CT | 13 (77) | 35 (87) | 48 (84) |
| Ultrasonography | 4 (23) | 5 (13) | 9 (16) |
| Size distribution of the abscess in cm | |||
| Mean | 4 | 8 | 6 |
| SD | 2.6 | 2.7 | 3.1 |
| Clinical presentation:28 | |||
| Acute (≤ 2 months) | 8 (47) | – | – |
| Chronic (> 2 months) | 9 (53) | – | – |
| Associated risk factors (mutually inclusive):3 | |||
| Diabetes mellitus | 14 (82) | – | – |
| Alcohol dependence | 5 (29) | – | – |
| Urolithiasis | 1 (5) | – | – |
Thirty-four of 40 of the evaluated melioid abscesses (85%) manifested the honeycomb sign. Twenty-nine of these abscesses (85%) manifested the sign on contrast-enhanced CT, 1 (3%) on plain CT, and 4 (12%) on ultrasonography. The honeycomb sign in different patients is depicted in Figure 1. Ten of 40 (25%) of the non-melioid abscesses manifested multiloculations with appearance of honeycomb sign.
Figure 1.
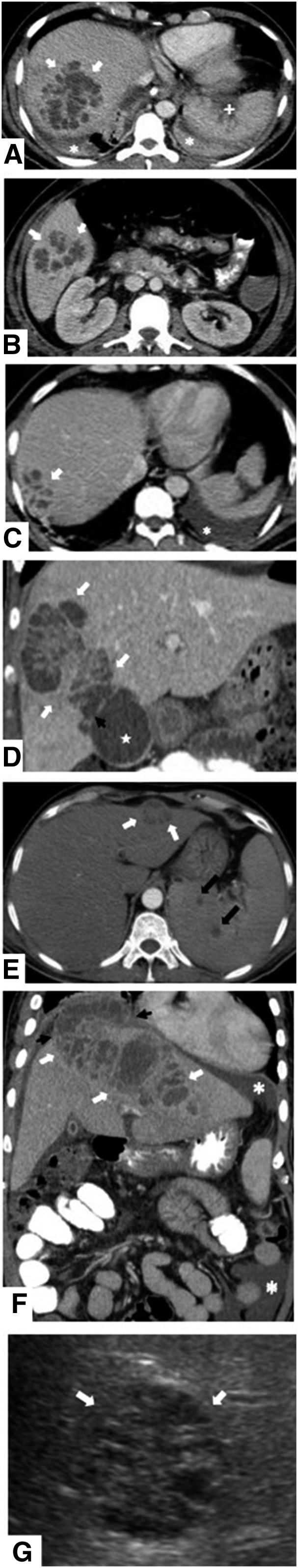
(A–G) The appearance of honeycomb liver abscess in different patients along with visualized organs of dissemination.
The kappa interobserver agreement for the presence of honeycomb sign in melioid liver abscess between two radiologists was found to be of almost perfect agreement (kappa = 0.92). Reader 1 and 2 disagreed on one melioid liver abscess on CT scan. Although reader 1 interpretation of the abscess was of a multiloculated abscess with thick septations qualifying a honeycomb sign, reader 2 has interpreted it as an abscess cavity with solid components within, thereby disqualifying the presence of honeycomb sign. For the control group, there was substantial agreement (kappa = 0.70). There were disagreements in five abscesses. Although reader 1 has considered the presence of multiple septations within the abscess dividing the abscess cavity into large loculations as not representative of honeycomb sign, reader 2 has considered the presence of any septations dividing the abscess cavity into multiloculations, regardless of size of the loculations, as honeycomb sign.
The presence of the honeycomb sign was found to be significantly associated with melioid liver abscess (P < 0.001). The sensitivity of honeycomb sign in diagnosis of melioid liver abscess was calculated to be 85% (95% CI: 70–94%) and specificity was 75% (95% CI: 59–87%). The positive predictive value of the sign was 77% (95% CI: 62–88%) and the negative predictive value of the sign was 83% (95% CI: 67–94%). In abscesses measuring more than 3 cm in size, the sensitivity was 91% (95% CI: 77–98%), specificity was 75% (95% CI: 59–87%), with a positive predictive value of 76% (95% CI: 61–88%), and the negative predictive value of 91% (95% CI: 76–98%).
Other imaging characteristics in the melioid liver abscess.
Among the 34 abscesses with honeycomb sign, 82% (N = 28) involved the right lobe of the liver, 15% (N = 5) involved the left lobe, and one large abscess extended across to involve both lobes. Abscesses of larger size were more likely to occur in the right lobe of the liver than in the left. The largest abscess manifesting the sign measured 15 cm in largest diameter. The smallest abscess manifesting the sign measured 2 cm. Five abscesses showed signs of rupture at the time of diagnosis. Of the five abscesses, four measured more than 5 cm and one measured 4 cm and was in the subcapsular location. In three patients, regional vein thrombosis was noted involving the portal system. The abscesses in all three measured more than 5 cm. None of the abscesses showed calcification. Eight patients with abscesses manifesting the honeycomb sign also had discrete very small (< 1 cm) abscesses in varying numbers (one to numerous).
Course of illness and follow-up.
Sixteen patients made uneventful recovery. Four patients required ventilation and inotropes in the intensive care unit. One of the patients succumbed to overwhelming infection. This patient also had accompanying vascular thrombosis and numerous smaller discrete abscesses in the liver.
Follow-up scan with ultrasonography for a total of 12 patients after treatment initiation was available. The shortest interval between treatment initiation and follow-up was 10 days and the longest was 1,095 days (Table 2).
Table 2.
Follow-up by ultrasonography
| S. No. | Size of abscess in maximum dimension at the time of diagnosis (cm) | Interval time at follow-up | Management | Outcome of liver abscess on ultrasonogram follow-up |
|---|---|---|---|---|
| 1 | 10 | 10 days | Medical | 8 cm; minimal interval decrease in size with honeycomb sign |
| 2 | 2.5 | 1 month | Medical | Resolved |
| 3 | 5 | 1 month | Aspiration and medical | Resolved |
| 4 | 9 | 1 month | Drainage and medical | Resolved |
| 5 | 4 | 1.5 months | Aspiration and medical | Resolved |
| 6 | 7 | 2 months | Medical | Resolved |
| 7 | 9 | 3 months | Medical | Resolved |
| 8 | 15 | 6 months | Aspiration and medical | Resolved |
| 9 | 4 | 7 months | Medical | Resolved |
| 10 | 4 | 9 months | Medical | Resolved |
| 11 | 4 | 12 months | Aspiration and medical | Resolved |
| 12 | 3 | 3 years | Medical | Resolved |
DISCUSSION
The role of imaging, apart from identification of the presence of abscess in the liver, can potentially be extended to help in early appropriate treatment of melioidosis by identifying the causative organism with the help of the honeycomb sign before microbiological confirmation as delayed or ineffective treatment can lead to fatal outcome.19–21 The treatment of melioidosis differs from other pyogenic infections in that an intensive phase of treatment with effective antibiotics lasting for 10 days or more followed by an eradication phase of at least 12 weeks, potentially 20 weeks is required to completely eliminate the organism from the bloodstream, thus avoiding relapses.22 Effective antibiotics for intensive therapy include parenteral ceftazidime, amoxicillin–clavulanic acid, or meropenem; for the eradication phase, oral trimethoprim–sulfamethoxazole is used.22,23 The utilization of the honeycomb sign in a case of suspected melioid liver abscess would help to initiate treatment earlier and before clinical specimen cultures becoming positive, resulting in avoiding unnecessary ineffective antimicrobial administration, probable dissemination, or septicemia, thus improving overall disease outcome and possibly even overall treatment expenditure.
We have demonstrated that the honeycomb sign has a strong kappa interobserver agreement between two radiologists. Overall, interobserver variations can be improved by paying attention to the size of the multiloculations within the abscess. Usually, the multiloculations are small in size and more or less of equal size in the honeycomb sign. The honeycomb sign was found diagnostic for melioid liver abscess size of more than or equal to 2 cm with 85% sensitivity and 75% specificity. In abscesses measuring more than 3 cm in size, the sensitivity and negative predictive value increase to 91%. We also found that majority of the melioid liver abscess measured more than 3 cm in our cohort of patients, mean being 4 cm. This finding increases the significance of the sign for larger abscesses. For abscesses measuring less than 2 cm in size, the challenge in differentiating it from any other pyogenic abscess could be resolved with the help of accompanying imaging spectrum involving other organs such as the spleen4,10 and image-guided aspiration for microbiological culture. A non-pyogenic infective etiology of liver lesion with multilocular cysts within, an imaging differential for melioid liver abscess, is a hydatid cyst with multiple daughter vesicles (World Health Organisation classification cystic echinococcosis 2).24,25 On imaging, multiple daughter cysts are arranged in the periphery of the mother cyst with intervening hydatid matrix in between.26 Presenting symptoms may be mass effect–related, allergic, incidental discovery, or complications such as rupture or superadded bacterial infection of the cyst.27 Imaging wise, an important point to differentiate the two conditions is that 85–90% of hydatidosis have single organ involvement,27 which is contrary to melioidosis, where disseminated disease is seen in 94% of our patient population.
In our cohort of patients, contrast-enhanced CT scan had been the more popular modality of imaging as the patients had clinical presentation of multisystem involvement, and a one-time imaging of the chest and abdomen with computed tomography was considered more appropriate than evaluation of the abdomen by ultrasound alone. In a single case, liver abscess was an isolated manifestation of the infection. Disseminated infection with coexisting abscesses in the spleen and lung was predominant in our patient population; this proportion is in consensus with previous observations.22,23 Findings in our cohort suggest that the route of infection in liver abscess is mostly by arterial hematological seeding. Most of the liver abscesses were found to be located in the right lobe of the liver which is larger and receive more blood supply than the left lobe and the caudate lobe; this is in consensus with previous observations about melioid liver abscess and other pyogenic liver abscesses.2,10,11,24 Our study agrees with previous observations in the discrete distribution of melioid liver abscesses.10,11 The mean size of melioid liver abscess in our cohort was, however, larger than said previous observations.
Of the 17 patients, one patient with disseminated disease had extensive liver involvement and portal vein thrombosis, succumbing to overwhelming infection while in the hospital. Follow-up imaging with ultrasonography was available for 12 of the remaining patients with a mean duration of 17 weeks after treatment initiation, documenting resolved or resolving abscesses.
Tuberculosis and melioidosis are endemic in southeastern countries and can mimic one another when presenting as chronic disease.25,26 Points that favor melioid liver abscesses are the presence of honeycomb sign in the liver and splenic abscess involvement.10 Typical CT features of hepatic tuberculosis are multiple lesions in different pathologic stages including tuberculous granuloma, liquefaction necrosis, fibrosis, or calcification.27 Calcification was not seen in any of our patients.
The limitation of this study was in its retrospective nature. We were not able to demonstrate typical previously described enhancement pattern of melioid liver abscess because of the absence of standardization in scan protocol. Tuberculoid liver abscess, an important differential diagnosis, is very rare and imaging features differentiating one from the other could not be addressed in our study. Although ideal follow-up imaging would be after treatment completion, many of the follow-up ultrasounds in our patient populations were performed while still on treatment. Studies comparing imaging findings of liver abscesses of various causative organisms in a prospective manner may help to further strengthen the role of this important sign.
CONCLUSION
In imaging of liver abscess, the presence of the honeycomb sign is 85% sensitive and 75% specific in the diagnosis of melioid etiology with strong interobserver agreement as seen in our cohort of patients. The sensitivity of the test increases for abscesses measuring more than or equal to 3 cm. The described sign, therefore, serves as an important imaging marker. Increased awareness of this imaging feature has the potential to alert the radiologist and treating physician in early initiation of appropriate antibiotics.
Acknowledgments:
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.Mohsen AH, Green ST, Read RC, McKendrick MW, 2002. Liver abscess in adults: ten years experience in a UK centre. QJM 95: 797–802. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh S, Sharma S, Gadpayle AK, Gupta HK, Mahajan RK, Sahoo R, Kumar N, 2014. Clinical, laboratory, and management profile in patients of liver abscess from northern India. J Trop Med 2014: 142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng AC, Currie BJ, 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18: 383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White N, 2003. Melioidosis. Lancet 361: 1715–1722. [DOI] [PubMed] [Google Scholar]
- 5.Pal P, Ray S, Moulick A, Dey S, Jana A, Banerjee A, 2014. Liver abscess caused by Burkholderia pseudomallei in a young man: a case report and review of literature. World J Clin Cases 2: 604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhya A, Balaji V, Jesudason MV, Amte A, Jeyamani R, Kurian G, 2007. Isolated liver abscesses in melioidosis. Indian J Med Microbiol 25: 150–151. [DOI] [PubMed] [Google Scholar]
- 7.Huang CJ, Pitt HA, Lipsett PA, Osterman FA, Lillemoe KD, Cameron JL, Zuidema GD, 1996. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg 223: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thrall JH, 2016. Trends and developments shaping the future of diagnostic medical imaging: 2015 annual oration in diagnostic radiology. Radiology 279: 660–666. [DOI] [PubMed] [Google Scholar]
- 9.Goldman L, 2012. Goldman’s Cecil Medicine: Expert Consult Premium Edition 24 Barnes & Noble. Available at: https://www.barnesandnoble.com/w/goldmans-cecil-medicine-lee-goldman/1101445576?type=eBook. Accessed March 28, 2018.
- 10.Laopaiboon V, Chamadol N, Buttham H, Sukeepaisarnjareon W, 2009. CT findings of liver and splenic abscesses in melioidosis: comparison with those in non-melioidosis. J Med Assoc Thai 92: 1476–1484. [PubMed] [Google Scholar]
- 11.Apisarnthanarak A, Apisarnthanarak P, Mundy LM, 2006. Computed tomography characteristics of Burkholderia pseudomallei liver abscess. Clin Infect Dis 42: 989–993. [DOI] [PubMed] [Google Scholar]
- 12.Ong SCL, Alemam MMM, Zakaria NA, Abdul Halim NA, 2017. Honeycomb and necklace signs in liver abscesses secondary to melioidosis. BMJ Case Rep 2017: 222342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong VH, 2006. Is a honeycomb appearance on computer tomography characteristic for Burkholderia pseudomallei liver abscess? Clin Infect Dis 43: 265–266. [DOI] [PubMed] [Google Scholar]
- 14.Mori N, Murakami K, 2017. Honeycomb liver abscess. IDCases 8: 66–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaosmanoglu AD, Onur MR, Sahani DV, Tabari A, Karcaaltincaba M, 2016. Hepatobiliary tuberculosis: imaging findings. AJR Am J Roentgenol 207: 694–704. [DOI] [PubMed] [Google Scholar]
- 16.Kakkar C, Polnaya AM, Koteshwara P, Smiti S, Rajagopal KV, Arora A, 2015. Hepatic tuberculosis: a multimodality imaging review. Insights Imaging 6: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muttarak M, Peh WC, Euathrongchit J, Lin SE, Tan AG, Lerttumnongtum P, Sivasomboon C, 2009. Spectrum of imaging findings in melioidosis. Br J Radiol 82: 514–521. [DOI] [PubMed] [Google Scholar]
- 18.Viera AJ, Garrett JM, 2005. Understanding interobserver agreement: the kappa statistic. Fam Med 37: 360–363. [PubMed] [Google Scholar]
- 19.Princess I, Ebenezer R, Ramakrishnan N, Daniel AK, Nandini S, Thirunarayan MA, 2017. Melioidosis: an emerging infection with fatal outcomes. Indian J Crit Care Med 21: 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young A, Tacon C, Smith S, Reeves B, Wiseman G, Hanson J, 2017. Case report: fatal pediatric melioidosis despite optimal intensive care. Am J Trop Med Hyg 97: 1691–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munckhof WJ, Mayo MJ, Scott I, Currie BJ, 2001. Fatal human melioidosis acquired in a subtropical Australian city. Am J Trop Med Hyg 65: 325–328. [DOI] [PubMed] [Google Scholar]
- 22.Dance D, 2014. Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents 43: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweizer HP, 2012. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 7: 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apisarnthanarak P, Thairatananon A, Muangsomboon K, Lu DS, Mundy LM, Apisarnthanarak A, 2011. Computed tomography characteristics of hepatic and splenic abscesses associated with melioidosis: a 7-year study. J Med Imaging Radiat Oncol 55: 176–182. [DOI] [PubMed] [Google Scholar]
- 25.Kjossev KT, Losanoff JE, 2005. Classification of hydatid liver cysts. J Gastroenterol Hepatol 20: 352–359. [DOI] [PubMed] [Google Scholar]
- 26.Pedrosa I, Saíz A, Arrazola J, Ferreirós J, Pedrosa CS, 2000. Hydatid disease: radiologic and pathologic features and complications. RadioGraphics 20: 795–817. [DOI] [PubMed] [Google Scholar]
- 27.Duman K, Girgin M, Hamcan S, 2016. Uncomplicated hydatid cysts of the liver: clinical presentation, diagnosis and treatment. J Gastrointest Dig Syst 6: 430. [Google Scholar]
- 28.Zueter A, Yean CY, Abumarzouq M, Rahman ZA, Deris ZZ, Harun A, 2016. The epidemiology and clinical spectrum of melioidosis in a teaching hospital in a north-eastern state of Malaysia: a fifteen-year review. BMC Infect Dis 16: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]


