Abstract
Background
To update vertebral augmentation literature by comparing outcomes between vertebroplasty (VP), balloon kyphoplasty (BKP), vertebral augmentation with implant (VAI), and nonsurgical management (NSM) for treating vertebral compression fractures (VCFs).
Methods
A PubMed literature search was conducted with keywords kyphoplasty, vertebroplasty, vertebral body stent, and vertebral augmentation AND implant for English-language articles from February 1, 2011, to November 22, 2016. Among the results, 25 met the inclusion criteria for the meta-analysis. Inclusion criteria were prospective comparative studies for mid-/lower-thoracic and lumbar VCFs enrolling at least 20 patients. Exclusion criteria included studies that were single arm, systematic reviews and meta-analyses, traumatic nonosteoporotic or cancer-related fractures, lack of clinical outcomes, or non–Level I and non–Level II studies. Standardized mean difference between baseline and end point for each outcome was calculated, and treatment groups were pooled using random effects meta-analysis.
Results
Visual analog scale pain reduction for BKP and VP was −4.05 and −3.88, respectively. VP was better than but not significantly different from NSM (−2.66), yet BKP showed significant improvement from both NSM and VAI (−2.77). The Oswestry Disability Index reduction for BKP showed a significant improvement over VAI (P < .001). There was no significant difference in changes between BKP and VP for anterior (P = .226) and posterior (P = .293) vertebral height restoration. There was no significant difference in subsequent fractures following BKP (32.7%; 95% confidence interval [CI]: 8.8%–56.6%) or VP (28.3%; 95% CI: 7.0%–49.7%) compared with NSM (15.9%; 95% CI: 5.2%–26.6%).
Conclusions/Level of Evidence
Based on Level I and II studies, BKP had significantly better and VP tended to have better pain reduction compared with NSM. BKP tended to have better height restoration than VP. Additionally, BKP had significant improvements in pain reduction and disability score as compared with VAI.
Clinical Relevance
This meta-analysis serves to further define and support the safety and efficacy of vertebral augmentation.
Keywords: meta-analysis, vertebral augmentation, vertebral compression fractures, vertebroplasty, balloon kyphoplasty
INTRODUCTION
Vertebral compression fractures (VCFs) are costly and are becoming even more common as more than 10 000 Americans turn 65 years old each day. In the United States, there are 1.5 million VCFs annually, and worldwide a vertebral fracture occurs every 22 seconds.1–3 Symptomatic fractures usually present with sudden onset of back pain and functional debilitation in an elderly patient with osteoporosis, though many fractures may be asymptomatic. VCFs are expensive to treat, costing around $17 billion per year.4,5 Morbidities associated with VCFs are substantial and can result in permanent loss of mobility and quality of life and lead to substantial disability.6
In addition, the deconditioning that affects patients with VCFs leads to mortality at a far higher rate than in age-matched controls.7,8 Increased mortality associated with VCFs has been well established for quite some time, but effects on mortality when patients undergo treatment with vertebral augmentation has only been described recently.9–12 Edidin et al13 reported significant reduction in morbidity and mortality in over a million patients with VCFs treated with vertebral augmentation as compared with patients treated with nonsurgical management (NSM).
Vertebral augmentation, including kyphoplasty and vertebroplasty (VP), have been accepted treatments for VCFs for decades. Balloon kyphoplasty (BKP) has had a large body of data supporting its use since receiving 510(k) clearance in 1998. More recently, interventionalists have started using implants and vertebral body stenting (VBS) in vertebral augmentation. One such implant is Kiva (Benvenue Medical, Inc, Santa Clara, California), a polyetheretherketone implant placed over a nitinol wire. Kiva was approved by the US Food and Drug Administration in 2014 after a randomized controlled trial (RCT) showed noninferiority to BKP, while maintaining an optimal safety profile and significantly improving patients' pain and function.16 VBS uses an expandable metal stent to restore vertebral height and currently is only available in Europe. Level I evidence concludes that although VBS is noninferior to BKP, in terms of patient outcomes, it is associated with a higher number of material related complications.15 The SpineJack (Vexim, SA, Balma, France), CE marked for use in Europe and other countries around the world, showed superiority to BKP in restoring VCF heights in a single-center trial16 and in a cadaver study.17 SpineJack is currently undergoing a comparison study with BKP in Europe and may be approved for use in the United States as early as 2018.
In 2009, 5 major societies developed a consensus statement on percutaneous vertebral augmentation,18 concluding that “percutaneous vertebral augmentation with vertebroplasty and kyphoplasty is a safe, efficacious, and durable procedure in appropriate patients with symptomatic osteoporotic and neoplastic fractures when performed in a manner in accordance with published standards.” Also in 2009, 2 randomized trials on VP as treatment for osteoporotic vertebral fractures (Buchbinder et al19 and Kallmes et al20) were published in the New England Journal of Medicine (NEJM). These studies found no significant difference between vertebroplasty and sham treatment and prompted a debate on the effectiveness of surgical treatment of VCFs, as well as numerous changes in clinical recommendations and adverse decisions on procedure reimbursement. Later, 2 blinded RCTs demonstrated statistically significant benefits in pain improvement and functional improvement of vertebroplasty when compared with sham treatments.21,22 The impact of these studies is being evaluated concurrently.
The purpose of this meta-analysis was to update the existing body of literature using recent highest-quality data to assess the effectiveness of BKP and VP, including vertebral augmenation with implant, compared with NSM in the treatment of patients with painful VCFs. Intertreatment analysis was also peformed. This meta-analysis also provided an updated review to guide an evidence-based approach to the use of vertebral augmentation procedures. This will provide an organizational framework to better define the heterogenous body of vertebral augmentation literature by analyzing the newest Level I and Level II studies.
MATERIALS AND METHODS
Information Source
PubMed, for articles published from February 1, 2011, to November 22, 2016.
Search
A PubMed search was performed with the assistance of a research librarian using keywords kyphoplasty, vertebroplasty, vertebral body stent, and vertebral augmentation AND implant for articles published in the English language from February 1, 2011, to November 22, 2016, resulting in 937 articles. The electronic search strings used by the research librarian to perform this PubMed search are included in Appendix I.
Eligibility Criteria and Study Selection
Inclusion criteria were as follows: prospective comparative studies of vertebral augmentation procedures, studies enrolling at least 20 patients, and studies performed for mid-/lower-thoracic and lumbar vertebral fractures (T5 through L5) due to osteoporosis.
Exclusion criteria were as follows: single-arm studies, kyphoplasty studies not using inflatable balloons, non-English language studies, systematic reviews and meta-analyses, traumatic nonosteoporotic or cancer-related fracture studies, studies without clinical outcomes, non–Level I and non–Level II studies, and studies involving sacroplasty. Vertebral body stenting and KIVA procedures were grouped as a separate treatment group (vertebral augmentation with implants, or “VAI”).14,23–25
Review Protocol and Data Collection Process
A systematic review protocol was established to determine which papers satisfied the inclusion/exclusion criteria and qualified for the meta-analysis. Abstracts for the 937 articles were reviewed by each of 2 reviewers to identify those that failed to meet the inclusion/exclusion criteria. For studies in which the abstracts were not adequate to reach a determination for inclusion/exclusion, the articles were reviewed for further assessment. Any discrepancies for qualification for the meta-analysis between the 2 reviewers were discussed and resolved together. After systematic review, 28 of these studies satisfied the inclusion/exclusion criteria. Follow-up length for these studies ranged from 6 months to 5 years.
Data Items
Outcomes of interest for this meta-analysis were as follows:
Optimal intervention time/age of compression fracture
Cost/benefit of surgical intervention versus NSM
Economic considerations
Quality of life (QOL) improvement as measured by the Short-Form 36 Survey Physical Component Summary (SF-36 PCS), SF-36, EuroQol-5D (EQ-5D), Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO), and low-back pain
Disability improvement as measured by the Roland Morris Disability Questionnaire (RMDQ) score, disability days, Oswestry Disability Index (ODI), and the time up-and-go (TUG) metric
Pain scores (10-point back pain, SF-36 bodily pain, visual analog scale [VAS], numeric rating scale, numerical visual scale)
Subsequent adjacent fractures and overall subsequent fractures
Cement extravasation
Spinal canal extravasation
Vertebral height restoration (anterior, midline, posterior)
Kyphotic angle
Serious adverse events (complications)
STATISTICAL METHODS
Data Collection Process
Data was extracted from each study from the provided tables and figures as well as the text in the articles. Extracted data was confirmed for accuracy by 2 reviewers. For each study, the standardized mean difference between baseline and endpoint for each outcome was calculated, as the scale for some outcomes varied across studies. For outcomes measured over time, the last time observed was considered the endpoint.
Synthesis of Results
Treatment groups were pooled using random effects meta-analysis to provide an overall estimate of effect. Pairwise comparisons were implemented using the z test.26 Summary statistics were used to calculate mean and standard deviation if unknown.27 To assess heterogeneity, I2 statistics were calculated for each summary effect size.27 A minimum of 3 studies in each treatment group were required to perform analysis to estimate a within-group effect. Mean effect sizes with 95% confidence intervals in parentheses are reported. The analysis was completed in SAS v.9.4 (SAS Inc, Cary, North Carolina).
Summary Measures
In addition to applying the random effects model for a pooled estimate, response ratios of standardized means between treatment groups within studies were calculated for outcomes of interest. This only included studies that had specific outcome measures for both treatment groups that were being compared.
RESULTS
Of the 28 studies, 3 did not report any outcomes of interest and were excluded, leaving 25 studies for meta-analysis. Six studies compared BKP to NSM (4 randomized versus 2 nonrandomized). Six studies compared VP to NSM (5 randomized versus 1 nonrandomized). Nine studies compared BKP to VP (5 randomized versus 4 nonrandomized). Four studies compared BKP to VAI (3 randomized versus 1 nonrandomized). Of the 25 studies included in the meta-analysis, 10 were Level I studies and 15 were Level II studies, based on the American Academy of Orthopaedic Surgeons guidelines for level of evidence.28 Due to the requirement of 3 studies to estimate within-group effect, some outcomes of interest (optimal intervention time/age of fracture, cost/benefit analysis, economic considerations, cement extravasation, spinal canal extravasation, and serious adverse events) could not be reported, as the minimum number of studies was not reached. Table 1 summarizes the findings of each study included in the meta-analysis. Table 2 summarizes the treatment comparison results across pooled studies, showing standardized mean differences of change from baseline for each treatment group and P values for treatment comparisons.
Table 1.
Summary of study findings.
|
Author/Year of Publication |
Baseline Characteristics |
Pain Relief |
Disability |
| Boonen et al28 | 1. BKP (149 patients, average age 72.2 y) versus NSM (151 patients, average age 74.1 y) at 24 mo. 2. Acute No. 3. Baseline/screening, 1, 3, 6, 12, 24 mo. Back pain also at 7 d. Randomized, nonblinded trial. Level II study. |
10-point back pain: BKP superior to NSM (−1.49, P < .0001) over 24 mo. BKP superior to NSM at all time points (SS). SF-36 subscale for bodily pain: BKP superior to NSM (9.75 points, P < .0001) over 24 mo. BKP superior to NSM at all time points (SS). |
24-point RMDQ: BKP superior to NSM (−3.01, P < .0001) over 24 mo. BKP superior to NSM (−2.81, P = .0001) at 12 mo, superior by −1.43 points, but NSS (P = .051) at 24 mo. Disability days: BKP superior to NSM (2.62 d, P < .0001) over 24 mo, SS at 12 mo (−2.04, P = .009), NSS at 24 mo |
| Borgström et al26 | 1. BKP (149 patients, average age 71.7 y) versus NSM (151 patients, average age 73.6 y). 2. Acute No. 3. Baseline/screening, 1, 3, 6, 12, 24 mo. Back pain at 7 d. Randomized, nonblinded trial. Level II study. |
60% of comparative gain of BKP is driven by improvements in pain. VAS: BKP superior to NSM (−2.12 points, P = .00) over 24 mo. |
RMDQ: BKP superior to NSM (−3.42, P = .02) over 24 mo. |
| Chen et al9 | 1. Total No. patients: 96, from Jan 2007 to Dec 2012. 2. PVP (46 patients, 69 vertebral bodies, average age 64.63 y) versus CT (43 patients, average age 66.49 y). 3. Baseline, 1d, 1 wk, 1, 3, 6 and 12 mo follow-up. Level I study. |
VAS: At baseline PVP similar to CT. Improved pain relief after 1 wk, 1 mo, 3 mo, 6 mo, and 1 y of PVP. Complete pain relief at final follow-up higher in PVP (SS). Use of analgesics after PVP reduced after 1 wk, 1 mo, 3 mo, 6 mo, 1 y (SS). |
ODI: improved after 1 wk, 1 mo, 3 mo, 6 mo, and 1 y of PVP (SS). |
| Clark et al21 | 1. Total No. patients: 120. 2. Placebo (59 patients) versus VP (61 patients). 3. Follow-up: baseline, 3 d, 14 d, 1 mo, 3 mo, 6 mo. Level I study. |
NRS score reduction: Placebo: −4.8 VP: −6.1 P = .043 (SS, VP versus placebo) VAS reduction: Placebo: −48 VP: −58 P = .050 (NSS, VP versus placebo) |
RMDQ reduction: Placebo: −7.4 VP: −11.7 P = .0022 (SS, VP versus placebo) TUG (baseline): Placebo: 29 VP: 26 |
| Dohm et al29 | 1. BKP (191 patients, average age 75.5 y) versus VP (190 patients, average age 75.7 y) 2. Acute No. 3. Baseline/screening 1, 3, 12, 24 mo. Back pain at 7 d. Randomized, nonblinded trial. Level II study (follow-up at final time point < 80%). |
BKP and VP both −4.0 for back pain at 24 mo, NSS across treatments. | BKP −26.9 and VP −25.9 for ODI at 24 mo. NSS across treatments. |
| Dong et al30 | 1. BKP (51 patients, average age 69.8 y) versus VP (35 patients, average age 70.5 y). 2. “Gradual collapse of the vertebral body.” 3. Follow-up of 7 to 36 mo (average: 21.3 mo). Level II study. |
VAS: BKP and VP both decreased pain scores (SS) after operation. No comment made on difference across procedures. |
NR |
| Endres and Badura23 | 1. Total No. patients: 59. 2. BKP (20 patients, average age 63.3 y) versus VP (21 patients, average age 71.3 y) versus SKP (18 patients, average age 67.1 y). 3. Presurgery, directly postsurgery, and 6 mo postsurgery. Level II study. |
VAS: NSS between groups, at presurgery and 6 mo postsurgery. |
ODI: NSS between groups, at presurgery and 6 mo postsurgery. |
| Farrokhi et al31 | 1. Total No. patients: 82. 2. PVP (40 patients, average age 72 y) versus OMT (42 patients, average age 74 y), from Sep 2004 to Jan 2006. 3. Follow-up at 1 wk, 2, 6, 12, 24, 36 mo postoperatively. Level I study. |
VAS: PVP reduced from 8.4 to 1.8. OMT reduced from 7.2 to 3.7. Better in PVP than OMT (SS at 6 mo, NSS at 12–36 mo). |
NR |
| Folman and Shabat32 | 1. Total No. patients: 45. 2. CV (14 patients, average age 75.6 y) versus SK (31 patients, average age 70.74 y). 3. Follow-up at 1, 6, and 12 mo after procedure. Level II study. |
VAS: Pretreatment and posttreatment NSS. Reduced pain: SK superior to CV. Both SK and CV were SS in reducing pain from pre-op. |
NR |
| Fritzell et al33 | 1. Total No. patients: 67. 2. BKP (35 patients, average age 72 y) versus control (32 patients, average age 75 y) 3. Acute/subacute (< 3 mo) No. 4. Follow-up at 1, 3, 6, 12, 24 mo. Level I study. |
NR | NR |
| Korovessis et al24 | 1. Total No. patients: 168. 2. KIVA (82 patients, average age 69 y) versus BKP (86 patients, average age 72 y) from May 2010 to Oct 2011. 3. Baseline, follow-up at 14 mo postoperatively. Level I study. |
VAS: Improvement in both groups postoperatively. NSS between groups. |
ODI: Improvement in both groups postoperatively. NSS between groups. |
| Kroon et al34 | 1. Total No. patients: 57. 2. VP (29 patients, average age 76.7 y) versus sham (28 patients, average age 77.7 y) from Apr 2004 to Oct 2008. 3. Baseline, follow-up at 12 mo, 24 mo postoperatively. Level II study. |
Overall pain: 12 mo: VP improved by 2.4 units versus 1.9 units in sham. 24 mo: VP improved by 3.0 units versus 1.9 units in sham. No beneficial effects of VP over sham. |
RMDQ: 12 mo: VP 2.0 units versus 2.6 units in sham. 24 mo: VP 2.6 units versus 2.7 units in sham. |
| Lee et al35 | 1. Total No. patients: 231. 2. CV—conservative treatment (149 patients, average age 66.2 y) versus BKP (82 patients, average age 76.8 y) from Mar 2005 to May 2009. 3. Baseline, follow-up at 1 y postoperatively. Level II study. |
VAS: Baseline similar and improved after 1 y in both groups. BKP only lower VAS up to 1 mo: 3.2 versus 4.8 in CV (SS). Afterwards NSS. |
ODI: Baseline similar and improved after 1 y in both groups. BKP only lower ODI up to 1 mo: 10.3 versus 17.08 in CV (SS). Afterwards NSS. |
| Li et al36 | 1. Total No. patients: 85. 2. BKP (45 patients, average age 68.5 y) versus VP (40 patients, average age 67.1 y). 3. Painful osteoporotic VCFs. 4. Follow-up at 3, 6, 12 mo. Level II study. |
VAS: Both BKP and VP had significantly (SS) less painful scores 1 d after of surgery and 12 mo afterwards. There was no SS difference between BKP and VP. |
ODI: Both BKP and VP had significantly (SS) lower disability score after 3 and 12 mo. There was no SS difference between BKP and VP. |
| Liu et al37 | 1. Total No. of patients: 100. 2. BKP (50 patients, average age 72.3 y) versus VP (50 patients, average age 74.3 y) 3. Osteoporotic VCF. 4. Follow-up period of 5 y. 5. Randomized controlled trial. Level I study. |
VAS: SS lower VAS scores after 3 d, 1 y, 2 y, and 5 y for both BKP and VP. No SS difference in VAS scores for BKP versus VP. |
NR |
| Movrin38 | 1. Total No. patients: 107. 2. BKP (46 patients, average age 67.8 y) versus CT (61 patients, average age 73.8 y) from Jan 2007 to Dec 2008. 3. Follow-up at 1 d, 6 wk, 12 wk, 1 y postoperatively. Level II study. |
VAS: BKP 2.0 versus CT 3.8. BKP superior to CT (SS). |
NR |
| Nakano et al39 |
1. Total No. patients: 80. 2. VP (40 patients, average age 79 y) versus CT (40 patients, average age 78 y) from Jun 2002 to Feb 2007. 3. Follow-up: 6, 12, 24 mo. VP 18.9 mo. CT 26.2 mo. Level II study. |
VAS (cm): VP: 0.5 CT: 2.0 Both VP and CT had SS lower VAS score from baseline, and VP was superior to CT for pain reduction at 24 mo (SS). |
NR |
| Otten et al25 | 1. Total No. patients: 52. 2. Kiva VP (26 patients, average age 73.6 y) versus BKP (26 patients, average age 66.4 y) 3. Pathological compression fractures. 4. Follow-up period of 6 mo 5. Matched pairs between Kiva VP and BKP based on vertebral body treated, age. Level II study. |
VAS: Both Kiva VP and BKP lowered VAS scores. But Kiva has SS lower VAS score 6 mo after treatment than BKP group (P < .0001). |
ODI: Both Kiva VP and BKP lowered ODI scores. No SS difference between Kiva and BKP. |
| Staples et al40 | 1. Total No. patients: 78. 2. Placebo (40 patients) versus VP (38 patients) from Apr 2004 to Oct 2010. 3. Follow-up: 1 wk, 1 mo, 3 mo, 6 mo, 1 y, 2 y. Level II study. |
NR | NR |
| Tutton et al14 | 1. Total No. patients: 253. 2. KIVA (127 patients, average age 76.03 y) versus BKP (126 patients, average age 75.09 y) from Aug 2010 to May 2013. 3. Follow-up: 12 mo. Level I study. |
VAS: KIVA: −70.8 BKP: −71.8 Both improved VAS score from baseline (SS), but NSS compared with each other. |
ODI: KIVA: −38.1 BKP: −42.2 Both improved VAS score from baseline (SS), but NSS compared with each other. |
| Van Meirhaeghe41 | 1. Total No. patients: 300. 2. BKP (149 patients, average age 72.2 y) versus NSM (151 patients, average age 74.1 y). 3. Follow-up: 24 mo. Level II study. |
VAS: BKP more back pain relief. BKP 2.82 versus NSM 3.65 (SS BKP superior to NSM). |
RMDQ: Less in BKP than in NSM. BKP 8.87 versus NSM 10.3. Mobility: TUG: BKP 13.8 versus NSM 16.9. NSS between groups. |
| Wang et al42 | 1. Total No. patients: 107. 2. HVCV (50 patients, average age 69.43 y) versus BKP (51 patients, average age 68.63 y) from Jan 2012 to Feb 2014. 3. Preoperatively, postoperatively, 3 mo and 1 y follow-up. Level I study. |
VAS: NSS between groups preoperatively, postoperatively, at 3 mo and at 1 y follow-up. |
ODI: NSS between groups preoperatively, at 3 mo and at 1 y follow-up. |
| Werner et al15 | 1. Total No. patients: 65 2. BKP (32 patients, average age 66 y) versus VBS (33 patients, average age 73 y). 3. Follow-up: NR. Level I study. |
NR | NR |
| Yang et al43 | 1. Total No. patients: 221. 2. VP (109 patients, average age 73.39 y) versus BKP (112 patients, average age 73.48 ) from Jan 2008 to Oct 2012. 3. Follow-up: NR. Level I study. |
NR | NR |
| Yokohama et al44 | 1. Total No. patients: 66. 2. VP (28 patients, average age 74 y) versus BKP (38 patients, average age 75.5 y) from Jan 2008 to Apr 2012. 3. Follow-up: NR. Level II study. |
VAS: VP 1.59 BKP 2.39 NSS |
NR |
Abbreviations: AE, adverse event; AP, anterior-posterior; APHC, anteroposterior height comparison; BF, bone filler; BKP, balloon kyphoplasty; CABG, coronary artery bypass grafting; CPC, calcium phosphate cement; CT, conservative therapy; CV, confidence vertbroplasty; EQ-5D, EuroQol five dimensions; HVCV, high viscosity cement vetebroplasty; LBP, low-back pain; NR, not relevant; NRS, numerical rating scale; NSM, nonsurgical management; NSS, not statistically significant; ODI, Oswestry Disability Index; OMT, optimal medical therapy; OVCF, osteoporotic vertebral compression fracture; PCS, physical component summary; PVP, percutaneous vertebroplasty; QALY, quality-adjusted life year; QOL, quality of life; QUALEFFO, Quality of Life Questionnaire of the European Foundation for Ostoporosis; RMDQ, Roland-Morris Disability Questionnaire; SF-36, 36-Item Short Form Health Survey; SI, sagital index; SK, sky kyphoplasty; SKP, shield kyphoplasty; SS, statistically significant; THA, total hip arthroplasty; TKA, total knee arthroplasty; TUG, time up-and-go; UTI, urinary tract infection; VAS, visual analog scale; VBH, vertebral body height; VBS, vertebral body stenting; VCF, vertebral compression fracture; VP, vertebroplasty.
Table 2.
Treatment comparison results across pooled studies indicating standardized mean differences of change from baseline and respective P value. (Note: n/a indicates insufficient number of studies for analysis.)
|
Outcome |
Mean Change from Baseline |
P
Values |
||||||
|
BKP |
VP |
VAI |
NSM |
BKP-VP |
BKP-NSM |
VP-NSM |
BKP-VAI |
|
| Pain VAS score | −4.05 | −3.88 | −2.77 | −2.66 | .774 | .041 | .085 | .018 |
| Oswestry Disability Index | −4.20 | −6.61 | −1.91 | n/a | .140 | < .001 | ||
| Roland Morris Disability Questionnaire | n/a | −2.41 | n/a | −2.86 | .763 | |||
| Kyphotic angular correction | −1.17 | −1.07 | n/a | n/a | .658 | |||
| Anterior vertebral height restoration | 1.23 | 0.98 | n/a | n/a | .226 | |||
| Posterior vertebral height restoration | 0.54 | 0.38 | n/a | n/a | .293 | |||
| Subsequent fracture rate | 0.33 | 0.28 | n/a | 0.16 | .790 | .207 | .307 | |
Abbreviations: BKP, balloon kyphoplasty; NSM, nonsurgical management; VAI, vertebral augmentation with implants; VAS, visual analog scale; VP, vertebroplasty.
Table 1.
Extended.
|
Quality of Life |
Kyphotic Angle (Grades) |
Vertebral Height |
Cement Extravasation |
| PCS: BKP superior to NSM (+3.24, P = .0004) over 24 mo. SS at 6 mo but not at 12 or 24 mo. At 24 mo, BKP superior (+1.68, NSS P = .15). EQ-5D: BKP superior to NSM (+0.12 points, P = .0002) over 24 mo. BKP superior to NSM at all time points (SS). |
NR | NR | Compression fracture serious adverse event showed cement migrated anteriorly |
| BKP gained on average more than NSM patients for all instruments (P < .05). EQ-5D: BKP superior to NSM (+0.20, P = .00) over 24 mo. SF-36: BKP superior to NSM (+0.11, P = .03) over 24 mo. |
NR | NR | NR |
| RMDQ: improved after 1 wk, 1 mo, 3 mo, 6 mo, and 1 y of PVP (SS). |
NR | NR | AP and lateral spinal X-ray showed leakage in 36 of 69 vertebral bodies. |
| EQ-5D: Placebo: 0.74 VP: 0.8 P = .012 (SS, VP versus placebo) QUALEFFO: Placebo: 45 VP: 38 P = .032 (SS, VP versus placebo) |
NR | Height loss: VP: 27% Placebo: 63% |
VP: Cement leakage at 21 of 61 patients (34%). |
| BKP +7.6 and VP +7.5 for SF-36 PCS at 24 mo. NSS across treatments. BKP +0.28 and VP +0.31 for EQ-5D at 24 mo. NSS across treatments. |
BKP superior to VP by 1.42° at 24 mo, P = .036. | NR | BKP lower (P = .047) than VP for cement extravasation. |
| NR | Mean improvement in local kyphotic angle was 2.31° and 8.32° after VP and BKP, respectively (SS). BKP superior to VP in differences of improvement (SS). |
VP: Restoration of anterior/posterior height was 1.93 mm and 0.47 mm, respectively (SS). BKP: Mean restoration of anterior/posterior height was 5.44 mm and 1.36 mm, respectively (SS). BKP superior to VP in differences of restoration of vertebral height (P < .001) |
NR |
| NR | NR | None of the groups improved vertebral body height in the anterior and central portion. | Measured directly postsurgery and 6 mo postsurgery. NSS differences: VP: 4 lateral leakages and 4 in the disk. BKP: 3 laterals and 1 anterior. SKP: 1 in the disk. |
| LBP: PV reduced from 52.2 to 8.0. OMT reduced from 50.4 to 22.0. Better in PV than OMT (SS at all time points). |
SI: PV reduced from 20.0° to 8.9°. OMT increased from 21.0° to 23.0°. PV superior to OMT (SS at all time points). |
VBH: PV increased from 2.8 to 3.0. OMT reduced from 2.5 to 2.0. PV restored VBH and prevented spinal deformity versus OMT. (SS at all time points) . |
PV: epidural (1), discal (5), paravertebral space (8). |
| NR | Pretreatment and post treatment: SK superior to CV. |
NR | NR |
| EQ-5D: Improved significantly within both groups, most significant within 3 mo. BKP had 0.085 difference compared with control over 24 mo (NSS). |
NR | NR | 1 BKP patient, cement in index vertebra migrated toward aorta in thoracic region but without clinical consequences. |
| SF-36: Physical functioning and mental health improved postoperatively. NSS between groups. |
KIVA: Reduced Gardner kyphotic angle. 84% of spines had residual kyphosis > 5° measured. BKP: Reduced Gardner kyphotic angle. 100% of spines had residual kyphosis > 5° measured. |
Anterior vertebral body height: NSS. Posterior vertebral body height: restored by KIVA. Midline vertebral body height: NSS. |
KIVA: 4 patients BKP: 12 patients |
| QUALEFFO: 12 mo: VP 6.7 units versus 8.8 units in sham. 24 mo: VP 5.9 units versus 4.6 units in sham. QOL: 12 mo: VP 0.1 units versus 0.2 units in sham. 24 mo: VP 0.1 units versus 0.1 units in sham. EQ-5D: 12 mo: VP 0.2 units versus 0.2 units in sham. 24 mo: VP 0.2 units versus 0.2 units in sham. TUG: 12 mo: VP −2.6 s versus 4.3 s in sham. 24 mo: VP 3.5 s versus 4.7 s in sham. |
NR | NR | Higher risk with higher volume of injected median cement. |
| NR | NR | NR | NR |
| NR | Both BKP and VP reduced kyphotic angle at follow-up of 12 mo. BKP had SS greater reduction (P < .01) than VP group. | Both BKP and VP had SS increase of mean vertebral heights after 12 mo. Higher degrees of height restoration were achieved in BKP versus VP (SS, P < .05). | BKP had 6 of 66 treated fractures with cement leaks, while VP had 18 of 52 with cement leaks. |
| NR | SS lower kyphotic wedge angles after 3 d, 1 y, 2 y, and 5 y for both BKP and VP. | SS higher vertebral body height after 3 d, 1 y, 2 y, and 5 y for both BKP and VP. | Asymptomatic cement leakage in some cases. |
| NR | Cobb's technique to calculate segmental kyphotic angle across fractured level: BKP 5.4 versus CT 10.6. |
NR | BKP: 8.7% |
| NR | NR | Deformity index: VP: 1.61 CT: 1.37 APHC: VP: 70.2 CT: 49.1 |
VP: 1 patient (intervertebral) |
| NR | NR | Both Kiva and BKP showed SS (P < .001) increase in anterior and midwall height after operations, but no SS difference after 6 mo compared with post-op. | 6 cases of cement extravasation in Kiva and 8 cases in BKP. No SS difference across groups. |
| NR | NR | NR | VP: Cement leakage at 18 of the 45 treated levels (40%). |
| NR | NR | NR | Lower in the KIVA group (64.6%) versus BKP (64.5%). |
| EQ-5D: SS improvement with BKP. BKP 0.61 versus NSM 0.53. |
Index fracture kyphotic angulation: SS, BKP 3.13° versus NSM 0.82°. |
Anterior vertebral height: BKP improved 6.7% versus NSM improved 1.1%. Midline vertebral height: BKP improved 5.9% versus NSM worsened 1.9%. BKP superior (SS) to NSM. |
51 bodies (27.1%) with cement leakage. |
| NR | NR | Lower anterior height restoration rate in HVCV (30.04) versus BKP (42.65), SS. At 1 y follow-up, NSS loss of height in both groups. |
Lower in HVCV (9 of 68) versus BKP (22 of 72). |
| NR | BKP: 4.5° VBS: 4.7° NSS |
NR | Major cement leakage (No. patients): BKP: 4 VBS: 5 NSS |
| NR | BKP: 7.82° VP: 3.82° BKP superior to VP (SS). |
Preadjusted Postoperative: VP: 23.48 BKP: 21.42 Adjusted Postoperative: VP: 21.988 BKP: 22.869 BKP superior to VP (SS). |
VP: 56 cases BKP: 49 cases NSS |
| NR | Kyphotic change: VP: 7.0 BKP: 6.9 NSS |
Anterior: VP: 3.6 BKP: 3.6 NSS Central: VP: 2.5 BKP: 2.0 NSS Posterior: VP: 0.9 BKP: 0.5 NSS |
Cement leak: VP: 16 vertebral bodies BKP: 9 vertebral bodies SS |
Table 1.
Continued. Extended.
|
Spinal Canal/ Foramen Extravasation |
New VCF |
Adverse Event |
Optimal Intervention Time |
| NR | New fractures: no SS difference (56 of 118 BKP fractures versus 45 of 102 NSM, P = .68). No SS for adjacent radiographic fractures, painful fractures. |
11 patients in BKP (7.4%) had new clinical fractures possibly caused by cement. One serious AE from recurrent compression fracture. 1 hematoma and 1 exacerbation of recurrent UTI. All deaths considered unrelated to treatment (23 total deaths, 12 kyphoplasty, 11 NSM). |
NR |
| NR | NR | NR | NR |
| NR | NSS differences: 3 of 46 PVP patients versus 7 of 43 CT patients |
NR | NR |
| NR | VP: 3 patients Placebo: 2 patients |
VP: 2 patients (respiratory arrest, humerus fracture) Placebo: 2 patients (spinal cord compression) |
NR |
| BKP lower intravascular extravasation (P = .028) than VP. | BKP had 8.6% fewer radiographic fractures versus PVP (P = .23). NSS clinically identified fractures. BKP had longer fracture-free survival (P = .0596), NSS. |
Common: procedural pain, back pain, new symptomatic fracture. Also: bronchitis, pneumonia, UTI, etc. 1 cement embolism for BKP and VP each. |
NR |
| NR | NR | NR | NR |
| NR | No adjacent fractures were observed. | NR | NR |
| NR | PV: 1 patient OMT: 6 patients |
Postoperative complications: 1 patient with severe right lower extremity pain and weakness. | NR |
| NR | NR | NR | NR |
| NR | 5 BKP and 4 control patients had new painful VCF in 1 or 2 adjacent levels and were treated either with new BKP or continuous standard treatment. | 1 cement extravasation in BKP group, 1 infection in BKP group. | NR |
| KIVA: NR BKP: 2 patients |
KIVA: 10 patients BKP: 11 patients |
NR | NR |
| NR | VP: 14 Sham: 13 |
New clinical fractures (hip, ribs, pelvis, sternum, shoulder, waist, elbow) up to 24 mo. | NR |
| NR | NR | NR | NR |
| NR | 9 patients with BKP and 7 patients with VP experienced new fractures. | NR | NR |
| NR | 12 BKP patients with new VCF, 10 VP patients with new VCF. No SS difference in incidence of new VCF between groups. | None | NR |
| NR | Adjacent fractures: BKP: 3 CT: 10 |
NR | NR |
| VP: 4 patients | VP: 5 CT: 11 |
VP: 1 patient suffered from urinary tract infection. CT: 6 patients had gastritis and anorexia, 2 patients with leg and facial edema. |
NR |
| NR | New fractures in 3 patients in Kiva and 14 patients in BKP. New fractures occur SS lower in Kiva (P < .0001) than in BKP after 6 mo. |
NR | Mean operation time per vertebra was 12.7 min for Kiva and 46.5 min for BKP. |
| NR | New adjacent level: Placebo: 3 VP: 6 New nonadjacent: Placebo: 7 VP: 10 New level: Placebo: 0 VP: 1 |
NR | NR |
| NR | NR | Only procedure-related devices. KIVA: 3 patients, herpes zoster, postprocedural pain, pruritus. BKP: 4 patients, airway complication of anesthesia, back pain, ischemic stroke, rash. |
NR |
| NR | BKP: 11 patients versus NSM 7 patients. | All AEs occurred in the first 30 d. Back pain: BKP 20 patients versus NSM 11 patients. UTI: BKP 10 patients versus NSM 3 patients. Nausea/vomiting: BKP 12 patients versus NSM 4 patients, Hematomas: BKP 4 patients. |
NR |
| NR | 1 HVCV patient versus 4 BKP patients. Subsequent fractures NSS. |
BKP group: 1 patient experienced severe discogenic back pain related to a disc leak; 1 patient asymptomatic cement emboli in the right lung related to venous leakage. |
NR |
| NR | NR | Material-related complication: Cannula: 5 VBS Balloon: 1 BKP, 1 VBS Stent: 3 VBS |
NR |
| NR | NR | NR | NR |
| NR | NR | NR | NR |
Table 1.
Continued. Extended.
|
Cost/Benefit of Surgical Intervention |
Economic Considerations |
QOL/SF-36 for CABG/ THA/TKA |
Conclusion |
| NR | NR | NR | BKP has improved QOL, pain relief, disability improvement, patient satisfaction for 2 y over NSM. Overall better performance of BKP over 1 y, but patients improve around 2-y mark for NSM. BKP has improved disability (RMDQ) and activity days at 12 mo but not at 24 mo. |
| NR | NR | NR | BKP improves QOL, pain, disability compared to NSM over 2 y. Pain relief shown to have most contribution to QOL measures/scales. |
| NR | NR | NR | PVP was found to be associated with greater pain relief and improved functional outcomes at 1 y compared with CT. |
| NR | NR | NR | Vertebroplasty was shown to reduce pain from osteoporotic spinal fractures of less than 6 wk when compared with a true placebo control. |
| NR | NR | NR | BKP and VP have SS less pain, disability, QOL, but not SS between groups. Both have similar AE profiles. Kyphoplasty had fewer cement leakages, a trend of longer fracture-free survival. |
| NR | NR | NR | VP and BKP both improve pain scores, kyphotic angle, and vertebral heights. BKP superior to VP in improving kyphotic angle and vertebral height restoration. |
| NR | NR | NR | Determined that the type of cement augmentation system used for primary osteoporosis patients does not matter. Overall, the vertebroplasty technique may be considered the surgical procedure of choice. |
| NR | NR | NR | Compared with patients who received OMT, patients who received PV had statistically significant improvements in QOL for 36 mo, VAS for 6 mo. |
| CV has a clear advantage. | NR | NR | SK system is technically superior in reconstructing the collapse and repair of the local kyphotic deformity, but this advantage is not manifest in the main index of procedure success—namely, pain relief. Both systems have a high level of safety. |
| Cost/QALY: BKP cost gain is $134 043. But counting for sensitivity analysis, it does not show cost-effectiveness compared with control. |
NR | NR | Study could not demonstrate cost-effectiveness of BKP over control. |
| NR | NR | NR | Supports KIVA implant as a reliable alternative technique to BKP for treating fresh ( < 3 mo) osteoporotic fractures. The better radiological reduction of posttraumatic kyphosis associated with KIVA may at least theoretically influence the medium- and long-term results (less back pain, less frequent adjacent segment fractures). |
| NR | NR | NR | Found no beneficial effects of vertebroplasty over a sham procedure at 12 or 24 mo among patients with painful osteoporotic vertebral fractures. |
| NR | NR | NR | When patients have no risk factors, conservative treatment for an initial 3 wk will be helpful in the treatment of acute OVCFs. However, if the patient failed conservative treatment, kyphoplasty also resulted in excellent results at 1 y after trauma. |
| NR | NR | NR | Both BKP and VP improved disability and pain scores, but BKP offered better spinal deformity correction and resulted in less cement leakage than VP. |
| NR | NR | NR | BKP and VP have SS less pain, kyphotic angle, greater vertebral heights. No SS difference between BKP and VP for VAS pain scores after 5 y. |
| NR | NR | NR | Correction of the vertebral morphology and prevention of further deterioration achieved with BKP probably has a positive effect on the spinal biomechanics and thus reduces the incidence of subsequent fracture. |
| NR | NR | NR | Vertebroplasty using CPC following cavity formation of VB provided better clinical and radiological results than conservative treatment for osteoporotic burst fracture in patients without neural deficit. |
| NR | NR | NR | Kiva is superior to BKP with pain VAS scores. Disability improvement was similar across Kiva and BKP. Both groups had vertebral height restoration and same risk of cement extravasation. Kiva operation time is shorter. |
| NR | NR | NR | VP in placebo-controlled studies has failed to provide superior pain relief or functional benefit compared with placebo, but the study did not observe an increase in subsequent fracture risk beyond that experienced by those with vertebral fractures. |
| NR | NR | NR | Kiva should be seriously considered to reduce pain, decrease disability, and improve quality of life in patients with painful VCFs. The Kiva system is noninferior to BKP in its ability to safely relieve pain and improve function in the treatment of osteoporotic VCFs. |
| NR | NR | NR | Compared with NSM, BKP rapidly reduces pain and improves function, disability, and QOL during the course of 2 y and the reduction in pain. EQ-5D QOL, patient satisfaction, and kyphotic angulation remain statistically significant at all time points. |
| NR | NR | NR | HVCV has a lower cement leakage rate. HVCV is recommended for the treatment of OVCFs. BKP is more effective in vertebral height restoration. |
| NR | BKP: 1 level: $5300 2 levels: $5700 3 levels: $6300 VBS: 1 level: $3750 2 levels: $6950 3 levels: $10 400 |
NR | No beneficial effect of vertebral body stenting over balloon kyphoplasty with regard to kyphotic correction, cement leakage. |
| NR | NR | NR | Vertebroplasty-BF: Bone cement distribution was more physiological. Lumped distribution was avoided. Sufficient bone cement injection was possible without increasing the rate of bone cement leakage. It can be considered as a compatible option for the osteoporotic compression fracture and has the advantages of both conventional vertebroplasty and kyphoplasty. |
| NR | NR | NR | The vertebral height restoration and kyphotic changes that were achieved after surgery largely depended on the preoperative vertebral mobility in not only the patients treated by VP but also those treated by BKP. The use of the balloon in BKP contributed little to the resolution of the vertebral deformities following surgery. |
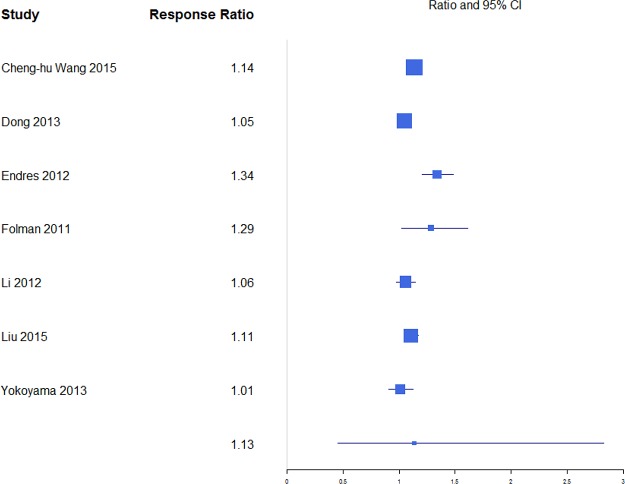
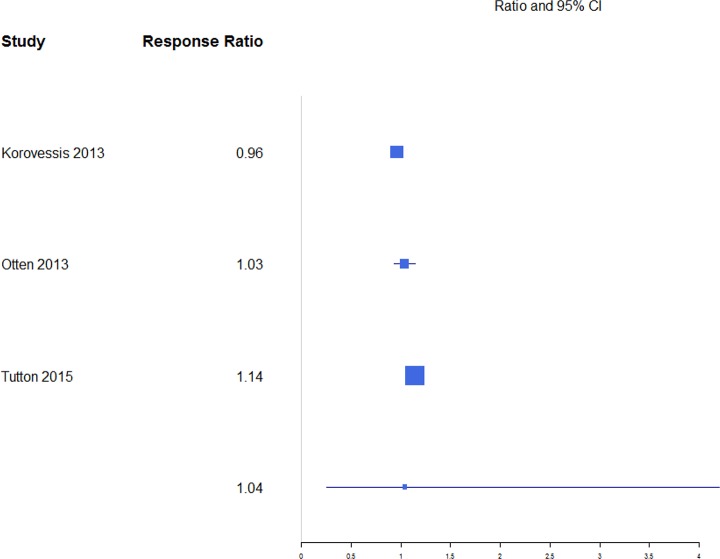
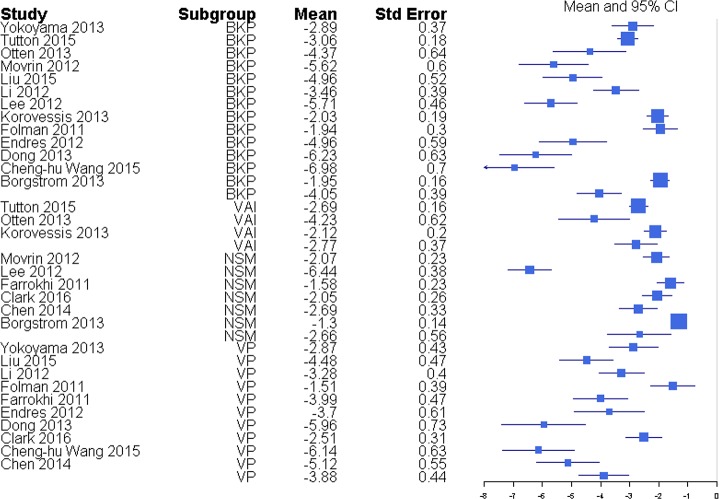
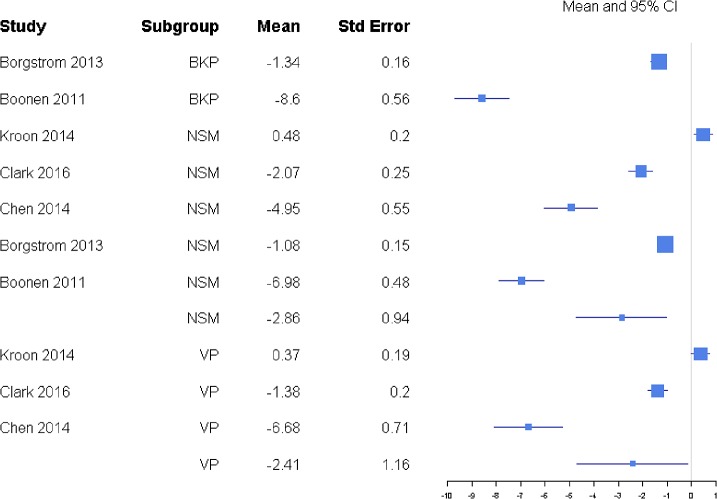
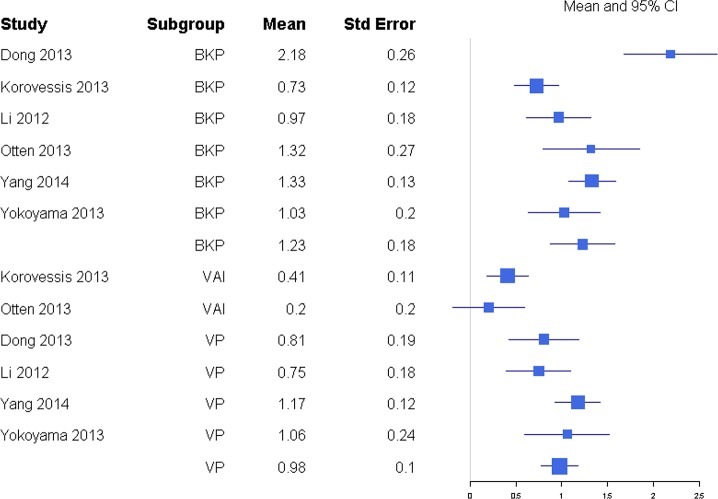
Pain was reported through multiple metrics, including 10-point back pain scores, SF-36 bodily pain score, and VAS score (Table 1), but there was a sufficient number of studies to only compare VAS scores (Table 2). BKP showed some tendency for slightly higher change in VAS scores from baseline than VP and VAI for the majority of studies with an overall response ratio of 1.13 (0.45, 2.83; Figure 1) and 1.04 (0.26, 4.20; Figure 2), respectively. In terms of pain reduction from baseline, BKP (−4.05 [−4.81, −3.29]) was not significantly different than VP (−3.88 [−4.74, −3.02], P = .774) but showed significantly greater improvement compared with NSM (−2.66 [−3.75, −1.56], P = .041; Figure 3) and VAI (−2.77 [−3.51, −2.04], P = .018). The difference between VP and NSM in terms of pain reduction was not significant (P = .085). There was substantial heterogeneity in the BKP (I2 = 95), VP (I2 = 86), VAI (I2 = 84), and NSM (I2 = 96) arms.
Figure 1.
Response ratios of standardized mean differences for visual analog scale pain scores comparing balloon kyphoplasty with vertebroplasty treatment.
Figure 2.
Response ratios of standardized mean differences for visual analog scale pain scores comparing balloon kyphoplasty with vertebral augmentation with implants treatment.
Figure 3.
Standardized mean differences of change from faseline for visual analog scale pain scores. Abbreviations: BKP, balloon kyphoplasty; VAI, vertebral augmentation with implants; NSM, nonsurgical management; VP, vertebroplasty.
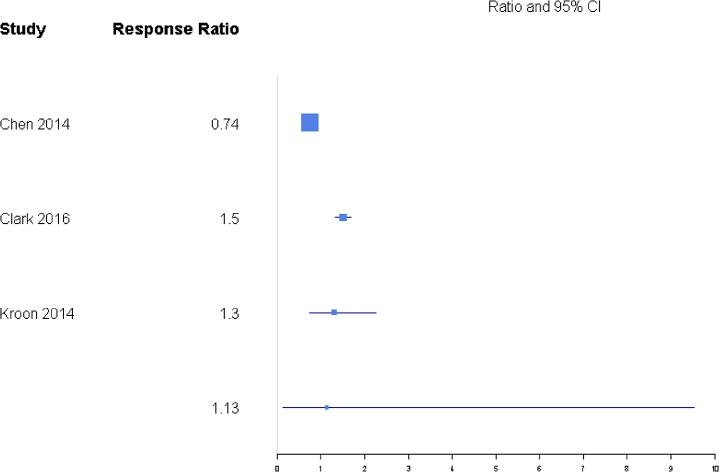
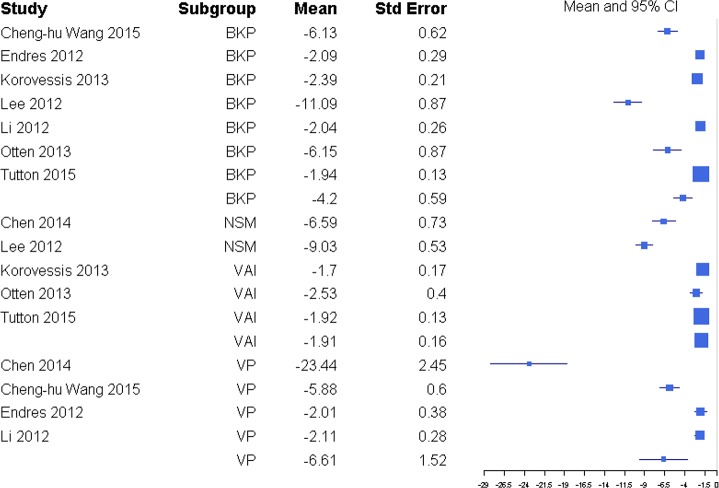
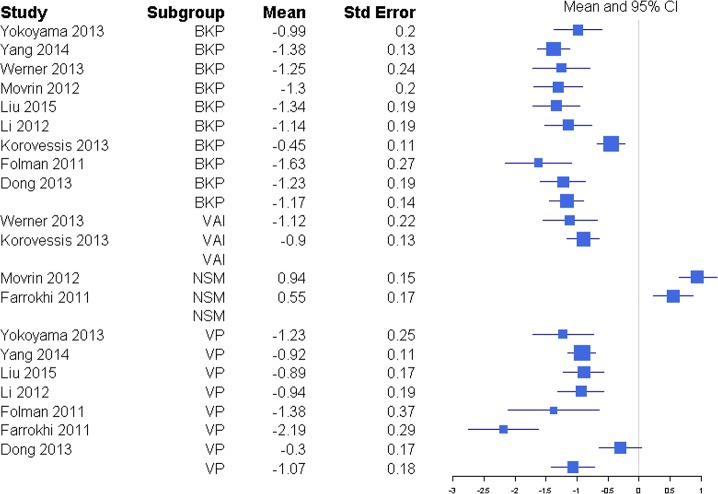
Disability was reported through RMDQ score, disability days, ODI, and TUG metrics (Table 1), but there was a sufficient number of studies to only compare ODI and RMDQ (Table 2). The response ratio plot of RMDQ scores showed NSM had some tendency for slightly higher changes from baseline compared with VP with a response ratio of 1.13 (0.13, 9.53; Figure 4). The overall mean effect of RMDQ for the NSM group was −2.86 (−4.71, −1.01) and for the VP group was −2.41 (−4.68, −0.15) with no significant difference (P = .763; Figure 5). A response ratio plot of ODI scores showed that BKP treatment groups had some tendency to have slightly higher changes from baseline compared with VP groups with a response ratio of 1.02 (0.18, 5.76; Figure 6) and compared with VAI with a response ratio of 1.51 (0.27, 8.58; Figure 7). For disability index (ODI) reduction, BKP (−4.20 [−5.35, −3.05]) showed slightly less improvement versus VP (−6.61 [−9.59, −3.63], P = .140; Figure 8). The reduction in ODI was significantly higher for BKP than VAI (−1.91 [−2.22, −1.61], P < .001). There was substantial heterogeneity in the BKP (I2 = 96) and VP (I2 = 97) arms but less observed heterogeneity in the VAI group (I2 = 47).
Figure 4.
Response ratios of standardized mean differences for Roland Morris Disability Questionnaire comparing nonsurgical management with vertebroplasty treatment.
Figure 5.
Standardized mean differences of change from baseline for Roland Morris Disability Questionnaire. Abbreviations: BKP, balloon kyphoplasty; NSM, nonsurgical management; VP, vertebroplasty.
Figure 6.
Response ratios of standardized mean differences for Oswestry Disability Index comparing balloon kyphoplasty with vertebroplasty treatment.
Figure 7.
Response ratios of standardized mean differences for Oswestry Disability Index comparing balloon kyphoplasty with vertebral augmentation with implants treatment.
Figure 8.
Standardized mean differences of change from baseline for Oswestry Disability Index. Abbreviations: BKP, balloon kyphoplasty; VAI, vertebral augmentation with implants; VP, vertebroplasty.
Quality of life (QOL) was reported through SF-36 PCS, EQ-5D score, and generally through the SF-36 metric. Due to the limited number of studies, overall mean effects were not calculated for BKP and VP. For quality of life (EQ-5D), NSM showed a mean improvement of 1.41 (−0.29, 3.11). There was substantial heterogeneity in the NSM (I2 = 98) arm.
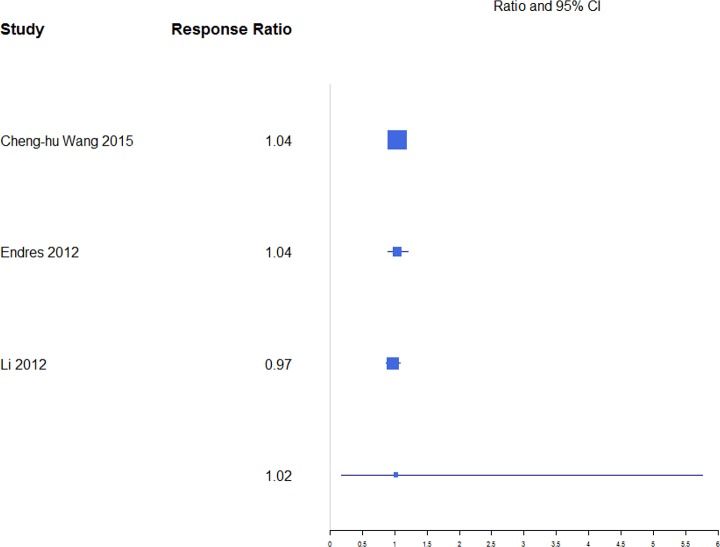
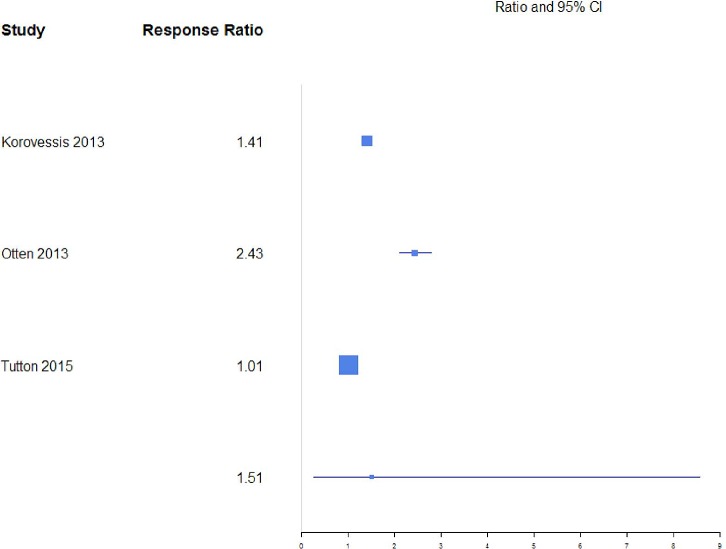
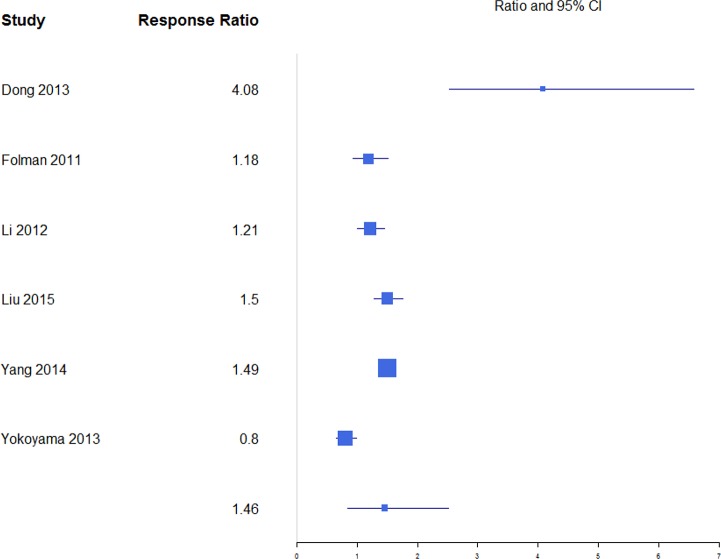
A response ratio plot comparing BKP with VP for kyphotic angular correction (KAC) (Figure 9) showed high variability across studies, with an overall mean estimate of 1.46 (0.84, 2.51) for response ratios. A forest plot of standardized mean differences from baseline for KAC is shown in Figure 10. There were not enough studies to complete an analysis of the NSM or VAI group. For KAC, BKP (−1.17 [−1.45, −0.89]) showed more kyphotic angle reduction than VP (−1.07 [−1.42, −0.72]; P = .658), although the difference is not significant. There was substantial heterogeneity in the BKP (I2 = 82) and VP (I2 = 83) arms.
Figure 9.
Response ratios of standardized mean differences for kyphotic angular correction comparing balloon kyphoplasty with vetebroplasty treatment.
Figure 10.
Standardized mean differences of change from baseline for kyphotic angular correction. Negative values indicate reduction in kyphosis angle. Abbreviations: BKP, balloon kyphoplasty; VAI, vertebral augmentation with implants; NSM, nonsurgical management; VP, vertebroplasty.
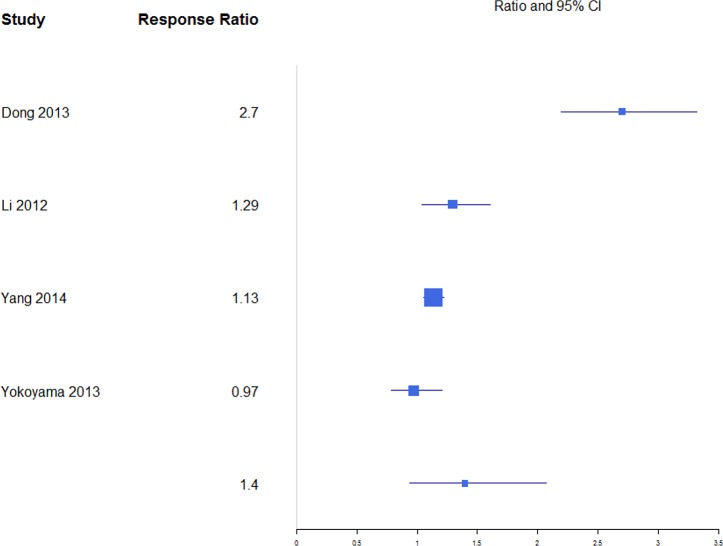
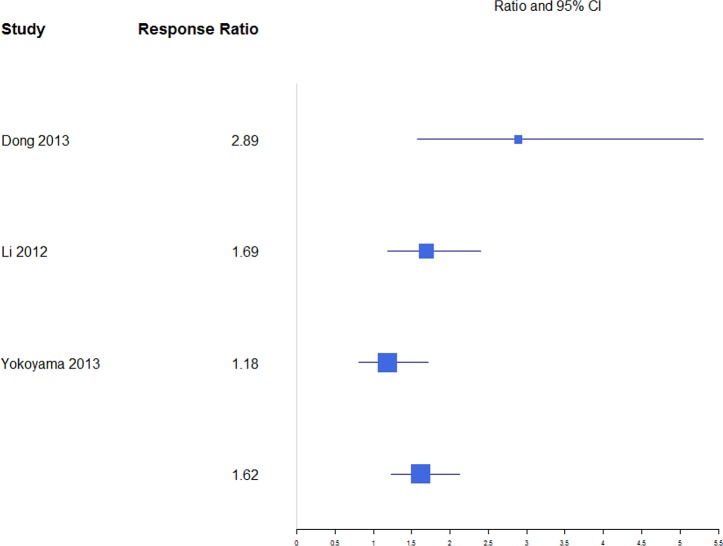
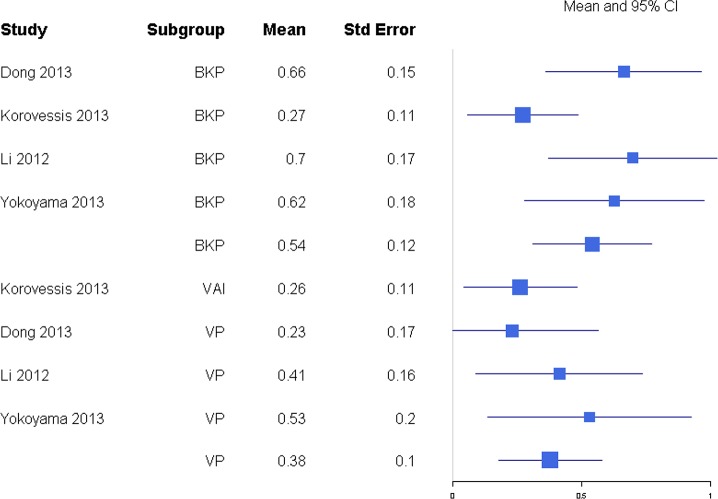
For anterior and posterior vertebral height restoration, BKP showed higher positive change in response compared with VP. Mean estimates for response ratios for anterior and posterior are 1.40 (0.94, 2.07; Figure 11) and 1.62 (1.23, 2.12; Figure 12), respectively, for BKP over VP. Forest plots of standardized mean differences from baseline for vertebral heights are shown in Figures 13 14. Only BKP and VP were compared due to the number of eligible studies.
Figure 11.
Response ratios of standardized mean differences for anterior vertebral height comparing balloon kyphoplasty with vertebroplasty treatment.
Figure 12.
Response ratios of standardized mean differences for posterior vertebral height comparing balloon kyphoplasty with vetebroplasty treatment.
Figure 13.
Standardized mean differences of change from baseline for anterior vertebral height. Abbreviations: BKP, balloon kyphoplasty; VAI, vertebral augmentation with implants; VP, vertebroplasty.
For anterior vertebral height change in response, both treatments showed improvement by increasing restoration. BKP had a mean response change of 1.23 (0.87, 1.58), which was not significantly higher than VP at 0.98 (0.78, 1.17; P = .226). There was substantial heterogeneity in the BKP (I2 = 81) arm but not the VP (I2 = 20) arm.
For midline vertebral height change in response, BKP was 1.12 (0.87, 1.38). There were not enough studies for VP or VAI. There was midheterogeneity in the BKP (I2 = 37) arm.
For posterior vertebral height restoration, BKP (0.54 [0.31, 0.77]) had slightly higher change than VP (0.38 [0.18, 0.58]), but the difference was not significant (P = .293). There was moderate and minimal heterogeneity in the BKP (I2 = 60) and VP (I2 = 0) arms, respectively.
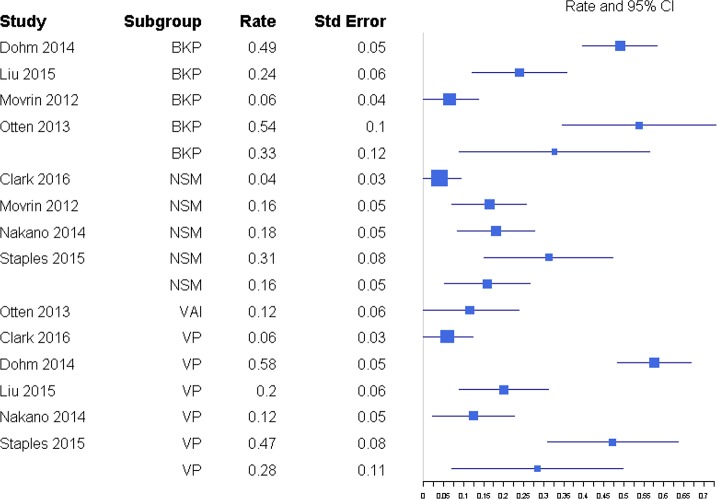
The percentages of patients with subsequent fractures were not significantly different for BKP (32.7%; 95% confidence interval [CI] 8.8%, 56.6%) and VP (28.3%; 95% CI 7.0%, 49.7%; P = .790) or for BKP and NSM (15.9%; 95% CI 5.2%, 26.6%; P = .207; Figure 15). There was also no significant difference between VP and NSM (P = .307). There was substantial heterogeneity in the BKP (I2 = 95), VP (I2 = 96) and NSM (I2 = 81) arms.
Figure 15.
Rate of subsequent fractures. Abbreviations: BKP, balloon kyphoplasty; NSM, nonsurgical management; VAI, vertebral augmentation with implants; VP, vertebroplasty.
Figure 14.
Standardized mean differences of change from baseline for posterior vertebral height. Abbreviations: BKP, balloon kyphoplasty; VAI, vertebral augmentation with implants; VP, vertebroplasty.
DISCUSSION
The drive to produce and collect data in recent years has been enhanced by the controversy initiated by the 2009 sham trials (Buchbinder et al19, Kallmes et al20) published in NEJM. Approximately 250 manuscripts dedicated to vertebral augmentation have been published annually. Much of the output attempts to further define the safety and efficacy of these procedures and investigate differences between the clinical experience with these procedures and the results of the sham trials.
Due to the aforementioned sham trials, there has been a significant decline in the number of vertebral augmentation procedures performed,46–49 and various health technology assessments (HTAs) have used this information to recommend against vertebral augmentation in other countries.
These HTA recommendations are at direct odds with medical societies whose recommendations support vertebral augmentation, including a consensus statement50 from 8 major medical societies that vertebral augmentation remains a proven, medically appropriate therapy for treatment of painful VCFs refractory to nonoperative medical therapy when performed for medical indications outlined in published standards.
An outlier in society recommendations was issued in 2010 by the American Academy of Orthopaedic Surgeons (AAOS).51 This was based on an algorithm using levels of evidence to perform hierarchal qualification of data. At the time the AAOS guidelines were issued, far less data were available, and the existing level of evidence hierarchy placed the Buchbinder et al19 and Kallmes et al20 studies as the highest level of evidence. As a consequence, the AAOS guidelines provided a weak recommendation for kyphoplasty and a strong recommendation against VP.
After the NEJM articles, HTA recommendations, and AAOS guidelines, Papanastassiou et al52 conducted a meta-analysis on the effects of kyphoplasty, VP, and NSM to treat VCFs that reported on 27 Level I and Level II studies. Papanastassiou et al52 concluded that BKP and VP provided better pain relief, resulted in fewer additional VCFs than NSM, and concluded that BKP is favored over VP for QOL improvements and cement extravasation. The authors noted that 2 RCTs in their analysis showed no difference between VP and a sham procedure and pointed to significant data heterogeneity recommending further trials to delineate some confounding variables. Since then, there have been several publications, including double-blind RCTs, other Level I studies on vertebral augmentation, and 2 additional VP versus sham studies, that have added to the literature on vertebral augmenation.
A 2016 sham study trial by Clark et al21 compared VP to sham in patients with acute vertebral compression fractures and concluded that VP significantly reduces pain, improves disability scores, and achieves greater vertebral height restoration. The findings support use of VP over sham surgery in patients with acute VCFs, which differs from the equivocal result of previous sham trials19,20 that concluded no significant difference in pain, function, and QOL between sham patients and patients undergoing VP. A second RCT comparing VP to sham, published in 2016 by Hansen et al,22 concluded that there was a statistically signficant VAS score reduction in the VP group as compared with the sham group during their trial period. An explanation for differences in the conclusions is found in one notable publication since the Papanastassiou et al52 study. Anderson et al53 conducted a meta-analysis in 2013 comparing vertebral augmentation with NSM and analyzed the study quality of the 2009 sham trials19,20 with the levels of evidence of primary research as adopted by the North American Spine Society.53,54 Anderson et al53 found the 2009 sham trials should have been classified as providing Level II evidence instead of Level I, based on inclusion and exclusion criteria and the Kallmes et al20 trial's unacceptably high crossover rate. Both trials were therefore downgraded to Level II evidence based on objective measures of quality for primary research.53
Although the most recent highest-quality literature supports vertebral augmentation over NSM to treat painful VCFs, many HTAs and several national treatment guidelines continue to make outdated, inconsistent recommendations regarding vertebral augmentation. For example, a recent (2015) HTA publication by the Cochrane Collaboration recommended against using VP to treat VCFs in routine clinical practice.55 This recommendation disregarded 11 RCTs and 1 “quasi RCT,” classifying all non–placebo-controlled trials as having high risk of bias, and therefore based the conclusion against VP on only the 2 NEJM sham trials, which were classified as Level I rather than Level II data. This arbitrary classification and selective information culling is one example of how significant discrepancies can arise between HTA reviews and high-quality meta-analyses.
Our present meta-analysis showed differences in outcomes between BKP, VP, and NSM, but fewer than seen in the Papanastassiou et al52 meta-analysis. More pain reduction for both BKP and VP were observed in comparison with NSM, although this was significantly different from BKP to NSM but not statistically significant from VP to NSM. Our analysis also included VAI as a subgroup. We found a significant difference in VAS scores between BKP and VAI, with BKP having more reductions in pain score. BKP had some tendency for slightly better pain reduction than VP, but the difference in this meta-analysis was not statistically significant. This evaluation also showed no difference in disability improvement between BKP and VP and no difference in rates of subsequent fractures. BKP showed significantly better outcomes than VAI in terms of disability improvement (ODI). Patients undergoing BKP were found not to have significantly greater amounts of anterior and posterior vertebral height restoration than VP patients, nor was a difference seen in kyphotic angle correction between VP and BKP groups.
Along with the various outcome measures, a few studies in this meta-analysis examined economic costs of BKP and VP. Folman and Shabat32 concluded that BKP and VP had similar success levels of pain relief, but due to higher costs of BKP, the cost-benefit advantage of VP was clear. However, though an analysis of Medicare data showed no differences in adjusted, cumulative treatment costs for VP and BKP patients in the first 9 months postsurgery, BKP patients were subsequently associated with significantly lower adjusted treatment costs in remaining periods through 2 years postsurgery.56 A survival and cost analysis by Lange et al12 comparing BKP and VP using German claims data showed 4-year mean overall costs were lower for the BKP group than for VP (BKP: €39 014 versus VP: €42 510), due to higher initial BKP costs being offset by pharmacy costs in VP patients. In a multicenter, randomized, controlled cost-effectiveness analysis, Fritzell et al33 determined that it was not possible to demonstrate that BKP was cost effective compared with standard medical treatment in VCF patients. Three other studies (Ström et al,57 Svedbom et al,58 and Klazen et al59) analyzed cost effectiveness using quality adjusted life years and confirmed vertebral augmentation was cost effective compared with NSM.
In a comparison of BKP and VBS, Werner et al15 found no substantial differences in cost, even though costs of vertebral fracture treatment were generally lower for BKP than VBS for treating 1 to 3 vertebral levels.
This meta-analysis yielded insufficient data to determine the QOL differences and optimal intervention time relative to age of the VCF. There were also insufficient data to determine differences in extravasation rates or serious adverse events. We demonstrated comparable findings to those of a recent meta-analysis comparing NSM with VP by Mattie et al.60 When evaluating 11 studies containing 1048 participants, Mattie et al60 reported VP was better than NSM with respect to pain relief up to 1 year after VP. This provides additional information supportive of the efficacy of VP, similar to previous meta-analyses by Anderson et al53 and Papanastassiou et al52 and trends shown in several of the 25 articles included in this meta-analysis. The level of evidence of the meta-analysis of Mattie et al60 was designated as Oxford Level I, which should be taken into account by not only treatment guidelines but also policy recommendations and HTAs. The meta-analysis also focused specifically on percutaneous vertebroplasty as compared with conservative therapy, while only evaluating studies in which the primary outcome was pain relief. No requirements were made of retrospective or prospective studies, minimum number of patients, or site of the procedure.
CONCLUSION
This meta-analysis revealed a large number of high-quality articles (25 Level I and Level II studies), including 17 randomized trials since February 2011. We were unable to include findings from a recently completed prospective, multicenter evaluation of the safety and efficacy of BKP in Medicare patients (EVOLVE trial61), as it had not yet been published in a peer-reviewed journal. The results of this EVOLVE trial included statistically significant improvements in patients' pain, function, quality of life, self-care indices, and vertebral deformity correction. Our current meta-analysis showed statistically superior pain reduction for BKP in comparison with NSM, but pain reduction was not statistically significant for VP in comparison with NSM. There was significantly improved pain reduction and disability improvement for BKP over VAI. BKP tended to show greater anterior and posterior vertebral height change than VP, though this was not statistically significant. The overall number of statistically significant categories was less than in a previous 2011 meta-analysis. It is noteworthy that the analysis includes a VP versus sham trial that offers Level I evidence of statistically significant differences in pain reduction and disability improvements in a study with higher statistical power than previous sham trials, which showed no difference between VP and sham. Also notable is the refinement of evidence, including reclassification of 2 previous sham trials to Level II evidence and conclusions of best-quality literature that serve to further define the safety and efficacy of vertebral augmentation. Despite the preponderance of high-quality data, all endpoints demonstrated substantial heterogeneity in the treatment arms even between randomized trials.
REFERENCES
- 1.Baaj AA, Downes K, Vaccaro AR, Uribe JS, Vale FL. Trends in the treatment of lumbar spine fractures in the United States: a socioeconomics perspective: clinical article. J Neurosurg Spine. 2011;15(4):367–370. doi: 10.3171/2011.5.SPINE10934. [DOI] [PubMed] [Google Scholar]
- 2.Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa 1976) 2000;25(8):923–928. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 4.Melton LJ., III Adverse outcomes of osteoporotic fractures in the general population. J Bone and Miner Res. 2003;18(6):1139–1141. doi: 10.1359/jbmr.2003.18.6.1139. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Atkinson EJ, O'Fallon WM, Melton JL., III Incidence of clinically diagnosed vertebral fractures: a population based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7(2):221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 6.Babayev M, Lachmann E, Nagler W. The controversy surrounding sacral insufficiency fractures: to ambulate or not to ambulate? Am J Phys Med Rehabil. 2000;79(4):404–409. doi: 10.1097/00002060-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90(7):1479–1486. doi: 10.2106/JBJS.G.00675. [DOI] [PubMed] [Google Scholar]
- 8.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 9.Chen AT, Cohen DB, Skolasky RL. Impact of nonoperative treatment, vertebroplasty, and kyphoplasty on survival and morbidity after vertebral compression fracture in the medicare population. J Bone Joint Surg Am. 2013;95(19):1729–1736. doi: 10.2106/JBJS.K.01649. [DOI] [PubMed] [Google Scholar]
- 10.Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res. 2011;26(7):617–626. doi: 10.1002/jbmr.353. [DOI] [PubMed] [Google Scholar]
- 11.Lange A, Kasperk C, Alvares L, Sauermann S, Braun S. Survival and cost comparison of kyphoplasty and percutaneous vertebroplasty using German claims data. Spine (Phila Pa 1976) 2014;39(4):318–326. doi: 10.1097/BRS.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 12.Zampini JM, White AP, McGuire KJ. Comparison of 5766 vertebral compression fractures treated with or without kyphoplasty. Clin Orthop Relat Res. 2010;468(7):1773–1780. doi: 10.1007/s11999-010-1279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edidin AA, Ong KL, Lau E, Kurtz SM. Morbidity and mortality after vertebral fractures: comparison of vertebral augmentation and nonoperative management in the medicare population. Spine (Phila Pa 1976) 2015;40(15):1228–1241. doi: 10.1097/BRS.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 14.Tutton SM, Pflugmacher R, Davidian M, Beall DP, Facchini FR, Garfin SR. KAST. Study: the Kiva system as a vertebral augmentation treatment—a safety and effectiveness trial: a randomized, non-inferiority trial comparing the Kiva system to balloon kyphoplasty in treatment of osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 2015;40(12):865–875. doi: 10.1097/BRS.0000000000000906. [DOI] [PubMed] [Google Scholar]
- 15.Werner CM, Osterhoff G, Schlickeiser J, et al. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures. J Bone Joint Surg Am. 2013;95(7):577–584. doi: 10.2106/JBJS.L.00024. [DOI] [PubMed] [Google Scholar]
- 16.Noriega DC, Ramajo RH, Lite IS, et al. Safety and clinical performance of kyphoplasty and SpineJack(®) procedures in the treatment of osteoporotic vertebral compression fractures: a pilot, monocentric, investigator-initiated study. Osteoporos Int. 2016;27(6):2047–2055. doi: 10.1007/s00198-016-3494-x. [DOI] [PubMed] [Google Scholar]
- 17.Krüger A, Oberkircher L, Figiel J, et al. Height restoration of osteoporotic vertebral compression fractures using different intravertebral reduction devices: a cadaveric study. Spine J. 2015;15(5):1092–1098. doi: 10.1016/j.spinee.2013.06.094. [DOI] [PubMed] [Google Scholar]
- 18.Jensen ME, McGraw JK, Cardella JF, Hirsch JA. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, and American Society of Spine Radiology. J Neurointerv Surg. 2009;1(2):181–185. doi: 10.1016/j.jvir.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–568. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 20.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569–579. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark W, Bird P, Gonski P, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10052):1408–1416. doi: 10.1016/S0140-6736(16)31341-1. [DOI] [PubMed] [Google Scholar]
- 22.Hansen EJ, Simony A, Rousing R, Carreon LY, Tropp H, Andersen MO. Double blind placebo-controlled trial of percutaneous vertebroplasty (VOPE) Global Spine J. 2016;6(suppl 1) doi: 10.1055/s-0036-1582763. GO106. [DOI] [Google Scholar]
- 23.Endres S, Badura A. Shield kyphoplasty through a unipedicular approach compared to vertebroplasty and balloon kyphoplasty in osteoporotic thoracolumbar fracture: a prospective randomized study. Orthop Traumatol Surg Res. 2012;98(3):334–340. doi: 10.1016/j.otsr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Korovessis P, Vardakastanis K, Repantis T, Vitsas V. Balloon kyphoplasty versus KIVA vertebral augmentation—comparison of 2 techniques for osteoporotic vertebral body fractures: a prospective randomized study. Spine (Phila Pa 1976) 2013;38(4):292–299. doi: 10.1097/BRS.0b013e31826b3aef. [DOI] [PubMed] [Google Scholar]
- 25.Otten LA, Bornemn R, Jansen TR, et al. Comparison of balloon kyphoplasty with the new Kiva® VCF system for the treatment of vertebral compression fractures. Pain Physician. 2013;16(5):E505–E512. [PubMed] [Google Scholar]
- 26.Borgström F, Olafsson G, Ström O, et al. The impact of different health dimensions on overall quality of life related to kyphoplasty and nonsurgical management. Osteoporos Int. 2013;24(7):1991–1999. doi: 10.1007/s00198-012-2237-x. [DOI] [PubMed] [Google Scholar]
- 27.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(00):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boonen S, Van Meirhaeghe J, Bastian L, et al. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res. 2011;26(7):1627–1637. doi: 10.1002/jbmr.364. [DOI] [PubMed] [Google Scholar]
- 29.Dohm M, Black CM, Dacre A, et al. A randomized trial comparing balloon kyphoplasty and vertebroplasty for vertebral compression fractures due to osteoporosis. AJNR Am J Neuroradiol. 2014;35(12):2227–2236. doi: 10.3174/ajnr.A4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong R, Chen L, Tang T, et al. Pain reduction following vertebroplasty and kyphoplasty. Int Orthop. 2013;37(1):83–87. doi: 10.1007/s00264-012-1709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine. 2011;14(5):561–569. doi: 10.3171/2010.12.SPINE10286. [DOI] [PubMed] [Google Scholar]
- 32.Folman Y, Shabat S. A comparison of two new technologies for percutaneous vertebral augmentation: confidence vertebroplasty vs. sky kyphoplasty. Isr Med Assoc J. 2011;13(7):394–397. [PubMed] [Google Scholar]
- 33.Fritzell P, Ohlin A, Borgström F. Cost-effectiveness of balloon kyphoplasty versus standard medical treatment in patients with osteoporotic vertebral compression fracture: a Swedish multicenter randomized controlled trial with 2-year follow-up. Spine (Phila Pa 1976) 2011;36(26):2243–2251. doi: 10.1097/BRS.0b013e3182322d0f. [DOI] [PubMed] [Google Scholar]
- 34.Kroon F, Staples M, Ebeling PR, et al. Two-year results of a randomized placebo-controlled trial of vertebroplasty for acute osteoporotic vertebral fractures. J Bone Miner Res. 2014;29(6):1346–1355. doi: 10.1002/jbmr.2157. [DOI] [PubMed] [Google Scholar]
- 35.Lee HM, Park SY, Lee SH, et al. Comparative analysis of clinical outcomes in patients with osteoporotic vertebral compression fractures (OVCFs): conservative treatment versus balloon kyphoplasty. Spine J. 2012;12(11):998–1005. doi: 10.1016/j.spinee.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Yang H, Tang T, et al. Comparison of kyphoplasty and vertebroplasty for treatment of painful osteoporotic vertebral compression fractures: twelve-month follow-up in a prospective nonrandomized comparative study. J Spinal Disord Tech. 2012;25(3):142–149. doi: 10.1097/BSD.0b013e318213c113. [DOI] [PubMed] [Google Scholar]
- 37.Liu JT, Li CS, Chang CS, Liao WJ. Long-term follow-up study of osteoporotic vertebral compression fracture treated using balloon kyphoplasty and vertebroplasty. J Neurosurg Spine. 2015;23(1):94–98. doi: 10.3171/2014.11.SPINE14579. [DOI] [PubMed] [Google Scholar]
- 38.Movrin I. Adjacent level fracture after osteoporotic vertebral compression fracture: a nonrandomized prospective study comparing balloon kyphoplasty with conservative therapy. Wien Klin Wochenschr. 2012;124(9–10):304–311. doi: 10.1007/s00508-012-0167-4. [DOI] [PubMed] [Google Scholar]
- 39.Nakano M, Kawaguchi Y, Kimura T, Hirano N. Transpedicular vertebroplasty after intravertebral cavity formation versus conservative treatment for osteoporotic burst fractures. Spine J. 2014;14(1):39–48. doi: 10.1016/j.spinee.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Staples MP, Howe BM, Ringler MD, et al. New vertebral fractures after vertebroplasty: 2-year results from a randomised controlled trial. Arch Osteoporos. 2015;10:229. doi: 10.1007/s11657-015-0229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Meirhaeghe J, Bastian L, Boonen S, et al. A randomized trial of balloon kyphoplasty and nonsurgical management for treating acute vertebral compression fractures: vertebral body kyphosis correction and surgical parameters. Spine (Phila Pa 1976) 2013;38(12):971–983. doi: 10.1097/BRS.0b013e31828e8e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CH, Ma JZ, Zhang CC, Nie L. Comparison of high-viscosity cement vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Pain Physician. 2015;18(2):E187–E194. [PubMed] [Google Scholar]
- 43.Yang DH, Cho KH, Chung YS, Kim YR. Effect of vertebroplasty with bone filler device and comparison with balloon kyphoplasty. Eur Spine J. 2014;23(12):2718–2725. doi: 10.1007/s00586-014-3379-7. [DOI] [PubMed] [Google Scholar]
- 44.Yokoyama K, Kawanishi M, Yamada M, et al. In not only vertebroplasty but also kyphoplasty, the resolution of vertebral deformities depends on vertebral mobility. AJNR Am J Neuroradiol. 2013;34(7):1474–1478. doi: 10.3174/ajnr.A3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright JG. Levels of evidence and grades of recommendations. http://www2.aaos.org/bulletin/apr05/fline9.asp Accessed Month DD, YYYY.
- 46.Morrison WB, Parker L, Frangos AJ, Carrino JA. Vertebroplasty in the Unites States: guidance method and provider distribution, 2001–2003. Radiology. 2007;243(1):166–170. doi: 10.1148/radiol.2431060045. https://doi.org/10/1148.radiol.2431060045. [DOI] [PubMed] [Google Scholar]
- 47.Brett AS. Use of vertebroplasy and kyphoplasty declined in 2010. J Watch General. 2013;201(00):237. [Google Scholar]
- 48.Long SS, Morrison WB, Parker L. Vertebroplasty and kyphoplasty in the United States: provider distribution and guidance method, 2001–2010. AJR Am J Roentgenol. 2012;199(6):1358–1364. doi: 10.2214/AJR.12.8733. [DOI] [PubMed] [Google Scholar]
- 49.Leake CB, Brinjikji W, Cloft HJ, Kallmes DF. Trends in inpatient spine augmentation: 2001–2008. AJNR Am J Neuroradiol. 2011;32(8):1464–1468. doi: 10.3174/ajnr.A2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barr JD, Jensen ME, Hirsch JA, et al. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the Society of Interventional Radiology (SIR), American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS) J Vasc Interv Radiol. 2014;25(2):171–181. doi: 10.1016/j.jvir.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Esses SI, McGuire R, Jenkins J, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the treatement of osteoporotic spinal compression fractures. J Bone Joint Surg Am. 2011;93(20):1934–1936. doi: 10.2106/JBJS.9320ebo. [DOI] [PubMed] [Google Scholar]
- 52.Papanastassiou ID, Phillips FM, Van Meirhaeghe J, et al. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur Spine J. 2012;21(9):1826–1843. doi: 10.1007/s00586-012-2314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson PA, Froyshteter AB, Tontz WL., Jr Meta-analysis of vertebral agumenation compared with conservative treatement for osteoporotic spinal fractures. J Bone Miner Res. 2013;28(2):372–382. doi: 10.1002/jbmr.1762. [DOI] [PubMed] [Google Scholar]
- 54.Bono CM, Ghiselli G, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders. Spine J. 2011;11(1):64–72. doi: 10.1016/j.spinee.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Buchbinder R, Golmohammadi K, Johnston RV, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst Rev. 2015;(4) doi: 10.1002/14651858.CD006349.pub2. CD006349. [DOI] [PubMed] [Google Scholar]
- 56.Ong KL, Lau E, Kemner JE, Kurtz SM. Two-year cost comparison of vertebroplasty and kyphoplasty for the treatement of vertebral compression fractures: are initial surgical costs misleading? Osteoporos Int. 2013;24(4):1437–1445. doi: 10.1007/s00198-012-2100-0. [DOI] [PubMed] [Google Scholar]
- 57.Stöm O, Borgström F, Kanis JA, et al. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2011;6(1–2):59–155. doi: 10.1007/s11657-011-0060-1. [DOI] [PubMed] [Google Scholar]
- 58.Svedbom A, Alvares L, Cooper C, Marsh D, Ström O. Balloon kyphoplasty compared to vertebroplasty and nonsurgical management in patients hospitalised with acute osteoporotic vertebral compression fracture: a UK cost-effectiveness analysis. Osteoporos Int. 2013;24(1):355–367. doi: 10.1007/s00198-012-2102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376(9746):1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 60.Mattie R, Laimi K, Yu S, Saltychev M. Comparing percutaneous vertebroplasty and conservative therapy for treating osteoporotic compression fractures in the thoracic and lumbar spine: a systematic review and meta-analysis. J Bone Joint Surg Am. 2016;98(12):1041–1051. doi: 10.2106/JBJS.15.00425. [DOI] [PubMed] [Google Scholar]
- 61.Beall DP, Chambers MF, Thomas SM, et al. EVOLVE: A prospective multicenter evaluation of quality of life, pain & activities of daily living outcomes for balloon kyphoplasty in the treatment of medicare patients with vertebral compression fractures. Evidence-Based Spine Interventions Seminar. 2017. , Palm Springs, CA, January 27–28,