Abstract
Preclinical studies in animals often require frequent blood sampling over prolonged periods. A preferred method in rats is the implantation of a polyurethane catheter into the jugular vein, with heparinized glycerol as a lock solution. However, analysis of various biologic compounds (for example, microRNA) precludes the use of heparin. We used sodium citrate as an alternative to heparin but observed more frequent loss of catheter patency. We hypothesized that this effect was due to evaporation of lock solution at the exteriorized portion of the catheter, subsequent blood infiltration into the catheter, and ultimately clot formation within the catheter. We therefore tested evaporation and its variables in vitro by using 5 common catheter materials. We used the migration of dye into vertically anchored catheters as a measure of lock displacement due to evaporation. Exposure to dry room-temperature air was sufficient to cause dye migration against gravity, whereas a humid environment and adding glycerol to the lock solution mitigated this effect, thus confirming loss of the lock solution from the catheter by evaporation. We tested 4 catheter treatments for the ability to reduce lock evaporation. Results were validated in vivo by using male Sprague–Dawley rats (n = 12) implanted with polyurethane jugular vein catheters and randomized to receive a nitrocellulose-based coating on the exteriorized portion of the catheter. Coating the catheters significantly improved patency, as indicated by a Kaplan–Meier log-rank hazard ratio greater than 5 in untreated catheters. We here demonstrate that a simple nitrocellulose coating reduces evaporation from and thus prolongs the patency of polyurethane catheters in rats.
Polyurethane catheters are a preferred tool for the collection of repeated blood samples necessary for preclinical pharmacokinetic studies in rats.7,16 Indwelling catheters provide the ability for frequent sampling (often 7 to 10 samples) over an extended time course (minutes to days) within the same host animal to allow for appropriate determination of pharmacokinetic parameters such as AUC, half-life, and clearance. Such preclinical studies often use a ‘locking solution’ consisting of the anticoagulant heparin in a glycerol or dextrose solution.20,22,25,26
Heparin is a broadly compatible anticoagulant for the accurate quantification of drugs in blood,14 but it is unsuitable for the study of some biologics. Blood-borne microRNA and extracellular vesicles are studied for their potential as both treatments for and biomarker predictors of human disease, and determining their kinetic parameters is an emerging field in human research. The International Society for Extracellular Vesicles discourages the use of heparin as a locking solution24 because it blocks the uptake of microRNA and exosomes from the blood into cells.1,4 Heparin also interferes with downstream analysis of microRNA extracted from the blood2,12 by inhibiting the RNA and DNA polymerases necessary for PCR amplification and next-generation sequencing assays. The use of heparinase can partially overcome this inhibition. 2,12,13,15
Alternative anticoagulants to heparin that interfere less with both the endogenous behavior of exosomes and downstream analysis of microRNA are the calcium chelators EDTA and sodium citrate.13 One minor limitation is that calcium chelation of stored blood may negatively influence the recovery of exosomes from samples.11 Sodium citrate is a weaker calcium chelator than EDTA, resulting in improved recovery. Sodium citrate has limited effects on the analysis of microRNA.13 Moreover, unlike EDTA, sodium citrate is commercially available as a sterile solution. Of all available anticoagulants, sodium citrate has the fewest limitations for the study of biologics.
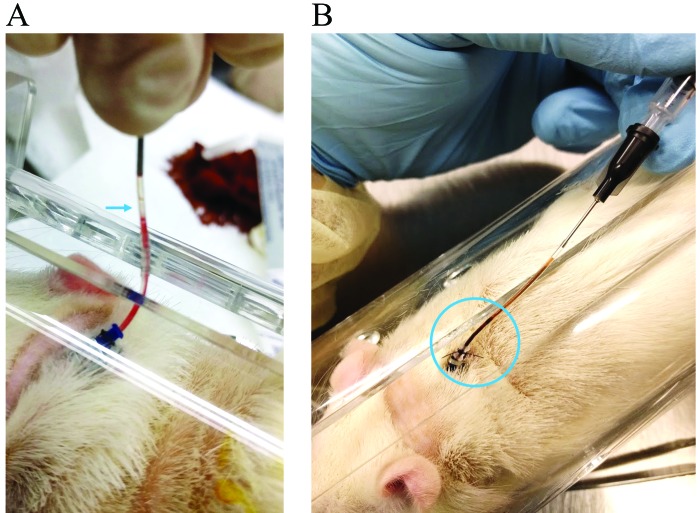
In our preliminary work studying microRNA kinetics in rats, blood samples were drawn over a 1-wk period, at 15 and 30 min and 1, 2, 4, 8, 12, 24, 72, and 128 h. We found that catheters locked with sodium citrate solution frequently prematurely (that is, between the 24- to 72-h collections) lost patency due to blood clots (Figure 1). Although citrate is viewed as comparable to heparin in its anticoagulant properties, other investigators have reported a nonsignificant increase in thromboses in citrate-locked hemodialysis catheters in humans.19 This effect was in agreement with our observations involving polyurethane catheters in rats. Furthermore, we noted that clotted blood became visible in the externalized catheter even before we removed the anticipated volume of locking solution (Figure 1 B), and sometimes, prior to sample withdrawal, we noticed that much of the external portion of the catheter was devoid of locking solution (Figure 1 A). This situation suggested that catheters perhaps were damaged through repeated sampling; however, removed catheters were intact, and no leaks were discovered when they were flushed with saline.
Figure 1.
(A) Air bubble located in a sodium citrate-locked polyurethane jugular vein catheter 48 h after flushing and locking the catheter. (B) Clotted blood visible in a sodium-citrate locked catheter 72 h after flushing and locking.
We hypothesized that loss of patency was due to direct evaporation of the sodium citrate locking solution from the porous polyurethane catheters. To test this hypothesis, we compared 5 common catheter materials (polyurethane, renathane, polyethylene, silicone, and polytetrafluoroethylene) under 2 in vitro conditions to influence the rate of lock evaporation. The evaporation of water is dependent on the humidity of the surrounding air, and we tested this condition by placing catheters in artificially constructed humidity chambers. Glycerol, a common locking solution additive, is hygroscopic,8 so we tested whether the condition of adding 30% glycerol to 4% sodium citrate rendered locks resistant to evaporation. We judged polyurethane to be the best pragmatic material for the jugular vein catheterizations needed in our laboratory, and we proceeded to test several catheter coatings to reduce evaporation. We then compared several external catheter treatments by evaluating polyurethane catheters in vitro to identify a practical method of reducing the evaporation of lock solution. To confirm our in vitro findings, we performed a study in Sprague–Dawley rats. Here we present a simple means of extending the patency of sodium-citrate–locked catheters.
Materials and Methods
Animals.
Adult male Hsd:Sprague–Dawley rats (pilot study, n = 2; main study, n = 12; weight, greater than 350 g; Envigo, Indianapolis, IN) were housed individually or in pairs under standard environmental conditions (22 ± 2 °C; 12:12-h light:dark cycle [lights on, 0700]; 30% to 70% relative humidity). Rats were acclimated for 14 d after arrival, prior to surgical manipulations. After surgical implantation of catheters, all rats were individually housed in individually ventilated microisolation shoebox caging with direct-contact bedding (Sani-Chip, Envigo, Indianapolis, IN). Food (6.2% fat, 18.6% protein, 3.5% fiber, catalog no. 2018SX, Teklad, Madison, WI) and water were provided without restriction. The colony was screened quarterly for the following pathogens by using indirect sentinels and serology or PCR testing: rat parvoviruses (Kilham rat virus, rat parvovirus, Toolan H1 virus, rat minute virus), rat coronavirus, rat theliovirus, Clostridium piliforme, ectoparasites (Radfordia ensifera, Ornithonyssus bacoti), and endoparasites (Aspiculuris tetraptera, Syphacia spp.). Random colony samples of feces were tested quarterly by PCR analysis for Mycoplasma pulmonis. All serology and PCR were performed by IDEXX BioResearch (Columbia, MO). The colony was considered free of all listed pathogens during this study. All animal procedures were approved by the Indiana University School of Medicine IACUC and were performed in an AAALAC-accredited facility in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.10,17
Catheter materials and locking solutions.
We used 5 catheter materials (Table 1) in this study. Polyurethane, polyethylene, polytetrafluoroethylene, and silicone catheter tubing samples were obtained from Access Technologies (Skokie, IL), and microrenathane tubing was a gift from Amanda Fisher (Department of Anesthesia, IN University School of Medicine, Indianapolis, IN). The 4% sodium citrate–30% glycerol lock solution was formulated by Custom Med Apothecary (Indianapolis, IN); 0.5% lidocaine was obtained through Indiana University's Laboratory Animal Resource Center Distribution Center; 4% sodium citrate (catalog no. 4B7867Q, Fenwal, Lake Zurich, IL) was obtained from the Investigational Drug Services Pharmacy at Indiana University Health.
Table 1.
Catheters used in this study
| Material | Vendor | Product no. | Lot no. | Interior diameter | Exterior diameter |
| Polyurethane | Access Technologies | BC-3.5P | 111816NS | 0.025 in. (0.6 mm) | 0.044 in. (1.1 mm) |
| Renathane | Braintree Scientific | MRE-040 | — | 0.025 in. (0.6mm) | 0.040 in. (1.0 mm) |
| Silicone | Access Technologies | BC-4S | 101316NS | 0.025 in. (0.6 mm) | 0.047 in. (1.2 mm) |
| Polyethylene (Tygon PE50) | Access Technologies | BC-PE50 | 101716NS | 0.023 in. (0.6 mm) | 0.038 in. (1.0 mm) |
| Polytetrafluoroethylene (PTFE) | Access Technologies | BC-T21 | — | 0.022 in. (0.6 mm) | 0.042 in. (1.1 mm) |
Surgery.
After a 2-wk acclimation period, rats were implanted with jugular vein catheters. The animals were anesthetized by using isoflurane inhalation (5% induction, 2% to 3% maintenance). Body weight was measured on a digital scale, and ketoprofen (5 to 10 mg/kg SC; Zoetis, Kalamazoo, MI) was administered. Fur was shaved from the incision sites, and the sites were scrubbed aseptically 3 times with the alternating use of povidone–iodine and 70% isopropyl alcohol. A ventral cervical skin incision (1.5 cm) was made right of the midline of the necks and lightly anterior to the level of the clavicle. The jugular vein was located by blunt dissection. The vein was isolated and tied off to occlude blood flow from the head region, and 2 small drops of 0.5% lidocaine were applied. A small cut was made into the vein by using microscissors, and a round-tip catheter (polyurethane, 3.5 French, cat. no. RJVC0612A, Access Technologies) filled with saline (0.9% bacteriostatic sodium chloride USP grade, Hospira, IL) was inserted into the vein and threaded 2.5 cm toward the heart. The catheter was secured to the vein by using 4-0 silk suture (Oasis, Mettawa, IL) on both sides of the insertion site. After patency was confirmed, the catheter was tunneled subcutaneously and exteriorized at the nape of the neck. The initial surgery incision was closed with nonabsorbable 4-0 nylon suture, and the exteriorized catheter was secured with a 9-mm Autoclip (catalog no. 205016, MikRon Precision, Gardena, CA) placed between subcutaneous and exterior polyethylene dumbbell anchors. A final nonabsorbable nylon suture was used to secure the exterior anchor to the skin, and the catheter was locked with 4% sodium citrate.
Measurement of capillary uptake in vitro.
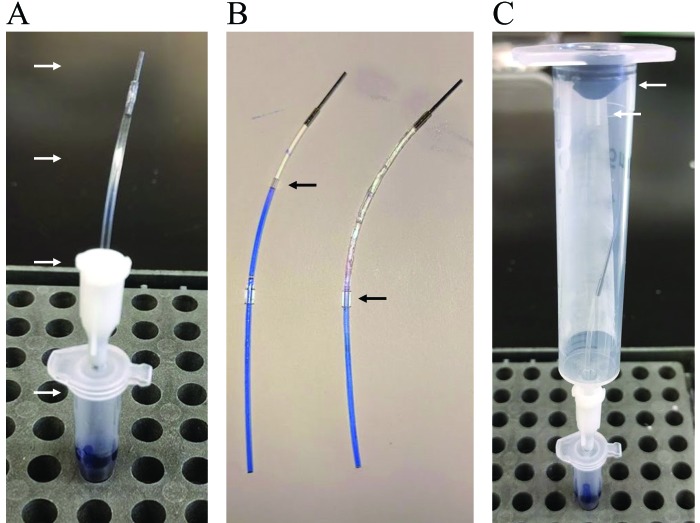
To measure capillary uptake, catheter tubing samples were cut into 7-cm segments, and each segment was fitted with a small polyurethane bead at 3 cm from one end. Each segment was filled with its appropriate locking solution, and an aluminum plug was inserted into the long end (Figure 2 A, top arrow). Fluid was lightly wicked from the short end by using laboratory tissue paper, so as to leave a minimal air gap (0.5 to 1.0 mm) between locking solution and dye (Figure 2 B, top arrow), and then the tubing was gently threaded through a blunt 17-gauge luer dispensing needle (Jensen Global, Santa Barbara, CA). The needle was likewise inserted into the top of a punctured microcentrifuge tube containing a glycerol-based dye (4× XT Sample Buffer, Bio-Rad, Hercules, CA), and the tubing was lowered so that the tip was immersed into the dye. The air gap between locking solution and dye provided a clear demarcation to measure the movement of dye into the catheter lumen; direct contact of the dye with locking solution sometimes resulted in a hazy boundary due to diffusion. Each assembled apparatus was secured in a vertical position (Figure 2 A) and remained undisturbed for the duration of the experiment, except when measurements were taken. To measure the distance of dye migration, catheters were gently removed one at a time and placed on a benchtop (Figure 2 B). A metric-scale ruler was used to measure the length of blue dye, rounded to the nearest half millimeter. Dye migration upward into the catheter against gravity represented the extent of lock solution evaporation, given that dye could migrate into the plugged catheter only to replace the lost volume.
Figure 2.
In vitro experimental design to test locking solution evaporation in catheter segments. (A) Arrows indicate from top-down: Aluminum plug, 7 cm length of catheter, luer stub needle, microcentrifuge tube containing blue glycerol-based dye. (B) Catheters after incubation at room temperature. Catheter on the left was exposed directly to air, whereas catheter on the right was given a white petrolatum-mineral oil coating. Left arrow indicates air gap purposefully placed between locking solution and dye to prevent mixing. Right arrow indicates polyurethane cuff used to mount the catheter within the luer stub. (C) Catheter placed within a constructed humidity chamber. Top arrow, rubber gasket; bottom arrow, polyethylene vent.
In vitro test conditions.
Experiments were performed under standard environmental conditions for our laboratory (22.2 ± 1 °C; 30% to 70% relative humidity). Ambient temperature and relative humidity were not actively monitored. To account for daily variability in the ambient environment, all variables were tested concurrently within each replicate experiment (n = 3 or 4).
We tested 5 different catheter materials (polyurethane, renathane, silicone, polyethylene, and polytetrafluoroethylene; Table 1) for evaporation of 4% sodium citrate lock solution by measuring displacement of an indicator dye after 24, 48, and 72 h. Two variables relevant to the evaporation process—environmental humidity and hygroscopicity of the locking solution—were altered and tested. The basic setup for measuring in vitro capillary uptake (Figure 2), as described earlier, was modified as appropriate to test various conditions. Catheters were locked with 4% sodium citrate or 4% sodium citrate–30% glycerol solution. Humidity chambers were constructed by using disposable 10-mL slip-tip syringes (Becton Dickinson, Franklin Lakes, NJ) containing laboratory tissues (Kimwipes, Kimberly-Clark, Neenah, WI) saturated with water, so that they were thoroughly damp but not dripping (Figure 2 C). The wet tissue was carefully positioned flush against the inner wall of the syringe, where it adhered. Catheters did not come into direct contact with the tissue. To ensure saturation of humidity for the duration of the experiment, syringes were sealed with rubber gaskets removed from their respective plungers, and the tissues were monitored at each measurement to confirm that they remained damp. To equilibrate air pressure, the rubber plungers were vented by puncturing and placement of a short length of Intramedic polyethylene tubing (catalog no. 427436, Clay Adams, Becton Dickinson; Figure 2 C, arrows). Where indicated, catheters were coated with white petrolatum–mineral oil (Artificial Tears, Rugby Laboratories, Livonia, MI), a nitrocellulose-based polymer (Liquid Bandage, CVS, Woonsocket, RI), or cyanoacrylate adhesive (Krazy Glue, Elmer's Products, Westerville, OH); in some cases, a 3-cm length of Intramedic polyethylene tubing was used as a protective sheath by sliding it over the wet liquid bandage or Krazy Glue.
In vivo catheter patency study.
To validate our in vitro findings in a real-world context, we opted to test the application of liquid bandage in extending the patency of rodent polyurethane jugular vein catheters subjected to intermittent blood draws. To minimize the number of animals used, we performed a pilot study using 2 male Sprague–Dawley rats to determine the appropriate timing to check for patency after each flush. The first animal was implanted with a catheter that was locked with sodium citrate and coated with liquid bandage on the exteriorized portion. The first catheter patency check was performed after a 2-d surgical recovery period, and then the catheter was flushed with saline, and locked with fresh sodium citrate. Next, this process was repeated, extending the duration between each subsequent patency check by 1 d (for example, the second patency check occurred 3 d after the first), until the catheter lost patency. In total, the catheter was checked for patency on days 2, 5, 9, and 14 after surgery. Because the catheter remained patent between days 5 and 9 (4 d between flushes) but lost patency between days 9 and 14 (5 d between flushes), we determined that a catheter coated with liquid bandage could reasonably be expected to remain patent for 4 d between flushes.
To validate the selection of 4 d between patency checks, we implanted the second rat under similar conditions, but we checked for patency on a modified schedule: days 2, 6, and 11. The catheter remained patent between days 2 and 6 (4 d between flushes) but lost patency between days 6 and 11 (5 d between flushes). Because we anecdotally had observed in previous experiments that untreated catheters lost patency after 2 d without maintenance, we determined that 4 d between patency checks would allow us to detect statistically significant differences between treated and untreated catheters.
For the main study, male Sprague–Dawley rats (n = 12) were randomly assigned to 2 groups and implanted with polyurethane jugular vein catheters that were locked with 4% sodium citrate, with 3.5 cm exteriorized at the nape of the neck. The first group of rats (n = 5) received no additional treatment and acts as controls. The catheters of the second group (n = 7) received a liquid bandage coating prior to withdrawal of anesthetic. After 2 d of surgical recovery, catheter patency was verified by placing each animal into a rodent restrainer (Stoelting, Wood Dale, IL, US), drawing 0.1 mL blood into a discarded syringe, flushing with 0.4 mL bacteriostatic saline, and locking with 0.15 mL 4% sodium citrate. All subsequent patency checks occurred at 4-d intervals until loss of patency or 24 d (26 d after surgery). Subjective difficulty was noted when additional manipulation was required to remove clotted blood from the catheter to restore patency, and clot length was estimated when possible. In the experimental group, the liquid bandage was reapplied after each patency check. To minimize backflow of blood into the catheter lumen during patency checks, positive pressure technique was used. Particular care was taken on reinserting the aluminum plug into the catheter: after infusing the 4% sodium citrate lock and clamping the catheter, we first inserted the plug approximately 1 mm, followed by unclamping of the catheter and insertion of the plug an additional 7 mm. This technique helps to clear the small amount of blood drawn into the interior tip of the catheter by the negative pressure from unclamping.
Statistics and data analysis.
For in vitro experiments, results were analyzed with Excel (Microsoft, Redmond, WA) and Prism 6 (GraphPad Software, La Jolla, CA) by using descriptive statistics, 2-way repeated measures ANOVA, and Tukey posthoc testing for multiple comparisons. For the in vivo study, animals were randomized by using an original random-integer script (using the randint function in random.py module) in Python 3.4.1. According to sample size calculations of 2 proportions using a one-sided equality with 80% power and 5% α, we determined that 5 animals per arm was sufficient to detect a 60% change in patency. Two additional animals were included to account for surgical complications and other unexpected circumstances. Results were analyzed with Prism 6, and the Kaplan–Meier log-rank test was used to compare catheter patency between the 2 groups.
Results
Comparison of catheter materials and variables pertinent to evaporation.
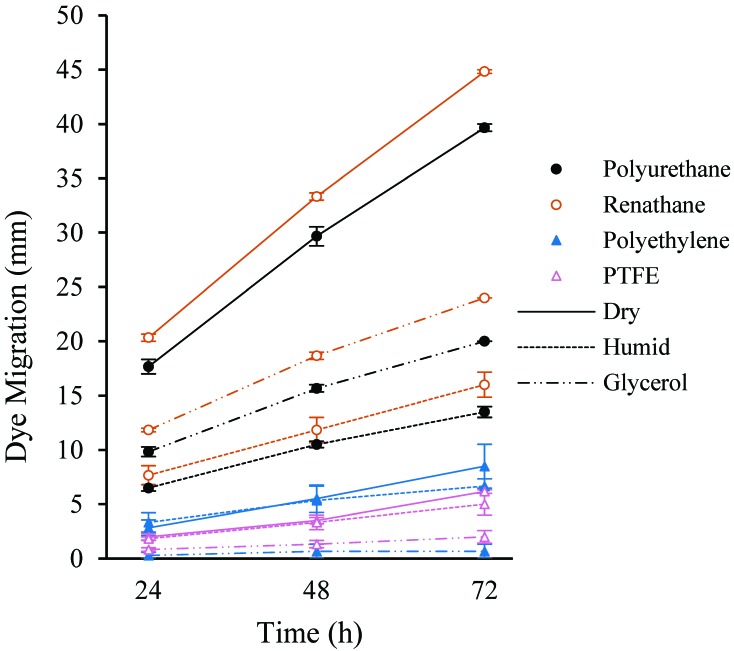
Over the entire 72-h time course, dye migrated 39.7 ± 0.3 mm (mean ± SE) in the polyurethane catheters (Figure 3, Table 2). Silicone failed to consistently retain lock solution (data not shown) and was excluded from measurement and analysis for the remainder of the study. Renathane performed comparably to polyurethane for the first 48 h but demonstrated significantly greater migration (44.8 ± 0.2 mm, P < 0.05) than all other materials by 72 h. Polyethylene and polytetrafluoroethylene performed similarly, demonstrating significantly less migration than both polyurethane and renathane over the entire time course, culminating at 8.5 ± 2.0 mm and 6.2 ± 0.2 mm, respectively (P < 0.05). In both polyurethane and renathane catheters, humidity decreased dye migration by about 35% (P < 0.05), and glycerol locking solution reduced dye migration by more than 50% (P < 0.05). Glycerol locking solution reduced dye migration by 30% to 50% in polyethylene and polytetrafluoroethylene catheters (P < 0.05).
Figure 3.
Dye migration over time (mm; mean ± SE, n = 3) in catheters of 4 different materials (PTFE, polytetrafluoroethylene) under 3 different conditions. Catheters locked with 4% sodium citrate and exposed to ambient air (Dry) were compared with those exposed to saturated humidity (Humid) and those locked with 4% sodium citrate–30% glycerol and exposed to ambient air (Glycerol).
Table 2.
Dye migration (mm) against gravity over time in catheters exposed to 3 conditions
| Polyurethane |
Polyethylene |
PTFE |
Renathane |
|||||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Dry (control) | ||||||||||||
| Days 1–3 | 17 | 30 | 40 | 3 | 6.5 | 11 | 1.5 | 3.5 | 6 | 21 | 34 | 45 |
| Days 4–6 | 17 | 28 | 39 | 1.5 | 3 | 4.5 | 2 | 3 | 6 | 20 | 33 | 45 |
| Days 7–9 | 19 | 31 | 40 | 4 | 7 | 10 | 2.5 | 4 | 6.5 | 20 | 33 | 44.5 |
| Mean | 17.7 | 29.7 | 39.7 | 2.8 | 5.5 | 8.5 | 2 | 3.5 | 6.2 | 20.3 | 33.3 | 44.8 |
| SE | 0.7 | 0.9 | 0.3 | 0.7 | 1.3 | 2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.2 |
| Humid | ||||||||||||
| Days 1–3 | 6.5 | 10.5 | 13 | 2 | 4 | 6 | 2 | 4 | 6 | 6 | 10 | 14 |
| Days 4–6 | 6 | 10 | 13 | 3 | 4 | 6 | 1.5 | 2 | 3 | 8 | 11.5 | 16 |
| Days 7–9 | 7 | 11 | 14.5 | 5 | 8 | 8 | 2 | 4 | 6 | 9 | 14 | 18 |
| Mean | 6.5 | 10.5 | 13.5 | 3.3 | 5.3 | 6.7 | 1.8 | 3.3 | 5 | 7.7 | 11.8 | 16 |
| SE | 0.3 | 0.3 | 0.5 | 0.9 | 1.3 | 0.7 | 0.2 | 0.7 | 1 | 0.9 | 1.2 | 1.2 |
| Glycerol lock | ||||||||||||
| Days 1–3 | 9 | 15 | 20 | 1 | 2 | 1 | 2 | 2 | 3 | 11.5 | 18 | 24 |
| Days 4–6 | 10 | 16 | 20 | 1.5 | 2 | 1 | 1 | 1 | 2 | 12 | 19 | 24 |
| Days 7–9 | 10.5 | 16 | 20 | 2 | 4 | 0.5 | 1 | 1 | 1 | 12 | 19 | 24 |
| Mean | 9.8 | 15.7 | 20 | 1.5 | 2.7 | 2.7 | 0.8 | 1.3 | 2 | 11.8 | 18.7 | 24 |
| SE | 0.4 | 0.3 | 0 | 0.3 | 0.7 | 0.7 | 0.2 | 0.3 | 0.6 | 0.2 | 0.3 | 0 |
In the Dry condition, catheter was locked with sodium citrate and exposed to ambient temperature and humidity. In the Humid condition, catheter was locked with 4% sodium citrate and exposed to ambient temperature in a humidity chamber . In the Glycerol Lock condition, catheter was locked with 4% sodium citrate, 50% glycerol and exposed to ambient temperature and humidity. All variables were tested concurrently within each of 3 separate experiments (n = 3; days 1–3, 4–6, and 7–9).
Treatments to reduce intralumenal evaporation in polyurethane catheters.
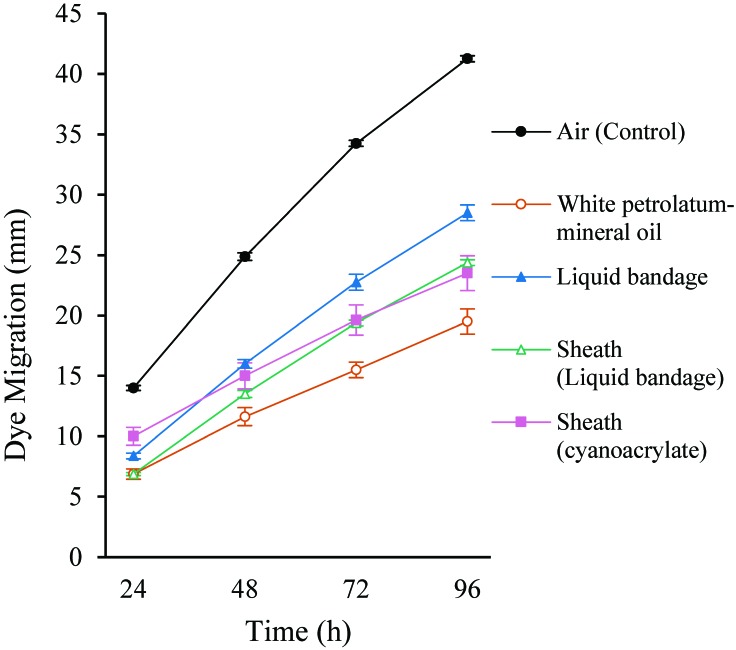
Because polyethylene and polytetrafluoroethylene are unsuitable for jugular vein implantation in rodents, and because renathane resulted in the greatest migration of dye among the materials tested, we proceeded with polyurethane as our preferred catheter material. Using the same in vitro system, we tested several treatments intended to reduce evaporation by acting as barriers to the dry-air environment (Figure 4, Table 3). All treatments were considered to be safe and convenient to perform in a surgical setting. White petrolatum–mineral oil is used to lubricate the eyes of animals under anesthesia, and liquid bandage is a topical, commercially available nitrocellulose-based polymer that is water-resistant. Cyanoacrylate adhesive is safe and quick-drying but proved unsuitable due to its fluidity and inability to coat the catheters evenly. Because cyanoacrylate did not provide a consistent layer on polyurethane and renathane catheters, it was not used as a sole treatment. Given that polyethylene demonstrated significantly less dye migration than polyurethane, we used polyethylene tubing as an external ‘sheath,’ with either liquid bandage or cyanoacrylate adhesive acting as an intracatheter sealant. In addition, we extended the time course to 96 h, with measurements taken every 24 h.
Figure 4.
Dye migration over time (mm; mean ± SE, n = 4) in polyurethane catheters locked with 4% sodium citrate exposed to ambient air. Control catheters (Air) received no other modifications; other catheters were coated with white petrolatum–mineral oil, liquid bandage, or polyethylene sheath (Sheath). Polyethylene sheaths were held in place by using liquid bandage or cyanoacrylate adhesive.
Table 3.
Dye migration (in mm) against gravity over time in 4% sodium citrate-locked polyurethane catheters exposed to ambient temperature and humidity
| Untreated (control) |
White petrolatum– mineral oil |
Liquid bandage |
Sheath with liquid bandage |
Sheath with cyanoacrylate |
||||||||||||||||
| 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | |
| Days 1–3 | 14 | 25 | 34 | 41 | 8 | 13.5 | 17 | 22 | 9 | 17 | 24.5 | 30 | 7 | 14 | 20 | 25 | 8.5 | 14 | 19 | 23 |
| Days 4–6 | 14 | 25 | 34 | 41 | 7 | 12 | 16 | 20 | 8 | 15.5 | 22 | 28 | 7 | 14 | 19.5 | 24.5 | 9.5 | 13 | 17 | 20 |
| Days 7–9 | 14.5 | 25.5 | 35 | 42 | 6.5 | 11 | 15 | 19 | 8 | 15.5 | 21.5 | 27 | 7 | 13 | 19 | 24 | 10 | 15 | 19.5 | 24 |
| Days 10–12 | 13.5 | 24 | 34 | 41 | 6 | 10 | 14 | 17 | 8.5 | 16 | 23 | 29 | 6.5 | 13 | 19 | 24 | 12 | 18 | 23 | 27 |
| Mean | 14 | 24.9 | 34.3 | 41.3 | 6.9 | 11.6 | 15.5 | 19.5 | 8.4 | 16 | 22.8 | 28.5 | 6.9 | 13.5 | 19.4 | 24.4 | 10 | 15 | 19.6 | 23.5 |
| SE | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | 0.7 | 0.6 | 1.0 | 0.2 | 0.4 | 0.7 | 0.6 | 0.1 | 0.3 | 0.2 | 0.2 | 0.7 | 1.1 | 1.2 | 1.4 |
Untreated catheters received no external coating. Treated catheters received an external layer of white petrolatum–mineral oil or liquid bandage or a polyethylene sleeve (Sheath) affixed with either liquid Bandage or cyanoacrylate. All variables were tested concurrently within each of 4 separate experiments (n = 4; days 1–3, 4–6, 7–9, and 10–12).
All 4 experimental methods resulted in significantly (P < 0.05) decreased migration of dye into the catheters. White petrolatum–mineral oil reduced dye migration to just under half the distance of untreated control catheters (19.5 ± 1. 0 mm compared with 41.3 ± 0.3 mm; P < 0.05). Liquid bandage reduced dye migration by 31% (28.5 ± 0.6 mm at 96 h; P < 0.05) compared with untreated controls but was still significantly (P < 0.05) less effective than white petrolatum–mineral oil. Placing a polyethylene sheath over the liquid bandage coating further reduced the migration of dye (24.4 ± 0.2 mm; P < 0.05) to nearly the level of white petrolatum–mineral oil, but white petrolatum–mineral oil was still more effective (P < 0.05). A polyethylene sheath with cyanoacrylate adhesive was not significantly better than sheath with liquid bandage. In summary, white petrolatum-mineral oil performed better than a polyethylene sheath, which performed better than liquid bandage, and all treatments performed better than the control.
Patency of surgically implanted jugular vein catheters with and without liquid bandage application.
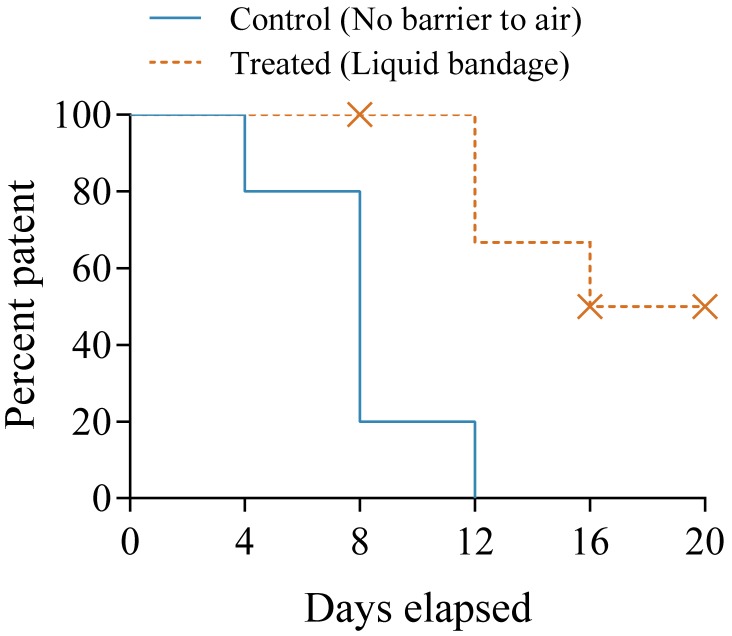
Using the pilot study results, we randomized 12 male Sprague–Dawley rats into 2 groups: an untreated control group (weight, 316.6 to 361.9 g) and a treatment group (weight, 313.5 to 363.2 g) that received a coating of liquid bandage on the exteriorized portion of the implanted catheters. After the initial 2-d surgical recovery period and flush, catheters were checked for patency every 4 d thereafter (Table 4). Kaplan–Meier estimates revealed a significant loss of patency in the control group compared with the treatment group (Figure 5), with a log-rank hazard ratio of 5.22 (95% CI, 3.74 to 95.06; P < 0.05) for untreated catheters to lose patency compared with liquid bandage-coated catheters. Four animals from the liquid bandage group were excluded from this analysis (Table 4). In one rat, the surgical clip became detached, and the catheter was pulled out approximately 1.5 cm. In another animal, the catheter became irreparably damaged during a patency check. Two catheters in the liquid bandage group remained patent and undamaged at the conclusion of the study (20 d), and were excluded from the final analysis because they did not reach the measured endpoint (loss of patency).
Table 4.
Number of patent catheters in control (n= 5) and liquid-bandage–treated (n= 7) groups and events excluded from analysis
| Days elapsed | No. patent catheters |
No. excluded events |
||
| Control | Treated | Control | Treated | |
| 0 | 5 | 7 | 0 | 0 |
| 4 | 4 | 7 | 0 | 0 |
| 8 | 1 | 7 | 0 | 1 |
| 12 | 0 | 6 | 0 | 0 |
| 16 | 0 | 4 | 0 | 1 |
| 20 | 0 | 2 | 0 | 2 |
Figure 5.
Kaplan–Meier curve for time to loss of patency in rats implanted with standard polyurethane jugular vein catheters (Control) or catheters coated with liquid bandage (Treated). X, excluded data point. Every 4 d, the catheters were flushed, and lock solution was replaced.
Discussion
Our preclinical studies involve the analysis of biologics, thus necessitating the use of sodium citrate anticoagulant locking solution in the jugular vein catheters used to obtain blood samples. We observed that locking polyurethane catheters with sodium citrate resulted in a premature loss of patency. Here we report that evaporation of lock solution causes blood to infiltrate into the catheter tip, where it can clot. Our in vitro data show that locking solution evaporates from catheters exposed to air, allowing displacement of the lost volume against gravity by a dense indicator dye. The type of catheter materials used affect evaporation, which can be reduced by placing the exposed catheter in a saturated humid environment, replacing the aqueous locking solution with a hygroscopic alternative, or by coating the catheter with a hydrophobic barrier, such as liquid bandage. We evaluated these variables to determine optimal conditions for extending the duration of patency in our in vivo studies.
In regard to catheter material, polyurethane and renathane exhibited the most migration of dye, whereas polyethylene and polytetrafluoroethylene exhibited the least. In our in vitro studies, silicone failed to retain any lock solution, despite our use of multiple sizes of aluminum plugs to ensure the ends of the catheters were sealed appropriately. Together, these results point to a relationship between the rigidity of a catheter material and its apparent susceptibility to evaporation, which may be explained by its permeability. Polyurethane has a water-vapor permeability more than 80 times higher than that of polyethylene,9 and we observed that dye migration was substantially greater in polyurethane than polyethylene catheters. Because both humidity and an external hydrophobic coating effect reduce evaporation through permeable catheter materials, our results contribute to an explanation for why subcutaneous access ports result in extended long-term patency compared with externalized catheters.6,23 Our results also support practices by research animal vendors who extend patency during shipment by tucking the exposed ends of rodent catheters into skin pockets and holding them in place with wound clips. By limiting the exposure of a permeable catheter to dry air, evaporation is reduced and blood is not displaced into the catheter, where it can clot.
However, for repeated blood sampling or intravenous injections, both the subcutaneous access port and skin-tuck method unnecessarily distress the animal. In the case of subcutaneous access ports, multiple needle punctures through the skin result in episodes of slight or momentary pain or distress. For skin-tucked catheters, the catheter must be removed from the skin pocket and replaced for each procedure; this repeated sequence of loosening the wound clip, removing the catheter, replacing the catheter, and reclamping the wound clip likewise results in multiple episodes of slight or momentary pain or distress. For practical and safety reasons, polyurethane cannot be replaced with a less-permeable material, such as polyethylene and polytetrafluoroethylene. These less-permeable materials are unsuitable for placement into the fragile jugular vein of a rat because of their rigidity. Given these limitations, we concluded that polyurethane remained the superior material among those we tested.
Supporting the role of evaporation in reducing catheter patency, we demonstrated that glycerol added to sodium citrate reduces the evaporation of locking solution compared with sodium citrate alone. Glycerol is found in locking solution formulations, where its hygroscopicity8 likely confers resistance to evaporation. Our results suggest that glycerol may very well extend catheter patency. We observed early in our studies, however, that the mixture of glycerol with blood made it difficult to visually assess the precise volume of blood drawn. This situation is problematic, because it is difficult to directly quantify microRNA or exosomes in small volumes of plasma, so the volume itself is used for normalization.2,3 For quantification of small amounts of biologics (typical in blood analysis) that require volume normalization, we recommend using citrated saline without glycerol to improve visual guidance of blood collection.
We showed that coating catheters with different hydrophobic barriers delayed the evaporation process. In selecting the best option for in vivo use, the liquid bandage had practical advantages over both the application of white petrolatum–mineral oil and the incorporation of a polyethylene sheath. First, although white petrolatum-mineral oil performed best at reducing dye migration in vitro, initial testing with a single rat suggested that the natural movements of the animal in its cage bedding caused the oil to dissociate from the catheter. Second, although the polyethylene sheath seemed promising, we were concerned that sheaths may not be practical for long-term repeated blood samplings. The process of repeated blood sampling (for example, clamping with a hemostat and removal of the aluminum plug) exposes the end of the polyurethane catheter to mechanical strain, weakening its ability to securely retain the plug. To manage this drawback, the catheter must be trimmed so that the plug can be inserted into an undistorted portion. When the polyurethane is trimmed to the point at which it is flush with the polyethylene sheath, the plug is extremely difficult to insert into the catheter. Therefore, we chose to use polyurethane catheters without sheaths for the in vivo experiments.
Our in vivo study validated the conclusions drawn from our in vitro experiments: the application of liquid bandage was able to extend the patency of sodium citrate-locked polyurethane catheters flushed at 4-d intervals. Although thromboses were still observed in the treated catheters during patency checks, they were smaller and easier to remove (data not shown). It is worth noting that the patency of indwelling catheters may also be compromised by the growth of fibrin around the intravascular catheter,5 which begins at the site of vascular incision21,25 and progresses to the catheter tip.25 The formation of fibrin sleeves is an inflammatory repair response to the insertion of the catheter, which is dependent on variables such as catheter material and shape of the catheter tip. For example, the tips of external jugular vein catheters in rats in one study exhibited surrounding tissue growth by 2 wk after implantation, which progressed to thick tissue growth by the end of the third week.25 Twice-weekly flushing maintained patency by introducing many small holes in the tissue.25 Preliminary necropsies of our own rats prior to our study reported here revealed no gross occlusion due to the growth of tissue around the catheter tip after 10 d, instead indicating intraluminal thrombosis as the chief cause for loss of patency. Taken together, we expected the loss of patency in externalized jugular vein catheters within 3 wk (21 d) to be driven primarily by thromboses. Our in vivo experiments terminated at 20 d after implantation, within this window of time.
We propose that liquid bandage is a safe and effective means of reducing the frequency at which a catheter must be flushed to maintain long-term patency. Although daily catheter maintenance is adequate for maintaining patency of polyurethane catheters locked with sodium citrate, reducing the number of times a catheter must be flushed has advantages. Unlike heparin, which may be flushed directly into the blood circulation of the animal, sodium citrate can be toxic to the animal at high exposure18 and must therefore be removed, with a small volume of blood, from the catheter and discarded. Because institutional approval typically limits the amount of blood that can be sampled from a single animal during a survival study, the cumulative loss of discarded blood from daily catheter maintenance limits the amount of blood available for sampling over an extended time course. In addition, consistent blood loss might cause unwanted hematopoietic effects. Limiting the number of times a catheter is accessed has additional benefits, including preserving the physical integrity of the line and reducing stress on the animal. Extending the patency of externalized polyurethane catheters with liquid bandage maximizes the data obtained from rodents as it minimizes pain or distress and is therefore consistent with the guiding principles (3 Rs) for the ethical use of animals in research.
In addition to these advantages, the study was associated with some noteworthy limitations. First, we performed this study by using only male rats, and we note that the potential for the rate of thrombosis might differ according to sex. Second, we observed that liquid bandage dehydrates over time, requiring repeated applications to preserve its ability to prevent sodium citrate evaporation. After multiple applications of liquid bandage, we observed that the catheter became less pliable and more difficult to visually inspect for internal (air gaps, blood) and more prone to external (physical damage) problems. After clamping and unclamping, coated catheters had a tendency to remain pinched due to the rigidity of the accumulated polymer coating. We managed this drawback by perpendicularly pinching the catheter with a hemostat to recover its shape. As a minor detail, we speculated that a polyethylene sheath might alleviate these problems with polymer accumulation by eliminating the need for multiple applications. In one animal, we attempted to place a polyethylene sheath over an implanted polyurethane catheter coated with liquid bandage. Overnight, the sheath became detached and was unable to be recovered. This effect, in addition to the concerns mentioned earlier, may render the sheath unviable.
In conclusion, by identifying evaporation of sodium citrate solution as the potential cause for decreased patency of catheters, we developed an in vitro methodology to test for ways to extend patency and reduce the frequency of maintenance checks. This feature is especially important for researchers studying heparin-sensitive biologics, such as microRNAs and exosomes, in a preclinical setting. By adopting these methods to their own protocols, other researchers might be able to extend their studies and reduce animal numbers by collecting more samples from each single animal, eliminate the confounding step of adding heparinase treatments to blood samples, and minimize animal pain and distress through refined catheter maintenance procedures.
Acknowledgment
This work was supported by NIH grant K08GM119006.
References
- 1.Atai NA, Balaj L, van Veen H, Breakefield XO, Jarzyna PA, Van Noorden CJ, Skog J, Maguire CA. 2013. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol 115:343–351. 10.1007/s11060-013-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson EA, Skaar TC. 2013. Incubation of whole blood at room temperature does not alter the plasma concentrations of microRNA-16 and -223. Drug Metab Dispos 41:1778–1781. 10.1124/dmd.113.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson EA, Skaar TC, Liu Y, Nephew KP, Matei D. 2015. Carboplatin with decitabine therapy, in recurrent platinum-resistant ovarian cancer, alters circulating miRNAs concentrations: a pilot study. PLoS One 10:1–12. 10.1371/journal.pone.0141279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. 2013. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA 110:17380–17385. 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Costanzo J, Sastre B, Choux R, Kasparian M. 1988. Mechanism of thrombogenesis during total parenteral nutrition: role of catheter composition. JPEN J Parenter Enteral Nutr 12:190–194. 10.1177/0148607188012002190. [DOI] [PubMed] [Google Scholar]
- 6.Farrow HA, Rand JS, Burgess DM, Coradini M, Vankan DM. 2013. Jugular vascular access port implantation for frequent, long-term blood sampling in cats: methodology, assessment, and comparison with jugular catheters. Res Vet Sci 95:681–686. 10.1016/j.rvsc.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt H, Fandrich F, Schaube H, Schroder J, Deltz E. 1995. A novel technique for long-term vascular access in the unrestrained rat. J Invest Surg 8:425–431. 10.3109/08941939509031608. [DOI] [PubMed] [Google Scholar]
- 8.Glycerine Producers’ Association. 1963. Physical properties of glycerine and its solutions. New York (NY): Glycerine Producers’ Association. [Google Scholar]
- 9.Hamilton RL. 1967. Water vapor permeability of polyethylene and other plastic materials. Bell Labs Tech J 46:391–415. 10.1002/j.1538-7305.1967.tb01064.x. [DOI] [Google Scholar]
- 10.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 11.Jayachandran M, Miller VM, Heit JA, Owen WG. 2012. Methodology for isolation, identification, and characterization of microvesicles in peripheral blood. J Immunol Methods 375:207–214. 10.1016/j.jim.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson ML, Navanukraw C, Grazul-Bilska AT, Reynolds LP, Redmer DA. 2003. Heparinase treatment of RNA before quantitative real-time RT-PCR. Biotechniques 35:1140–1142, 1144. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Linnstaedt S, Palma J, Park JC, Ntrivalas E, Kwak-Kim JY, Gilman-Sachs A, Beaman K, Hastings ML, Martin JN, Duelli DM. 2012. Plasma components affect accuracy of circulating cancer-related microRNA quantitation. J Mol Diagn 14:71–80. 10.1016/j.jmoldx.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni P, Karanam A, Gurjar M, Dhoble S, Naik AB, Vidhun BH, Gota V. 2016. Effect of various anticoagulants on the bioanalysis of drugs in rat blood: implication for pharmacokinetic studies of anticancer drugs. Springerplus 5:1–8. 10.1186/s40064-016-3770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Chen H, Song J, Lee C, Geng Q. 2016. Avoiding heparin inhibition in circulating microRNAs amplification. Int J Cardiol 207:92–93. 10.1016/j.ijcard.2016.01.129. [DOI] [PubMed] [Google Scholar]
- 16.Nolan TE, Klein HJ. 2002. Methods in vascular infusion biotechnology in research with rodents. ILAR J 43:175–182. 10.1093/ilar.43.3.175. [DOI] [PubMed] [Google Scholar]
- 17.Office of Laboratory Animal Welfare. 2015. Public health service policy on humane care and use of laboratory animals. Bethesda (MD): National Institutes of Health. [Google Scholar]
- 18.Oudemans-van Straaten HM, Kellum JA, Bellomo R. 2011. Clinical review: anticoagulation for continuous renal replacement therapy—heparin or citrate? Crit Care 15:1–9. 10.1186/cc9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passero BA, Zappone P, Lee HE, Novak C, Maceira EL, Naber M. 2014. Citrate versus heparin for apheresis catheter locks: an efficacy analysis. J Clin Apher 30:22–27. 10.1002/jca.21346. [DOI] [PubMed] [Google Scholar]
- 20.Peternel L, Skrajnar S, Cerne M. 2010. A comparative study of 4 permanent cannulation procedures in rats. J Pharmacol Toxicol Methods 61:20–26. 10.1016/j.vascn.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Sinno MC, Alam M. 2012. Echocardiographically detected fibrinous sheaths associated with central venous catheters. Echocardiography 29:E56–E59. 10.1111/j.1540-8175.2011.01582.x. [DOI] [PubMed] [Google Scholar]
- 22.Thrivikraman KV, Huot RL, Plotsky PM. 2002. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Brain Res Protoc 10:84–94. 10.1016/S1385-299X(02)00185-X. [DOI] [PubMed] [Google Scholar]
- 23.Tsai HL, Chang JW, Liu CS, Chin TW, Wei CF, Lee OK, Wang SJ. 2013. A newly designed total implantable venous access device in rats for research with high efficiency and low cost. J Surg Res 187:36–42. 10.1016/j.jss.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F. 2013. Standardization of sample collection, isolation, and analysis methods in extracellular vesicle research. J Extracell Vesicles 2:1–25. 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Maarek JM, Holschneider DP. 2005. In vivo quantitative assessment of catheter patency in rats. Lab Anim 39:259–268. 10.1258/0023677054307033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoburn BC, Morales R, Inturrisi CE. 1984. Chronic vascular catheterization in the rat: comparison of 3 techniques. Physiol Behav 33:89–94. 10.1016/0031-9384(84)90018-0. [DOI] [PubMed] [Google Scholar]