Abstract
Commonly used in biomedical research, vervets (Chlorocebus aethiops) are omnivorous but primarily meet their vitamin A requirements from provitamin A carotenoids. Hypervitaminosis A has occurred in vervets that consume feed high in preformed vitamin A. We investigated the vitamin A status of vervets supplemented daily with various antioxidants derived from palm oil. Male vervets (n = 40) were placed for 23 wk on a high-fat diet (34.9% energy) containing 645 μg retinol activity equivalents (RAE), with 515 μg RAE from preformed vitamin A. Vervets were randomized to 5 treatments (duration, 20 mo): control; 100 mg d-α-tocopheryl acetate; 100 mg oil palm (Elaeis guineensis)-derived vitamin E; 50 mg oil palm-derived vitamin E + 50 mg carotenoid complex + unrestricted palm-derived water-soluble antioxidants; and 5) unrestricted water-soluble antioxidants. Livers (n = 38) were analyzed for vitamin A, α-retinol (α-vitamin A), and carotenoids. Median hepatic vitamin A and total carotenoid concentrations were 6.49 μmol/g and 4.30 nmol/g, respectively. Compared with controls, vervets fed the carotenoid complex had higher hepatic vitamin A (11.9 ± 5.1 μmol/g), α-vitamin A (1.3 ± 0.7 μmol/g), α-carotene (11.5 ± 5.3 nmol/g), β-carotene (15.6 ± 8.6 nmol/g), and total carotenoids (28.1 ± 13.9 nmol/g) but lower lutein (0.66 ± 0.28 nmol/g) and zeaxanthin (0.24 ± 0.06 nmol). NHP may benefit from replacement of preformed vitamin A with carotenoids in feeds; however, bioconversion efficiency in these models should be investigated to determine optimal levels.
Abbreviations: αC, α-carotene; αRE, α-retinyl ester; βC, β-carotene; BCO1, β-carotene 15, 15′-oxygenase; HFD, high-fat diet; OPC, oil palm carotenoids; RAE, retinol activity equivalents; RE, retinyl esters
Vitamin A is a fat-soluble vitamin important for normal growth and development, immune function, and vision.35 Preformed vitamin A (that is, retinol and retinyl esters [RE]), can be obtained by consuming animal-sourced foods, fortified foods, and supplements.35 When consumed in amounts exceeding daily needs, vitamin A is stored primarily in the liver. The central cleavage of the provitamin A carotenoids, such as α-carotene (αC), β-cryptoxanthin, and β-carotene (βC), by the cytosolic enzyme β-carotene-15, 15′-oxygenase (BCO1) yields 1, 1, and 2 molecules of retinal, respectively, which is subsequently reduced to retinol. BCO1 and scavenger receptor B1 are regulated by dietary vitamin A by a negative feedback loop involving the transcription factor intestine-specific homeobox.26,43
Vervets (Chlorocebus aethiops) are commonly used in biomedical research2 because they are not endangered, are easily handled, are a good model for hypertension,8 and have been used in studying the effects of dietary lipids on plasma lipoprotein metabolism and atherosclerosis.1,53,54 Unlike other NHP species, they have done relatively well in weathering the loss of habitat perhaps due to their ability to adapt their diets to suit their environment,18 such as raiding vegetable, cereal, and fruit crops.40 Vervets are naturally omnivorous, consuming leaves, seeds, grasses, fruits, bird eggs, birds, lizards, rodents, and invertebrates, with a preference for fruits and flowers.10,19
The vitamin A requirements of vervets and other NHP have not been defined precisely and are based partly on the Institute of Medicine's estimated average daily requirement for adult male humans (625 μg retinol activity equivalents [μg RAE]).20 Diets containing 10,000 IU (3000 μg RAE as preformed vitamin A) per kilogram of dry matter are generally considered to be adequate; however, little evidence is available to justify this concentration.34 Very high vitamin A hepatic stores have been reported in NHP consuming common feeds formulated with preformed vitamin A.31,37,38 Considering that the bioconversion of provitamin A carotenoid to vitamin A is regulated in humans (and presumably in NHP species as well) and that free-living vervets likely consume most of their vitamin A as provitamin A carotenoids in nature, some authors suggest that providing vitamin A as carotenoids in feeds may be safer.5 Moreover, the potential effects of hypervitaminotic but subtoxic vitamin A hepatic stores on biomarkers of status and physiology, such as elevated circulating RE and catabolism, are largely unknown.
Here we investigated the vitamin A status of vervets on a high-fat diet (HFD) supplemented with various antioxidant preparations derived from the palm fruit, one of which included a natural mixed carotenoid complex, for an extended period. Carotenoid intake may protect against many chronic diseases, including atherosclerosis.6,21 Liver samples from these vervets were analyzed for vitamin A, α-retinol species (α-vitamin A), and carotenoid concentrations to further investigate the vitamin A status of NHP housed for biomedical research. In addition to βC, red palm oil contains αC that produces α-retinol upon central cleavage in addition to retinol.
Materials and Methods
Research animals.
Adult male vervets (Chlorocebus aethiops; n = 40 total) were recruited from the South African Medical Research Council Primate Unit's inhouse breeding colony; 30 of these animals were bred inhouse, and the remaining 10 originally were wild-caught with permission from Nature Conservation as part of a study examining the effect of dietary antioxidants on the progression of atherosclerosis. The treatment protocols were reviewed and approved by the Ethics Committee for Research on Animals of the South African Medical Research Council. The holding facilities and animal health and welfare were inspected twice annually by the local Society for the Prevention of Cruelty to Animals. In addition, the Ethics Committee performed postapproval monitoring ensuring adherence to the procedures and activities outlined in the study protocol.
Vervets were housed in a closed sterile environment with 15 to 20 air changes hourly. The room air temperature was maintained at 23 to 26 °C, with 45% to 50% humidity and a 12:12-h photoperiod; fresh water was available automatically and without restriction. For the duration of the study, housing was in single cages (900 × 700 × 1200 cm), which are fitted with full perches and mesh grooming panels to allow social interaction with adjacent animals. In addition, each vervet had access to a large exercise cage for 24 h weekly. Separate foraging devices were offered 3 times each week to encourage foraging. All procedures carried out with the vervets, including handling and care, were performed by the same qualified people. All vervets were housed according to the Primate Unit's standard operating procedures and in accordance with the revised South African National Standard for the Care and Use of Animals for Scientific Purposes.45 The Primate Unit at the South African Medical Research Council is accredited by the South African Veterinary Council (registration no. FR15/13973), and its research and veterinary care are regulated by the South African Bureau of Standards to ensure compliance with legislation and international guidelines for animal welfare.
Experimental feeds.
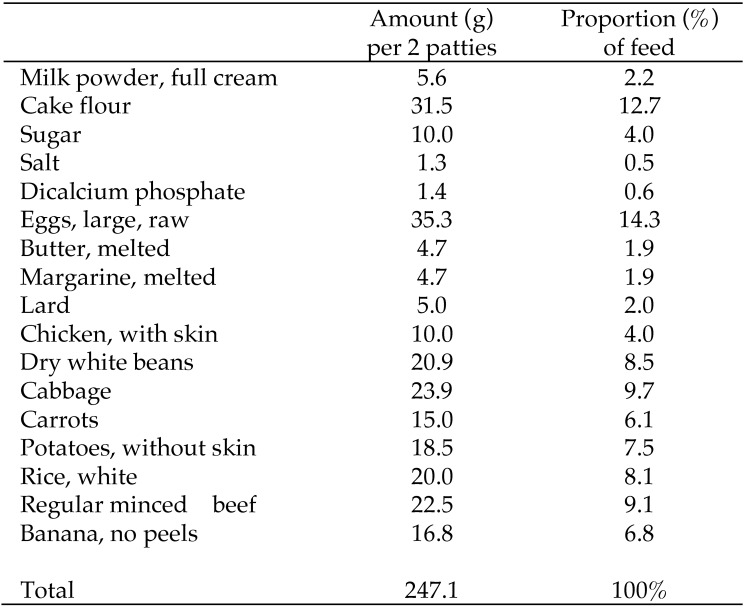
Typical experimental feed details have been published elsewhere.53 Briefly, all 40 vervets were placed on a high-fat diet (HFD; 34.9% energy from fat), beginning in December 2003, for 23 wk until baseline (May 2004) as a run-in period to acclimate them to procedures. The HFD was formulated inhouse from household ingredients and vegetables; including bananas, lard, margarine, butter, beef mince, chicken with skin, full-cream milk powder, egg, rice, dried beans, cake flour, sugar, salt, cabbage, potato, carrot, and rice (Figure 1). HFD ingredients were baked into ‘patties’ (92.2 ± 6.7 g each) and stored at –20 °C until use. Patties were served immediately after thawing at room temperature; vervets received one patty twice daily. Vervets typically consumed the entire meal, with minimal wastage on high-fat formulated diets (between 4.1% and 6.1%).54 In addition, the HFD was supplemented daily with minerals and vitamins to optimize the micronutrient intake of the vervets. A piece of raw apple (approximately 70 g) was supplied daily to each vervet as part of the foraging enrichment. Suppliers of the food ingredients were kept constant.
Figure 1.
Composition of the high-fat diet composition. Batches were mixed and baked into patties and stored at -20°C until use. Adult male vervets (Chlorocebus aethiops) consumed 2 patties daily.
Vervets were randomized to 1 of 5 (n = 8 per group) experimental treatments, with 2 of the wild-caught vervets assigned to each group: 1) HFD control (no supplements), 2) HFD + 100 mg d-α-tocopheryl acetate (commercial product; Hoffman-La Roche, Basel, Switzerland) daily, 3) HFD + 100 mg vitamin E derived from oil palm (Tocomin 50% oil suspension; natural full-spectrum tocotrienol–tocopherol complex; Carotech, Naples, FL) daily, 4) HFD + 50 mg oil palm vitamin E + 50 mg oil palm-derived carotenoid complex (OPC; Caromin 20% concentrate, Carotech; natural mixed carotenoid complex consisting mainly of αC, βC, and lycopene with approximately 240,000 IU vitamin A/g) daily + free-choice oil-palm–derived water-soluble antioxidants (as an E. guineensis juice) provided after thawing from the frozen state in addition to the drinking water supply, and 5) HFD + free-choice oil-palm–derived water-soluble antioxidants as undiluted palm juice (as for treatment 4) in addition to the water supply. Vervets consumed these treatments for 20 mo. For experimental patties containing commercial vitamin E, oil palm-derived vitamin E, and OPC, supplements were mixed with the fat portion of the food before incorporation into the full recipe to ensure optimal mixing. Water-soluble supplements were chosen because they could be purified and recovered from the palm-waste manufacturing process. Palm juice is a water-soluble extract rich in phenolic acids recovered from the vegetation liquor generated from the milling of oil-palm fruits46 and contains substantial amounts of caffeic, p-hydroxybenzoic, and caffeoylshikimic acids.41 For treatments 4 and 5, palm juice was initiated 1 wk after initiation of study treatments. Palm juice, Tocomin 50%, and Carmin 20% (both from Carotech) were couriered to the South African Medical Research Council in batches as needed. Palm juice was stored at –20 °C and thawed as needed, whereas the fat-soluble supplements were kept at 4 °C until used to formulate study treatments.
Dietary vitamin A and provitamin A carotenoid content.
The Medical Research Council's Food Composition Tables25 were used to compile the diets and calculate the quantities and nutrient energy of the experimental HFD. Estimates of vitamin A and carotenoid contents were calculated by using ingredient equivalents compiled from the US Department of Agriculture's National Nutrient Database for Standard Reference Release 28 (Table 1).51 The HFD contained 645 μg RAE per vervet daily (amount in 2 patties), with 145 μg as preformed vitamin A from the food and 370 μg as preformed vitamin A from the vitamin mix (thus, 515 μg RAE as total preformed A each day). The HFD provided 1290 μg βC, 530 μg αC, 3 μg β-cryptoxanthin, and 240 μg lutein + zeaxanthin daily.
Table 1.
Dietary vitamin A (VA) and carotenoid content as estimated by ingredients in the USDA National Nutrient Database for Standard Reference Release 28a
| Database no. | Amount (μg, except VA [RAE]) in 100 g of ingredient |
Amount (μg, except VA [RAE]) per vervet daily (2 patties) |
|||||||||||
| Retinolb | VA | βC | αC | βCX | L+Z | Retinolb | VA | βC | αC | βCX | L+Z | ||
| Milk, dry, whole, without added vitamin D | 01212 | 253 | 258 | 55 | 0 | 0 | 0 | 14 | 14 | 3 | 0 | 0 | 0 |
| Wheat flour, white, cake, enriched | 20084 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 |
| Granulated sugar | 45105765 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Salt | 45079336 | 0 | 0 | 0 | 0 | 0 | 0 | . | . | . | . | . | . |
| Egg, whole, raw, fresh | 01123 | 160 | 160 | 0 | 0 | 9 | 503 | 57 | 57 | 0 | 0 | 3 | 178 |
| Butter, salted | 01001 | 671 | 684 | 158 | 0 | 0 | 0 | 32 | 32 | 7 | 0 | 0 | 0 |
| Margarine, regular, hard, soybean (hydrogenated) | 04073 | 768 | 819 | 610 | 0 | 0 | 0 | 36 | 39 | 29 | 0 | 0 | 0 |
| Lard | 04002 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chicken, skin (drumsticks and thighs), cooked, roasted | 05675 | 54 | 54 | 0 | 0 | 1 | 91 | 5 | 5 | 0 | 0 | 0 | 9 |
| White kidney beans | 45122317 | . | 0 | . | . | . | . | . | 0 | . | . | . | . |
| Cabbage, raw | 11109 | 0 | 5 | 42 | 33 | 0 | 30 | 0 | 1 | 10 | 8 | 0 | 7 |
| Carrots, raw | 11124 | 0 | 835 | 8285 | 3477 | 0 | 256 | 0 | 125 | 1240 | 520 | 0 | 38 |
| Potatoes, boiled, cooked without skin, flesh, without salt | 11367 | 0 | 0 | 1 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 2 |
| Rice, white, medium-grain, cooked, unenriched | 20451 | 0 | 0 | . | . | . | . | 0 | 0 | . | . | . | . |
| Beef, ground, unspecified fat content, cooked | 23220 | 7 | 7 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| Bananas, raw | 09040 | 0 | 3 | 26 | 25 | 0 | 22 | 0 | 1 | 4 | 4 | 0 | 4 |
| Total | 1913 | 2825 | 9177 | 3535 | 10 | 914 | 145 | 275 | 1293 | 532 | 3 | 239 | |
αC, α-carotene; βC, β-carotene; βCX, β-cryptoxanthin; L+Z, lutein and zeaxanthin
Available at: https://ndb.nal.usda.gov/ndb/
The vitamin mix added to the patties contained an additional 370 µg preformed vitamin A.
Euthanasia and sample collection.
Vervets were euthanized in May 2006 according to standard, routine necropsy procedures at this facility.12,13,15,54 After premedication (such as that used for routine blood sampling: 10 mg/kg IM ketamine), animals were deeply anesthetized by using 2.5% fluothane and 1% oxygen. During exsanguination, the vasculature was flushed with heparinized saline. Liver samples (site not specified) were collected before perfusion fixation, frozen at –80 °C, and shipped on dry ice to the Department of Nutritional Sciences at the University of Wisconsin-Madison, where they were stored at –80 °C until analysis. For perfusion fixation, fixative containing 1% glutaraldehyde and 4% formaldehyde in 0.2 M phosphate buffer (pH 7.2) was applied through the left ventricle at physiologic pressure for 30 min.
One vervet in the group fed oil palm vitamin E and one in the group fed commercial vitamin E were euthanized at 4 and 7 mo after baseline, respectively, due to a ‘twisted colon;’14 a liver sample for the vervet in the oil palm vitamin E group was not obtained. In addition, one vervet was euthanized 11 mo after baseline due to weight loss resulting from a kidney cyst discovered postmortem; a liver sample was not collected. Therefore, the control and oil palm vitamin E treatment groups each had 7 vervets, bringing the final number analyzed to 38.
Extraction and detection of hepatic vitamin A and α-vitamin A species.
Samples were thawed twice: once for analysis of retinol and RE by HPLC and then for concurrent analysis by ultraHPLC and HPLC for optimization of α-retinyl esters (αRE) and carotenoids, respectively. α-Retinol was detected in the first HPLC analysis, but the HPLC method was not optimized for quantification of α-retinol. Therefore, samples were extracted again and analyzed by using ultraHPLC for α-vitamin A because of the high amounts of αC in treatment 4. Liver samples were extracted as described previously,49 with minor modifications. Briefly, 100 mg liver tissue, collected from different locations of the provided sample, was ground with approximately 5 g anhydrous sodium sulfate. C23 β-apo-carotenol was added as an internal standard, and the sample was repeatedly extracted with dichloromethane and filtered into a 50-mL volumetric flask. One mL extract was dried under nitrogen and resuspended in 200 μL 50:50 (v:v) methanol:dichloroethane; 1 μL was then injected into an ultraHPLC H-class system with a photodiode array detector and an HSS C18 column (2.1 × 150 mm; 1.8 µm; Acuity, Waters, Milford, MA). Samples were eluted at 0.4 mL/min with 70:25:5 (v:v:v) acetonitrile:water:2-propanol with 10 mM ammonium acetate (solvent A) and 75:25 (v:v) methanol:2-propanol (solvent B) using the following gradient: 7 min hold at 100% A, 4-min linear gradient to 95% B, 12-min linear gradient to 99% B, 2-min reverse gradient to 100% A, and then a 4-min hold at 100% A for reequilibration. Chromatograms were generated at 325 and 311 nm to quantify RE and αRE, respectively. RE and αRE were verified by using HPLC-purified standards isolated from pig and rat livers (animals previously dosed with α-retinyl acetate). Retinyl oleate and retinyl palmitate, as well as α-retinyl oleate and α-retinyl palmitate, could not be resolved and are reported together.
Extraction and detection of hepatic carotenoids.
Liver (300 mg) was extracted and reconstituted as described earlier, except that 200 µL trans-β-apo-8′-carotenal (Sigma, St Louis, MO) was used as internal standard. Carotenoids were analyzed as described previously3 with modifications. Briefly, 50 µL was injected into a reversed-phase HPLC system (Waters) consisting of a guard column, 5-µm YMC carotenoid column (250 mm × 4.6 mm), binary pump (model 1525), and a 2996-photodiode array detector. Samples were eluted by using the same gradient method as described previously.3 Chromatograms were obtained at 450 nm to quantify carotenoids, which were confirmed by using HPLC-purified standards.
Statistical analyses.
Unless otherwise noted, evaluation of model assumptions and statistical analyses were performed by using SAS (version 9.4; SAS Institute, Cary, NC). Normality of residuals and the homogeneity of variance were determined by using the Shapiro–Wilk (univariate procedure) and Levene tests, respectively. Data satisfying these assumptions were analyzed by one-factor ANOVA by using the generalized linear model procedure with subsequent Fisher protected Least Significant Difference (LSD) tests for group comparisons when variables were compared among treatments. When residuals were not normally distributed, the data were log-transformed (or an inverse was taken, as with weight) and analyzed as described. If transformed residuals were not normally distributed, the original data were analyzed by using a nonparametric test on ranked data. When residuals were normally distributed but Levene testing revealed nonconstant variance, P values were obtained from Welch ANOVA. The mixed procedure was used to assess a potential association with hepatic vitamin A by using diet as a categorical variable because provitamin A intake was different for the OPC group. As for other analyses, when residuals were not normally distributed, data were transformed (logarithm: hepatic carotenoid or vitamin A species, inverse: weight). A P value of 0.05 or less was considered significant. Baseline liver samples were not available; therefore, ending weight and change in weight across the study were compared with liver vitamin A concentrations. In the text, summarized data are reported as mean ± 1 SD, whereas data in tables are reported as medians (1st, 3rd quartiles) unless otherwise noted, because several variables had nonnormally distributed residuals. Data in tables are presented in original (nontransformed) form; however, where noted, group comparisons were performed on transformed or ranked data.
Results
Baseline characteristics.
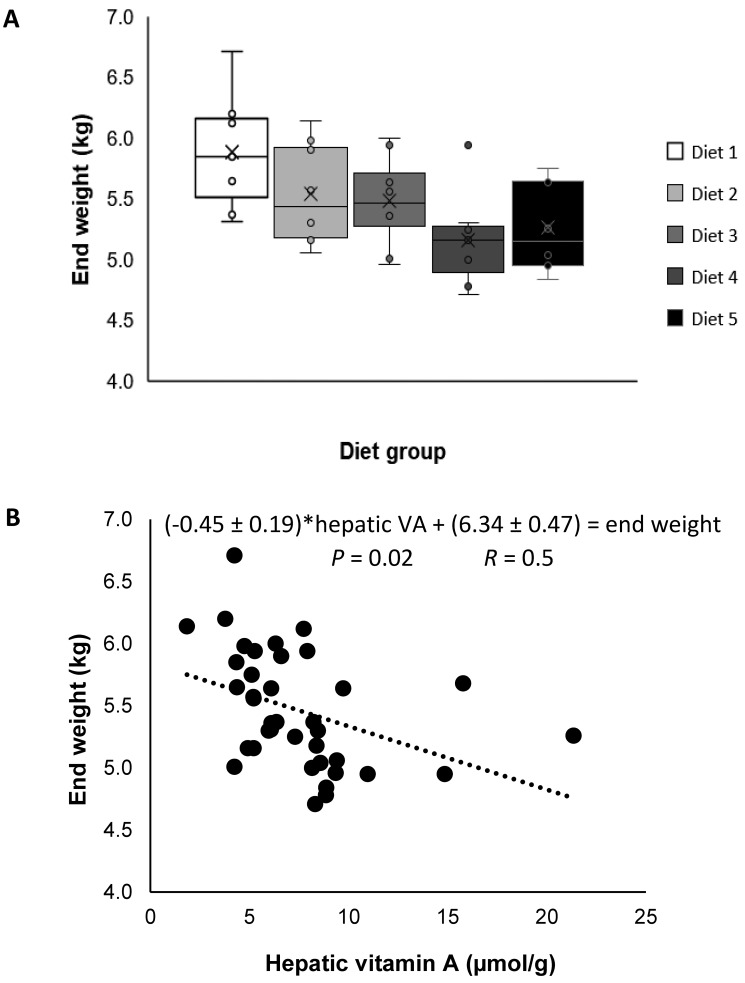
Vervets were 6.46 ± 1.33 y old (mean ± 1 SD) and weighed 5.58 ± 0.53 kg at the initiation of experimental treatments (baseline). Age and weight did not differ by treatment group (P = 0.8 and P = 0.1, respectively), although vervets given oil palm vitamin E and OPC appeared to weigh less at the end of the study (Figure 2 A). Relative to baseline weights, vervets lost 0.13 ± 0.36 kg over the study, and weight loss did not differ by diet group (P = 0.9).
Figure 2.
(A) Box plots of end weights (kg) of adult male vervets (Chlorocebus aethiops; n = 39) in 5 diet groups, which did not differ (P = 0.1). Left to right, high-fat diet was supplemented with: 1) no supplements (control); diet 2) free-choice palm-derived water-soluble antioxidants; 3) 100 mg d-α-tocopheryl acetate; 4) 100 mg oil palm (Elaeis guineensis)-derived vitamin E; and 5) 50 mg oil palm vitamin E + 50 mg oil-palm–derived carotenoid complex + free-choice palm-derived water-soluble antioxidants. (B) Weight of adult male vervets (n = 38) was negatively associated (P = 0.02) with hepatic vitamin A (μmol/g) concentration. P values reflect analyses with transformations (A, 1/kg; B, log[vitamin A]) to address nonconstant variance.
Hepatic vitamin A and α-vitamin A.
Hepatic vitamin A concentration, defined as retinol + RE, ranged from 1.9 to 21.4 µmol/g for individual vervets. Thus, all vervets had hypervitaminosis A, defined in humans as 1 µmol/g or greater.50 Overall, the hepatic concentration of vitamin A (retinol + RE; mean ± 1 SD) was 7.6 ± 3.8 µmol/g liver and was significantly (P < 0.05) different among treatment groups (Table 2). Hepatic free-retinol concentrations were not different among groups; however, individual and summed RE concentrations were higher (P < 0.05) in the OPC group compared with other treatment groups (Table 2). Weight at the end of the study was negatively associated with hepatic vitamin A concentrations after accounting for the effect of diet (P = 0.02, log-transformed to address nonconstant variance; Figure 2 B); however, the change in weight (at study end – baseline) was not significant after accounting for the effect of diet (P = 0.9).
Table 2.
Hepatic vitamin A (VA) and αVA concentration (µmol/g liver) of adult male vervets (Chlorocebus aethiops; n= 38) enrolled in an antioxidant study
| Control (no supplement) (n = 7) | Commercial vitamin E (n = 8) | Oil-palm– derived vitamin E (n = 7) | Oil-palm– derived vitamin E + carotenoids + antioxidants (n = 8) | Palm-derived antioxidants (n = 8) | Total (n = 38) | P | |
| Vitamin A | |||||||
| Retinol | 0.33 (0.26, 0.46) | 0.40 (0.24, 0.54) | 0.44 (0.42, 0.54) | 0.52 (0.33, 0.58) | 0.32 (0.26, 0.39) | 0.40 (0.27, 0.56) | 0.5 |
| Retinyl laurated | 0.15 (0.14, 0.26)c | 0.26 (0.19, 0.37)b,c | 0.35 (0.28, 0.36)b | 0.51 (0.45, 0.70)a | 0.27 (0.15, 0.30)b,c | 0.29 (0.20, 0.39) | 0.0009 |
| Retinyl oleate+retinyl palmitatee | 3.51 (3.40, 4.86)b | 5.12 (4.63, 6.09)b | 6.29 (4.76, 6.81)b | 8.14 (6.96, 12.19)a | 4.45 (4.03, 5.60)b | 5.25 (4.08, 6.88) | 0.002 |
| Retinyl stearatee | 0.47 (0.41, 0.68)b | 0.62 (0.59, 1.00)b | 0.84 (0.65, 0.90)b | 1.25 (1.06, 1.61)a | 0.65 (0.51, 0.76)b | 0.68 (0.57, 1.03) | 0.0008 |
| Total retinyl esterse | 4.11 (3.97, 5.80)b | 5.93 (5.49, 7.46)b | 7.53 (5.75, 7.98)b | 9.83 (8.47, 14.50)a | 5.28 (4.82, 6.68)b | 6.12 (4.93, 8.26) | 0.001 |
| Total VA (all)e | 4.38 (4.29, 6.22)b | 6.22 (5.88, 8.00)b | 8.16 (6.29, 8.39)b | 10.35 (8.80, 15.09)a | 5.60 (5.09, 7.05)b | 6.49 (5.20, 8.55) | 0.002 |
| α-Vitamin A | |||||||
| α-Retinolf | 0.01 (0.00, 0.01)b | 0.02 (0.01, 0.02)b | 0.01 (0.01, 0.02)b | 0.04 (0.03, 0.05)a | 0.01 (0.01, 0.01)b | 0.01 (0.01, 0.03) | 0.004 |
| α-Retinyl oleate+α- retinyl palmitatee | 0.22 (0.20, 0.25)c | 0.32 (0.28, 0.36)b,c | 0.37 (0.28, 0.44)b | 0.87 (0.77, 1.42)a | 0.29 (0.27, 0.32)b,c | 0.30 (0.23, 0.46) | <0.0001 |
| α-Retinyl stearated,f | 0.01 (0.01, 0.02)c | 0.02 (0.01, 0.04)b,c | 0.04 (0.03, 0.05)b | 0.13 (0.10, 0.18)a | 0.03 (0.02, 0.03)b | 0.03 (0.01, 0.05) | <0.0001 |
| Total α-retinyl esterse | 0.22 (0.20, 0.27)c | 0.35 (0.31, 0.38)b,c | 0.41 (0.30, 0.51)b | 1.00 (0.87, 1.61)a | 0.32 (0.29, 0.35)b,c | 0.34 (0.26, 0.52) | <0.0001 |
| Total α-VA (all)e | 0.23 (0.20, 0.29)c | 0.35 (0.33, 0.39)b,c | 0.43 (0.31, 0.52)b | 1.04 (0.90, 1.65)a | 0.33 (0.29, 0.36)b,c | 0.35 (0.28, 0.53) | <0.0001 |
| Total VA and α-VA speciese | 4.82 (4.69, 6.81)b | 6.95 (6.65, 9.01)b | 9.08 (7.12, 9.43)b | 12.05 (10.22, 17.70)a | 6.28 (5.62, 7.86)b | 7.20 (5.86, 9.81) | 0.009 |
Data are reported as median (1st quartile, 3rd quartile).
Different superscript letters within a row denote significant differences between groups (one-factor ANOVA with Fisher protected least significant difference testing or a nonparametric [ranked] test), with a > b > c. P values result from testing the null hypothesis that all groups are not statistically different for a given variable.
Nonnormally distributed residuals; P value reflective of a non-parametric (ranked) analysis. The original (untransformed) data are reported here.
Statistical analysis carried out on the log transform of the variable, that is, log(y). The original (untransformed) data are reported here.
Not all species were detected in all samples, therefore the number reported varies from 5 to 8.
Total hepatic α-vitamin A species ranged from 0.10 to 2.7 µmol/g, with a mean of 0.5 ± 0.5 µmol/g (Table 2). All verified αRE as well as the ratio of α-vitamin A to vitamin A were higher (P < 0.05) in the OPC group compared with other treatment groups. In addition, hepatic concentrations of free α-retinol were higher in the OPC group than all other treatment groups (Table 2).
Overall, the concentration of total hepatic vitamin A and α-vitamin A species was 8.7 ± 4.6 µmol/g (mean ± 1 SD), with values ranging from 1.98 to 25.5 µmol/g for individual vervets. Vervets given the OPC supplement exhibited the highest hepatic concentrations of vitamin A among all groups (P < 0.05; Table 2).
Hepatic carotenoids.
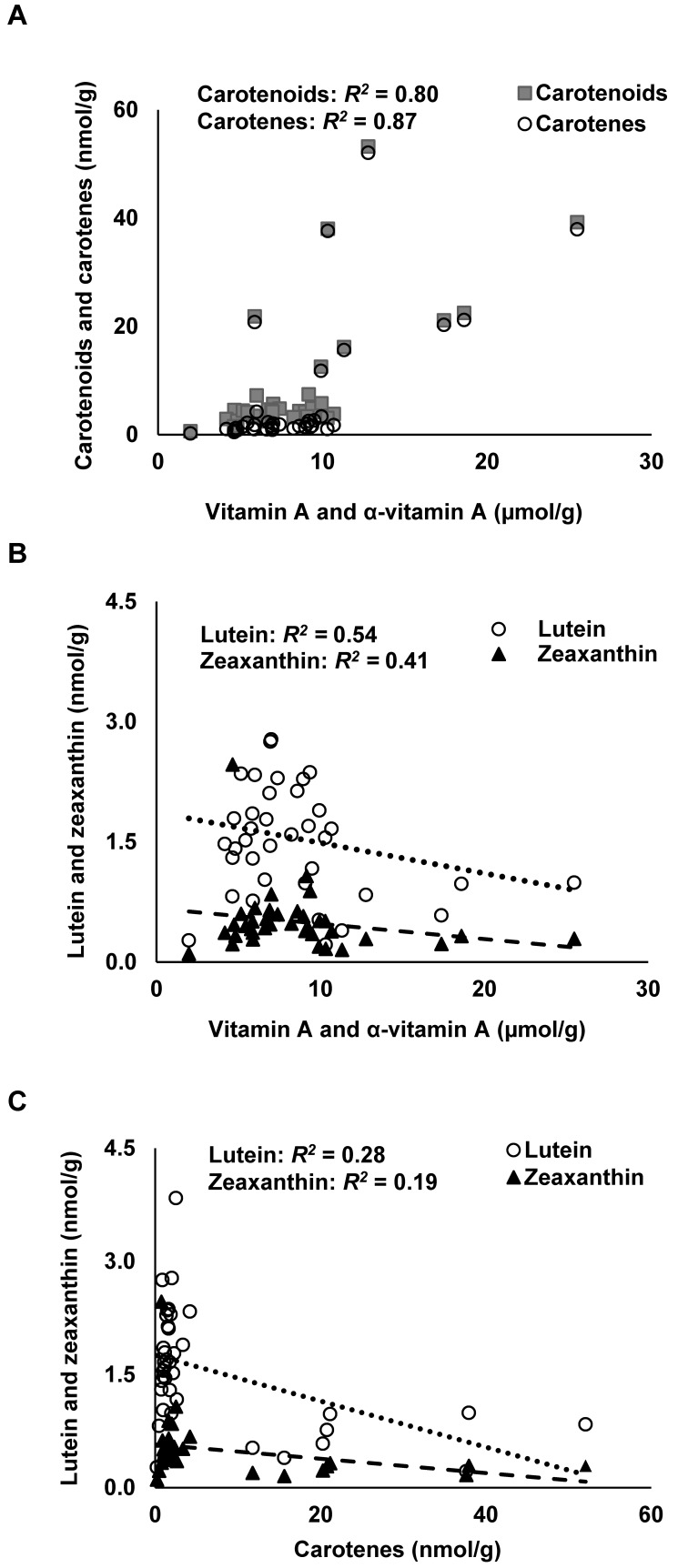
Total hepatic carotenoid concentration ranged from 0.6 to 53.2 nmol/g, with a mean of 9.02 ± 11.7 nmol/g for all treatment groups (Table 3). Hepatic concentrations of αC and βC, 9-cis- and 13-cis-βC, total carotenoids, and carotenes were higher in the OPC group compared with all other treatment groups (Table 3). After the effect of diet was included, hepatic carotenoids (log-transformed) were positively associated with hepatic vitamin A + α-vitamin A (P = 0.04; R2 = 0.80); the same was true for hepatic carotenes (log-transformed; P = 0.04; R2 = 0.87). Mean total hepatic βC (all-trans + 9-cis + 13-cis) concentration for the OPC group was 15.6 ± 8.6 nmol/g liver, which was significantly (P < 0.0001) higher than in all other groups. Interestingly, hepatic concentrations of lutein and zeaxanthin were lowest in the OPC group (Table 3). Hepatic carotenoid concentration varied greatly among individual vervets in the OPC group; however, coefficients of variation were similar to those in other groups, with a range of 27% to 55% among carotenoids in the OPC group. Hepatic carotene and carotenoid concentration appeared to increase with total hepatic vitamin A (vitamin A+α-vitamin A) concentration (Figure 3 A). After the effect of diet was included, hepatic lutein was associated with hepatic liver vitamin A + α-vitamin A (P = 0.01; R2 = 0.54; log-transformed); however, no clear pattern emerged (Figure 3 B). Hepatic zeaxanthin concentration showed no association with total hepatic vitamin A concentration (Figure 3 B). Hepatic concentrations of lutein and zeaxanthin decreased with increasing hepatic carotene concentrations (Figure 3 C).
Table 3.
Hepatic carotenoid concentration (nmol/g liver) of adult male vervets (Chlorocebus aethiops; n = 38)
| Control (no supplement) (n = 7) | Commercial vitamin E (n = 8) | Oil-palm–derived vitamin E (n = 7) | Oil-palm– derived vitamin E + carotenoids + antioxidants (n = 8) | Palm-derived antioxidants (n = 8) | Total (n = 38) | P | |
| Lutein | 1.47 (1.36, 1.96)a | 1.83 (1.41, 2.40)a | 1.59 (1.34, 2.11)a | 0.67 (0.49, 0.87)b | 1.74 (1.67, 1.96)a | 1.54 (0.99, 2.06) | 0.003 |
| Zeaxanthina | 0.45 (0.40, 0.74)a | 0.54 (0.45, 0.63)a | 0.48 (0.42, 0.59)a | 0.25 (0.19, 0.29)b | 0.46 (0.41, 0.56)a | 0.44 (0.33, 0.59) | 0.004 |
| α-Caroteneb | 0.62 (0.50, 0.79)c | 0.77 (0.55, 0.97)c | 1.27 (1.03, 1.66)b | 9.69 (8.06, 15.42)a | 0.84 (0.67, 1.10)c | 1.00 (0.67, 1.83) | <0.0001 |
| All trans-β-carotenea | 0.26 (0.22, 0.37)c | 0.34 (0.24, 0.43)c | 0.56 (0.46, 0.91)b | 7.78 (6.42, 13.99)a | 0.37 (0.26, 0.40)c | 0.43 (0.27, 0.91) | <0.0001 |
| β-carotene + isomersa | 0.43 (0.37, 0.58)c | 0.53 (0.43, 0.70)c | 0.91 (0.75, 1.34)b | 11.7 (10.4, 21.2)a | 0.60 (0.42, 0.69)c | 0.70 (0.45, 1.38) | <0.0001 |
| Total hepatic carotenesb | 1.02 (0.88, 1.37)c | 1.30 (0.98, 1.67)c | 2.17 (1.79, 3.00)b | 21.0 (19.1, 37.7)a | 1.44 (1.08, 1.79)c | 1.69 (1.12, 3.18) | <0.0001 |
| Total hepatic carotenoidsb | 3.45 (2.70, 4.43)b | 3.84 (3.13, 4.29)b | 4.15 (3.74, 5.32)b | 22.2 (19.9, 38.3)a | 3.67 (3.32, 4.47)b | 4.30 (3.35, 6.86) | <0.0001 |
Data are reported as median (1st quartile, 3rd quartile).
Different superscript letters within a row denote significant (P < 0.05, one-factor ANOVA or chi-squared testing) differences between groups; a > b > c. P values result from testing the null hypothesis that all groups are not statistically different for a given variable.
Statistical analysis done on the log transform of the variable, that is, log(y). The original (untransformed) data are reported here.
Nonnormally distributed residuals; P value reflective of a nonparametric (ranked) analysis. The original (untransformed) data are reported here.
Figure 3.
Hepatic carotenoid species and total vitamin A (vitamin A + α-vitamin A) concentration in adult male vervets (Chlorocebus aethiops; n = 38). The effect of diet was included in the statistical analysis. (A) Hepatic carotenoids (all identified carotenoids) and carotenes (cis- and trans α- and β-carotenes) × hepatic vitamin A and α-vitamin A concentration. (B) Lutein and zeaxanthin × hepatic vitamin A and α-vitamin A concentration. (C) Lutein and zeaxanthin × hepatic carotenes. Lutein differed by hepatic carotene content and by diet group (P = 0.001 for both). Squares in panel A represent carotenoids. Circles in panels B and C indicate hepatic lutein, whereas triangles represent hepatic zeaxanthin.
Discussion
In this study, liver samples from adult male vervets maintained on 5 experimental diets were analyzed for vitamin A, α-vitamin A, and carotenoid concentrations. Hepatic vitamin A stores in all of the vervets in this study exceeded 1 µmol/g, the cutoff defining hypervitaminosis A in humans.50 Furthermore, hepatic vitamin A concentrations of 4 of the 8 vervets in the OPC group exceeded 10 μmol/g, which is considered toxic in humans.50 Hepatic vitamin A concentrations in our vervets were high (range, 1.9 to 21.4 μmol/g), but the mean was lower than previously reported for wild-caught vervets consuming laboratory feed for 2 y (14.6 ± 2.3 μmol/g)31 and in colony-bred rhesus macaques (17.0 ± 6.3 μmol/g).37 By comparison, human hepatic RE concentrations ranged from 0.008 to 2.86 μmol/g in 12 surgical biopsies from patients in France,47 0.049 to 0.56 μmol/g in 11 healthy surgical patients in the United States,16 0.13 to 6.31 μmol/g in 77 generally healthy men and women at autopsy,42 and 0 to 1.97 μmol/g in 15 children and adults at autopsy.36 Overall, hepatic α-vitamin A concentrations in our vervets ranged from 0.1 to 2.7 μmol/g liver, a generally similar range as for hepatic vitamin A concentrations in humans;16,42,46 however, α-vitamin A concentrations were not measured in cited human studies.
When vitamin A and α-vitamin A species are considered together as an estimate of total hepatic vitamin A stores, vervets in our study had combined vitamin A stores 4 to 10 times greater than the current cutoff for hypervitaminosis A in humans (that is, 1 μmol vitamin A/g liver).50 Although we did not analyze these samples histologically to evaluate potential hepatotoxicity and consequent adverse health effects, a study that included histologic evaluation showed hepatic stellate cell hypertrophy and hyperplasia at hepatic vitamin A concentrations of approximately 14 μmol/g in vervets.31 Lactate dehydrogenase was elevated in half of the experimental rhesus macaques with a mean hepatic vitamin A concentration of 16.4 μmol/g,9 likely indicating hepatotoxicity. Given the high hepatic vitamin A concentrations of vervets in the current study, liver function in at least some of our vervets likely was compromised, because hypervitaminosis A leads to hepatic injury.39 Interestingly, although weight did not differ by diet group, vervet weight at study end was inversely associated with hepatic vitamin A concentrations, perhaps suggesting decreased food intake with increasing vitamin A intake and liver vitamin A concentration. In addition, change in weight did not differ among study groups. Anorexia and subsequent weight loss have been described in clinical hypervitaminosis A in humans,20 and piglets dosed with 25,000, 50,000 or 200,000 IU vitamin A within 12 h of birth did not grow as well as placebo-dosed piglets.17 Although these previous studies cannot be taken as definitive evidence for vitamin A toxicity, the potential for inadvertent vitamin A toxicity in captive NHP populations (and its unknown effects) has particular relevance for studies evaluating nutritional requirements or pharmacologic agents in NHP models.
Whereas hepatic concentrations of free retinol were not different among treatment groups, hepatic concentrations of RE, αRE, αC, βC, 9-cis-βC, and 13-cis-βC were higher but concentrations of lutein and zeaxanthin were lower in the OPC group compared with other groups. Furthermore, vervets in the OPC group had higher hepatic vitamin A concentrations (range, 5.1 to 21.4 μmol/g) than any other treatment group, and the concentrations in the OPC vervets were similar to those in other species of NHP.31,37 The magnitude of the difference in total hepatic vitamin A in the OPC group compared with vervets on other supplements is somewhat surprising. Although the OPC group received a large dose of provitamin A carotenoids (equivalent to approximately 12,000 IU vitamin A, according to the manufacturer's estimate of vitamin A equivalents), provitamin A conversion is regulated by dietary vitamin A in humans and Mongolian gerbils (Meriones unguiculatus).4,26,27 To our knowledge, regulation of BCO1 by dietary vitamin A has not been examined specifically in vervets; however, Chlorocebus aethiops sabaeus,7 along with other NHP, have an isx ortholog.28,32,33 Because our vervets consumed the same HFD for several weeks prior to vitamin A treatment, differences may have been due to continued uptake of carotenoid through scavenger receptor B1, passive diffusion, or (albeit unlikely) continued species-specific BCO1-induced cleavage of provitamin A carotenoids, a phenomenon that has been observed in mice but which do not exhibit substantial regulation of BCO1 by dietary vitamin A.48
Differences in carotenoid absorption or provitamin A carotenoid conversion among primate species may exist. Female rhesus monkeys (n = 5) given 50 μCi 14C-labeled βC in oil absorbed βC intact, albeit with high variability.24 The majority of radioactivity was associated with the liver retinol fraction, and only approximately 2% to 8% of radioactivity was found in the liver βC fraction, suggesting that almost all of the βC was cleaved to vitamin A.24 Chimpanzees (Pan spp.) and orangutans (Pongo spp.) circulated more lutein and RE compared with humans, regardless of dietary carotenoid profile,18 suggesting differences even among primate species. Although not fully evaluated in vervets, a previous study observed circulation of lutein—but not RE—during a hypervitaminotic state.31 Moreover, a cross-sectional survey of serum carotenoid concentrations at a United States zoo found substantial variation in serum carotenoids among 13 captive NHP species.44 As expected, some of the variation probably was due to dietary preference. Sooty mangabeys (Cercopithecus ascanius schmidti) consumed more carotenoid than golden lion tamarins (Leontopithecus rosalia rosalia), which had no detectable serum carotenoid.44 However, spider (Ateles spp.) and capuchin (Cebinae spp.) monkeys had similar dietary consumptions, but spider monkeys accumulated more serum carotenoid,44 suggesting possible differences in carotenoid absorption or bioconversion of provitamin A carotenoids among New World primate species.
Hepatic carotenoid concentrations in the OPC group (median total carotenoid concentration, 22.2 nmol/g) were similar to concentrations observed in human biopsies.47 Mongolian gerbils given 35 nmol αC or 17.5 nmol βC had hepatic concentrations of 1.2 nmol βC/g and 3.2 nmol αC/g, respectively.49 In the current study, vervets had a median total hepatic βC concentration of 0.70 nmol/g liver, but that of vervets in the OPC group was 11.7 nmol/g, reflecting their increased dose. Hepatic lutein and zeaxanthin concentrations were lowest in the OPC group. Given that all vervets were fed the same HFD, this finding might suggest that supplemental carotenes compete with lutein and zeaxanthin for inclusion in micelles or for scavenger receptor B1. Evidence for such an interaction among carotenoids is available, because in healthy humans, simultaneous single-dose supplementation with βC and lutein23 and supplementation with βC for 6 wk both decreased serum lutein.30 High βC doses decreased serum lutein and zeaxanthin concentrations and decreased retinal zeaxanthin in chickens after 14 d.56 This effect has not been observed consistently, because simultaneous dosing with carotenoids and lutein did not significantly affect plasma lutein AUC in healthy young men.52
Hepatic carotenoid concentrations were variable, with coefficients of variation ranging from 27% to 96%, depending on the carotenoid analyzed and treatment group; however, no clear pattern emerged. Other than reflecting biologic variation, this finding might be due to differences between methods or the fact that we were working with small liver samples. Vitamin A concentrations do vary throughout the liver,36 but our recent analyses of whole human livers yielded coefficients of variation of 8% to 14% within and among liver lobes. Like vitamin A concentrations, carotenoid levels probably vary, but little is known regarding their distribution among hepatic locations. Nonetheless, hepatic concentrations of free retinol did not differ among treatment groups, and all liver RE values were high. Finally, variations in BCO1 activity due to single-nucleotide polymorphisms have been identified in chickens22 and humans11 and can affect plasma27,55 and tissue response to dietary carotenoids.22,29 Although little is known about BCO1 activity in vervets, given the existence of bco1 polymorphisms and their effects on BCO1 activity in the human population,27 such polymorphisms may occur in NHP as well.
In conclusion, hepatic vitamin A and α-vitamin A concentrations in these vervets were elevated, especially when the animals were supplemented with provitamin A carotenoids, and were consistent with previous reports for captive-bred NHP. Weight was negatively associated with hepatic vitamin A concentrations and may have been a result of decreased food intake. Given the effects of decreased weight with elevated liver vitamin A associated with hepatotoxicity, researchers using NHP should consider reducing the vitamin A content of research feeds. One option for vervets is to replace preformed vitamin A with βC,5 which more closely mimics what they typically consume in nature. However, our vervets who consumed increased amounts of dietary supplemental carotenoids developed very high hepatic vitamin A concentrations. Research examining provitamin A bioconversion among NHP species is needed.
Acknowledgments
We thank Bryan Gannon, Devika Suri, and Peter Crump for statistical consultation; Dr Charon de Villiers for management of the vervets; and Amelia Damons for preparation of the food. Manuscript preparation and hepatic sample analysis were supported by Global Health Funds at the University of Wisconsin-Madison and the Friday Chair for Vegetable Processing Research endowment (SAT). The experimental procedures were originally supported by a research grant from the Malaysian Palm Oil Board, and all supplements derived from oil palm fruit (that is, oil-palm–derived vitamin E and OPC, and oil palm-derived water-soluble antioxidants (which were recovered from the vegetation liquor generated from the milling of oil palm fruits) were generously donated.
References
- 1.Benadé AJS, Fincham JE, Smuts CM, Weight MJ, van Jaarsveld PJ, Kruger M. 1997. Vervet monkeys and whole-food diets for studying the effects of dietary lipids on plasma lipoprotein metabolism and atherosclerosis. Asia Pac J Clin Nutr 6:17–21. [PubMed] [Google Scholar]
- 2.Carlsson HE, Schapiro SJ, Farah I, Hau J. 2004. Use of primates in research: a global overview. Am J Primatol 63:225–237. 10.1002/ajp.20054. [DOI] [PubMed] [Google Scholar]
- 3.Davis C, Jing H, Howe JA, Rocheford T, Tanumihardjo SA. 2008. β-Cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to β-carotene supplements. Br J Nutr 100:786–793. 10.1017/S0007114508944123. [DOI] [PubMed] [Google Scholar]
- 4.Deming DM, Erdman JW., Jr 1999. Mammalian carotenoid absorption and metabolism. Pure Appl Chem 71:2213–2223. 10.1351/pac199971122213. [DOI] [Google Scholar]
- 5.Dever JT, Tanumihardjo SA. 2009. Hypervitaminosis A in experimental nonhuman primates: evidence, causes, and the road to recovery. Am J Primatol 71:813–816. 10.1002/ajp.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Odorico A, Martines D, Kiechl S, Egger G, Oberhollenzer F, Bonvicini P, Sturniolo GC, Naccarato R, Willet J. 2000. High plasma levels of α- and β-carotene are associated with a lower risk of atherosclerosis: results from the Bruneck study. Atherosclerosis 153:231–239. 10.1016/S0021-9150(00)00403-2. [DOI] [PubMed] [Google Scholar]
- 7.Ensembl. [Internet]. 2017. Vervet–AGM, Chl.Sab1.1, release 88; Gene:ISX. [Cited 11 April 2017]. Available at: http://useast.ensembl.org/Chlorocebus_sabaeus/Gene/Compara_Tree?anc=13261430;db=core;g=ENSCSAG00000008226;g1=ENSG00000175329;r=19:17828177-17852069;t=ENSCSAT00000006278.
- 8.Ervin F, Palmour R. 2003. Primates for 21st century biomedicine: the St. Kitts Vervet (Chlorocebus aethiops, SK), p 49–53. In: Proceedings of the workshop “International perspectives: the future of nonhuman primate resources,” Washington, DC, 17–19 April, 2002. Washington (DC): National Academies Press: Available at: https://doi.org/10.17226/10774. [PubMed] [Google Scholar]
- 9.Escaron AL, Green MH, Howe JA, Tanumihardjo SA. 2009. Mathematical modeling of serum 13C-retinol in captive rhesus monkeys provides new insights on hypervitaminosis A. J Nutr 139:2000–2006. 10.3945/jn.109.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedigan L, Fedigan LM. 1988. Cercopithecus aethiops: a review of field studies, p 389–411. In: Gautier-Hion A, Bourlière F, Gautier JP, Kingdon J, editors. A primate radiation: evolutionary biology of the African guenons. Cambridge (United Kingdom): Cambridge University Press. [Google Scholar]
- 11.Ferrucci L, Perry JRB, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi AM, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM. 2009. Common variation in the β-carotene 15, 15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet 84:123–133. 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fincham JE, Gouws E, Woodroof CW, van Wyk MJ, Kruger M, Smuts CM, van Jaarsveld PJ, Taljaard JJF, Schall R, Strauss JA de W, Benadé AJS. 1991. Atherosclerosis: chronic effects of fish oil and a therapeutic diet in nonhuman primates. Arterioscler Thromb 11:719–732. 10.1161/01.ATV.11.3.719. [DOI] [PubMed] [Google Scholar]
- 13.Fincham JE, Quack G, Wülfroth P, Benadé AJS. 1996. Confirmation of efficacy of etofibrate against peripheral atherosclerosis in nonhuman primates which model human lesion types I–VII. Arzneimittelforschung 46:519–525. [PubMed] [Google Scholar]
- 14.Fincham JE, Seier JV, Lombard CJ. 1992. Torsion of the colon in vervet monkeys: association with an atherogenic Western-type of diet. J Med Primatol 21:44–46. [PubMed] [Google Scholar]
- 15.Fincham JE, Woodroof CW, van Wyk MJ, Capatos D, Weight MJ, Kritchevsky D, Rossouw JE. 1987. Promotion and regression of atherosclerosis in vervet monkeys by diets realistic for westernized people. Atherosclerosis 66:205–213. 10.1016/0021-9150(87)90064-5. [DOI] [PubMed] [Google Scholar]
- 16.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen HR, 3rd, Jones AD, Anderson DP, Olson JA. 1989. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally health adult humans. Am J Clin Nutr 49:713–716. 10.1093/ajcn/49.4.713. [DOI] [PubMed] [Google Scholar]
- 17.Gannon BM, Davis CR, Nair N, Grahn M, Tanumihardjo SA. 2017. Single high-dose vitamin A supplementation to neonatal piglets results in a transient dose response in extrahepatic organs and sustained increases in liver stores. J Nutr 147:798–806. 10.3945/jn.117.247577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García AL, Raila J, Koebnick C, Eulenberger K, Schweigert FJ. 2006. Great apes show highly selective plasma carotenoids and have physiologically high plasma retinyl esters compared to humans. Am J Phys Anthropol 131:236–242. 10.1002/ajpa.20428. [DOI] [PubMed] [Google Scholar]
- 19.Harrison MJ. 1984. Optimal foraging strategies in the diet of the green monkey, Cercopithecus sabaeus, at Mt. Assirik, Senegal. Int J Primatol 5:435–471. 10.1007/BF02692269. [DOI] [Google Scholar]
- 20.Institute of Medicine Panel on Micronutrients. 2001. Vitamin A, Chapter 4. p 82–161. In: Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): The National Academies Press; https://doi.org/10.17226/10026 [PubMed] [Google Scholar]
- 21.Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K. 2006. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol, and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev 7:533–546. [PubMed] [Google Scholar]
- 22.Jlali M, Graulet B, Chauveau-Duriot B, Chabault M, Godet E, Leroux S, Le Bihan-Duval E, Duclos MJ, Berri C. 2012. A mutation in the promoter of the chicken β,β-carotene 15, 15′-monooxygenase 1 gene alters xanthophyll metabolism through a selective effect on its mRNA abundance in the breast muscle. J Anim Sci 90:4280–4288. 10.2527/jas.2012-5240. [DOI] [PubMed] [Google Scholar]
- 23.Kostic D, White WS, Olson JA. 1995. Intestinal absorption, serum clearance, and interactions between lutein and β-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr 62:604–610. 10.1093/ajcn/62.3.604. [DOI] [PubMed] [Google Scholar]
- 24.Krinsky NI, Mathews-Roth MM, Welankiwar S, Sehgal PK, Lausen NCG, Russett M. 1990. The metabolism of [14C]β-carotene and the presence of other carotenoids in rats and monkeys. J Nutr 120:81–87. 10.1093/jn/120.1.81. [DOI] [PubMed] [Google Scholar]
- 25.Langenhoven ML, Kruger M, Gouws E, Faber M. 1991. MRC food composition tables, 3rd ed. Cape Town (South Africa): South African Medical Research Council. [Google Scholar]
- 26.Lee CM, Lederman JD, Hofmann NE, Erdman JW., Jr 1998. The Mongolian gerbil (Meriones unguiculatus) is an appropriate animal model for evaluation of the conversion of β-carotene to vitamin A. J Nutr 128:280–286. 10.1093/jn/128.2.280. [DOI] [PubMed] [Google Scholar]
- 27.Lietz G, Oxley A, Leung W, Hesketh J. 2012. Single-nucleotide polymorphisms upstream from the β-carotene 15, 15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr 142:161S–165S. 10.3945/jn.111.140756. [DOI] [PubMed] [Google Scholar]
- 28.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. 2010. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β-β-carotene absorption and vitamin A production. FASEB J 24:1656–1666. 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo R, Jr, Truitt B, Klein ML, Snodderly DM, Blodi BA, Gehrs KM, Sarto GE, Wallace RB, Robinson J, LeBlanc ES, Hageman G, Tinker L, Mares JA. 2013. Genetic determinants of macular pigments in women of the carotenoids in Age-Related Eye Disease Study. Invest Ophthalmol Vis Sci 54:2333–2345. 10.1167/iovs.12-10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, Beecher GR, Smith JC, Jr, Cecil J. 1992. Plasma carotenoid response to chronic intake of selected foods and β-carotene supplements in men. Am J Clin Nutr 55:1120–1125. 10.1093/ajcn/55.6.1120. [DOI] [PubMed] [Google Scholar]
- 31.Mills JP, Tanumihardjo SA. 2006. Vitamin A toxicity in wild-caught African green vervet monkeys (Chlorocebus aethiops) after 2 years in captivity. Comp Med 56:421–425. [PubMed] [Google Scholar]
- 32.National Center for Biotechnology Information. [Internet]. 2016. HomoloGene:28312. Gene conserved in Amniota. [Cited 6 October 2016]. Available at: https://www.ncbi.nlm.nih.gov/homologene/28312
- 33.National Center for Biotechnology Information. [Internet]. 2016. ISX intestine specific homeobox [Homo sapiens (human)] Gene ID: 91464. [Cited 6 October 2016]. Available at: https://www.ncbi.nlm.nih.gov/gene/91464/
- 34.National Research Council. 2003. Vitamins. Fat-soluble vitamins. Chapter 7. p 113–116. In: Nutrient requirements of nonhuman primates: 2nd revised ed. Washington (DC): National Academies Press. [Google Scholar]
- 35.O'Byrne SM, Blaner WS. 2013. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res 54:1731–1743. 10.1194/jlr.R037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson JA, Gunning D, Tilton R. 1979. The distribution of vitamin A in human liver. Am J Clin Nutr 32:2500–2507. 10.1093/ajcn/32.12.2500. [DOI] [PubMed] [Google Scholar]
- 37.Penniston KL, Tanumihardjo SA. 2001. Subtoxic hepatic vitamin A concentrations in captive rhesus monkeys (Macaca mulatta). J Nutr 131:2904–2909. 10.1093/jn/131.11.2904. [DOI] [PubMed] [Google Scholar]
- 38.Penniston KL, Thayer JC, Tanumihardjo SA. 2003. Serum vitamin A esters are high in captive rhesus (Macaca mulatta) and marmoset (Callithrix jacchus) monkeys. J Nutr 133:4202–4206. 10.1093/jn/133.12.4202. [DOI] [PubMed] [Google Scholar]
- 39.Russell RM, Boyer JL, Baheri SA, Hruban Z. 1974. Hepatic injury from chronic hypervitaminosis a resulting in portal hypertension and ascites. N Engl J Med 291:435–440. 10.1056/NEJM197408292910903. [DOI] [PubMed] [Google Scholar]
- 40.Saj TL, Sicotte P, Paterson JD. 2001. The conflict between vervet monkeys and farmers at the forest edge in Entebbe, Uganda. Afr J Ecol 39:195–199. 10.1046/j.0141-6707.2000.00299.x. [DOI] [Google Scholar]
- 41.Sambanthamurthi R, Tan Y, Sundram K, Abeywardena M, Sambandan TG, Rha C, Sinskey AJ, Subramaniam K, Leow SS, Hayes KC, Wahid MB. 2011. Oil palm vegetation liquor: a new source of phenolic bioactives. Br J Nutr 106:1655–1663. 10.1017/S0007114511002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schindler R, Friedrich DH, Kramer M, Wacker HH, Feldheim W. 1988. Size and composition of liver vitamin A reserves of human beings who died of various causes. Int J Vitam Nutr Res 58:146–154. [PubMed] [Google Scholar]
- 43.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, Seino S. 2007. Isx participates in the maintenance of vitamin A metabolism by regulation of β-carotene 15, 15′-monooxygenase (Bcmo1) expression. J Biol Chem 283:4905–4911. 10.1074/jbc.M707928200. [DOI] [PubMed] [Google Scholar]
- 44.Slifka KA, Bowen PE, Stacewicz-Sapuntzakis M, Crissey SD. 1999. A survey of serum and dietary carotenoids in captive wild animals. J Nutr 129:380–390. 10.1093/jn/129.2.380. [DOI] [PubMed] [Google Scholar]
- 45.South African National Standard. 2008. The care and use of animals for scientific purposes. Pretoria (South Africa): SABS Standards Division. [Google Scholar]
- 46.Sundram K, Sambanthamurthi R, Tan Y. 2001. FTN 29: Composition and nutritional characteristics of a water-soluble antioxidant-rich extract from oil palm processing, p 250–253. In: 2001 PIPOC International Palm Oil Conference Food Technology and Nutrition Conference, 20–22 August 2001. [Google Scholar]
- 47.Tanumihardjo SA, Furr HC, Amedee-Manesme O, Olson JA. 1990. Retinyl ester (vitamin A ester) and carotenoid composition in human liver. Int J Vitam Nutr Res 60:307–313. [PubMed] [Google Scholar]
- 48.Tanumihardjo SA. 2012. Mammalian models for understanding mechanisms of retinol and retinoid actions, p 93 –108. WHO technical consultation on vitamin A in newborn health: mechanistic studies. Geneva, Switzerland, 1–3 December 2009. Geneva (Switzerland): World Health Organization. [Google Scholar]
- 49.Tanumihardjo SA, Howe JA. 2005. Twice the amount of α-carotene isolated from carrots is as effective as β-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr 135:2622–2626. 10.1093/jn/135.11.2622. [DOI] [PubMed] [Google Scholar]
- 50.Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. 2016. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J Nutr 146:1816S–1848S. 10.3945/jn.115.229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.United States Department of Agriculture. [Internet]. 2015. USDA food composition database, standard reference release 28. [Cited 6 October 2016]. Available at: https://ndb.nal.usda.gov/ndb/
- 52.van den Berg H, van Vliet T. 1998. Effect of simultaneous, single oral doses of β-carotene with lutein or lycopene on the β-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr 68:82–89. 10.1093/ajcn/68.1.82. [DOI] [PubMed] [Google Scholar]
- 53.van Jaarsveld PJ, Smuts CM, Benadé AJS. 2002. Effect of palm olein oil in a moderate-fat diet on plasma lipoprotein profile and aortic atherosclerosis in nonhuman primates. Asia Pac J Clin Nutr 11 Suppl 7:S424–S432. 10.1046/j.1440-6047.11.s.7.8.x. [DOI] [PubMed] [Google Scholar]
- 54.van Jaarsveld PJ, Smuts CM, Tichelaar HY, Kruger M, Benadé AJS. 2000. Effect of palm oil on plasma lipoprotein concentrations and plasma low-density lipoprotein composition in nonhuman primates. Int J Food Sci Nutr 51 sup1:S21–S30. 10.1080/096374800111120. [DOI] [PubMed] [Google Scholar]
- 55.Wang TTY, Edwards AJ, Clevidence BA. 2013. Strong and weak plasma response to dietary carotenoids identified by cluster analysis and linked to beta-carotene 15, 15′-monooxygenase 1 single-nucleotide polymorphisms. J Nutr Biochem 24:1538–1546. 10.1016/j.jnutbio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Illingworth DR, Connor SL, Duell PD, Connor WE. 2010. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin, and β-carotene. Eur J Nutr 49:327–336. 10.1007/s00394-009-0089-8. [DOI] [PubMed] [Google Scholar]