Abstract
Facility-wide Corynebacterium bovis eradication was established using vaporized hydrogen peroxide (VHP) decontamination guided by C. bovis PCR surveillance. Prior attempts limited to culling PCR-positive mice and decontaminating affected rooms were ineffective in preventing recurrence. Because research aims often require trafficking to and use of procedural cores, a 12-mo facility-wide C. bovis PCR surveillance of 2064 specimens was performed and documented that, despite the presence of few clinically hyperkeratotic mice, 35% of the murine housing and use space was contaminated by C. bovis. The airways of IVC racks and air-handling units (AHU) provided a substantive niche for C. bovis survival, comparable to the primary enclosure, with 26% of murine and 22% of airway specimens PCR-positive for C. bovis. Equipment airway VHP sterilization in a ‘flex room’ required an ‘active–closed’ setting with the IVC rack connected to the AHU set to the VHP cycle, because 12% of specimens from ‘static–open’ VHP-exposed airways remained PCR-positive for C. bovis, whereas 0% of specimens from active–closed VHP exposures were positive. VHP decontamination of the 29,931-ft2 facility was completed in 2 mo. C. bovis PCR testing of IVC exhaust plenums for 200 d in previously C. bovis-affected rooms confirmed that none of the 259 specimens tested were PCR-positive for the organism. Monthly surveillance identified a single recurrence during June 2017 (month 9), ensuring rapid culling of C. bovis PCR-positive mice and acute VHP decontamination of equipment and rooms. Molecular persistence of C. bovis was resolved in procedural and personnel areas, and no murine or housing specimens tested C. bovis PCR-positive during study months 11 and 12. Furthermore, since the conclusion of the 12-mo study, none of the 452 additional murine, cell biologic, environmental, and monthly equipment surveillance specimens tested were C. bovis PCR-positive, documenting an 11-mo period of facility-wide C. bovis eradication to date. Study invalidation due to C. bovis can be avoided through PCR surveillance for the organism, immediate culling of PCR-positive mice, and acute VHP decontamination of affected areas.
Abbreviations: aHP, accelerated hydrogen peroxide; AHU, air handling unit; VHP, vaporized hydrogen peroxide
Corynebacterium bovis is a resilient, lipophilic, opportunistic, gram-positive coryneform bacteria associated with ‘scaly skin disease,’ a hyperkeratotic acanthotic dermatitis in nude mice2-4,18 that may also contribute to skin disease in haired Pkrdcscid mice19 and hairless immunocompetent SKH1-Hrhr mice.4,8 C. bovis-infected nude mice may remain asymptomatic and become a persistently infected source of agent dissemination, or they may develop clinical manifestations with variable morbidity, including hyperkeratosis and dehydration that persists for approximately 7 to 10 d and then resolves with low mortality.2-5,18,20 Clinically affected nude mice shed C. bovis-infected keratin flakes that are spread through airborne and fomite transmission and remain resistant to facility decontamination efforts.2-4,18 Consequently, environmental monitoring by PCR analysis for C. bovis is encouraged when immunodeficient strains are housed, especially of exhaust plenums venting IVC rack systems.12 In addition, the agent may be transmitted from C. bovis-infected immunodeficient mice to other immunodeficient mice in resected patient-derived xenografts (PDX) or allograft tumors; methods to avoid this transmission have been described.6,13,20
Alterations to xenograft growth and chemotherapeutic agent efficacy in C. bovis-infected nude mice have been suggested anecdotally.6,12,18 Acanthosis persists after hyperkeratosis is no longer apparent in infected nude mice.2,4,20 Ultimately, however, the difficulty with the presence of C. bovis in a murine facility, in addition to the obvious potential for morbidity in immunodeficient strains, is the invalidation of studies involving valuable mice and unique reagents, including primary human patient-derived explants, that arises when mice become unsuitable for use. Because C. bovis dissemination is presumed to be surreptitiously broad, not necessarily accompanied by an increase in clinical cases of hyperkeratotic acanthotic dermatitis,4,5,18 and resistant to facility decontaminating efforts,2-4,18,20 the threat of the organism's persistence remains unless a validated facility-wide decontamination effort is implemented, but such methods have yet to be described.
Previously, beginning in December 2013, several immunodeficient murine studies were invalidated when nude mice developed hyperkeratotic acanthotic dermatitis and nude, B6.C3Rl-Lystbg/J, NOD.CB17-Prkdcscid/J, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG), NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ (NSGS), RAG2-KO/MMP2-KO on a C57Bl/6 background, and RAG2-KO/MMP13-KO mice on a C57Bl/6 background; air handling unit (AHU) trolleys; and exhaust plenums of the IVC racks tested PCR-positive for C. bovis. During the 2013 outbreak, 4 of 7 (57%) of specimens from immunodeficient mice in the room and all 3 (100%) of the tested IVC exhaust plenums in the room were PCR-positive for C. bovis. Efforts were implemented to cull all clinically affected and C. bovis PCR-positive immunodeficient mice, decontaminate the affected housing equipment and rooms according to standard procedures at that time by using either Clidox (Pharmacal, Naugatuck, CT) or Sporicidin (Contec, Spartanburg, SC), and repopulate with unaffected mice.
Clinical cases of hyperkeratotic acanthotic dermatitis in nude mice recurred in March 2015. During the 2015 outbreak, nude, NOD.CB17-Prkdcscid/J, NSG, NSGS, B6.C3Rl-Lystbg/J, RAG2-KO/MMP2-KO, and RAG2-KO/MMP13-KO mice housed in 4 isolation housing rooms tested PCR-positive for C. bovis, confirmed twice by C. bovis aerobic microbial culture. During the 2015 outbreak, 10 of 11(91%) of the specimens from immunodeficient mice, 1 of 14 (7%) of specimens from immunocompetent mice housed in the same room, 15 of 26 (58%) of the tested IVC exhaust plenums in the room; and none (0%) of the 4 tumor cell line aliquots used in the room were PCR-positive for C. bovis. As in 2013, clinically affected and C. bovis PCR-positive immunodeficient mice were culled; all portable equipment, IVC racks, and AHU trolleys were sanitized; and all surfaces of the affected housing rooms were decontaminated by using either Clidox (Pharmacal) or Sporicidin (Contec). Clinically unaffected breeders of 3 irreplaceable immunodeficient murine lines (that is, B6.C3Rl-Lystbg/J Cancer Evolution, RAG2-KO/MMP2-KO, and RAG2-KO/MMP13-KO lines) were quarantined briefly and then returned to colony production after decontaminating efforts were completed. No further testing was done to determine whether breeder mice were C. bovis-infected prior to being returned to housing.
In October 2016, nude mice again presented with scaly skin disease. In addition, NSG mice appeared clinically affected by C. bovis, presenting with hyperkeratosis and acanthosis of the periocular skin. Immunodeficient mice and equipment tested C. bovis PCR-positive. Studies involving immunodeficient mice were invalidated. The prior responses to the presentation of nude mice with C. bovis-associated hyperkeratotic acanthotic dermatitis—that is, culling PCR-positive mice and decontaminating equipment in the affected isolation housing room—were deemed to have been ineffective for preventing C. bovis recurrence.
In recognition that achieving research aims often requires trafficking to and use of procedural cores and to further our understanding regarding the facility-wide prevalence of this opportunistic agent in an SPF murine facility based at a Comprehensive Cancer Center, processes of C. bovis PCR-based environmental and murine surveillance were implemented at this site. Immunodeficient mouse housing areas were secured by limiting trafficking, and handling procedures were improved. Equipment and room decontamination routines were refined, as described later. Although methods of PCR detection, culling of C. bovis PCR-positive mice, and agent eradication within a barrier room setting have been described,14 we were interested in documenting the extent to which C. bovis dissemination occurred throughout the entire murine facility, including in core resource procedural areas; whether and how C. bovis can be eradicated facility-wide, and whether and when after decontamination the agent recurs.
Although IVC rack washing alone is insufficient for eliminating C. bovis and although washing followed by autoclaving is effective to eliminate the organism from some IVC racks,12 other nonautoclavable IVC racks, the associated electronic AHU trolleys, and the plastic and rubber hose connections of these IVC systems can accumulate murine dander and potentially C. bovis microbial load and cannot be autoclaved. However, all of these components might be amenable to vaporized hydrogen peroxide (VHP) to eliminate C. bovis, but this hypothesis has not been confirmed.10,17 In addition, murine housing rooms can be topographically complex: animal transfer stations, biosafety cabinets, shelving units, electronic scales, calipers, gavage needles, computers, and disposable supplies all provide potential but undocumented niches for agent survival. Furthermore, murine research settings frequently require the shared use of imaging, microscopy, irradiation, phenotyping, surgical, and other core resources that rely on computers and electronics not readily amendable to some methods of decontamination (for example, chlorine dioxide, autoclave sterilization); these areas likewise might provide potential niches for C. bovis survival within the murine facility.
Here we document the facility-wide prevalence of C. bovis by using PCR-based environmental and murine monitoring during an outbreak of scaly skin disease in C. bovis-infected nude mice. In addition, we describe a comprehensive, systematic method of facility-wide C. bovis eradication. All murine housing rooms; use, quarantine, storage, common procedural, surgical, imaging, genetic engineering, and pharmacy areas; and offices of the facility were decontaminated by using VHP (Bioquell, Horsham, PA) with room equipment left in place; this process was verified through the use of biologic and chemical indicators applied in healthcare,1,11,15,16 biocontainment,9 and animal facilities.10,17 In addition, decontamination of surfaces, including benchtops, biosafety cabinets, animal transfer stations, gloved hands, imaging and anesthesia equipment, and the external surfaces of the occupied IVC before and after each use was accomplished by treatment with accelerated hydrogen peroxide (aHP) typically used in healthcare settings and validated as an effective, safe sanitizing agent (OxivirTb, Diversey, Charlotte, NC).7,14,15 The prevalence, eradication, and potential for recurrence of C. bovis was documented by using C. bovis-specific PCR analysis and aerobic microbial culture as the facility-wide aHP and VHP-decontamination efforts began, progressed, and concluded.
Materials and Methods
Murine facility.
The 350,000-ft2 research building was constructed in 2004 and houses the 29,931-ft2 vivarium, comprising 7117-ft2 of murine housing space outside of quarantine and 4510 ft2 of procedural, surgical, imaging, irradiation, and genetic engineering space (Figure 1). Mice are used only within the facility, unless written IACUC approval is granted for outside-facility use (for example, multiphoton microscopy); afterward, mice are housed separately under quarantine conditions and outside of the vivarium itself for the duration of study. The program and facilities for animal care and use are fully AAALAC-accredited. All animals are housed and used in accordance with IACUC-approved protocols.
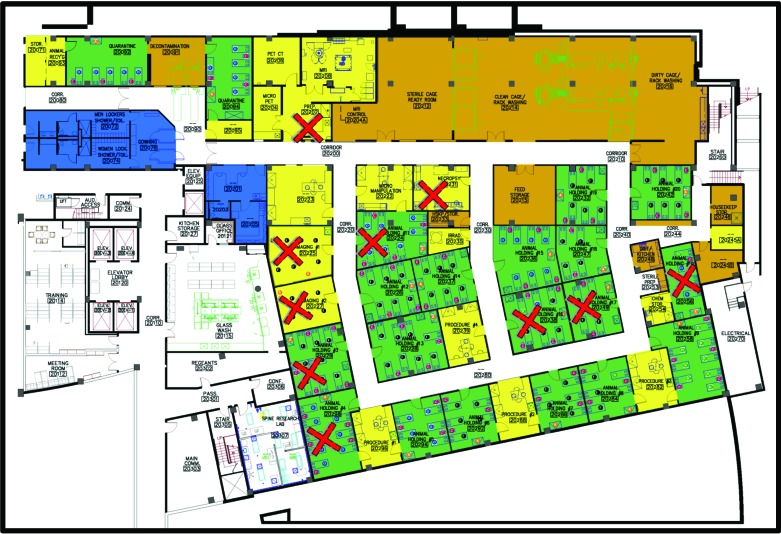
Figure 1.
Environmental, equipment, and murine C. bovis PCR surveillance during October 2016 through February 2017 (that is, months 1 through 5) revealed that 35% of the total housing space (green) and 30% of the total procedural space (yellow) was PCR-positive for C. bovis (red X). Storage and cage processing spaces are shown in brown, and personnel space is blue.
Quarantine.
The murine facility is SPF and viral-antibody-free and contains a separate 6-room, 1593-ft2 quarantine area outside of the vivarium proper. Quarantine and sentinel-testing standard procedures exclude murine norovirus, Helicobacter spp., Syphacia spp., Aspiculuris tetraptera, Parainfluenza virus type 1 (Sendai), coronavirus, Mycoplasma pulmonis, paramyxovirus, parvovirus, poliovirus, reovirus type 3, lymphocytic choriomeningitis virus, Mouse adenovirus (types 1 and 2), poxvirus (ectromelia), rotavirus, papovavirus, Hantaan virus, cilia-associated respiratory bacillus, Clostridium piliforme, and Encephalitozoon cuniculi. Quarantine housing empties into a quarantine soiled-cage processing room, within which 2 prevacuum sterilizers with bioseals (Amsco Eagle SV-3053, Steris, Mentor, OH) are used for decontaminating quarantine soiled microisolators by passage through these autoclaves; autoclaved goods are then carted into the main murine facility for cage washing. Before VHP decontamination of the facility, including quarantine, as described herein, standard procedures to exclude or to monitor and not tolerate C. bovis were not in place.
Entry.
Personnel entry space into the murine facility includes separate bathrooms with locker and changing areas. Personnel proceed to a common gowning room, where they don personal protective equipment and then enter 1 of 2 air showers that lead into the facility. Corridors are bidirectional. As described later, all common areas, including corridors, foyers, and air showers, not readily exposed to VHP were surface-decontaminated by using aHP (OxivirTb, Diversey), as were all corridor walls, floors, and ceilings.
Procedural cores.
Integral within the murine facility are the Mouse Models Core and the Small Animal Imaging Laboratory. The Mouse Models Core is a 303-ft2 laboratory that provides pronuclear microinjection services to generate transgenic or CRISPR/Cas9 founders and blastocyst microinjection and morula aggregation services to generate germline chimera from gene-targeted embryonic stem cells. The Small Animal Imaging Laboratory is composed of 6 procedural rooms totaling 2035 ft2 and providing 3D, high-resolution anatomic and molecular imaging in mice by using a 7-T MRI scanner (Varian, Agilent, Oxford, United Kingdom), 13C 15N 29Si hyperpolarizer (Oxford Hypersense), a CT–PET–SPECT system (Inveon, Siemens, Erlangen, DE), ultrahigh-frequency ultrasound imager (Vevo 2100, VisualSonics, Toronto, Ontario, Canada), several in vivo imaging systems (IVIS 100, IVIS 200, and FMT 2500, Perkin-Elmer, Waltham, MA; In Vivo Xtreme II, Bruker, Billerica, MA), a molecular imaging system (Optix MX3, Advanced Research Technologies, Montreal, Quebec, Canada), a SurgVision system (‘t Harte, Netherlands), and an open in vivo fluorescence imaging system (Fluobeam 700, Fluoptics, Cambridge, MA). In addition, integral within the murine facility are a 137Cs γ-irradiator (JL Shepherd Associates, San Fernando, CA) and a 320-kV x-irradiator (Pantak Therapax X-Rad, Precision X-ray, North Branford, CT) for total-body or focal murine exposure, and 2 solar simulators (Oriel, Newport, Irvine, CA) for murine cutaneous UV exposure. Surface decontamination of all surfaces in procedural areas before and after each use was accomplished by using OxivirTb spray (Diversey) in accordance with newly established standard procedures (described later herein).
Murine procedural space includes the Mouse Models Core, the Small Animal Imaging Laboratory, 3 procedural rooms in each of 3 housing-and-use suites, and 2 corridor-accessible procedural rooms; each of these procedural areas contains class 2A2 biologic safety cabinets, anesthesia machines, surgical photomicroscopes, centrifuges, refrigerators, and a –80 °F freezer. A separate necropsy room is equipped with a fume hood, vented specimen storage, hematology and serum chemistry analyzers, and a 2-station, dual-draft murine necropsy workstation. Each fully equipped procedural room, including the Mouse Models Core, the Small Animal Imaging Laboratory, all suite- and corridor-accessible procedural rooms, and necropsy were VHP decontaminated by leaving the equipment in place, after consultation with equipment manufacturers to confirm that VHP exposure would not alter equipment functions.
Housing.
Murine housing is in corridor-accessible housing rooms. IVC are changed in animal transfer stations or in class 2A2 biologic safety cabinets.
IVC sterilization.
In the murine facility, cage wash movement is one-way, from soiled to clean to sterile. IVC are sanitized through a 180 °F final rinse in redundant tunnel washers with dryers (Basil 6000, Steris, Mentor, OH). IVC racks are sanitized at 180 °F final rinse in a rack washer with dryer (Basil 9500, Steris). Washed and dried caging equipment exit these machines and enter into a clean IVC preparation room. Clean IVC are stocked with bedding, reassembled, and clamped shut. Prepared IVC and filled water bottles are sterilized in separate loads by passage through 2 roll-in prevacuum bulk steam sterilizers (Century SLH Scientific, Steris) with bio-seals; appropriate processing is ensured using by using chemical indicator strips (Verify, Steris) and biologic indicators. Sterile IVC and filled sterile water bottles are labeled with the date sterilized, covered, and stored in the sterile cage-ready room.
IVC racks and AHU trolleys.
IVC units (Blueline, Tecniplast, Buguggiate, Italy) were used for all murine housing, including in immunodeficient strain colony production and use, in ABSL2 containment for patient-derived xenographs or recombinant DNA, and in quarantine during imported strain testing and rederivation. These cages can be used under either positive or negative pressurization and revert to 0.2-μm efficiency static microisolation caging for approximately 72 h in the event of power failure. A double-sided rack configuration with each rack holding 126 microisolation cages (that is, 63 cages per rack side) was used uniformly throughout the murine facility, and each pair of racks was ventilated and exhausted by a single AHU trolley.
Individual, mobile, low-energy-consumption (that is, 0.45 W per cage), HEPA-filtered air supply and exhaust AHU trolleys (that is, 4 models: SystemBox-110, SlimLine, Touchslim-Plus, and SmartFlow; all from Tecniplast) were used. Each AHU trolley served 4 IVC rack sides (that is, 252 microisolators). Baffling in each IVC top ensures a correct air mix without short-circuiting or blowing directly on animals. AHU delivered a low airspeed (that is, less than 0.02 m/s), and exhaust air was actively removed from the cage, not merely pushed out. AHU trolley exhaust prefilters were changed every 2 wk by using a bag-out procedure and OxivirTb spray (Diversey), unless described otherwise later.
IVC racks, plenums, and connecting hoses were washed in a mechanized rack or tunnel washer, and a final rinse temperature of 180 °F was ensured. The exterior surfaces and interior prefilter chambers and hose connecting ports of all AHU were hand sanitized by using OxivirTb during AHU change-outs, unless described otherwise later. All washed IVC racks, connecting hoses, and hand-sanitized AHU were then VHP decontaminated in a flex room, as described later.
Decontamination methods.
OxivirTb (Diversey) is an aHP and a broad-spectrum virucidal, bactericidal, tuberculocidal, and fungicidal surface cleaner. OxivirTb disinfects in 1 min, kills methicillin-resistant Staphylococcus aureus (MRSA) and norovirus, meets bloodborne pathogen standards for blood and body fluid decontaminations, and is tuberculocidal in 5 min. All surfaces, including animal transfer stations, biosafety cabinets, procedural countertops, gloved hands, core imaging equipment, and the external surfaces of the occupied IVC, were decontaminated before and after each use by saturate spraying with OxivirTb.
All murine housing and use rooms were decontaminated by using VHP (Bioquell, Horsham, PA) with equipment left in place, a process recently validated at our institution by using biologic indicators, chemical indicators, and PCR-supported evidence of opportunistic bacteria.17 Briefly, effective equipment VHP sterilization requires an ‘active–closed’ VHP exposure of IVC racks assembled, sealed, and connected to AHU set to the VHP cycle. Biologic indicators detected VHP levels in equipment airways capable of a 6-log kill of Geobacillus stearothermophilus spores sealed inside pouches (Bioquell), incubated for 7 d. In addition, chemical indicators were placed inside equipment airways and comprised a semiquantitative visual ink-on-card indication of 2-, 4-, or 6-log kill levels of VHP.17
Herein, VHP decontaminating efforts were systematic, comprehensive, room-by-room, and facility-wide by using a Z2 generator (Bioquell), an R30 aeration catalytic accelerator (Bioquell), and a ‘flex room’ (that is, an empty housing room) as an equipment decontaminating chamber. Early ineffective efforts to VHP decontaminate IVC racks and AHU, evaluated during months 1 through 5 of the present report, were ‘static–open’ VHP exposures, with AHU off, hose connections unsealed, and IVC rack plenums unassembled and unsealed (Figure 2). All subsequent equipment VHP decontaminations, during months 6 to 12 of the present report, were active–closed VHP exposures, wherein IVC rack plenums were fully assembled and sealed, with air supply and exhaust hoses in place and connecting IVC racks to AHU trolleys set on to the VHP cycle (Figure 2), an assembly that ensures that VHP-saturated room air is drawn through the airways of the AHU trolley, connecting hoses, and IVC rack plenums, both supply and exhaust, previously confirmed by using biologic and chemical indicators, by testing with a handheld hydrogen peroxide monitor (Dräger Safety, Lubeck, Germany), and by PCR testing of airway interior surfaces for opportunistic bacteria.17
Figure 2.
(A) IVC racks and AHU were VHP decontaminated during months 1 through 5 in the static–open setting, such that AHU were off, hoses were disconnected, and rack plenums were unassembled (arrows). (B) In contrast, during months 6 through 12, IVC racks and AHU were VHP exposed in the active–closed setting, with IVC rack plenums assembled, sealed, and hoses connected (arrows) to AHU set to the VHP cycle. (C) Airways (here an exhaust plenum) were sampled either when soiled, after they were washed, or after VHP exposure by using a sterile swab to trace a circular pattern for 3 circumferences as the tip was rolled. Sample-bearing swabs then were assessed by using C. bovis PCR analysis.
VHP flex room.
To facilitate VHP decontamination routines, the program invested in additional IVC racks and AHU so that even the largest housing room could be completely changed out at once and VHP exposed in its entirety at the same time on a single day semiannually. Scheduled dates of murine housing room change-outs and procedural room VHP decontaminations were announced and posted in advance. The day before a housing room was scheduled for VHP decontamination, the flex room was equipped with sufficient IVC racks hose-connected to AHU set to the VHP cycle, for an active–closed VHP decontamination (Figure 3). Other portable equipment (for example, anesthesia machines, animal transfer stations) was included in flex room VHP decontaminating cycles, as required. Then the flex room was sealed by using HVAC supply and exhaust vent covers and thimble covers, the door was taped closed, and all equipment needed for the scheduled housing room change-out the next day was VHP decontaminated, a process requiring approximately 4 h. At the completion of the active–closed VHP decontaminating exposure, when flex room VHP levels detected by using the hydrogen peroxide monitor (Dräger Safety) that were below the long term exposure limit 8-h time-weighted average of less than 1 ppm, the HVAC supply and exhaust vents were reopened, and the AHU were reset to a typical supply and exhaust cycle used to ventilate 2 doubled-sided IVC racks, so as to expel any residual VHP from the rack plenums and AHU ducts and filters, as previously described.17 The next morning, this inventory of VHP-decontaminated IVC racks, hoses, and AHU were used in the scheduled housing room change-out, as follows.
Figure 3.
The flex room where assembled IVC racks connected to AHU were VHP exposed in the active–closed setting, with AHU set to the VHP cycle, during months 6 through 12. All equipment required for a scheduled housing room change-out in its entirety was VHP decontaminated the day before by using the Z2 generator and R30 accelerator. Other portable equipment (here, an animal transfer station with its work surface propped open) was VHP decontaminated in the flex room also.
VHP decontamination of housing and procedural rooms.
On the morning of a scheduled housing room change-out, the Z2 generator (Bioquell), R30 aeration catalytic accelerator (Bioquell), HVAC vent and thimble covers, and AHU with hoses connected—all of which had been active–closed VHP decontaminated in the flex room the day before—were parked by the scheduled housing room door, ready for room VHP decontamination. The IVC racks that had been active–closed VHP decontaminated in the flex room the day before were left in that room. The floor scrubber (S20, Clark-Encore, Mechanicsville, VA) filled with OxivirTb (Diversey) was run in the scheduled housing room and in the corridors between the scheduled housing room, the flex room, and the soiled side of cage wash (Figure 4). Each occupied IVC rack in the scheduled housing room was disconnected from hoses, plenums capped, and taken to the flex room, where all occupied IVC were transferred to the VHP-sterilized IVC racks. After being emptied of occupied IVC, the soiled IVC racks were taken to soiled cage wash for washing. While the scheduled housing room was VHP decontaminated, the murine inventory was left in the flex room in static microisolation until the next morning. Similarly, soiled AHU in the scheduled housing room had their hoses capped, were sprayed with OxivirTb, and were taken directly to soiled cage wash for hand cleaning with OxivirTb and prefilter change.
Figure 4.
(A) The housing room scheduled for VHP decontamination, from which occupied IVC racks were taken to the flex room, where occupied IVC were transferred to (B) VHP-sterilized racks. (C) In the now unoccupied housing room, storage unit drawers were opened, the floor scrubber was run (arrow), (D) thimbles were covered, (E) VHP-sterilized AHU with hoses were positioned (arrow), (F) HVAC supply and exhaust vents were covered (left arrow) and animal transfer stations were left powered on (right arrow), and the Z2 generator and R30 accelerator were positioned. (G) The next morning, murine occupied IVC racks were brought from the flex room to the VHP-decontaminated housing room and connected to AHU.
In the scheduled housing room, now devoid of soiled IVC racks, AHU, and murine inventory, all disposable porous materials were discarded, prefilters were changed in animal transfer stations, surfaces were sanitized by using OxivirTb, and AHU were left powered on in the scheduled housing room for VHP exposure. Storage drawer units (Flexline, InterMetro, Ontario, Canada) or shelving units (Lab Products, Seaford, DE) were either changed-out or drawers were opened. Shelving or drawer units were used in housing to hold minor supplies and equipment and were left in the housing room to be VHP decontaminated. Other portable equipment used in housing (for example, quad anesthesia machines, scales, calipers) were sanitized by using OxivirTb and left to be VHP decontaminated. HVAC diffusers and thimble connections were covered. The AHU with hoses connected that had been active–closed VHP sterilized the day before in the flex room, Z2 generator (Bioquell), and R30 aeration catalytic accelerator (Bioquell) were all placed inside the scheduled housing room (Figure 4). The next morning, after VHP cycle completion and after use of the hydrogen peroxide monitor confirmed that VHP levels were less than 1 ppm in the housing room and therefore safe for reentry, the IVC racks occupied by murine inventory were moved from the flex room to the VHP-decontaminated housing room and connected to the AHU.
Procedural rooms were VHP decontaminated also (Figure 5), either by sealing procedural rooms that are contiguous with housing rooms and VHP exposing all of them simultaneously as a suite, or separately by sealing and VHP exposing procedural rooms that are accessed from the corridor. In either case, all fixed and portable equipment and supplies (for example, imaging equipment, biosafety cabinets, shelving units, quad anesthesia machines, microscopes, computers, calipers, scales, forceps) were VHP decontaminated.
Figure 5.
Procedure rooms were VHP decontaminated with all supplies and equipment present, centrifuges open, biologic safety cabinet sashes raised, anesthesia induction boxes open, and all casework drawers and shelving exposed.
PCR surveillance for C. bovis and Staphylococcus xylosus.
Sterile swabs (FLOQ, Copan Flock Technologies, Brescia, Italy) were used to collect environmental specimens from surfaces by tracing a circular pattern for 3 circumferences as its tip was rolled; samples were then analyzed in PCR assays. Swabs of room and corridor surfaces, IVC rack plenums, AHU exhaust prefilter chambers, connecting hoses, and fixed and portable equipment surfaces and fecal pellets of mice5 were submitted to a testing laboratory (IDEXX BioResearch, Columbia, MO) for C. bovis real-time PCR testing, an assay that targets a region of the 16S rRNA gene that is conserved among all C. bovis genomic sequences deposited in GenBank, by using a FAM/TAMRA-labeled hydrolysis probe. Hydrolysis probe-based real-time PCR assays targeting the bacterial gene (16S rRNA) were used to ensure DNA recovery and the absence of PCR inhibitors in extracted nucleic acids. Real-time PCR analysis was performed at standard primer and probe concentrations by using a master mix (LC480 ProbesMaster, Roche Applied Science, Indianapolis, IN) on a real-time PCR platform (LightCycler 480, Roche). The copy number estimate of C. bovis DNA for each PCR test was calculated by plotting the real-time crossing-point values from the C. bovis PCR assay on a standard curve of log-fold dilutions of a positive control having a known copy number.
Similarly, swabs of airway interior surfaces were submitted to the testing laboratory (IDEXX BioResearch) for S. xylosus real-time PCR testing, an assay that targets a region of the 16S rRNA gene conserved among all S. xylosus genomic sequences deposited in GenBank, by using a FAM/TAMRA-labeled hydrolysis probe. S. xylosus, a commensal of the murine skin, is broadly prevalent on IVC and AHU airway interior surfaces and becomes PCR detectable on airway surfaces approximately 7 to 12 d after the return of murine-occupied IVC to the VHP sterilized rack.17 In the present report, S. xylosus was used as an indicator species to ensure the sterility of VHP-exposed equipment and to serve as a positive control after the return of murine-occupied IVC to the VHP-sterilized rack during monitoring of whether PCR evidence of C. bovis recurs in the airways of VHP-sterilized equipment in previously C. bovis-affected housing rooms.
Statistics.
An unconditional exact test using the R 3.4 [R Core Team (2017)] statistical software (R Foundation for Statistical Computing, Vienna Austria) was performed to compare the percentage of C. bovis PCR-positive specimens obtained during months 1 through 5 with those obtained during months 6 to 12 from either mice, equipment that was soiled, equipment that was sanitized, or equipment VHP that was exposed as either static–open (that is, months 1 through 5) or active–closed (that is, months 6 to 12). A nonparametric Wilcoxon rank-sum test, based on the nonzero C. bovis PCR copy numbers determined for specimens from mice and soiled, washed, or VHP exposed equipment, was used to compare C. bovis PCR copy numbers of specimens from months 1 through 5 with those from months 6 through 12. A P value less than 0.05 was considered to define a statistically significant difference.
Results
The murine facility, at capacity, accommodates 62,496 mice in 15,624 IVC in 18 housing rooms, including 8064 immunodeficient mice in 2,016 IVC in 4 isolation housing rooms separate from immunocompetent strain housing. From its 2005 opening through mid 2016, procedures to exclude C. bovis were not in place, and neither immunodeficient murine inventories nor the surfaces of the facility environment were monitored for C. bovis. Two prior attempts (December 2013 and March 2015), implemented at the level of the affected isolation room only, had failed to eliminate study invalidations due to scaly skin disease in nude mice. Prior to October 2016, despite a substantive immunodeficient murine inventory, diagnostic PCR testing and aerobic microbial cultures for C. bovis were conducted only in response to immunodeficient mice appearing scaly or scruffy, with only 66 specimens submitted for C. bovis PCR testing and 2 specimens submitted for C. bovis aerobic microbial culture, thus totaling only approximately 7 tests for C. bovis annually for 10 y.
In contrast, beginning October 2016 through September 2017 (that is, months 1 through 12 of the present report), 2064 specimens were submitted for C. bovis PCR testing, and 8 specimens were submitted for C. bovis aerobic microbial culture. Of these 2064 specimens for PCR testing, 498 specimens were murine feces or fur swabs of mice, 6 specimens were of tumor cell lines, and 1560 specimens were environmental swabs of fixed and portable equipment and facility surfaces. Environmental PCR surveillance for C. bovis was broadly implemented to document the facility-wide prevalence of C. bovis and to assess the efficacy of improved aHP and VHP decontaminating measures.
The extent of the 2016 C. bovis outbreak was characterized, and effective aHP and VHP decontaminating procedures were developed October 2016 through February 2017 (that is, months 1 through 5), during which nude mice developed hyperkeratotic acanthotic dermatitis, NSG mice presented with hyperkeratosis and acanthosis of the periocular skin,21 and nude, NOD.CB17-Prkdcscid/J, NSG, B6.C3Rl-Lystbg/J, and RAG2-KO/MMP2-KO, and RAG2-KO/MMP13-KO mice tested PCR-positive for C. bovis in 6 of 18 housing rooms, thus affecting 2472 ft2 (35%) of 7117 ft2 of housing space (Figure 1). In addition, surfaces in 4 of 16 procedural rooms tested PCR-positive for C. bovis, thus affecting 1356 ft2 (30%) of 4510 ft2 of procedural space. During the 5-mo characterization of the extent of the C. bovis outbreak, 39 (26%) of 153 specimens from mice, 30 (22%) of 138 specimens from soiled IVC racks and AHU, and 0 (0%) of 6 tumor cell line aliquots were PCR-positive for C. bovis (Table 1). Procedural rooms that were PCR-positive for C. bovis included the optical imaging rooms, necropsy room, and preparatory lab that led into the MRI and CT–PET–SPECT imaging rooms in the Small Animal Imaging Laboratory. Specifically, in these rooms, the imaging chamber of the IVIS 200 system (Perkin-Elmer), the imaging chamber of the Optix MX3 system (Advanced Research Technologies), the imaging chamber of the FMT 2500 system (Perkin-Elmer), an anesthesia induction box, the chamber of a biosafety cabinet, a procedural tabletop, and the tabletop of the hematology analyzer in necropsy were all PCR-positive for C. bovis, thus documenting widespread C. bovis contamination.
Table 1.
PCR-based detection of C. bovis DNA
| Equipment surfaces |
||||
| Mice | Soiled | Washed | VHP-exposed as static–opena | |
| October | 14/45 (31%) | 5/12 (42%) | ||
| November | 6/14 (43%) | 9/15 (60%) | 4/9 (44%) | 4/24 (17%) |
| December | 2/2 (100%) | 5/41 (12%) | 1/7 (14%) | 1/11 (9%) |
| January | 14/69 (20%) | 6/22 (27%) | 1/11 (9%) | 1/8 (13%) |
| February | 3/23 (13%) | 5/48 (10%) | 1/9 (11%) | 0/6 (0%) |
| Total for months 1–5 | 39/153 (26%) | 30/138 (22%) | 7/36 (19%) | 6/49 (12%) |
| VHP-exposed as active–closeda | ||||
| March | 0/2 (0%) | 2/379 (1%) | 1/86 (1%) | 0/64 (0%) |
| April | 0/10 (0%) | 1/103 (1%) | 0/18 (0%) | 0/70 (0%) |
| May | 0/11 (0%) | 1/59 (2%) | 0/2 (0%) | |
| June | 10/264 (4%) | 1/96 (1%) | 0/1 (0%) | |
| July | 0/11 (0%) | 2/113 (2%) | 0/1 (0%) | |
| August | 0/46 (0%) | 3/234 (1%) | ||
| September | 0/1 (0%) | 0/111 (0%) | ||
| Total for months 6–12 | 10/345 (3%) | 10/1,095 (1%) | 1/104 (1%) | 0/138 (0%) |
| P < 0.0001b | P < 0.0001b | P = 0.0005b | P = 0.0004b | |
Data are given as no. of specimens that were PCR-positive for C. bovis/no. tested (% positive)
’Static–open’ indicates that AHU were off, and IVC rack plenums and hoses were disconnected; dactive–closed’ indicates that AHU were on VHP cycle, and IVC rack plenums and hoses were connected to AHU
Total for months 1 through 5 compared with total for months 6 through 12.
During November 2016, a Z2 VHP generator (Perkin-Elmer), an R30 aeration catalytic accelerator (Bioquell) and additional IVC racks and AHU were procured, so that even the largest housing room could be changed-out in its entirety in a single day. A flex room was established for VHP exposures of portable equipment. Because identification and culling of C. bovis PCR-positive mice continued, efforts to improve decontaminating procedures using VHP were evaluated. Initially, during November 2016 through February 2017 (that is, months 2 through 5) VHP exposures of IVC racks and AHU were done in the static–open configuration (Figure 2), with all IVC rack plenums left open, hoses left disconnected, and AHU left off, as previously described in detail.17
OxivirTb for surface decontamination was implemented; this product was selected for its broad efficacy, short kill time, and safe, noncorrosive, nonirritant ease of use, to ensure compliance by all research and animal care staff. After checking with the manufacturers of imaging, anesthesia, microscopy, and other portable equipment, surface-decontaminating procedures using OxivirTb were implemented facility-wide, in imaging chambers, in anesthesia induction chambers and nosecones, on gloved hands and sleeves, on benchtops, in animal transfer stations, in biosafety cabinets, and on the exterior surfaces of the closed occupied IVC. All research and animal care staff were trained to use OxivirTb to saturate-spray all chamber surfaces of the biosafety cabinet or changing station prior to use, their gloves and sleeves, and the external surfaces of the occupied primary enclosure prior to placing it into the biosafety cabinet or changing station for use. Furthermore, improved AHU sanitizing methods were implemented with OxivirTb to ensure that all accessible surfaces had been cleaned prior to VHP exposure.
During November 2016 through February 2017 (that is, months 2 through 5), IVC racks and AHU were assessed by C. bovis PCR testing when soiled, after mechanized washing or hand-sanitizing, and after static–open VHP exposure. During this initial 5-mo effort to understand and then improve decontaminating procedures, 30 (22%) of 138 specimens from soiled equipment were PCR-positive for C. bovis, 7 (19%) of 36 remained C. bovis PCR-positive after mechanized washing racks or hand-sanitizing AHU, and 6 (12%) of 49 remained C. bovis PCR-positive after static–open VHP exposure (Table 1). Because only newer models of AHU trolleys (for example, SmartFlow [Tecniplast]) are programmable to a VHP decontaminating cycle, which operates AHU fans at a slower airspeed to ensure sufficient VHP decontaminating contact time of interior duct and prefilter chamber surfaces, all older model AHU were replaced with VHP-cycle-programmable SmartFlow AHU. All subsequent VHP exposures used the active–closed configuration, with plenums fully assembled and sealed, hoses in place and connecting IVC racks to AHU, and AHU set to the VHP cycle; this assembly ensures that VHP-saturated air is drawn through the airway interiors, as previously described.17
To limit personnel traffic into immunodeficient mice housing rooms, security card access was installed on the doors of 4 immunodeficient mice housing and use rooms during January 2017, with one room limited to immunodeficient colony production and accessible to caretakers only, a second room limited to unidirectional immunodeficient mice housing and use and equipped with a dedicated IVIS 100 system (Perkin-Elmer) for imaging, therefore forbidding immunodeficient mice to exit the room until euthanasia, and the third and fourth isolation rooms established as bidirectional isolation housing and use rooms from which immunodeficient mice were permitted to travel to common procedural areas within the murine facility (for example, the Small Animal Imaging Laboratory) and return to the security-card–accessible isolation housing room.
During February 2017 (month 5), culling of all C. bovis PCR-positive mice was completed. Colonies of all replaceable immunodeficient strains (for example, nude, NSG, SCID) were euthanized regardless of C. bovis testing status and not replaced for a period of 30 d to permit an opportunity for effective facility-wide VHP decontamination using active–closed exposure. On-study immunodeficient research cohorts and irreplaceable intramural immunodeficient lines were relocated to a neighboring vivarium to complete to study endpoint or for rederivation. All housing and procedural areas and all portable and fixed equipment were scheduled for VHP decontamination. The entire facility was VHP decontaminated in 2 mo, during March and April 2017 (months 6 and 7), with all IVC racks and AHU VHP exposed in the active–closed setting, and all housing and procedural rooms, including the Small Animal Imaging Laboratory and Mouse Models Core, VHP exposed. C. bovis PCR testing of equipment continued, before and after washing and hand sanitizing, and after active–closed VHP exposure. C. bovis-affected housing rooms identified during months 1 through 5 were changed-out and VHP decontaminated first, each changed in its entirety in a single day.
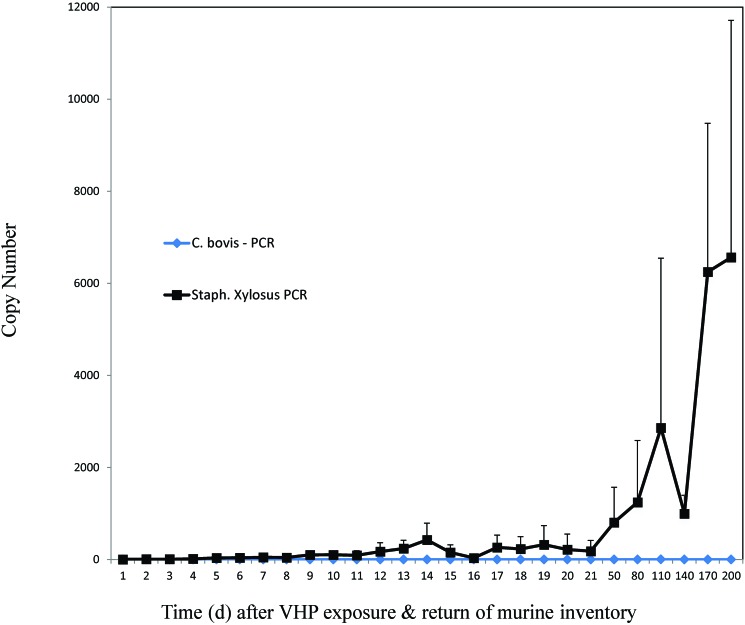
During March and April 2017 (months 6 and 7), after C. bovis PCR-positive mice had been culled, after older-model AHU had been replaced with VHP-cycle–programmable units, and after improved OxivirTb sanitizing methods and active–closed VHP decontaminating processes had been implemented, 0 (0%) of 12 murine specimens, 3 (1%) of 482 specimens from soiled IVC racks and AHU trolleys, 1 (1%) of 104 specimens from washed racks or sanitized AHU, and 0 (0%) of 134 specimens from active–closed VHP-exposed racks and AHU were C. bovis PCR-positive (Table 1). Each of the C. bovis PCR-positive specimens from soiled or washed equipment was from previously C. bovis-affected housing rooms that had been culled of C. bovis PCR-positive mice. Neither of the 2 PCR-positive specimens obtained from washed equipment resulted in aerobic microbial growth. In each of the previously C. bovis-affected rooms, murine inventories were monitored for C. bovis by PCR testing of pooled fecal pellets, with 0 (0%) of 24 specimens positive, and by daily C. bovis PCR testing of airways for 200 d, with 0 (0%) of 259 environmental specimens C. bovis PCR-positive (Figure 6). In contrast, S. xylosus was detectable in airways by PCR analysis on days 9 through 200, as previously described.17
Figure 6.
Mean quantitative PCR copy numbers of S. xylosus (black squares) or C. bovis (blue diamonds) detected in the IVC rack exhaust plenum after active–closed VHP exposure and the return of murine inventory to a previously C. bovis-affected housing room.
After effective active–closed VHP exposures were completed in April 2017, facility-wide PCR surveillance for C. bovis on rack exhaust plenum interiors during May (month 8) found 0 (0%) of 56 specimens C. bovis PCR-positive. A single C. bovis PCR-positive specimen was detected in the interior chamber of the optical imaging equipment (FMT 2500, Perkin-Elmer) in the Small Animal Imaging Laboratory, which the manufacturer had recommended to be closed during VHP exposure. Regardless, this specimen yielded a low C. bovis DNA copy number of 3, and resulted in no aerobic microbial growth; after additional hand-washing, the equipment later tested PCR-negative in a subsequent month. Indeed, after the culling of C. bovis PCR-positive and clinically affected mice was completed in February 2017 (month 5), no clinically unaffected nude mice developed hyperkeratotic acanthotic dermatitis during the rest of the study (months 6 through 12). Regardless, during June 2017 (month 9), a single rack in a previously affected housing room tested C. bovis PCR-positive, with a copy number of 4. Subsequent testing of every cage in the room found 10 (4%) of 264 specimens from asymptomatic nude mice to be C. bovis PCR-positive, with a copy number of 2 to 2421 (mean ± 1 SD, 468 ± 706). As before, C. bovis PCR-positive mice were culled, and the room and its equipment were changed-out and VHP decontaminated in their entirety in a single day. In addition, one strain of mice on a C57BL/6 background with a keratin promoter deficiency tested positive, suggesting that sampling mice on all backgrounds, regardless of suspected immune status, is recommended.
After resolution of the June 2017 C. bovis recurrence, 0 (0%) of 58 murine specimens and 0 (0%) of 447 equipment airway specimens were C. bovis PCR-positive during July through September 2017 (months 10 through 12). Still, of the 11 environmental specimens taken during July and August 2017 (months 10 and 11), 5 detected molecular C. bovis persistence. During July 2017 (month 10), a single specimen from the benchtop of the procedural room across the hall from the June C. bovis recurrence, to which C. bovis-positive mice had been trafficked for euthanasia to resolve the recurrence, tested PCR-positive for C. bovis. Also during July, a single specimen from a computer keyboard in the facility office tested C. bovis PCR-positive. Both the affected office and procedural rooms were again VHP decontaminated. During August 2017 (month 11), PCR evidence of residual C. bovis was again found in 3 specimens from computer keyboards in the office. None of these 3 specimens resulted in aerobic microbial growth. Keyboards were replaced, and again, the office was VHP decontaminated. During September 2017 (month 12), 0 (0%) of 111 equipment and environmental specimens were C. bovis-positive (Table 1).
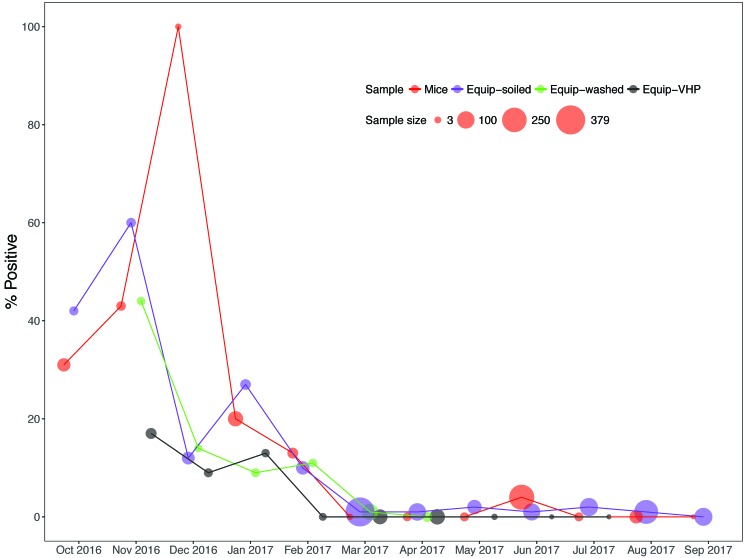
The cumulative effect of culling C. bovis-positive mice and implementing improved aHP and active–closed VHP decontaminating exposures during months 6 through 12 significantly reduced the prevalence of C. bovis compared with months 1 through 5 (Figure 7), including significantly reducing C. bovis PCR-positive specimens obtained from mice (P < 0.0001), from soiled equipment (P < 0.0001), washed equipment (P = 0.0005), and VHP-exposed equipment (P = 0.0004; Table 1). As an additional indication of improved VHP decontaminating efficacy, the nonzero C. bovis DNA copy numbers of specimens obtained from mice or soiled or washed equipment during months 6 through 12 were significantly (P < 0.0001) less than those obtained during months 1 through 5 (Table 2). No specimen derived from active–closed VHP-exposed IVC racks and AHU trolleys tested C. bovis PCR-positive.
Figure 7.
Percentage of environmental, equipment (Equip), and murine specimens that were PCR-positive for C. bovis during the 12-mo study.
Table 2.
C. bovis PCR copy numbers
| Equipment surfaces |
||||
| Months | Mice | Soiled | Washed | VHP exposed |
| 1–5 | 68 (2–12,884) | 121 (3–2858) | 6 (3–22) | 16.5 (8–1209)a |
| 6–12 | 12.5 (3–62) | 5 (2–11) | 6 | —b |
| <0.0001 | <0.0001 | <0.0001 | ||
Mean (range) of nonzero C. bovis PCR copy numbers only
Static–open configuration
Active–closed configuration
Furthermore, since the conclusion of the 12-mo study, none of the 452 additional murine, cell biologic, environmental, and monthly equipment surveillance specimens tested were C. bovis PCR-positive, documenting a consistent 11-mo period of facility-wide C. bovis eradication to date.
Discussion
Although long presumed to be a wide-spread murine facility contaminant during an outbreak of scaly skin disease,2-4,18 herein we document for the first time the molecular prevalence and persistence of C. bovis in an affected murine facility, beginning when nude mice first presented clinically with hyperkeratosis. During the outbreak, C. bovis was detected in 10 (29%) of 34 of the murine housing and use rooms, affecting 3828 ft2 (33%) of the 11,627 ft2 of the murine housing and use space, or 13% of the total 29,931-ft2 facility. Additional space affected by C. bovis during the 12-mo study included, during the June C. bovis recurrence, a 286-ft2 procedural room and the 255-ft2 personnel write-up room, so that a total of 4114 ft2 (35%) of the murine housing and use space and 4369 ft2 (15%) of the total 29,931-ft2 facility space became C. bovis contaminated during the 12-mo study. Our findings support the prior controlled demonstration that increasing prevalence of C. bovis is not accompanied by an increase in clinical signs of scaly skin disease.5
Although security-card access restriction of immunodeficient housing and use areas was established for the enhanced isolation of immunodeficient strains, intrafacility trafficking of immunodeficient mice to procedural cores (for example, irradiation, imaging) remained an essential aspect of many research aims. Consequently, the entire facility was decontaminated by using a combination of 2 products new to the program, OxivirTb (an aHP) and VHP by using a commercially available generator and accelerator. The OxivirTb aHP surface cleaner eliminated the prior requirement to rinse surfaces after the use of a corrosive cleaner (Clidox-S, Pharmacal), avoided the sticky residue left by another prior surface cleaner (Sporocidin, Contec), and has resulted in greater use compliance by staff because it is safe, noncorrosive, nonirritating; has broad efficacy and a short kill time; and can be used liberally to saturate surfaces before and after murine uses.
Although initially requiring 5 mo of evaluation and refinement to develop,17 VHP decontamination by using the Z2 generator and R30 aeration catalytic accelerator is now accomplished facility-wide efficiently in 2 mo with the documented effective outcome. To avoid study invalidations due to C. bovis, we now routinely monitor by monthly C. bovis PCR assay the airways of all IVC racks and AHU and anticipate being immediately responsive by complete equipment and room VHP decontamination to any C. bovis PCR detection. Furthermore, even in the absence of C. bovis PCR evidence in airways, we now schedule facility-wide VHP decontamination every 6 mo. We also have implemented C. bovis PCR environmental monitoring of procedural and personnel areas, C. bovis PCR monitoring of immunodeficient mice, and the quarantine exclusion of C. bovis, consequently anticipating the continued absence of study invalidations due to C. bovis. Although biologics have been shown to be a source of C. bovis murine contamination,6,14,20 none of the biologics tested during any of the outbreaks in 2013, 2015, or 2016 were found to be C. bovis-contaminated. Regardless, such characterizations continue.
Contributing to effective facility-wide VHP decontamination were prior confirmations from manufacturers of procedural equipment that OxivirTb use and VHP exposure could be implemented without substantial risk to equipment functional integrity. This implementation included the VHP exposure of imaging, irradiating, surgical, microinjection, anesthetic, and other research equipment. Also contributing to C. bovis eradication was the use of a flex room in advance of room change-outs, which required a sufficient inventory of IVC racks and AHU that could be active–closed VHP exposed in advance of the scheduled housing room VHP exposure. The flex room also was used as a VHP decontamination chamber for other portable equipment. Indeed, the flex room is now considered the fourth room of the equipment sanitization–sterilization process, such that staff now ensure that when equipment cannot go through mechanized washing and bulk autoclave steam-sterilizing, it is VHP sterilized. Another contribution to C. bovis eradication was the recognition that merely VHP exposing IVC racks and AHU did not ensure airway sterilization given that 6 (12%) of 49 of specimens from static–open VHP-exposed airways were C. bovis PCR-positive during November 2016 through February 2017 (months 2 to 5). In contrast, using the validated active–closed VHP exposure17ensured that 0 (0%) of 138 specimens from airways were C. bovis PCR-positive during March through July 2017 (months 6 through 10), a significantly improved VHP decontaminating outcome (P = 0.0004).
Scheduled housing rooms were each changed-out in 24 h, mitigating concerns that a niche for agent survival may be left unexposed to VHP as murine inventory returned to housing. Although occupied IVC were shifted from soiled to active–closed VHP-exposed IVC racks in the flex room, this single soiled–sterile equipment overlap did not affect the outcome. We reasoned, and results demonstrated, that because the interior of the primary enclosure was documented as sterile due to routine bulk autoclave sterilization and because the exterior of the microisolation was now routinely saturate sprayed with OxivirTb each time it was handled, including during regular IVC change-outs every other week, the principle niches for C. bovis survival in housing rooms were the secondary supporting equipment. Indeed, during characterization of the outbreak, soiled IVC racks and AHU provided a safe niche for C. bovis survival, comparable to the interior of the primary enclosure, with 39 (26%) of 153 of murine specimens and 30 (22%) of 138 equipment airway specimens C. bovis PCR-positive during October 2016 through February 2017 (months 1 through 5).
Prior efforts in 2013 and 2015 to mitigate C. bovis study invalidations had been implemented only at the level of the affected housing room. Although C. bovis PCR testing was conducted in the affected housing room, with culling of clinically affected and C. bovis PCR-positive mice and change-out of racks and AHU, facility-wide PCR environmental monitoring had not been implemented, and the extent of C. bovis contamination had not been determined. On the basis of the present report, efforts implemented only at the affected housing room level are unlikely to eradicate C. bovis facility-wide, given that procedural and personnel areas also offer niches for C. bovis persistence.
The present report demonstrates that critical to the eradication of C. bovis is the implementation of regular facility-wide environmental and murine C. bovis PCR surveillance to guide and direct regular facility-wide VHP decontamination to ensure efforts effectively contribute to agent eradication. After culling C. bovis clinically affected and PCR-positive mice, implementing routine C. bovis PCR surveillance, and implementing effective aHP and VHP decontaminating routines by February 2017 (month 5), no mice became C. bovis clinically affected for the rest of the study (months 6 through 12). Furthermore, routine C. bovis PCR surveillance allowed the quick identification of a single C. bovis PCR-positive rack during June 2017 (month 9) and the discovery of C. bovis PCR-positive mice at the cage level, ensuring the rapid culling of these mice, the immediate VHP decontamination of equipment, the subsequent PCR detection of C. bovis in the procedural room across the hall, and its VHP decontamination. Preventing study invalidations due to C. bovis requires regular C. bovis PCR surveillance and an acute VHP decontaminating response.
In addition, because the June 2017 recurrence affected mice of a single laboratory and because all murine specimens had been PCR-negative during the prior 3 mo (March through May), the basic laboratory space outside of the murine facility was tested for PCR evidence of C. bovis, but none of the 9 specimens taken in various locations throughout the basic laboratory were C. bovis-positive.
Our observations confirm that despite the absence of marked, wide-spread morbidity attributable to C. bovis, the agent can be harbored by a number of immunodeficient strains, is PCR identifiable among unaffected immunocompetent strains, and is broadly disseminated in housing, procedural, and personnel areas during an outbreak. Although relatively few nude mice presented with clinical signs during the outbreak, owing to the large inventory of immunodeficient mice housed, 35% of the housing and procedural space became contaminated. After resolution of the C. bovis recurrence in June 2017, 0 (0%) of 58 murine specimens and 0 (0%) of 447 IVC rack plenum and AHU specimens were C. bovis PCR-positive, indicating that monthly surveillance, early identification and culling of C. bovis PCR-positive mice, and immediate VHP decontamination of all equipment and the affected room contributes to the eradication of C. bovis. It is our impression that C. bovis must be considered an opportunistic agent to be excluded, monitored by routine C. bovis PCR environmental and murine surveillance, not tolerated when detected, and acutely addressed through VHP decontamination when immunodeficient strains are housed.
Although a degree of granulocytic–monocytic infiltration, associated dermal inflammation, and epithelial acanthosis may persist after hyperkeratosis is no longer clinically apparent in C. bovis-infected nude mice2,4,20 and although it may be tempting to suggest that such lesions alter measurements of subcutaneous tumor xenograft growth, and by extrapolation, alter interpretations regarding tumor chemotherapeutic responsiveness,6,12,18 only recently has any evidence supported these anecdotal observations that C. bovis infection jeopardizes xenograft tumor data integrity.21 Regardless, C. bovis must be excluded, monitored, and not tolerated in murine facilities supporting research involving immunodeficient strains to avoid study invalidations and the loss of valuable mice and unique reagents.
Herein we describe for the first time a comprehensive, systematic method of facility-wide C. bovis eradication using VHP decontamination that was guided and monitored by C. bovis-specific PCR environmental and murine surveillance. Documenting the extent to which C. bovis dissemination occurs during an outbreak, including in core procedural and personnel areas, ensures agent eradication and diminishes concerns of its recurrence. Topographically complex murine facilities require decontaminating capabilities (such as VHP) to avoid study invalidations caused by opportunistic agents. Programs that house immunodeficient strains that rely on the absence of morbidity due to scaly skin disease, that lack validated decontaminating processes, and that lack C. bovis PCR surveillance are likely at risk of a residual and accumulating C. bovis prevalence that will lead to study invalidations involving immunodeficient mice and unique reagents, including primary human patient-derived explants.
Acknowledgments
We thank Dr Livingston (IDEXX Bioresearch) for his technical assistance and Dr Ram Thapa (biostatistician, Department of Biotatistics and Bioinformatics, H Lee Moffitt Cancer Center) for statistical analysis.
References
- 1.Alfa MJ, Lo E, Wald A, Dueck C, DeGagne P, Harding GK. 2010. Improved eradication of Clostridium difficile spores from toilets of hospitalized patients using an accelerated hydrogen peroxide as the cleaning agent. BMC Infect Dis 10:268–277. 10.1186/1471-2334-10-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burr HN, Lipman NS, White JR, Zheng J, Wolf FR. 2011. Strategies to prevent, treat, and provoke Corynebacterium-associated hyperkeratosis in athymic nude mice. J Am Assoc Lab Anim Sci 50:378–388. [PMC free article] [PubMed] [Google Scholar]
- 3.Burr HN, Wolf FR, Lipman NS. 2012. Corynebacterium bovis: epizootiologic features and environmental contamination in an enzootically infected rodent room. J Am Assoc Lab Anim Sci 51:189–198. [PMC free article] [PubMed] [Google Scholar]
- 4.Clifford CB, Walton BJ, Reed TH, Coyle MB, White WJ, Amyx HL. 1995. Hyperkeratosis in athymic nude mice caused by a coryneform bacterium: microbiology, transmission, clinical signs, and pathology. Lab Anim Sci 45:131–139. [PubMed] [Google Scholar]
- 5.Dole VS, Henderson KS, Fister RD, Pietrowski MT, Maldonado G, Clifford CB. 2013. Pathogenicity and genetic variation of 3 strains of Corynebacterium bovis in immunodeficient mice. J Am Assoc Lab Anim Sci 52:458–466. [PMC free article] [PubMed] [Google Scholar]
- 6.Field K, Greenstein G, Smith M, Herrman S, Gizzi J. 1995. Hyperkeratosis-associated coryneform in athymic nude mice. Lab Anim Sci 45:469. [Google Scholar]
- 7.Gawaziuk JP, Alfa MJ, Olson N, Logsetty S. 2013. Intermediate-level disinfection with accelerated hydrogen peroxide prevents accumulation of bacteria in Versajet tubing during repeated daily debridement using simulated-use testing with an inoculated pork hock. Burns 40:460–465. 10.1016/j.burns.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Gobbi A, Crippa L, Scanziani E. 1999. Corynebacterium bovis infection in immunocompetent hirsute mice. Lab Anim Sci 49:209–211. [PubMed] [Google Scholar]
- 9.Kaspari O, Lemmer K, Becker S, Lochau P, Howaldt S, Nattermann H, Grunow R. 2014. Decontamination of a BSL3 laboratory by hydrogen peroxide fumigation using 3 different surrogates for Bacillus anthracis spores. J Appl Microbiol 117:1095–1103. 10.1111/jam.12601. [DOI] [PubMed] [Google Scholar]
- 10.Krause J, McDonnell G, Riedesel H. 2001. Biodecontamination of animal rooms and heat-sensitive equipment with vaporized hydrogen peroxide. Contemp Topics 40:18–21. [PubMed] [Google Scholar]
- 11.Lemmen S, Scheithauer S, Hafner H, Yezli S, Mohr M, Otter JA. 2015. Evaluation of hydrogen peroxide vapor for the inactivation of nosocomial pathogens on porous and nonporous surfaces. Am J Infect Control 43:82–85. 10.1016/j.ajic.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Manuel CA, Pugazhenthi U, Leszczynski JK. 2016. Surveillance of a ventilated rack system for Corynebacterium bovis by sampling exhaust-air manifolds. J Am Assoc Lab Anim Sci 55:58–65. [PMC free article] [PubMed] [Google Scholar]
- 13.Manuel CA, Bagby SM, Reisinger JA, Pugazhenthi U, Pitts TM, Keysar SB, Arcaroli JJ, Leszczynski JK.2017. Procedure for horizontal transfer of patient-derived xenograft tumors to eliminate Corynebacterium bovis. J Am Assoc Lab Anim Sci 56:166–172. [PMC free article] [PubMed] [Google Scholar]
- 14.Manuel CA, Pugazhenthi U, Spiegel SP, Leszczynski JK. 2017. Detection and elimination of Corynebacterium bovis from barrier rooms by using an environmental sampling surveillance program. J Am Assoc Lab Anim Sci 56:202–209. [PMC free article] [PubMed] [Google Scholar]
- 15.Otter JA, Cummins M, Ahmad F, van Tonder C, Drabu YJ. 2007. Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapor decontamination. J Hosp Infect 67:182–188. 10.1016/j.jhin.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Otter JA, Mepham S, Athan B, Mack D, Smith R, Jacobs M, Hopkins S. 2016. Terminal decontamination of the Royal Free London's high-level isolation unit after a case of Ebola virus disease using hydrogen peroxide vapor. Am J Infect Control 44:233–235. 10.1016/j.ajic.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Ragland NH, Miedel EL, Gomez JM, Engelman RW. 2017. Staphylococcus xylosus PCR-validated vaporized hydrogen peroxide decontamination of murine individually ventilated cage racks and air handling units using an ‘active-closed’ exposure to vaporized hydrogen peroxide. J Am Assoc Lab Anim Sci 56:742–751. [PMC free article] [PubMed] [Google Scholar]
- 18.Scanziani E, Gobbi A, Crippa L, Giusti AM, Giavazzi R, Cavalletti E, Luini M. 1997. Outbreaks of hyperkeratotic dermatitis of athymic nude mice in northern Italy. Lab Anim 31:206–211. 10.1258/002367797780596310. [DOI] [PubMed] [Google Scholar]
- 19.Scanziani E, Gobbi A, Crippa L, Giusti AM, Pesenti E, Cavalletti E, Luini M. 1998. Hyperkeratosis-associated coryneform infection in severe combined immunodeficient mice. Lab Anim 32:330–336. 10.1258/002367798780559239. [DOI] [PubMed] [Google Scholar]
- 20.Treuting PM, Clifford CB, Sellers RS, Brayton CF. 2011. Of mice and microflora: considerations for genetically engineered mice. Vet Pathol 49:44–63. 10.1177/0300985811431446. [DOI] [PubMed] [Google Scholar]
- 21.Vedder A, Miedel EL, Ragland NH, Balasis M, Letson C, Engelman RW, Padron E. 2017. Primary patient derived xenografts in NSG-S mice show variable engraftment when infected with Corynebacterium bovis. Abstract presented at the 68th meeting of American Association of Laboratory Animal Science, Austin, Texas, 16–19 October 2017. J Am Assoc Lab Anim Sci 56:691–692. [Google Scholar]