Abstract
It is almost ten years since the Banerjee 2009 report established that inappropriate prescribing of antipsychotics in the elderly was occurring in the UK and such patients had an 85% increased risk of adverse events and greater mortality. This report was a critical analysis addressing the outcomes of treatment practices for dementia in UK patients and globally, aimed at reducing prescribing of antipsychotic drugs for dementia. Since 2009, many significant studies worldwide (including several more recent large retrospective studies) provide more extensive longitudinal data for the adverse impacts of antipsychotic drugs in dementia. We have used the data in these studies including from over 380,000 dementia patients, with 85,069 prescribed antipsychotic agents as well as from 359,235 non-dementia antipsychotic drug users to provide an up-dated meta-analysis. This is the first meta-analysis to include evidence from general mental health studies showing that antipsychotic drugs precipitate excessive mortality across the spectrum. Prescribing of antipsychotic drugs for dementia or for other mental health care should be avoided and alternative means sought for handling behavioral disorders of such patients.
Keywords: Antipsychotic agents, causes of death, clinical governance, dementia, deprescriptions, excess mortality, health care reform, meta-analysis, psychotropic drugs, review, risk
INTRODUCTION
Many studies have shown that Alzheimer’s disease patients with psychoses or agitation–aggression (behavioral and psychological symptoms of dementia or BPSD) respond to chemical restraint, reducing the severity of their behavioral problems. However, such drugs are being widely used beyond these indications and not only for particular symptoms such as anger, aggression or paranoid ideas [1]. Moreover, their use does not improve functioning, care needs, or quality of life [1]. In addition, problems can arise if antipsychotic medication is discontinued, when dementia patients may frequently relapse with their behavioral issues [2]. From the UK Banerjee report in 2009 [3], it was concluded that although the antipsychotic drugs can be effective at reducing psychotic problems such as delusions, they were also linked with serious adverse events, provided moderate benefit and failed to address the underlying causes of BPSD. This has led to the need for change in the current practice for dealing with BPSD by using the antipsychotic drugs as chemical restraints.
In the 2009 UK Department of Health nationwide study of Banerjee [3], of the 180,000 prescriptions for people with dementia, the majority (140,000) were considered inappropriate, with prescribing antipsychotic drugs considered to be extremely harmful. Such inappropriate overprescribing of antipsychotic drugs was estimated to contribute to 1,800 deaths in the UK per year [3]. Studies the same year had shown up to a 9-fold risk of stroke in the first 30 days after commencing antipsychotics in elderly patients [4].
Despite the Banerjee report [3], a more recent study in 2015 [5] from the UK showed that the number of patients at doctors’ clinics with cognitive impairment also being treated with psychotropic drugs had remained far in excess of those with mental illness. The authors concluded that people without mental illness, but who had dementia (with BPSD) were being given potent antipsychotic drugs which should only be approved for use in people with schizophrenia or bipolar disorder, and hence, were being used more to manage and control the behavior of aged dementia patients [5]. The authors added that the risks and benefits must be carefully considered before prescribing antipsychotics to dementia patients without severe mental illness because the research evidence did not support use of antipsychotics to manage their behavioral problems. Furthermore, it was concluded that many people with such dementia related behavioral disturbances have complex needs and that any medications should not be prescribed lightly and were no substitute for more comprehensive patient care.
Early studies worldwide about all-cause mortality led to the black label warning on antipsychotic use for dementia
In the United States of America (USA), analysis by the federal based Centers for Medicare and Medicaid Services (CMS) databases of over 75,000 patients aged≥65 from 2001–2005 in nursing homes showed a direct dose-response relationship for all-cause mortality with all antipsychotic drugs given over 6 months, except for quetiapine [6–8]. Unfortunately, the comparator used in these early studies was against another common atypical antipsychotic drug, risperidone, as the reference control and not the more appropriate controls, which should have been drug non-treated or non-user dementia patients. In 2005, based on the published greater risk of cerebrovascular events (with risperidone, olanzapine, and aripiprazole) from meta-analysis of 17 randomized placebo controlled trials, the Federal Food and Drug Administration (FDA) in the USA noted that such atypical antipsychotics were associated with a 60–70% increased risk of death compared to placebo in many randomized trials among aged dementia patients [9, 10]. It should be noted that most of these trials were analyzing effects of usage over a short duration for up to 6 months. At this point, the FDA required black box warnings be added onto the labels for such atypical antipsychotic drugs [11]. However, subsequent studies found equal risks, at least as high, even with the more conventional, older, more typical antipsychotics [9, 12, 13], resulting in similar warnings for all of these drugs being issued by the FDA in 2008 [14]. This strong safety warning was also consistent with other reports of little to no distinction in the toxicities and adverse events either with the first/typical or the second generation/atypical antipsychotic drugs used for treating a range of neurological diseases, including dementia [15–19].
Worldwide, many similar studies over 2005–2009 were reported contraindicating use of antipsychotic drugs for dementia, with the majority clearly establishing and raising a serious red flag regarding their drug safety and adverse effects [9, 10, 12, 13, 20, 21]. Most of these earlier studies were based on data from antipsychotic drug short-term use in clinical trials before or shortly following their approvals for widespread use and as such, they significantly underestimate the relative risk (RR) of mortality which can result from higher doses and longer term usage.
Since 2009, the results from several large retrospective longitudinal studies have been reported, with five in 2017 [22–27], such that the present study encompasses data from over 380,000 dementia patients, including 89,514 prescribed antipsychotic drugs and whose data on RR of mortality are subjected to meta-analysis. The outcomes provide further support strengthening the continuing calls for increasing the stringent standards and restrictions governing the availability and prescribing of antipsychotic drugs for dementia. It also raises into question their use, in general, when it is becoming abundantly clear that de-prescribing and training in the use of alternative methods for healthcare of the elderly have been proven to provide greater longer term health and welfare benefits.
METHODS
Literature search
A search of the literature since 2009 and including up to the submission date in 2017 was performed, using the databases of PubMed and Google Scholar, with the Medical Subject Heading (MeSH) terms: Antipsychotic, and/or related, psychotropic, hypnotic combined with Dementia, Elderly and Mortality and Risk and variations used together with these headings. The search was restricted to human studies. Reference lists from retrieved articles were also used to find additional related studies.
To be included, studies were required to meet the criteria: 1) original research publications, reviews or prospective or retrospective analyses of databases or patient trials; 2) use of psychotropic drugs including either antipsychotics (first or second generation) or benzodiazepines or related drugs; 3) the outcomes based on all-cause mortality, seizures in dementia patients versus controls or the increased incidence of dementia in the elderly (generally age≥65 years old); 4) Effect sizes represented as RR, hazard ratios (HR), or odds ratios (OR), including 95% confidence intervals (95% CIs). HR was calculated using a 2×2 contingency table for risks of mortality based on background incidence in the matched control non-user population and the online calculator http://vassarstats.net/odds2x2.html.
Data assessment and meta-analysis
Studies were not included in our final meta-analysis under the following situations: 1) when based on only small numbers of patients or sample sizes (and hence causing high between-study heterogeneity, as shown previously in [28]) or 2) where analysis of large patient databases for mortality risk involved first making extensive adjustments during data extraction to exclude the majority with confounding factors such as advanced severity of dementia or multiple co-morbidities. Such studies were deemed unsuitable based on the rationale that they do not appropriately represent the target population, as outlined further in the results and discussion sections.
HRs were obtained or calculated as the effect sizes with variances for each study included in the meta-analysis along with their respective 95% CI. The study-specific value for the logn Hazard Ratio was weighted by the inverse of the variances to provide a summary estimate with its 95% CIs. The DerSimonian-Laird random effects model was applied [29] and compared to the Hunter-Schmidt model [30], the latter which in addition to using the inverse of the variances in effect sizes, also incorporates a weighting based on the sample sizes [30]. The Hedges-Vevea model for the mean effect size across the studies was also determined for comparison [31]. I2 of Higgins and Thompson was determined to assess for heterogeneity between the studies and to find inconsistencies [32]. The leave-one-out analysis [33] was used to evaluate those studies having a substantial effect on between-study heterogeneity. A sensitivity analysis was also undertaken by excluding one or more studies at a time to determine whether one or more of the studies with extreme results was causing an undue influence. A subgroup analysis by type of antipsychotic drugs (conventional versus atypical) was carried out where data was available. Small-study effect was assessed by visually inspecting the funnel plot for asymmetry and Egger’s regression intercept test [34].
Comparative statistical analyses were performed using a range of software programs including SPSS Vn24.0; Comprehensive Meta-Analysis (CMA; https://www.meta-analysis.com/) and the MIX 2.0 PRO (https://www.meta-analysis-made-easy.com) on Microsoft Excel. Syntax for SPSS Vn24.0 was prepared and constructed using the David B. Wilson macro: “MeanES.sps” (http://mason.gmu.edu/ dwilsonb/ma.html) included within the macro outlined by Field and Gillett [35]; (https://www.discoveringstatistics.com/repository/fieldgillett/how_to_do_a_meta_analysis.html).
RESULTS
More recent studies from many countries confirm that the antipsychotic drugs should not be prescribed for dementia patients, as they significantly increase the risk of seizures and all-cause mortality
Since the Banerjee report in 2009, many more recent analyses have been reported from around the world, including longitudinal studies from large databases of patient records and these are outlined and summarized in Table 1. The results of different meta-analysis models applied to the data from the reports in Table 1 are shown in Tables 2 and 3. Outcomes on a regional and global basis from these studies are also described in the next sections. Although a meta-analysis of nine studies pooling from 11,463 subjects was reported in 2016 [28], it included several with small sample sizes such that the resulting effect sizes from these studies contributed to a high between-study heterogeneity (I2 = 95%). Many studies use excessive or inappropriate data adjustment factors based on dementia severity or other co-morbidities to the point that they are no longer representative and result in greatly reduced numbers of patients (one with less than 16 subjects using antipsychotics included in the previous meta-analysis [28]). Inclusion of such restricted and heavily adjusted data sets can introduce flaws in the study design. Moreover, interpretations using qualitative assessments applied to individual studies [28] to assign a relative weighting of their study scores is also questionable. Added to this, since [28] was published, several much larger and longer term studies have recently emerged. These more recent studies are the focus of the next sections, allowing us to undertake a much larger meta-analysis encompassing over 380,000 dementia subjects, including data from 80,330 dementia patients receiving antipsychotic drugs (Table 1). The present meta-analysis also greatly enlarges the total number of patients analyzed who have received antipsychotic drugs globally, including dementia as well as non-dementia patients for meta-analysis, with the total number = 359,235 used for calculation.
Table 1.
Worldwide increased mortality in patients using antipsychotic agents
| Country | Study/Years | Patient No. | Event/duration | Drug Use | Hazard Ratio (HR); [95% CIs] | Reference |
| United Kingdom | General Practice Research Database (GPRD) 1995–2011 | 183,392 patients (115,491 typical; 67,901 atypical) versus 544,726 general population controls or 193,920 psychiatric non-users | All-cause mortality | Atypical and typical antipsychotics | Users matched to psychiatric non-users HR = 2.15; [2.10–2.21] and HR = 2.98; [2.93–3.03] matched with general population non-users. | Jones et al., 2013 [37]; Murray-Thomas et al., 2013 [36] |
| Sudden cardiac death was greatly increased in psychiatric users versus the psychiatric matched non-users with HR = 5.76; [2.90–11.45] | ||||||
| UK Clinical Practice Research Datalink 1998–2013 | 60,121 dementia patients | First-time seizures in Dementia patients over 15-year period | Atypical antipsychotics Olanzapine, quetiapine | *HR = 2.335 [1.35–4.01] | Bloechliger et al., 2015 [38] | |
| Low-to-medium potency typical antipsychotics | HR = 3.01 [1.34–6.64] | [38] | ||||
| Medium-to-high potency typical antipsychotics | HR = 2.21 [1.05–4.62] | [38] | ||||
| Welsh Secure Anonymised Information Linkage (SAIL) databank 2003–2011 | 9,674 newly diagnosed dementia patients aged≥65 years | All-cause Mortality and serious adverse events. 12 months prior compared to 12 months post starting antipychotic | Typical 1513 | Prior event rate ratio PERR used as HR = 2.07 [1.732–2.473]. Increased SAEs as Cardiac arrest, venous thromboembolism, stroke or hip fracture. Mainly in first 100 days of use. | Dennis et al., 2017 [40] | |
| Atypical 1687 | ||||||
| France | 16 French Memory Centres | 534 Alzheimer’s Dementia patients, 102 new users | All-cause Mortality in Alzheimer’s dementia 3.5-year follow-up | Antipsychotics (typical and atypical) new use versus non-user control | HR = 1.93; [1.15–3.25] | Gardette et al., 2012 [41] |
| Norway | 2014 Norwegian Prescription Database | 26,940 dementia outpatients | All-cause mortality in Dementia patients short (30 day) or long term use (730–2400 days), Follow up over more than 6 years | Antipsychotic (ATC code N05A) use versus other psychotropics: antidepressants (N06A), benzodiazepine (N03AE01, N05B, N05C), benzodiazepine-like agents (N05C), lithium (N05AN01), and anticonvulsive drugs (N03A). | HR30days = 2.1 [1.6–2.9] | Langballe et al., 2014 [42] |
| HR730 - 2,400days = 1.7 [1.6–1.9]. | ||||||
| Haloperidol HR 30 days = 1.7 [1.0–3.0], HR 730–2,400 days = 1.4 [1.0–1.9] compared to risperidone. | ||||||
| Denmark | Danish National Patient Registry 1997–2009 | 26,821 dementia patients versus 44,286 matched control non-users. | All-cause mortality in dementia patients or non-dementia patients | Psychotropics: | SSRIs HR = 1.355 (SD = 0.023), P = 0.001 in patients versus HR = 1.808 (0.033), p < 0.001 in non-dementia users), | Jennum et al., 2015 [43] |
| Also compared use in dementia versus non-dementia users. | 4 up to 12-year follow-up | Antidepressants (SSRI) Benzodiazepines, and Typical or Atypical Antipsychotics | Tricyclic antidepressants (HR = 1.004 (0.046), p = 0.925 versus HR = 1.406 (0.061), p < 0.001) in non-dementia users. | |||
| Versus non-user controls | Benzodiazepines HR = 1.131 (SD = 0.039), p = 0.060 patients versus HR = 1.362 (0.028), p < 0.001 in non-dementia users. | |||||
| Typical antipsychotics (HR = 1.183 (SD = 0.074), p = 0.022 versus HR = 2.026 (SD = 0.114) in non-dementia users, p < 0.001. Atypical antipsychotics (HR = 1.380 (SD = 0.042) patients, p < 0.001 versus HR = 1.785 (SD = 0.088), p < 0.001) in non-dementia users. | ||||||
| Danish National Patient Registries 2000–2011 | 45,894 Alzheimer’s Dementia patients | All-cause Mortality in Dementia patients: | Antipsychotics: Atypical and Typical | Current antipsychotic users (prior 12 months) HR = 2.31; [2.14–2.49], p < 0.001 | Nielsen et al., 2017 [44] | |
| Cardiovascular, infection, cancer | ATC Code = N05A except Lithium. | |||||
| 12-month follow-up | Versus non-user control | |||||
| Finland | Finnish National Prescription Register (FNPR) 2000–2008 | 332 community dwelling elderly (≥65 years, 0.5% dementia) | All-cause Mortality 9-year follow up | New users of antipsychotics | HR = 2.71; [2.3–3.2] | Gisev et al., 2012 [45] |
| MEDALZ 2005–2011 | 15,806 Alzheimer’s dementia from 57,755 community-dwelling newly diagnosed Alzheimer’s patients 2005–2011 | All-cause Mortality. | Antipsychotics | Antipsychotic monotherapy (HR = 1.61; [1.53–1.70]) compared to non-users. | Koponen et al., 2017 [46] | |
| Median follow-up 2.5 years | (HR1 - 30days = 1.74 to HR30 - 90days = 2.11). At 2 years, HR = 1.30; [1.16–1.46]. Haloperidol HR = 1.52; [1.14–2.02]) relative to risperidone. | |||||
| >1mg Haloperidol HR = 2.55; [1.7–3.85] relative to risperidone. | ||||||
| Japan | 2014 | 10,000 dementia patients | All-cause Mortality in Alzheimer patients. | Antipsychotics (typical and atypical) new use versus non-user control | New users | Arai et al., 2016 [47] |
| 11-24-week follow-up | *HR = 3.7; [1.57–8.3] | |||||
| Taiwan | Longitudinal Health Insurance Database 2000 (LHID2000) | 183,410 patients on antipsychotic monotherapy 1996–2011 | All-cause Mortality follow up varied from 2–6 years | Typical Antipsychotics Chlorpromazine (2133) | New users <30 day, 30–90 day and >90 days | Wang et al., 2016 [72] |
| Haloperidol (4454) | Haloperidol HR30 = 2.11; [1.87–2.39], risperidone HR30 = 1.79; [1.45–2.22] relative to chlorpromazine as reference. | |||||
| Atypical antipsychotics | ||||||
| Quetiapine (1513) | ||||||
| Risperidone (1046) | ||||||
| Italy | Milan health information database 2002–2008 | 4,369 dementia patients≥60 years | All-cause Mortality over 2-year follow up | typical (n = 156) versus atypical (n = 806) antipsychotic drugs | New users antipsychotics. | Musicco et al., [48] - 2011 |
| Atypical (HR = 2.5; [2.05–3.1]) | ||||||
| Typical (HR = 3.7; [2.6–5.1]) | ||||||
| Teramo Hospital 2007–2009 | 696 Alzheimer’s dementia patients≥65 years | All-cause Mortality follow up over 3 years; n = 375 users compared to non-user control | Atypical Antipsychotics (quetiapine, risperidone and olanzapine) | HR = 2.354; [1.704–3.279] | Piersanti et al., 2014 [49] | |
| REPOSI (Registro Politerapie Società Italiana Medicina Interna) database 2010–2012 | 135 dementia (≥65 years; mean age ∼80 years) | All-cause Mortality over 90 days | Antipsychotics promazine, chlorpromazine, olanzapine, levomepromazine, haloperidol, amisulpride, clotiapine, tiapride, zuclopenthixol, quetiapine, risperidone, periciazine, levosulpiride and clozapine. | New users | Chiesa et al., 2017 [50] | |
| Hr = 1.57; [0.95–2.61] | ||||||
| Spain | FEDRA Spanish system for Drug Surveillance 1995–2012 | N = 5,203 adverse event – exposed; N = 200 related deaths – all users | All-cause Mortality plus adverse events. All users versus users of other drugs | Antipsychotics versus any other drugs as control | Atypical antipsychotics | Martin Arias et al., 2017 [24] |
| HR = 2.47; [2.10–2.92] | ||||||
| Typical antispychotics | ||||||
| HR = 1.79; [1.47–2.18] | ||||||
| Particularly: zuclopenthixol (HR = 3.39; [1.75–6.59]) | ||||||
| risperidone HR = 2.07; [1.56–2.75) | ||||||
| Haloperidol HR = 2.71; 1.98–3.69]. No difference between elderly versus younger groups. | ||||||
| 7 European Union countries; including Israel | 59 Nursing homes 2009–2011 | Dementia patients n = 604≥65 years old | All-cause mortality in dementia patients | Antipsychotic users n = 278 versus other drugs as control. | HR = 1.71; [1.15–2.54] | Liperoti et al., 2017 [23] |
| Australia | Nationwide memory clinics | Community based dementia patients 779. Mostly >65 years | follow-up 3 years with mortality determined at 8 years | Atypical antipsychotic drugs versus non-users | Atypical HR = 1.61; [1.29–2.02] (p < 0.01) | Connors et al., 2016 [51] |
| U.S.A. | Tennessee Medicaid records 1990–2005 | Antipsychotic drug users 30–74 years old (mean age 46) | Sudden cardiac death from baseline. Median follow-up 2.5 years | Antipsychotic users (Typical 44218, atypical 44089) versus 186,600 non-users (sub-group to matched psychiatric non-users) | Typical HR = 1.99; [1.69–2.35] | Ray et al., 2009 [52] |
| Atypical HR = 2.26; [1.88–2.72]. Dose-related with high dose HR = 2.7 | ||||||
| 1998–2005 National VHA database Texas | Antipsychotics dementia users versus non-user control N = 8,867–32,996 | All-cause mortality, 5-year follow-up | Antipsychotics | 30 day first use: | Rossom et al., 2010 [53] | |
| ≥65 years old | Haloperidol, 2,217 | HR = 3.2; [2.2–4.5], p < 0.001 | ||||
| Quetiapine, 4,277 | HR = 1.2; [0.7–1.8], p = 0.5 | |||||
| Olanzapine, 3,384 | HR = 1.5; [1.1–2.0], p = 0.01 | |||||
| Risperidone, 8,249 | HR = 1.5; [1.1–2.2], p = 0.01 | |||||
| 1998–2000 Across 5 states. Nursing homes | 6,524 new users atypical and 3205 typical antipsychotics≥65 years old | All-cause mortality in dementia patients. | Typical Antipsychotics cf’d atypicals. | HR = 1.26; [1.13–1.42] | Liperoti et al., 2009 [6] | |
| 6-month follow-up | Except quetiapine or olanzapine cf’d risperdone. | HR = 1.06; [0.80–1.39] | ||||
| Haloperidol compared to risperidone. | HR = 0.95; [0.8–1.12] | |||||
| risperidone (n = 4,406); haloperidol (n = 1,413) | HR = 1.31; [1.13–1.53] | |||||
| 2001–2005 Nursing homes | 75,445≥65 years | All-cause mortality elderly in nursing homes. | Antipsychotic | HR = 2.07; [1.89–2.26] | Huybrechts et al., 2012 [7] | |
| 6-month follow-up | Haloperidol compared to risperidone | |||||
| Dept. Veteran Affairs 1999–2008 | 33,604≥65 years | All-cause mortality in dementia patients. | Antipsychotic | Haloperidol HR = 1.54; [1.38–1.73] compared to valproate | Kales et al., [8]- 2012 | |
| 6-month follow-up | (risperidone, olanzapine, quetiapine, or haloperidol) | |||||
| Dept. Veteran Affairs 1998–2009 | 90,786≥65 years | All-cause mortality in dementia patients. | New users of atypical antipsychotics (olanzapine, quetiapine, and risperidone, haloperidol). Dose-related increase in mortality compared to matched non-user controls or alternatively, antidepressants as reference group; | Haloperidol HR = 1.123; [1.086–1.16]; p < 0.01 cf’d antidepressant group. | Maust et al., 2015 [54, 55] | |
| 6-month follow-up | Atypical antipsychotics (olanzapine, quetiapine, and risperidone) dose-response increase in mortality risk, HR = 1.035; [1.005–1.065]; p = 0.02 in the high-dose subgroup relative to the low-dose group. |
*Relative risk (RR) calculated from adjusted Odds Ratio (OR) given incidence in controls using online convertor (http://clincalc.com/stats/convertor.aspx).
Table 2.
Meta-Analysis Data
| No. Study ID | Hazard ratio | 95% Lower Confidence Limit | 95% Upper Confidence Limit | Logn Hazard Ratio: Ln (hr) | Std Err | Year | Sample Size: patient users (N) |
| 1. Ray* FGA | 1.990 | 1.686 | 2.340 | 0.6881 | 0.085 | 2009 | 44,218 |
| 2. Ray* SGA | 2.260 | 1.879 | 2.720 | 0.8154 | 0.094 | 2009 | 46,089 |
| 3. Rossom | 1.674 | 1.062 | 2.637 | 0.5152 | 0.232 | 2010 | 18,127 |
| 4. Musicco FGA | 3.700 | 2.600 | 5.100 | 1.3083 | 0.172 | 2011 | 156 |
| 5. Musicco SGA | 2.500 | 2.033 | 3.100 | 0.9163 | 0.106 | 2011 | 806 |
| 6. Gisev | 2.070 | 1.730 | 2.470 | 0.7280 | 0.091 | 2012 | 332 |
| 7. Gardette | 1.930 | 1.148 | 3.245 | 0.6575 | 0.265 | 2012 | 102 |
| 8. Jones* FGA | 2.340 | 2.281 | 2.401 | 0.8502 | 0.013 | 2013 | 115,491 |
| 9. Jones* SGA | 1.760 | 1.711 | 1.811 | 0.5653 | 0.014 | 2013 | 67,901 |
| 10. Langballe | 2.052 | 1.693 | 2.486 | 0.7188 | 0.098 | 2014 | 8,214 |
| 11. Piersanti SGA | 2.354 | 1.697 | 3.265 | 0.8561 | 0.167 | 2014 | 375 |
| 12. Jennum FGA | 1.292 | 1.218 | 1.366 | 0.2562 | 0.029 | 2015 | 259 |
| 13. Jennum SGA | 1.442 | 1.400 | 1.484 | 0.3660 | 0.015 | 2015 | 832 |
| 14. Maust | 1.688 | 1.370 | 2.090 | 0.5235 | 0.108 | 2015 | 14,788 |
| 15. Arai | 1.675 | 0.900 | 3.118 | 0.5158 | 0.317 | 2016 | 4,873 |
| 16. Connors | 1.610 | 1.290 | 2.020 | 0.4762 | 0.114 | 2016 | 779 |
| 17. Nielsen | 2.310 | 2.142 | 2.492 | 0.8372 | 0.039 | 2017 | 16,976 |
| 18. Koponen | 1.610 | 1.530 | 1.700 | 0.4762 | 0.027 | 2017 | 13,576 |
| 19. Martin-Arias* | 2.030 | 1.760 | 2.330 | 0.7080 | 0.072 | 2017 | 5,206 |
| 20. Chiesa | 1.570 | 0.945 | 2.607 | 0.4511 | 0.259 | 2017 | 135 |
| TOTAL | 359,235 |
*values based on antipsychotic drug users in the general population.
Table 3.
Effect of excluding one study at a time on the meta-analysis
Hedges-Vevea Random-Effects Model¥:
Mean Effect Size (r = ln HR), ln (Lower & Upper 95% CI’s), and Z-test
| Mean r | Lower r | Upper r | Z | p | k |
| 0.741 | 0.669 | 0.798 | 13.059 | 0.000 | 20 |
HR = 2.098; [1.952–2.221]
Estimated Variance in Population (Fisher-Transformed) Correlations, τ= 0.1044, τ2 = 0.011 indicating a small between studies variance.
Homogeneity Test: Q Statistic (Goodness of Fit)
| Chi2 | df | p |
| 98.028 | 19 | 0.000 |
Hunter-Schmidt Random-Effects Model*:
Mean Effect Size (r = ln HR), ln (Lower & Upper 95% CI’s), and Chi2–test
| Mean r | Lower r | Upper r | Chi2 | p | df |
| 0.715 | 0.444 | 0.986 | 28743 | 0.000 | 19 |
HR = 2.044; [1.559–2.681]
Sample Correlation Variance = 0.0191
Sampling Error Variance = 0.0000
Estimated Variance in Population Correlations = 0.0191
¥SPS 24.0 Syntax based on the macro of Field and Gillett [35].
United Kingdom (UK)
In 2013, a large cohort study of the UK-General Practice Research Database (GPRD) from 1995–2011 assessed the risk of all-cause mortality (excluding suicide) for 183,392 users of the antipsychotic drugs with a mean age of 60.3 years (25,174 or 13.7% of which had dementia) and follow-up was on average for 4 years [36, 37]. The mean duration of antipsychotic use was 1.6 years, including 115,491 typical and 67,901 atypical users versus 544,726 general population controls or 193,920 psychiatric non-users [36, 37]. When a comparison matched on the basis of age, gender and location was analyzed relative to psychiatric non-users as control, the risk of all-cause mortality for users was HR = 2.15; [95% CI 2.10–2.21]. The RR increased to HR = 2.98; [2.93–3.03] comparing to the general population as control. A slight reduction in all-cause mortality and cardiac mortality was detected with the atypical relative to the typical antipsychotic drugs. Dementia patients were not specifically examined as a study group in this report, but given that they were nearly 14% of the user population, this data was included in the meta-analysis. Of note, the risk of sudden cardiac death was greatly increased in the psychiatric users versus the psychiatric matched non-users with HR = 5.76; [2.90–11.45]. Hence, this evidence further supports that antipsychotic drugs cause risks to not only dementia patients, but also to the general population prescribed these drugs [36].
Another large study was reported in 2015 examining the UK-based Clinical Practice Research Datalink (CPRD) over a very extensive 15-year time period as a retrospective population-based follow-up study with a nested case–control of 60,121 patients (79.6% affected disorders (depression, bipolar or anxiety); 11.2% dementia; 3% schizophrenia; and 6.2% with more than one disorder) [38]. This study showed a significantly (up to 3 fold) greater RR of first-time seizures with antipsychotic drug use compared to the patients on other medications for affective mood disorders or non-user dementia patients, irrespective of which antipsychotic (olanzapine, quetiapine, haloperidol) except for amisulpride, aripiprazole, risperidone, or sulpiride [38]. However, aripiprazole has been linked in meta-analyses and some individual trials with increased mortality in Alzheimer’s disease [39].
A recent 2017 population based retrospective dementia cohort study from Wales specifically focused on the risk of serious adverse events of those prescribed antipsychotic medication. The Welsh Secure Anonymised Information Linkage (SAIL) databank was examined over 2003–2011 using data from 9,674 dementia patients aged≥65 years, of whom 3,735 (n = 1,513 typical and n = 1,687 atypical, n = 535 both) were new antipsychotic drug users with 1–4 years (median 1.8 years) follow-up, including 12 months prior to first prescription, matched with 5,939 who were non-users [40]. This study analyzed and compared the prior event rate ratio (PERR) for risks of serious adverse events (SAEs) over the previous 12 months prior to starting versus the risk for the same SAEs after starting use of antipsychotics, including for all-cause mortality. The results confirmed the greater risks for those Alzheimer’s dementia patients prescribed the antipsychotics, including increased acute cardiac events (PERR = 1.65; [1.05–1.78]), venous thromboembolism (HR and PERR = 1.80; [1.67–1.89], stroke (HR = 1.5; PERR = 2.06; [1.97–2.13]) or hip fractures (PERR = 1.65; [1.61–1.73]). The highest mortality rate occurred within the first 100 days of starting on antipsychotics compared to non-exposed patients and subsided thereafter with an absence of any long-term mortality risk from antipsychotic use compared to non-user controls. However, the long-term data was adjusted and data excluded for many co-morbidities as well as being only based on a short-term follow-up of about two years.
France
In the 2012 REAL.Fr prospective study [41], 534 community dwelling, mild to moderate Alzheimer’s dementia patients were assessed from 2000 to 2002 with follow-up for four years for all-cause mortality comparing newly prescribed users of Anatomical Therapeutic Chemical (ATC) Code NO5A. They compared those prescribed the atypical antipsychotics (n = 44) and typical antipsychotics (n = 58) to 432 non-users as controls. Antipsychotic use remained a significant predictor for all-cause mortality (HR = 1.93; [1.15–3.25]) when adjusted only on age, gender and center. In addition, a cumulative dose-related increased risk was found (<50% cumulative drug exposure, HR = 1.81; [1.02–2.32], increasing with >50% cumulative drug exposure to HR = 2.05; [1.03–4.07]). However, by heavily adjusting the data based on dementia severity and other co-morbidities, the risk was greatly reduced (HR = 1.12; [0.59–2.12]).
Norway
In 2014, a population based study of 26,940 dementia outpatients in the Norwegian Prescription database by Cox survival analysis, adjusted for age, gender, mean daily defined dose, and severe medical conditions, showed that antipsychotic drug users (n = 8,214) had nearly twice the mortality risk compared to the other psychotropics (including ATC codes: antidepressants (N06A); benzodiazepines (N03AE01, N05B, N05C); benzodiazepine-like agents (N05C); lithium (N05AN01); and anticonvulsive drugs (N03A)), both for short (30 days) or longer term (730 to 2,400 days) use [42]. Furthermore, the RR held across all investigated time points after first dispensing the drugs (HR30days = 2.684 [95% CI; 1.991–3.618]; HR30 - 180days = 2.052; [1.693–2.486]; and HR730 - 2,400days = 1.7 [95% CI: 1.6–1.9]). Haloperidol was associated with the highest mortality risk (HR30days = 4.968 [95% CI: 3.437–7.183]; HR30 - 180days = 2.808; [2.047–3.852] and for risperidone, HR30 - 180days = 2.614; [1.960–3.486] compared to other psychotropic drugs.
Denmark
In 2015, a longitudinal analysis of the Danish National Patient Registry evaluating all-cause mortality of middle-aged and elderly subjects diagnosed with dementia (26,821 patients) and treated with psychotropic drugs as compared with control non-user subjects (44,286) was reported [43]. Their study had a minimum follow-up of 4 years, extending to 12 years, and was patient matched on age, gender, marital status, community location, and medication use (whether benzodiazepines, antidepressants and first- or second-generation antipsychotics) and showed all-cause mortality was higher in patients with dementia using antipsychotics (n = 1,091) as compared to the non-drug control subjects [43].
In a second, larger, independent study of Danish nationwide registries reported in 2016 [44] and 2017 [26], the mortality rates of 45,894 Alzheimer’s dementia patients were followed long term over 11 years from 2000–2011 [26]. Antipsychotic drug usage by 16,976 of these patients (40% risperidone, 36% quetiapine, 21% olanzapine, 15% haloperidol, 6% zuclopenthixol, 4% clorprothixene) was analyzed comparing those with or without current exposure (defined as over the previous year) to dementia patients not given antipsychotics as the reference control. The analysis showed increased, almost twice the RR of all-cause mortality (HR = 1.92–2.31) including cardiovascular, cancer and infection related deaths, particularly apparent with greater cumulative dosages linked to current exposure. Dose-response data such as this greatly increases the confidence levels assigned to such studies for an underlying cause-effect relationship and scientific validity that antipsychotic drug use increases the risks of all-cause mortality in dementia patients.
Studies on the effects of cumulative doses in patients with previous exposure showed increased mortality only in those on the lower dosage, but then decreasing mortality with the higher daily dosage [26, 44]. Although the reasons for this anomaly were not clear in these reports, it could be due to a survival bias with long term patients either selected for (in that those who were susceptible to these types of drugs had already succumbed to their toxicity) or survivors were becoming tolerant or adapting to the toxicity of these drugs over the longer exposure periods and higher doses. A consistently observed phenomenon is that the longer term users of the antipsychotics appear more resistant to their lethality, as other studies described below have also shown.
Finland
In 2012, an analysis of the Finnish National Prescription Register (FNPR) examined community dwelling elderly (≥65 years, only 0.5% with dementia) who were followed over 2000–2008, including 332 new users of antipsychotic drugs [45]. The users were matched to 2085 non-users and all-cause risk of mortality was compared along with comorbidities. The unadjusted HR = 2.71; [2.3–3.2] and after adjusting for baseline age, gender, antidepressant use, and diagnostic confounders, the HR was 2.07; [1.73–2.47]. The adjusted HR was the highest among antipsychotic users with baseline respiratory disease (HR = 2.21; [1.30–3.76]) as the most severely impacting co-morbidity covariate, possibly linked to increased risk of death from pneumonia. The next severe covariate was baseline cardiovascular disease, HR = 1.88; [1.49–2.37] [45].
In 2017, results of the MEDALZ study encompassing 15,806 antipsychotic drug users out of 57,755 community-dwellers newly diagnosed with Alzheimer’s dementia during 2005–2011 with a median follow-up of 2.4 years [22, 46]. The analysis showed that users had an overall greater risk of mortality associated with antipsychotic monotherapy (HR = 1.61; [1.53–1.70]) compared to non-users. The risk was even greater where polypharmacy was applied (≥2 antipsychotic drugs, HR = 2.88; [2.38–3.49]) [22]. The RR of mortality increased from the first days of use (HR1 - 30days = 1.74 to HR30 - 90days = 2.11) and attenuated gradually after 90 days, but remained increased even after 2 years of use (HR = 1.30; [1.16–1.46]). Moreover, compared with non-users, antipsychotic polypharmacy remained high at 2 years with a higher risk of mortality than for monotherapy (HR = 1.57; [1.49–1.66]). Haloperidol again showed the highest risk of mortality among the antipsychotics used as a single agent (HR = 1.52; [1.14–2.02]) relative to risperidone users as reference (HR = 1). With higher doses >1 mg per day, the risk for haloperidol increased to HR = 2.55; [1.7–3.85] relative to risperidone.
Japan
In 2014, a 10,079 Japanese Alzheimer’s disease patient (70.7% female) large-scale, prospective study analyzed 4,873 exposed to antipsychotics [71.4% (3479 of 4873) were taking atypical antipsychotics, whereas 21.6% (1054 of 4873) were taking conventional antipsychotics, and 7.0% (340 of 4873) were taking both] and were matched with 4,898 non-exposed controls across a large range of baseline characteristics including age, gender, severity of dementia and other co-morbidities [47]. As with the Finnish study above, the greatest risk was detected over the 30-90-day period for those current users with HR = 1.675; [0.90–3.118] at the 10-week follow-up time point compared to non-users as control. In particular, this study found that 85 newly prescribed users of antipsychotics had greatly increased risks of mortality (HR = 3.317; [1.681–6.544] over the follow-up period) compared to the non-user controls [47]. The authors concluded that antipsychotic drugs should be avoided or, if their use was absolutely necessary, then they should be limited to use only for very short periods [47], whereas the longer-term users over 6 months were a low-risk group (HR = 0.84).
Italy
In 2011, a retrospective study of 4,369 dementia patients from the Milan health information database prescribed anti-dementia drugs (donepezil, rivastigmine, or galantamine) during the period 2002–2008 were examined for those new users of the typical (n = 156) versus atypical (n = 806) antipsychotic drugs and their effects on mortality over a two year follow up since dementia diagnosis [48]. Their results showed an increased mortality over the two-year period for those prescribed the atypical (HR = 2.5; [2.05–3.1]) and conventional (HR = 3.7; [2.6–5.1]) antipsychotics, respectively compared to non-users as control.
In 2014, a second retrospective study of 696 Alzheimer’s dementia patients at Teramo hospital in Italy was reported [49]. Of these, 375 prescribed the atypical antipsychotics (quetiapine, risperidone or olanzapine) were followed up over a three-year period (2006–2009) and showed significantly higher risk of mortality than the non-user control patients (HR = 2.354; [1.704–3.279]) [49]. The greatest increase in mortality risk occurred close to the last drug supply, and in patients halting antipsychotics, mortality rates declined exponentially as time passed from the last drug supply. Quetiapine was the most commonly prescribed drug and increasing doses of this drug significantly correlated with increased mortality rates, up to 40% with the highest dose and most of these within the 4 months since last treatment [49]. Risperidone had an HR = 2.112; [1.152–3.871] compared to non-user controls.
In 2017, a study analyzed the REPOSI (Registro Politerapie Società Italiana Medicina Interna) database (≥65 years; mean age ∼80 years) from 80 internal medicine and geriatric wards in Italy from 2010 to 2012 for patients mainly with dementia, of which 135 were new antipsychotic drug users as hospitalized in-patients with subsequent 3-month follow-up [50]. For the overall risk of mortality from this study, the HR = 1.357; [0.913–2.013], comparing to 2498 age and gender matched non-user controls. Although mortality while hospitalized was low, the follow-up risk over the ensuing 90 days increased with HR = 1.57; [0.945–2.607].
Spain
A 2017 retrospective study of the FEDRA Spanish pharmacovigilance database examined 189,441 suspected adverse events over 1995 to 2012 across the general population, of which 5,206 were from antipsychotic drug use with 200 fatal outcomes [24]. In particular, the atypical antipsychotics showed increased all-cause risk of adverse events and excess mortality. The controls used for comparison were based on reports of adverse reactions to any other drugs without a fatal outcome and were sex and age matched to the users of antipsychotics. Such a spontaneous report database can be viewed as source data for a case-control study, where the reporting odds ratio (ROR) can be used to estimate RR of mortality. Overall the risk of mortality caused by antipsychotic drug use showed HR = 2.03 [1.76–2.33] and Relative Odds Ratios (ROR) = 2.07; [1.79–2.33]. The atypical antipsychotics had a higher RR for adverse events with a fatal outcome, HR = 2.47; [2.10–2.92] and for the typical antipsychotics, HR = 1.79; [1.47–2.18]. This study showed no disproportionality in risk between elderly versus the younger population and some of the specific types of antipsychotics analyzed included risperidone, quetiapine, olanzapine, haloperidol and zuclopenthixol [24]. For haloperidol, the HR = 2.71; [1.98–3.69] and for risperidone, the HR = 2.07; [1.56–2.75]. Again, this data supports that the antipsychotic drugs can be hazardous for all and not just for dementia patients.
European Union and Israel
A retrospective study was reported in 2017 analyzing data from 2009–2011 across 59 nursing homes in 7 different European Union countries and Israel for dementia patients aged 65 or older treated with antipsychotics (n = 604), comparing those only prescribed antipsychotics to those also using other potentially contraindicated drugs (n = 278), including cardiovascular or psychotropic medications, and known to cause a range of drug interactions [23]. After follow-up for 12 months, a significantly increased all-cause mortality was found in the group with potential drug interactions (HR = 1.71; [1.15–2.54]). Thus, polypharmacy in combination with antipsychotic drug use is advisable and should be very carefully monitored.
Australia
As part of the ‘PRIME’ study, 779 community based dementia patients across Australia were enrolled at baseline and observed with follow-up for three years with mortality determined at eight years [51]. The atypical antipsychotic drugs were found to have a HR = 1.61; [1.29–2.02] (p < 0.01) for mortality based on univariate analysis adjusting for age and sex. The numbers using typical antipsychotics were too small (p = 0.74) and hence, this latter data was excluded from the present meta-analysis.
United States of America
In 2009, a retrospective study assessed 6,524 new users of antipsychotics during 1998–2000 for all-cause mortality [6]. This study compared those prescribed the atypical antipsychotics (risperidone (n = 4,406), olanzapine (n = 1,563), quetiapine (n = 497), and clozapine (n = 58) and 3,205 new users of conventional antipsychotics (haloperidol (n = 1,413), phenothiazines (including thioridazine [n = 546], perphenazine [n = 314], promazine [n = 305], chlorpromazine [n = 220], fluphenazine [n = 103], trifluoperazine [n = 32], and mesoridazine [n = 24])), as well as other conventional antipsychotics (including loxapine [n = 127], chlorprothixene [n = 59], thiothixene [n = 37], and molindone [n = 25]) from nursing homes across 5 states in the USA [6]. The typical antipsychotic drugs were associated with a higher RR of mortality (HR = 1.26; [1.13–1.42]) compared to the atypical antipsychotic drugs. Relative to the atypical antipsychotic risperidone, a higher rate of death was documented for haloperidol (HR = 1.31; [1.13–1.53]). No other atypical antipsychotic drug was found associated with a disproportionate risk relative to risperidone [6]. Again, such a comparison between those prescribed different antipsychotics as opposed to more appropriate control groups would greatly underestimate the real risks.
Another retrospective cohort study from the Medicaid Tennessee database over 1990–2005 analyzed 44,218 and 46,089 given typical or atypical antipsychotic drugs respectively as monotherapy at baseline, matched on a 2:1 basis with 186,600 non-users [52]. This was a carefully controlled analysis with follow-up from first use over a median of 2.5 years, excluding in-hospital deaths. Control patients were matched by age, gender, race, location and their propensity score (i.e., the predicted probability that they would be users of antipsychotic drugs). The propensity matched groups included 3% diagnosed with dementia. Current users of the typical or atypical antipsychotic drugs had higher rates of sudden cardiac death compared to non-users, with adjusted HR = 1.99; [1.68–2.34] and 2.26; [1.88–2.72, p < 0.001] respectively. Former (prior long term) users of antipsychotic drugs had no significantly increased risks. For both classes of drugs, the risk for current users increased significantly with increasing daily doses such that for typical antipsychotic drugs (FGA), the incidence-rate ratios increased from 1.31; [0.97–1.77] for low dose up to HR = 2.42; [1.91–3.06] for high doses (p < 0.001). Among users of atypical agents (SGA), for low doses the HR = 1.59; [1.03–2.46] and increased to HR = 2.86; [2.25–3.65] for larger doses (p = 0.01). For the 24,589 users of risperidone alone, the HR = 2.91; [2.26–3.76] which at larger doses had a high HR = 3.56; [2.3–5.4]. The direct dose-response is a strength of this study because it highlights a significant cause-effect relationship between antipsychotic drug use and death.
In 2010, a retrospective study of the Veterans Health Administration (VHA) National Patient Care Database from Austin, Texas over a five-year follow-up period compared large numbers of dementia patients prescribed antipsychotics (haloperidol, n = 2,217; quetiapine, 4,277; olanzapine, 3,384 or risperidone, 8,249) for the RR of all-cause mortality compared to non-user dementia controls [53]. This report examined the dose-related risk of mortality. For any dose of haloperidol, the adjusted HR was 2.3; [1.6–3.3], but for the higher doses of haloperidol above 1 mg, the adjusted HR increased to 3.2; [2.2–4.5], p < 0.001 within the first 30 days of use [53], which declined to non-significant levels over longer term use beyond 30 days. For the other atypical antipsychotics, the any dose adjusted HR was 1.3; [1.0–1.7] for olanzapine and 1.2; [1.0–1.4] for risperidone whereas quetiapine did not show a higher risk (refer to Table 1; Rossom et al., 2010 [53]).
A more robust analysis was reported in 2015 by Lon Schneider’s group on use of antipsychotics or other psychotropics for 6 months and the risk of death in dementia patients. This was a retrospective cohort study, based on national data from the U.S. Department of Veterans Affairs (from 1999–2008) for 90,786 dementia patients age 65 and older [54]. In this study, 15,689 antipsychotic new drug users were compared by pair-matching to the non-drug user dementia patients or 29,704 prescribed antidepressants as controls [54, 55]. Analysis of their data for the antipsychotic drugs prescribed over a 6-month period adjusting for age, sex, years with dementia, presence of delirium, and other clinical and demographic characteristics showed that, the risk of mortality was HR = 2.457; [2.070–2.916] for haloperidol (95% CI: [2.33–3.45]) compared to that of the antidepressant drug users. Applying the inverse-variance–weighted random-effects model to pool the results provided an HR for all-cause mortality across the 4 antipsychotics haloperidol, risperidone, quetiapine, and olanzapine examined over this time as HR = 1.688 (95% CI: [1.366–2.086]) [54]. However, these authors further stratified the patient data by heavily adjusting on the basis of almost 40 factors (including comorbidities and dementia severity) such that the relative difference in mortality amongst dementia patients prescribed antipsychotics was greatly diminished. Nevertheless, the study authors concluded that the atypical antipsychotics (olanzapine, quetiapine, and risperidone) showed a dose-response increase in mortality risk. From the highly adjusted extracted data, haloperidol was associated with the greatest mortality risk, (12.3% higher; 95% CI: [8.6–16.0%]; p < 0.01) than for antidepressants. Of the antipsychotic drugs, quetiapine had the lowest effect on mortality, with a 3.2% (95% CI: [1.6–4.9%]; p < 0.01) higher risk relative to users of antidepressants.
Importantly, many of the more recent studies concluded that whilst their results were consistent with earlier reports (prior to 2009), it was considered that the earlier studies underestimated the increased risks associated, particularly with haloperidol and which in several studies (as identified above) has shown double the risk of all-cause mortality (predominantly cardiovascular, respiratory or stroke related causes). Disturbingly, death was occurring usually shortly after the start of treatment, revealing that a lack of attention was likely being given during the initial stages to dose administration and regulating the use of such a potent sedative. Thus, results reported in 2012 showed that the mortality risk with haloperidol was highest in the first 30 days (RR = 2.24 compared to risperidone), but decreased significantly and sharply thereafter [8] confirming the earlier reports [53]. Among the other antipsychotic agents, mortality risk differences were most significant in the first 120 days and declined in the subsequent 60 days during follow-up. Similar findings were reported in 2016 from a study of dementia patients in nursing homes (Medicare beneficiaries) with behavioral symptoms over 2007–2009 showing a lowering of mortality when using lower doses of antipsychotics, particularly over the longer duration [56]. Hence, many studies have shown highest risk occurs during first use and provide consistent support for proceeding cautiously whilst emphasizing the importance of starting with the lowest doses [8, 13, 42, 53, 57].
Meta-analysis results using different models
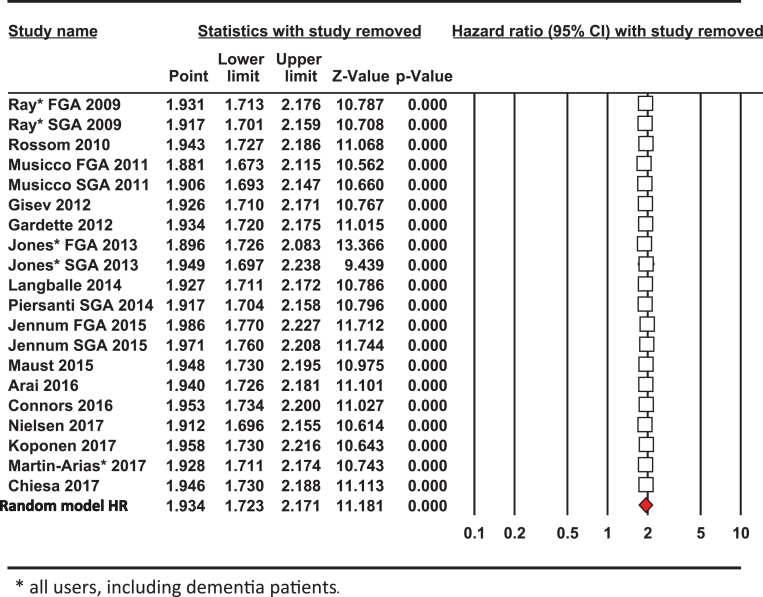
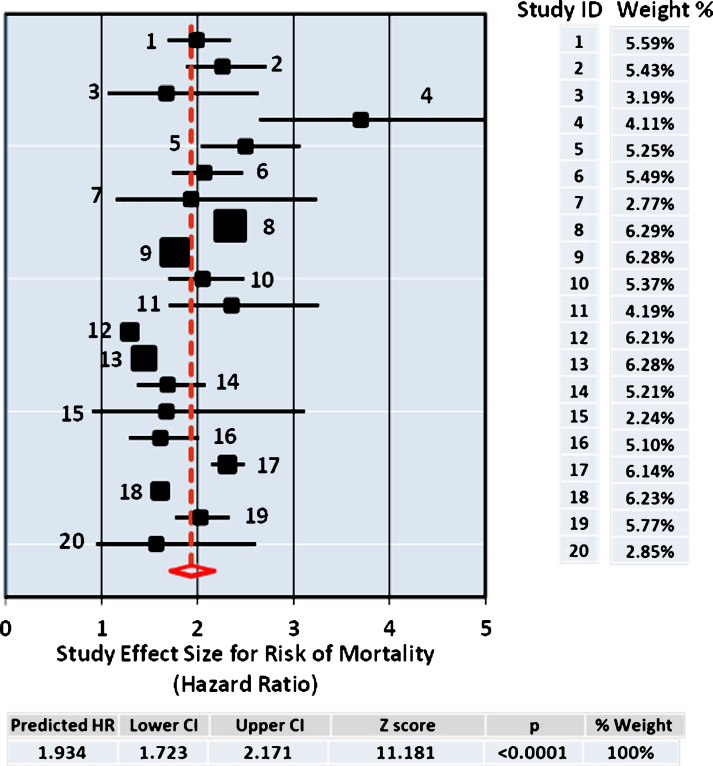
Several different methods for meta-analysis were used to compare each with resulting pooled estimate for effect size determined as the HR for risk of mortality from use of antipsychotic drugs. The data set from the 20 studies is summarized in Table 2. The DerSimonian and Laird procedure is the simplest, most commonly used method for fitting the random effects model [29] and the pooled results (HR = 1.934; [1.723–2.171], Supplementary Table 1) from this analysis are shown in Fig. 1, which also includes the Forest Plot. In addition, the Hedges and Vevea [31] as well as the Hunter and Schmidt [30] methods for calculating the pooled estimate from the 20 studies were compared, giving similar point effect sizes for the pooled HR, 2.098; [1.952–2.221] and HR = 2.044; [1.559–2.681] respectively, as shown in Table 2. All three methods of meta-analysis produced similar pooled effect sizes for the risks of mortality of antipsychotic drug users at close to twice that of the control non-user group.
Fig.1.
Forest Plot. The size of the squares represent point estimates for magnitude of effect size (HR), scaled based on their precision (1/Standard Error). The diamond displays the estimated overall mean based on the random-effects meta-analysis (REMA) model with the width defining the 95% CIs. The horizontal lines across each square show 95% CI’s. Sample % weight is indicated on the right for each study (ID) used in the estimation. The pooled estimate from the DerSimonium-Laird analysis for the predicted HR is shown underneath.
Sensitivity analysis
In a sensitivity analysis excluding one study at a time, the overall outcome for the pooled HR and risk of mortality altered only slightly (Table 3). Each of the point estimates was close to or within the 95% CI for the combined mean HR, indicating that no individual study had excessive influence on the pooled effect estimate for the risk of mortality from using antipsychotic drugs. Excluding one or more studies at a time to investigate whether the results were due to a large study or studies with an extreme result revealed that by omitting those with the largest impact by Z score or percentage weighting (Jones [36, 37], Jennum [43] or Nielsen [44]) individually shifted the HR value slightly from the mean effect size 1.934 to either 1.916, 2.033 or 1.958, respectively. Hence, no single study was unduly influencing the risk of mortality from the meta-analysis and the weighted average HR from the 20 studies.
Analysis of the heterogeneity statistics for the 20 studies gave values for deviation, Q = 837, inconsistency among the group of studies, I2 = 97.5% and variance, t2 = 0.06. Omitting all three (Jones [36, 37], Jennum [43], and Koponen [22]) studies lowered these heterogeneity values to Q = 32.8, I2 = 57.3% and t2 = 0.0135 and HR = 2.09; [1.92–2.98]. This shows that although these three large studies contributed extensively to the high level of study heterogeneity in effect sizes, regardless their exclusion did little to alter the overall outcomes for the point estimate and greater risk of mortality.
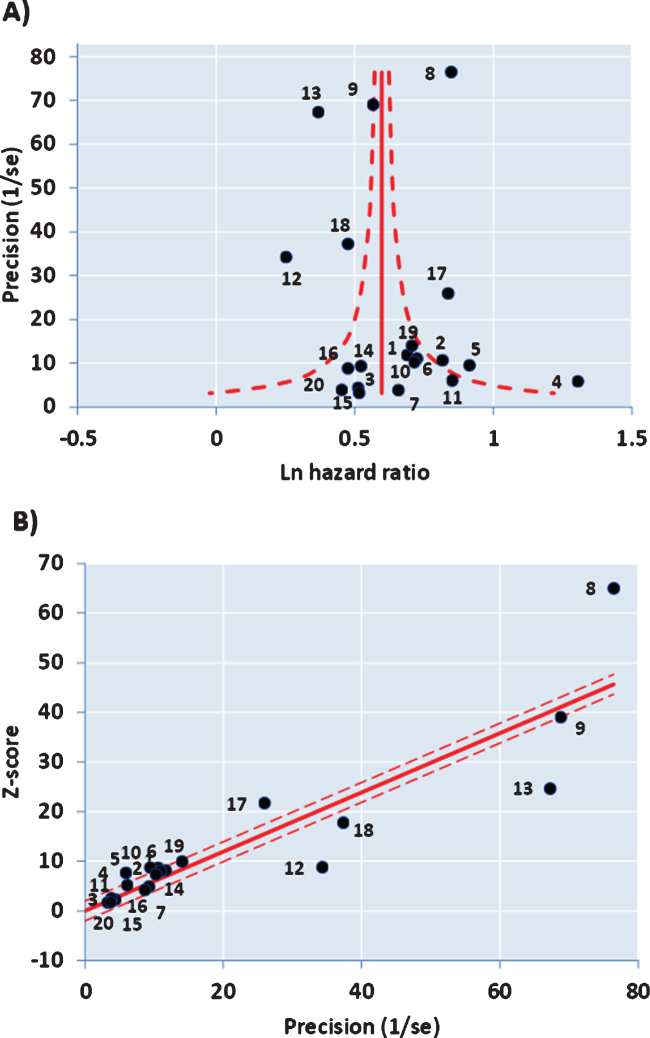
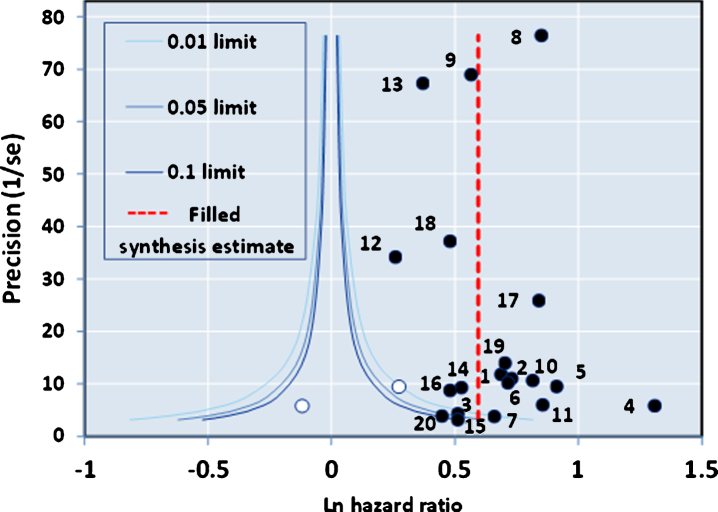
The funnel plot of precision (inverse of standard error of each estimate) versus the log value of effect size (Fig. 2A) for the 20 studies was approximately symmetrical around the pooled estimate. Similarly, the Galbraith plot for unweighted regression of z-scores versus the inverse of the standard error with the intercept constrained to zero showed few outliers (Fig. 2B). Hence analysis for small study effects and obvious asymmetry did not show the presence of publication bias. In addition, most of the studies were close to or within the pseudo-95% CI around the pooled estimate. Formalized testing using the Begg and Mazumdar’s rank correlation for Kendall’s tau (τ), with our without continuity correction, showed that this was not significant (p = 0.87 on two-tailed test). Likewise, Egger’s regression test did not detect publication bias (p = 0.411), further validating our meta-analysis. Analysis of the contour-enhanced funnel plot [58] with trim and fill (Fig. 3) showed that only two studies were required to be inserted for balancing the symmetry of the funnel plot, one of which fell within the area of statistical non-significance (p > 0.1), whereas the majority of the 20 studies used were in the area of significance (p < 0.01). This may indicate that the slight asymmetry in the funnel plot could be caused by publication bias based on statistical significance [58].
Fig.2.
A) Funnel Plot with 95% confidence limits and asymmetry from small study effects. B) Galbraith Plot.
Fig.3.
Contour-enhanced Funnel Plot with Trim and Fill.
Haloperidol
In all of the studies, the findings have consistently shown that use of haloperidol is consistently linked with the highest risk of all-cause mortality amongst the antipsychotics (see Table 4). Risks are particularly great when it is initiated as injections, added to which haloperidol is known to be a potent neurotoxic drug, with a call for its total ban in dementia patients [59]. The results from many studies of haloperidol use are summarized in Table 4 and the data is consistent such that by the random effects model, the haloperidol HR = 2.43; [2.25–2.61] for risk of mortality compared to non-user groups (Table 4A). This is supported by a further seven studies where haloperidol was compared with risperidone use as the reference and from this comparison, the pooled estimate for the HR = 1.71 (Table 4B), whereas from six more studies where risperidone was compared to non-user control, the risperidone HR = 1.65 (Table 4C). The risk of mortality from haloperidol compared to non-users can then be estimated by using this data to provide the HR = 1.71×1.56 or 2.67, in agreement with the HR = 2.43 from the five studies in Table 4A comparing haloperidol users directly to non-user control groups. Considered together, the data from the 12 studies with haloperidol all consistently points out the heavy risks associated with general use of haloperidol, particularly when doses are >1 mg.
Table 4.
Haloperidol or Risperidone and risk of all-cause mortality
| Study Name | HR | Lower 95% CI | Upper 95% CI | Drug use/days |
| A. Haloperidol current users relative to non-users or users of other drugs. | ||||
| Langballe | 2.938 | 2.324 | 3.714 | 0–180 |
| Martin-Arias* | 2.710 | 1.980 | 3.690 | Proportional Reporting Ratios |
| Jones¥ | 2.330 | 2.120 | 2.560 | 0–30 days |
| Rossom | 2.300 | 1.600 | 3.300 | 0–30 days |
| >1 mg/ day | 3.20 | 2.20 | 4.50 | 0–30 days |
| Maust | 2.457 | 2.070 | 2.916 | 0–180 days |
| D-L Random Model | 2.427 | 2.254 | 2.614 | 0–180 days |
| B. Haloperidol relative to Risperidone as reference (HR = 1). | ||||
| Kale 2012 | 2.24 | n.d. | n.d. | 0–30 days |
| 1.59 | 1.36 | 1.85 | 30–180 | |
| Huybrechts 2012 | 2.07 | 1.89 | 2.26 | 0–180 |
| Langballe | 1.700 | 1.00 | 3.00 | 0–30 |
| 2.007 | 1.464 | 2.751 | 180–365 | |
| Koponen ≤ 1 mg/day | 1.66 | 1.10 | 2.50 | 0–180 |
| >1 mg/day | 2.55 | 1.70 | 3.85 | 0–180 |
| Liperoti 2009 | 1.383 | 1.240 | 1.543 | 0–180 |
| D-L Random Model | 1.711 | 1.408 | 2.079 | 0–180 |
| C. Risperidone HR relative to non-users. | ||||
| Langballe | 1.976 | 1.504 | 2.595 | 0-180 days |
| Piersanti 2014 | 2.112 | 1.152 | 3.871 | Average use 7 months, follow-up to 3 years |
| Jones 2013 | 1.640 | 1.56 | 1.72 | 0–30 days |
| Maust 2015 | 1.641 | 1.483 | 1.816 | 0–180 days |
| Martin-Arias* | 2.07 | 1.56 | 2.75 | Proportional Reporting Ratio |
| Koponen 2017 | 1.428 | 1.336 | 1.525 | 0–180 days |
| D-L Random Model | 1.647 | 1.496 | 1.813 | 0–180 days |
| D. Risk of All-Cause Mortality from Antipsychotic drug use in the General User Population. | ||||
| Ray FGA | 1.99 | 1.68 | 2.34 | 44,218 users |
| Jennum FGA | 2.026 | 2.016 | 2.036 | 138/32,606 |
| Jones FGA | 2.34 | 2.28 | 2.41 | 15473 |
| D-L Random Model | 2.132 | 1.887 | 2.409 | |
| Ray SGA | 2.26 | 1.88 | 2.72 | 46,089 |
| Jennum SGA | 1.785 | 1.779 | 1.791 | 222/32606 |
| Jones SGA | 1.76 | 1.71 | 1.82 | 7356 |
| D-L Random Model | 1.788 | 1.728 | 1.851 | |
¥Antipsychotic users (incl. 13.7% dementia) compared to psychiatric non-users (24% dementia). *All users, including dementia patients.
DISCUSSION
Main findings
In this meta-analysis, we calculated aggregate HR from data for the antipsychotic drug users that was minimally adjusted when matched to the appropriate non-user control populations. Nevertheless, comparisons of our minimally adjusted risk estimates over the 20 studies showed closely related effect sizes across these studies for the association of antipsychotic drug use with all-cause risks of mortality. Consequently, this makes the calculations for the minimally adjusted summary HR in our meta-analyses all the more convincing. This is further supported by comparing the risks of mortality for dementia patients using antipsychotic drugs to that of the non-dementia patients in the population who are using antipsychotic drugs, with both showing similar HR around twice the risk levels for mortality compared to the non-user groups.
Sources of heterogeneity
In the current review, we found evidence of moderate between study heterogeneity for pooled estimate of the HRs from aggregate data for antipsychotic drug use and risk of death. As revealed by our sensitivity analyses, three studies with very small study variances [22, 36, 37, 43], two of which were of larger sample size from the general population of antipsychotic drug users [36, 37, 46] accounted for most of the observed heterogeneity between studies. Epidemiological studies with large sample sizes are less driven by confounding effects and chance sampling errors, consequently producing more precise risk estimates for their effect sizes, and hence, can have a much greater weighting contribution. In the majority (16 out of the 20), the variances in study effect sizes were <0.05, with 11 of 20 studies based on data from more than four thousand dementia patients each prescribed antipsychotic drugs such that variances became exceedingly low, <0.001. This supports the accuracy of the overall meta-analysis. In addition, we have aggregated a large data set from pooled estimates of studies with 80,330 dementia users of antipsychotics as a sub-group with that from 278,905 users of antipsychotic drugs in the general population when compared to non-users. The individual estimates from these studies are closely related such that the exclusion sensitivity test confirmed that no single study unduly influenced the overall pooled estimate for risk of mortality (see Table 3).
Limitations
Our review has some limitations. First, including three studies with data on users who were not categorized as either having dementia or other mental illness [24, 37, 52] restricts examining antipsychotic drug use association with risk of mortality from these three studies and its association to the dementia versus non-dementia sub-groups. However, excluding data from the three studies of general antipsychotic drug users, such that only those studies focused on dementia patient users (80,330) as a sub-group were considered in meta-analysis slightly lowered the HR from 1.93; [1.72–2.17] for the pooled estimate to 1.88; [1.66–2.12] for the dementia user sub-group compared to non-users. Hence, the difference when accounted for by user sub-groups did not greatly affect the overall estimate for the risk of mortality from antipsychotic drug use.
Alzheimer’s disease and vascular dementia are the two major subtypes of dementia with different incidence and etiology. Although we did not analyze whether the risk of mortality for those with Alzheimer’s disease differs from that of vascular dementia, due to the limited nature of the included studies, the majority of the studies were focused on Alzheimer’s dementia. During sensitivity analysis, we initially observed high heterogeneity (I2 = 97%) for the pooled estimate risk of mortality by all (dementia and non-dementia) users of antipsychotic drugs versus non-users. Such heterogeneity raises some concern about the reliability of the pooled results. However, the sources of heterogeneity were identified by sensitivity analyses and excluding three studies [37, 43, 46] reduced the heterogeneity to a more acceptable and moderate level (Q = 32.8; I2 = 57.3 %; t2 = 0.0135) without greatly affecting the pooled HR = 2.09; [1.92–2.98].
The studies incorporated into this meta-analysis were reported since the Banerjee study [3] from 2009 onwards and were mainly based on minimally adjusted effect sizes standardized across age, gender, marital status, and location of drug users matching to the control non-user population as the reference. These pooled results might be subject to uncontrolled or residual confounding, given our focus on minimally adjusted risk estimates. Nevertheless, when we pooled the minimally adjusted HRs, the increased risk of mortality in antipsychotic users remained consistent across studies as evidenced by the sensitivity analyses and the HR, which were highly significant in most cases (Fig. 1, Tables 2 and 3).
In this regard, it should be noted that several reports have claimed minimal effects of antipsychotic drugs on risk of mortality with dementia patients [41, 57, 60–67]. However, the latter studies have been based on older literature or culminated from extracting heavily selected and refined data, adjusted by many exclusions made for clinical factors deemed as confounders such as mortality risks and co-morbidities relating to terminal illness [65]. Amongst the more stringent criteria applied for exclusion have been factors such as the severity of dementia, gender, advanced age or co-morbidities including cardiovascular, diabetes, cancer, respiratory or other somatic disease burdens or neuropsychiatric behavioral problems with some of the studies involving nearly 40 exclusion criteria in their adjustments [41, 57, 60–66]. It is unreasonable and unjustified to greatly extract and refine the data in such an extreme manner. Often in these highly adjusted studies, the excluded population of patients are those very same individuals who will be, by their health conditions, those that are more likely to succumb from the all-cause risks of mortality including cardiovascular, stroke, seizure or respiratory illness related death after being prescribed antipsychotic drugs, and especially for haloperidol [68–71]. Hence, the practice of heavy adjustment and censoring is very debatable, particularly given that the selected populations in the majority of the other, much larger studies incorporated into the present meta-analysis were matched against controls by adjusting predominantly on age, gender and location, and which, as highlighted in this meta-analysis using the data of 20 studies from Table 1 have consistently shown greatly increased all-cause risk of mortality from antipsychotic drug use.
Examples of the significant effects on the outcomes which can result by adjusting and excluding data based on potential confounding factors, compared to data solely adjusted by age, gender, marital status and location matching to controls can be found in [41, 64]. An extreme example comes from comparing age-standardized death rates of the Polish National Health Fund (NHF) database, accumulated over the period of 2008 to 2012 [72]. The risks of all-cause mortality for the 66,744 users of typical (HR = 9.305) and atypical (HR = 6.468) antipsychotic drugs (including for mental illnesses of all types) was very high compared to that of the non-user general population. This study was not adjusted for modifiers of mortality risk such as concomitant diseases, psychosocial or lifestyle-related factors and was controlled only for age, location, and gender. It was excluded however, as an outlier from the present study given the extreme nature of their findings regarding risk of death from using antipsychotic drugs.
In relation to study size, it should be noted that many highly adjusted studies consequently also end up selecting much smaller relative numbers of patients (commonly with only a hundred or less drug users included) for their final analyses. In addition, little benefit arises from studies where the mean age is close to 90, because such patients are too frail and impending death rates are consequently in the extreme (close to 50%) in both user and non-user groups, obscuring any drug-related effects [67]. Thus, in such heavily “adjusted” or highly selective studies, the data becomes overly restrictive, comparing insufficient numbers of patients, in which effect sizes will no longer detect any significant differences in RR. For this reason, the results of heavily adjusted or censored studies were excluded from the present meta-analysis.
Antipsychotic drugs increase risk of mortality in other users, not just for dementia
Surprisingly, risk of all-cause mortality has shown itself to be high (HR = 1.4–2.06) in large retrospective studies of antipsychotic drug-treated non-dementia users compared to non-drug users or non-dementia controls [37, 43] (refer to Table 4D). This is one of the more disturbing points emerging from the present meta-analysis, which is consistent with all other studies, including from the UK [36, 37], USA [52], and Spain [24], confirming that control subjects (without cognitive impairment) prescribed the antipsychotic drugs can show even greater risks for severe adverse events leading to increased all-cause mortality. Hence, the frequently reported increased mortality from antipsychotic drugs in dementia is not restricted only to those with impaired cognition and neither is it restricted to only one class or type of antipsychotic drug. This latter data is of major concern and would indicate a need for more thorough studies to be carried out on the negative impacts and general safety of antipsychotic drugs prescribed for the general patient population in mental health care. This is backed up by studies focusing on using antipsychotics, particularly as treatments for mental health in patients with cardiovascular disease and obesity problems [73] or in schizophrenia [74].
A meta-analysis reported in late 2016 [28] examined only nine dementia-related studies (11,463 participants in total) and three effect sizes from amongst these studies were found to contribute to a very high between-study heterogeneity. After excluding these three studies, the total proportion of variance owing to heterogeneity between studies (I2 = 94.9%) decreased (I2 = 75.7%), while the pooled HR for all-cause mortality from antipsychotic drugs increased from HR = 1.36; [0.83–2.24] overall from the nine studies to HR = 2.08; [1.39–3.13]. The three highly heterogeneous studies in [28] were likely flawed by design because of only small dementia patient sample sizes [61, 75], or they concerned mild to moderate dementia patients in outpatient settings [62], not advanced/elderly patients residing in nursing homes. For these reasons, the three studies were excluded from the present meta-analysis, which extends well beyond [28] to include the data from much larger scale longitudinal studies. Our study adds significantly more recent data since 2009 from a total number of over 380,000 dementia patients, of which 80,330 were prescribed antipsychotics as well as data from 278,905 other antipsychotic drug users (mostly non-dementia patients).
In summary from the global evidence, it is clear that valuable lessons can be learned from the mistakes made over the past decades, and since the 2009 Banerjee report [3] calling for solutions to the problem of overprescribing antipsychotic drugs for dementia and the best way to enforce changes in practice [76]. Despite the extensive evidence that these drugs increase all-cause mortality in dementia and other patients, they remain commonly overprescribed and in some countries have seen little to no change in use or in many cases have been increasingly prescribed over the past 10 years, even for long term use and regardless of all the warnings to the contrary [46, 77, 78].
Clinical practice guidelines are not sufficient and central government intervention may be required
Whilst the evidence indicates that doctors should proceed with extreme caution when prescribing for new users, they also have a duty of care to dementia patients in de-prescribing use of antipsychotic drugs for dementia. However, the systematic review of worldwide reports together with meta-analyses considered here suggests that it will require stronger leading roles played by central government interventions. This is exemplified by the recent Centers for Medicare & Medicaid Services (CMS) in the USA instituting central regulation to ensure that greater reform takes place in clinical practice to reduce prescribing of antipsychotic drugs for dementia [79]. This necessitates monitoring doctor/nursing home compliance and accountability in controlling use of antipsychotic drugs in order to be effective. It is readily apparent based on the information and growing volume of evidence above that prescribing of antipsychotic drugs is in need of such further reform worldwide. Whether greater control can be exerted to include their off-label use based on the failure of clinical guidelines alone is uncertain. The search for better alternatives needs to be actively encouraged. However, in order to improve existing national based systems for health care, particularly concerning the elderly with dementia, further changes will have to be made because the current situation shows little signs of working in a highly effective manner.
Changes should include greater accountability by monitoring and regulations restricting use of the antipsychotic drugs for dementia, particularly in a way that better treatment alternatives can be actively sought and encouraged. The recently published clinical practice guidelines for dementia in Australia [80] and by the American Psychiatric Association (APA) in the USA [81] are a step forward, but on the basis of the facts presented from the comprehensive international range of sources and assessments as reviewed here, will unlikely have the desired outcomes. Disturbingly, increased prescribing of antipsychotic drugs has been reported [82], including a doubling in prescriptions over the period from 2007 to 2015 within Australia, predominantly with 47.5% of single antipsychotic prescriptions being for haloperidol [83]. Given the risk of all-cause mortality particularly from sudden cardiac death, stroke or seizures with the HR for low doses of <1 mg at more than twice that of non-users, increasing to more than three-fold over control for >1 mg doses, prescribing haloperidol should be halted or restricted altogether.
De-prescribing of antipsychotic drugs: Time for a federal mandate enforceable by health authorities
With the failures of clinical guidelines elsewhere (UK and USA) in exerting any major influence or bringing about modified practices or greater compliance, tighter federal regulation will be required. The impact of changing regulatory policies which have either failed or proven successful is discussed in detail elsewhere [84] and is outside the scope of the present systematic review and meta-analysis. The evidence is undeniable that the antipsychotic drugs, when first administered carry a very high risk of serious adverse events leading to increased all-cause mortality. Also, when used for any length of time, they can be detrimental to the health of dementia patients and de-prescribing has been proven successful when alternative programs are implemented to treat BPSD [85]. De-prescribing is likely to be highly beneficial, as has been supported by systematic review and meta-analysis [86–88] as well as by a recent randomized trial which showed no significant adverse effects on survival or other clinical outcomes after de-prescribing elderly patients in aged care [89].
An earlier randomized, placebo-controlled, parallel, two-group treatment discontinuation trial of dementia patients in an antipsychotic withdrawal study (DART-AD, 2009), examined 165 patients residing in aged care in the UK [90]. This trial showed reduced 12-month survival (70%; 95% CI [58–80%]) in those who continued on antipsychotics versus those who halted drug use as the control group (77%; 95% CI [64–85%]). However, of greater concern was the significantly increased mortality after continued longer term use at the 2 (46% survival only versus 71% in the control) and 3-year (30% survival only versus 59% in the control) follow-up time points [90, 91]. This study, with an almost two-fold greater RR of mortality at 3 years after continuing on the antipsychotics, provides additional support for halting the use of antipsychotic drugs to treat dementia.
An open question which remains is whether the use of alternatives such as sedatives in the GABAB receptor agonist group of drugs (including pregabalin or gabapentin) or opioids will increase the risk of dementia or cause greater mortality in dementia patients. At present, no published reports could be found relating to this area. Disturbingly, studies examining prevalence trends in the use of opioids, other analgesics and psychotropic medications have shown that the opioids and GABAB receptor agonists such as pregabalin/gabapentin are becoming more frequently used and would appear to be replacing that of the antipsychotic drugs as alternatives for nursing homes [92, 93]. This raises the prospect that restricting use of the antipsychotic and related drugs will promote a shift to alternative drugs whose use still remains questionable or unknown but which are being prescribed under the pretense of treating BPSD. There are very few studies on the short or long-term effects of opioids or GABAB receptor type sedative drugs on dementia. One large cohort study was reported in which a 10 year follow up period of 3,484 patients over 65 years old in elderly care showed that opioids were relatively safe, although heaviest opioid use increased the risk of dementia (HR = 1.29; [1.02–1.62]) [94]. Low to moderate opioid exposure was not associated with risk. The authors concluded that the evidence showed little long-term cognitive harm specific to opioids in relation to dementia risk, although more thorough studies will be required.
One final point to consider is the emerging concept of “Elder Abuse” which is gaining acceptance worldwide and is now recognized by the World Health Organization as a problem [95]. It is likely that it will not be long before misuse of chemical sedation with antipsychotics or other psychotropics/hypnotics such as the benzodiazepine anxiolytics for the elderly with dementia will become recognized and included under the list of criteria qualifying as having considerable potential during screening for “Elder Abuse” in the aged care setting [96]. Hence, the complex issues raised here should be tackled head-on as a worldwide health problem and perhaps will require legal reforms to introduce “Halting Antipsychotic use in Long-Term” care (HALT). At least, we should aim to attain the desired level of de-prescribing for this current widespread and often unnecessary practice as the easy fix option and simple remedy over the long term when dealing with the behavioral problems of dementia patients. Despite the facts, the message to de-prescribe such drugs is not getting through and a recent call that their sustained use as chemical restraints be included under “Elder Abuse” perhaps should be considered to help enforce law reform and the change necessary to regulate compliance [3, 97].
Conclusions
From the above meta-analysis including several large retrospective studies recently reported, the data is consistent for risk of increased all-cause mortality associated with dementia or other patients when prescribed the antipsychotic drugs. In agreement with the previous meta-analysis published in [28], excluding highly heterogeneous studies, from our meta-analysis, the facts remain indisputable and it can be summarily concluded:
-
1)
The RR for all-cause mortality of patients prescribed the antipsychotic drugs is closer to 2 (HR = 1.9–2.19) (Tables 1 and 2).
-
2)
The danger period for elevated risk of mortality is highest from the outset over the initial 0–180 days after starting their use.
-
3)
Risks of all-cause mortality are dose-related, increasing with greater doses.
-
4)
Little difference exists in risks when using either the typical or atypical antipsychotic drugs.
-
5)
Risk of mortality is significant and similarly high for all users, including dementia and non-dementia patients alike.
-
6)
Current practices in the prescribing and use of antipsychotics should be more restrictive and their use stringently monitored by the health authorities.
-
7)
De-prescribing of the antipsychotic drugs should be heavily encouraged and some, such as haloperidol should be placed on restricted drug schedules and regarded as highly neurotoxic drugs.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
Supplementary Material
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/ADR-170042.
REFERENCES
- [1]. Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, Rosenheck RA, Hsiao JK, Lieberman JA, Schneider LS (2008) Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: Phase 1 outcomes from the CATIE-AD Effectiveness Trial. Am J Psychiatry 165, 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, de la Pena D, Gupta S, Colon S, Schimming C, Pelton GH, Levin B (2012) Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med 367, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Banerjee S (2009) The use of antipsychotic medication for people with dementia: Time for action. Department of Health UK Government, London. [Google Scholar]
- [4]. Kleijer B, van Marum R, Egberts A, Jansen P, Knol W, Heerdink E (2008) Risk of cerebrovascular events in elderly users of antipsychotics. J Psychopharmacol 23, 909–914. [DOI] [PubMed] [Google Scholar]
- [5]. Sheehan R, Hassiotis A, Walters K, Osborn D, Strydom A, Horsfall L (2015) Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population based cohort study.. BMJ 351, h4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Liperoti R, Onder G, Landi F, Lapane KL, Mor V, Bernabei R, Gambassi G (2009) All-cause mortality associated with atypical and conventional antipsychotics among nursing home residents with dementia. J Clin Psychiatry 70, 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Huybrechts KF, Gerhard T, Crystal S, Olfson M, Avorn J, Levin R, Lucas JA, Schneeweiss S (2012) Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: Population based cohort study. BMJ 344, e977–e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Kales HC, Kim HM, Zivin K, Valenstein M, Seyfried LS, Chiang C, Cunningham F, Schneider LS, Blow FC (2012) Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry 169, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA (2005) Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 353, 2335–2341. [DOI] [PubMed] [Google Scholar]
- [10]. Schneider LS, Dagerman KS, Insel P (2005) Risk of death with atypical antipsychotic drug treatment for dementia. JAMA 294, 1934. [DOI] [PubMed] [Google Scholar]
- [11].Food and Drug Administration (FDA) Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances, Department of Health and Human Services, U.S. Government, www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171.htm, 4 Nov. 2005,
- [12]. Gill SS, Bronskill SE, Normand S-LT, Anderson GM, Sykora K, Lam K, Bell CM, Lee PE, Fischer HD, Herrmann N, Gurwitz JH, Rochon PA (2007) Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 146, 775. [DOI] [PubMed] [Google Scholar]
- [13]. Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang PS (2007) Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 176, 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Food and Drug Administration (FDA), Information for Healthcare Professionals—antipsychotics, Department of Health and Human Services, U.S. Government, www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm, 16 Jun., Accessed 09/12-2016.
- [15]. Leucht S, Arbter D, Engel RR, Kissling W, Davis JM (2008) How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 14, 429–447. [DOI] [PubMed] [Google Scholar]
- [16]. Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 373, 31–41. [DOI] [PubMed] [Google Scholar]
- [17]. Tyrer P, Kendall T (2009) The spurious advance of antipsychotic drug therapy. Lancet 373, 4–5. [DOI] [PubMed] [Google Scholar]
- [18]. Steinberg M, Lyketsos CG (2012) Atypical antipsychotic use in patients with dementia: Managing safety concerns. Am J Psychiatry 169, 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Ma H, Huang Y, Cong Z, Wang Y, Jiang W, Gao S, Zhu G (2014) The efficacy and safety of atypical antipsychotics for the treatment of dementia: A meta-analysis of randomized placebo-controlled trials. J Alzheimers Dis 42, 915–937. [DOI] [PubMed] [Google Scholar]
- [20]. Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, Tariot P, Yaffe K (2007) ACNP white paper: Update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 33, 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA (2006) Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 355, 1525–1538. [DOI] [PubMed] [Google Scholar]
- [22]. Koponen M, Taipale H, Lavikainen P, Tanskanen A, Tiihonen J, Tolppanen AM, Ahonen R, Hartikainen S (2017) Risk of mortality associated with antipsychotic monotherapy and polypharmacy among community-dwelling persons with Alzheimer’s disease. J Alzheimers Dis 56, 107–118. [DOI] [PubMed] [Google Scholar]
- [23]. Liperoti R, Sganga F, Landi F, Topinkova E, Denkinger MD, van der Roest HG, Foebel AD, Finne-Soveri H, Bernabei R, Onder G (2017) Antipsychotic drug interactions and mortality among nursing home residents with cognitive impairment. J Clin Psychiatry 78, e76–e82. [DOI] [PubMed] [Google Scholar]
- [24]. Martin Arias LH, Treceno Lobato C, Perez Garcia S, Sainz Gil M, Sanz Fadrique R, Garcia Ortega P (2017) Risk excess of mortality and use of antipsychotics: A case-noncase study. Int Clin Psychopharmacol 32, 1–5. [DOI] [PubMed] [Google Scholar]
- [25]. Maust DT, Langa KM, Blow FC, Kales HC (2017) Psychotropic use and associated neuropsychiatric symptoms among patients with dementia in the USA. Int J Geriatr Psychiatry 32, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Nielsen RE, Lolk A, Rodrigo-Domingo M, Valentin JB, Andersen K (2017) Antipsychotic treatment effects on cardiovascular, cancer, infection, and intentional self-harm as cause of death in patients with Alzheimer’s dementia. Eur Psychiatry 42, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Taipale H, Koponen M, Tanskanen A, Lavikainen P, Tolppanen AM, Sund R, Tiihonen J, Hartikainen S (2017) Use of benzodiazepines and related drugs is associated with a risk of stroke among persons with Alzheimer’s disease. Int Clin Psychopharmacol 32, 135–141. [DOI] [PubMed] [Google Scholar]
- [28]. Zhai Y, Yin S, Zhang D (2016) Association between antipsychotic drugs and mortality in older persons with Alzheimer’s disease: A systematic review and meta-analysis. J Alzheimers Dis 52, 631–639. [DOI] [PubMed] [Google Scholar]
- [29]. Jackson D, Bowden J, Baker R (2010) How does the DerSimonian and Laird procedure for random effects meta-analysis compare with its more efficient but harder to compute counterparts? J Stat Plan Inference 140, 961–970. [Google Scholar]
- [30]. Hunter JE, Schmidt FL (2004) Methods of Meta-Analysis: Correcting Error and Bias in Research Findings, SAGE Publications, Thousand Oaks, CA. [Google Scholar]
- [31]. Hedges LV, Vevea JL (1998) Fixed- and random-effects models in meta-analysis. Pyschol Methods 3, 486–504. [Google Scholar]
- [32]. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- [33]. Patsopoulos NA, Evangelou E, Ioannidis JP (2008) Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int J Epidemiol 37, 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Field AP, Gillett R (2010) How to do a meta-analysis. Br J Math Stat Psychol 63, 665–694. [DOI] [PubMed] [Google Scholar]
- [36]. Murray-Thomas T, Jones ME, Patel D, Brunner E, Shatapathy CC, Motsko S, Van Staa TP (2013) Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: A study with the general practice research database. Cardiovasc Psychiatry Neurol 2013, 247486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Jones ME, Campbell G, Patel D, Brunner E, Shatapathy CC, Murray-Thomas T, van Staa TP, Motsko S (2013) Risk of mortality (including sudden cardiac death) and major cardiovascular events in users of olanzapine and other antipsychotics: A study with the general practice research database. Cardiovasc Psychiatry Neurol 2013, 647476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Bloechliger M, Rüegg S, Jick SS, Meier CR, Bodmer M (2015) Antipsychotic drug use and the risk of seizures: Follow-up study with a nested case–control analysis. CNS Drugs 29, 591–603. [DOI] [PubMed] [Google Scholar]
- [39]. De Deyn PP, Drenth AFJ, Kremer BP, Oude Voshaar RC, Van Dam D (2013) Aripiprazole in the treatment of Alzheimer’s disease. Expert Opin Pharmacother 14, 459–474. [DOI] [PubMed] [Google Scholar]
- [40]. Dennis M, Shine L, John A, Marchant A, McGregor J, Lyons RA, Brophy S (2017) Risk of Adverse outcomes for older people with dementia prescribed antipsychotic medication: A population based e-cohort study. Neurol Ther 6, 57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Gardette V, Lapeyre-Mestre M, Coley N, Cantet C, Montastruc JL, Vellas B, Andrieu S (2012) Antipsychotic use and mortality risk in community-dwelling Alzheimer’s disease patients: Evidence for a role of dementia severity. Curr Alzheimer Res 9, 1106–1116. [DOI] [PubMed] [Google Scholar]
- [42]. Langballe EM, Engdahl B, Nordeng H, Ballard C, Aarsland D, Selbæk G (2014) Short- and long-term mortality risk associated with the use of antipsychotics among 26,940 dementia outpatients: A population-based study. Am J Geriatr Psychiatry 22, 321–331. [DOI] [PubMed] [Google Scholar]
- [43]. Jennum P, Baandrup L, Ibsen R, Kjellberg J (2015) Increased all-cause mortality with use of psychotropic medication in dementia patients and controls: A population-based register study. Eur Neuropsychopharmacol 25, 1906–1913. [DOI] [PubMed] [Google Scholar]
- [44]. Nielsen RE, Lolk A, Valentin JB, Andersen K (2016) Cumulative dosages of antipsychotic drugs are associated with increased mortality rate in patients with Alzheimer’s dementia. Acta Psychiatr Scand 134, 314–320. [DOI] [PubMed] [Google Scholar]
- [45]. Gisev N, Hartikainen S, Chen TF, Korhonen M, Bell JS (2012) Effect of comorbidity on the risk of death associated with antipsychotic use among community-dwelling older adults. Int Psychogeriatr 24, 1058–1064. [DOI] [PubMed] [Google Scholar]
- [46]. Koponen M, Taipale H, Tanskanen A, Tolppanen AM, Tiihonen J, Ahonen R, Hartikainen S (2015) Long-term use of antipsychotics among community-dwelling persons with Alzheimers disease: A nationwide register-based study. Eur Neuropsychopharmacol 25, 1706–1713. [DOI] [PubMed] [Google Scholar]
- [47]. Arai H, Nakamura Y, Taguchi M, Kobayashi H, Yamauchi K, Schneider LS (2016) Mortality risk in current and new antipsychotic Alzheimer’s disease users: Large scale Japanese study. Alzheimers Dement 12, 823–830. [DOI] [PubMed] [Google Scholar]
- [48]. Musicco M, Palmer K, Russo A, Caltagirone C, Adorni F, Pettenati C, Bisanti L (2011) Association between prescription of conventional or atypical antipsychotic drugs and mortality in older persons with Alzheimer’s disease. Dement Geriatr Cogn Disord 31, 218–224. [DOI] [PubMed] [Google Scholar]
- [49]. Piersanti M, Capannolo M, Turchetti M, Serroni N, De Berardis D, Evangelista P, Costantini P, Orsini A, Rossi A, Maggio R (2014) Increase in mortality rate in patients with dementia treated with atypical antipsychotics: A cohort study in outpatients in Central Italy. Riv Psichiatr 49, 34–40. [DOI] [PubMed] [Google Scholar]
- [50]. Chiesa D, Marengoni A, Nobili A, Tettamanti M, Pasina L, Franchi C, Djade CD, Corrao S, Salerno F, Marcucci M, Romanelli G, Mannucci PM, Investigators R (2017) Antipsychotic prescription and mortality in hospitalized older persons. Psychogeriatrics 17, 397–405. [DOI] [PubMed] [Google Scholar]
- [51]. Connors MH, Ames D, Boundy K, Clarnette R, Kurrle S, Mander A, Ward J, Woodward M, Brodaty H (2016) Predictors of mortality in dementia: The PRIME Study. J Alzheimers Dis 52, 967–974. [DOI] [PubMed] [Google Scholar]
- [52]. Ray WA, Chung CP, Murray KT, Hall K, Stein CM (2009) Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 360, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Rossom RC, Rector TS, Lederle FA, Dysken MW (2010) Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? J Am Geriatr Soc 58, 1027–1034. [DOI] [PubMed] [Google Scholar]
- [54]. Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, Kales HC (2015) Antipsychotics, other psychotropics, and the risk of death in patients with dementia. JAMA Psychiatry 72, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Maust DT, Schneider LS, Kales HC (2015) Estimating mortality associated with antipsychotics and other psychotropics—reply. JAMA Psychiatry 72, 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Simoni-Wastila L, Wei YJ, Lucas JA, Brandt N, Moyo P, Huang TY, Franey CS, Harris I (2016) Mortality risk of antipsychotic dose and duration in nursing home residents with chronic or acute indications. J Am Geriatr Soc 64, 973–980. [DOI] [PubMed] [Google Scholar]
- [57]. Chan TC, Luk JK, Shea YF, Lau KH, Chan FH, Yu GK, Chu LW (2011) Continuous use of antipsychotics and its association with mortality and hospitalization in institutionalized Chinese older adults: An 18-month prospective cohort study. Int Psychogeriatr 23, 1640–1648. [DOI] [PubMed] [Google Scholar]
- [58]. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2008) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61, 991–996. [DOI] [PubMed] [Google Scholar]
- [59]. Nasrallah HA (2013) Haloperidol clearly is neurotoxic. Should it be banned? Curr Psychiatry 12, 7–8. [Google Scholar]
- [60]. Raivio MM, Laurila JV, Strandberg TE, Tilvis RS, Pitkala KH (2007) Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: A two-year prospective study. Am J Geriatr Psychiatry 15, 416–424. [DOI] [PubMed] [Google Scholar]
- [61]. Suh GH, Shah A (2005) Effect of antipsychotics on mortality in elderly patients with dementia: A 1-year prospective study in a nursing home. Int Psychogeriatr 17, 429–441. [DOI] [PubMed] [Google Scholar]
- [62]. Lopez OL, Becker JT, Chang YF, Sweet RA, Aizenstein H, Snitz B, Saxton J, McDade E, Kamboh MI, DeKosky ST, Reynolds CF 3rd, Klunk WE (2013) The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry 170, 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Simoni-Wastila L, Ryder PT, Qian J, Zuckerman IH, Shaffer T, Zhao L (2009) Association of antipsychotic use with hospital events and mortality among medicare beneficiaries residing in long-term care facilities. Am J Geriatr Psychiatry 17, 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Selbaek G, Aarsland D, Ballard C, Engedal K, Langballe EM, Benth JS, Bergh S (2016) Antipsychotic drug use is not associated with long-term mortality risk in Norwegian nursing home patients. J Am Med Dir Assoc 17, 464 e461–467. [DOI] [PubMed] [Google Scholar]
- [65]. Luijendijk HJ, de Bruin NC, Hulshof TA, Koolman X (2016) Terminal illness and the increased mortality risk of conventional antipsychotics in observational studies: A systematic review. Pharmacoepidemiol Drug Saf 25, 113–122. [DOI] [PubMed] [Google Scholar]
- [66]. Hulshof TA, Zuidema SU, Ostelo RW, Luijendijk HJ (2015) The mortality risk of conventional antipsychotics in elderly patients: A systematic review and meta-analysis of randomized placebo-controlled trials. J Am Med Dir Assoc 16, 817–824. [DOI] [PubMed] [Google Scholar]
- [67]. Brannstrom J, Bostrom G, Rosendahl E, Nordstrom P, Littbrand H, Lovheim H, Gustafson Y (2017) Psychotropic drug use and mortality in old people with dementia: Investigating sex differences. BMC Pharmacol Toxicol 18, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Jackson JW, Schneeweiss S, VanderWeele TJ, Blacker D (2014) Quantifying the role of adverse events in the mortality difference between first and second-generation antipsychotics in older adults: Systematic review and meta-synthesis. PLoS One 9, e105376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Jackson JW, VanderWeele TJ, Viswanathan A, Blacker D, Schneeweiss S (2014) The explanatory role of stroke as a mediator of the mortality risk difference between older adults who initiate first- versus second-generation antipsychotic drugs. Am J Epidemiol 180, 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Shin JY, Choi NK, Lee J, Park MJ, Lee SH, Park BJ (2015) A comparison of risperidone and haloperidol for the risk of ischemic stroke in the elderly: A propensity score-matched cohort analysis. J Psychopharmacol 29, 903–909. [DOI] [PubMed] [Google Scholar]
- [71]. Wang LJ, Lee SY, Yuan SS, Yang KC, Yang CJ, Lee TL, Shyu YC (2016) Risk of mortality among patients treated with antipsychotic medications: A nationwide population-based study in Taiwan. J Clin Psychopharmacol 36, 9–17. [DOI] [PubMed] [Google Scholar]
- [72]. Zagozdzon P, Goyke B, Wrotkowska M (2016) Mortality rates in users of typical and atypical antipsychotics: A database study in Poland. Drugs Real World Outcomes 3, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, Thapa-Chhetri N, Fornaro M, Gallicchio D, Collantoni E, Pigato G, Favaro A, Monaco F, Kohler C, Vancampfort D, Ward PB, Gaughran F, Carvalho AF, Stubbs B (2017) Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 16, 163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Enger C, Weatherby L, Reynolds RF, Glasser DB, Walker AM (2004) Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis 192, 19–27. [DOI] [PubMed] [Google Scholar]
- [75]. Vilalta-Franch J, Calvo-Perxas L, Garre-Olmo J, Turro-Garriga O, Lopez-Pousa S (2013) Apathy syndrome in Alzheimer’s disease epidemiology: Prevalence, incidence, persistence, and risk and mortality factors. J Alzheimers Dis 33, 535–543. [DOI] [PubMed] [Google Scholar]
- [76]. Farrell B, Tsang C, Raman-Wilms L, Irving H, Conklin J, Pottie K (2015) What are priorities for deprescribing for elderly patients? Capturing the voice of practitioners: A modified Delphi process. PLOS One 10, e0122246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Taipale H, Koponen M, Tanskanen A, Tolppanen AM, Tiihonen J, Hartikainen S (2014) High prevalence of psychotropic drug use among persons with and without Alzheimer’s disease in Finnish nationwide cohort. Eur Neuropsychopharmacol 24, 1729–1737. [DOI] [PubMed] [Google Scholar]
- [78]. Hughes LD, Cochrane L, McMurdo ME, Guthrie B (2016) Psychoactive prescribing for older people–what difference does 15 years make? Int J Geriatr Psychiatry 31, 49–57. [DOI] [PubMed] [Google Scholar]
- [79]. Lucas JA, Bowblis JR (2017) CMS strategies to reduce antipsychotic drug use in nursing home patients with dementia show some progress. Health Aff (Millwood) 36, 1299–1308. [DOI] [PubMed] [Google Scholar]
- [80]. Laver K, Cumming RG, Dyer SM, Agar MR, Anstey KJ, Beattie E, Brodaty H, Broe T, Clemson L, Crotty M, Dietz M, Draper BM, Flicker L, Friel M, Heuzenroeder LM, Koch S, Kurrle S, Nay R, Pond CD, Thompson J, Santalucia Y, Whitehead C, Yates MW (2016) Clinical practice guidelines for dementia in Australia. Med J Aust 204, 191–193. [DOI] [PubMed] [Google Scholar]
- [81]. Yohanna D, Cifu AS (2017) Antipsychotics to treat agitation or psychosis in patients with dementia. JAMA 318, 1057–1058. [DOI] [PubMed] [Google Scholar]
- [82]. Berling I, Buckley NA, Isbister GK (2016) The antipsychotic story: Changes in prescriptions and overdose without better safety. Br J Clin Pharmacol 82, 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Brett J, Karanges EA, Daniels B, Buckley NA, Schneider C, Nassir A, Zoega H, McLachlan AJ, Pearson SA (2017) Psychotropic medication use in Australia, 2007 to 2015: Changes in annual incidence, prevalence and treatment exposure. Aust N Z J Psychiatry 51, 990–999. [DOI] [PubMed] [Google Scholar]
- [84]. Ralph SJ, Espinet AJ (2017) Use of antipsychotics and benzodiazepines for dementia: Time for action? What will be required before global de-prescribing? Dementia (London) 10.1177/1367549417708437 [DOI] [PubMed] [Google Scholar]
- [85]. Tjia J, Hunnicutt JN, Herndon L, Blanks CR, Lapane KL, Wehry S (2017) Association of a communication training program with use of antipsychotics in nursing homes. JAMA Intern Med 177, 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD (2016) The feasibility and effect of deprescribing in older adults on mortality and health: A systematic review and meta-analysis. Br J Clin Pharmacol 82, 583–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Bain KT, Schwartz EJ, Chan-Ting R (2017) Reducing off-label antipsychotic use in older community-dwelling adults with dementia: A narrative review. J Am Osteopath Assoc 117, 441–450. [DOI] [PubMed] [Google Scholar]
- [88]. Declercq T, Petrovic M, Azermai M, Vander Stichele R, De Sutter AI, van Driel ML, Christiaens T (2013) Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev, CD007726. [DOI] [PubMed] [Google Scholar]
- [89]. Potter K, Flicker L, Page A, Etherton-Beer C (2016) Deprescribing in frail older people: A randomised controlled trial. PLoS One 11, e0149984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, Gill R, Juszczak E, Yu L-M, Jacoby R (2009) The dementia antipsychotic withdrawal trial (DART-AD): Long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol 8, 151–157. [DOI] [PubMed] [Google Scholar]
- [91]. Ballard C, Creese B, Corbett A, Aarsland D (2011) Atypical antipsychotics for the treatment of behavioral and psychological symptoms in dementia, with a particular focus on longer term outcomes and mortality. Expert Opin Drug Saf 10, 35–43. [DOI] [PubMed] [Google Scholar]
- [92]. Pitkala KH, Juola AL, Hosia H, Teramura-Gronblad M, Soini H, Savikko N, Bell JS (2015) Eight-year trends in the use of opioids, other analgesics, and psychotropic medications among institutionalized older people in Finland. J Am Med Dir Assoc 16, 973–978. [DOI] [PubMed] [Google Scholar]
- [93]. Jensen-Dahm C, Gasse C, Astrup A, Mortensen PB, Waldemar G (2015) Frequent use of opioids in patients with dementia and nursing home residents: A study of the entire elderly population of Denmark. Alzheimers Dement 11, 691–699. [DOI] [PubMed] [Google Scholar]
- [94]. Dublin S, Walker RL, Gray SL, Hubbard RA, Anderson ML, Yu O, Crane PK, Larson EB (2015) Prescription opioids and risk of dementia or cognitive decline: A prospective cohort study. J Am Geriatr Soc 63, 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].World Health Organization (WHO), Elder Abuse, Department of Ageing and Life-Course, WHO, http://www.who.int/ageing/projects/elder_abuse/en/, Accessed 21/06-2016.
- [96]. Dong X (2015) Screening for elder abuse in healthcare settings: Why should we care, and is it a missed quality indicator? J Am Geriatr Soc 63, 1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Alzheimer’s Australia (2016), Submission to the Australian Law Reform Commission (ALRC). Response to the ALRC issues paper: Elder Abuse, https://www.fightdementia.org.au/files/NATIONAL/documents/AA-Submission-to-ALRC-Elder-Abuse-Inquiry.pdf, Accessed 08/09-2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.