Abstract
Endogenous circadian clocks regulate 24-h rhythms of physiology and behavior. Circadian rhythm disruption (CRD) is suggested as a risk factor for inflammatory bowel disease. However, the underlying molecular mechanisms remain unknown. Intestinal biopsies from Per1/2 mutant and wild-type (WT) mice were investigated by electron microscopy, immunohistochemistry, and bromodeoxyuridine pulse–chase experiments. TNF-α was injected intraperitoneally, with or without necrostatin-1, into Per1/2 mice or rhythmic and externally desynchronized WT mice to study intestinal epithelial cell death. Experimental chronic colitis was induced by oral administration of dextran sodium sulfate. In vitro, caspase activity was assayed in Per1/2-specific small interfering RNA–transfected cells. Wee1 was overexpressed to study antiapoptosis and the cell cycle. Genetic ablation of circadian clock function or environmental CRD in mice increased susceptibility to severe intestinal inflammation and epithelial dysregulation, accompanied by excessive necroptotic cell death and a reduced number of secretory epithelial cells. Receptor-interacting serine/threonine-protein kinase (RIP)-3-mediated intestinal necroptosis was linked to increased mitotic cell cycle arrest via Per1/2-controlled Wee1, resulting in increased antiapoptosis via cellular inhibitor of apoptosis-2. Together, our data suggest that circadian rhythm stability is pivotal for the maintenance of mucosal barrier function. CRD increases intestinal necroptosis, thus rendering the gut epithelium more susceptible to inflammatory processes.—Pagel, R., Bär, F., Schröder, T., Sünderhauf, A., Künstner, A., Ibrahim, S. M., Autenrieth, S. E., Kalies, K., König, P., Tsang, A. H., Bettenworth, D., Divanovic, S., Lehnert, H., Fellermann, K., Oster, H., Derer, S., Sina, C. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine.
Keywords: epithelial cells, necroptosis, inflammatory bowel disease
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis, is a chronic inflammatory disorder with an etiology that is not yet fully resolved. Various environmental triggers are thought to initiate and propagate mucosal inflammation in genetically susceptible individuals (1, 2). Sleep disruption and chronic fatigue are among the major complaints associated with IBD (3–7), and it has been suggested that these symptoms result from perturbations of the circadian timing system, as is widely present in modern 24-h societies (8). Circadian rhythms (CRs) are self-sustained ∼24 h oscillations in physiologic and behavioral processes controlled by endogenous clocks that are entrained by environmental cues such as the light–dark (LD) cycle or food intake (9). In mammals, a master clock localized in the suprachiasmatic nucleus of the hypothalamus synchronizes cellular clocks in other central nervous and peripheral tissues with each other and with external time. At the molecular level, these clocks are based on an interregulatory network of clock genes, including the 3 Period genes (Per1–3), that control CRs by rhythmic orchestration of 5–10% of the cellular transcriptome in a tissue-specific manner (10). In the gut, emerging evidence suggests an important role for circadian clocks in regulating digestive physiology and maintaining intestinal barrier function (11–13). Previous studies demonstrated that TLRs of the innate immune system, which are important in the detection of pathogens, are rhythmically expressed in the intestine to enable the coordination of antimicrobial responses with the cell cycle in intestinal epithelial cells (IECs) (14). Furthermore, the expression of defensins (12) or proteins that control IEC proliferation and migration (15–18), digestion, and absorption of nutrients (19–21), as well as gastrointestinal motility (22–24), has been found to be regulated in a CR-dependent manner. In line with this finding, repetitive phase shifts of the LD cycle or sleep restriction conditions render mice more susceptible to experimental colitis (25, 26). Moreover, a polymorphism of the core clock gene Per3 was recently found to be associated with increased susceptibility to and disease severity in patients with CD (27). Furthermore, Per1 and -3 mRNA levels were decreased in colonic biopsies from patients with IBD (28), again suggesting the existence of disturbed CRs in IBD. However, the molecular mechanisms linking CR and intestinal inflammation are still unknown. To address this question, we investigated Per1/2 double-mutant (Per1/2) mice, carrying a genetic ablation of the molecular circadian clock (29) under physiologic and inflammatory conditions. Per1/2 double-mutant mice carry a homozygous null mutation in the clock genes Per1 and -2. They exhibit a circadian activity profile that is not distinguishable from wild-type (WT) mice under a regular 12-h LD cycle. If, however, these animals are exposed to constant darkness, they show an immediate and complete loss of this rhythmic activity and become arrhythmic. WT animals, on the other hand, retain their circadian activity profile, even in the dark, with only a shorter period length (29, 30). As a second model system, we used externally desynchronized wild-type mice.
Our data reveal a molecular mechanism by which CRD impairs intestinal barrier function through the induction of necroptosis in proliferating IECs, resulting in self-sustained severe intestinal inflammation.
MATERIALS AND METHODS
Animal models and treatment
Experiments were conducted with circadian clock-deficient Per1Brd1/Per2Brd1 double-mutant (Per1/2) mice, generated from Per1 (Per1Brd1) (29) and Per2 (Per2Brd1) (31) mutant mice (both backcrossed to C57BL/6 for at least 10 generations), and C57BL/6J congenic WT controls. Mice were housed in a regular 12-h LD (rhythmic) cycle in standard conditions and were provided food and water ad libitum. Mice were either treated with recombinant murine TNF-α (ImmunoTools, Friesoythe, Germany; 200 ng/g body weight, i.p.) in the presence or absence of the necroptosis inhibitor necrostatin 1 (Nec-1; Enzo Life Sciences, Lörrach, Germany; intraperitoneal injection; 1.65 µg/g body weight) or were left untreated. All experiments were performed in accordance with the animal care guidelines of the University of Lübeck and after evaluation of the protocol ethics by the state’s Animal Welfare Committee [V 312-72241.122-1 (29-3/11)]. Procedures involving animals and their care were conducted in accordance with national and international laws and regulations.
Desynchronization of CRs
To externally disrupt the circadian clock, WT mice were kept individually under 400 lux constant-light conditions (arrhythmic) for 2–4 wk. Activity levels were determined by using the ClockLab software package (Actimetrics, Evanston, IL, USA) to monitor the usage of running wheels installed in each cage. Mice were regarded as desynchronized when χ2 periodogram analysis of running-wheel activity over 5 consecutive days revealed no significant rhythmicity in the circadian range (period, 20–28 h). Singly housed animals kept under LD conditions with running wheels were used as controls.
Induction of colitis and determination of clinical scores
Experimental chronic colitis was induced in mice aged 12–14 wk by administration of 2% dextran sodium sulfate [Batch DB001-27; dextran sodium sulfate (DSS), molecular mass 40 kDa; TdB Consultancy, Uppsala, Sweden] dissolved in the drinking water for 5 d, followed by 5 d of normal drinking water, repeated for a total of 3 cycles. Control mice received water without DSS. Mice were euthanized on d 17 and 30 of the experiment and clinical parameters were assessed. All animals were euthanized between Zeitgeber time 4 and 6. High-resolution mouse video endoscopy was used (Hopkins Optik 64019BA; Aida Vet; Karl Storz, Tuttlingen, Germany) to obtain the murine endoscopic index of colitis severity (MEICS) (32).
Bromodeoxyuridine incorporation experiment
Bromodeoxyuridine (BrdU) pulse–chase experiments were performed according to the manufacturer’s instructions (BrdU Labeling and Detection Kit I; Roche, Mannheim, Germany). In brief, BrdU was intraperitoneally injected (10 μl/g body weight) into control and Per1/2 mice (n = 6 each). Three mice were euthanized at any one time after 2 and 24 h, and incorporated BrdU was detected on colonic and ileal tissue biopsies.
Histologic evaluation of intestinal mouse tissue
Immunohistochemical and immunofluorescence techniques were performed according to standard protocols, and histologic scores were determined (33). In brief, deparaffinized tissue sections were fixed and stained with hematoxylin-eosin (HE), periodic acid-Schiff, Alcian blue, or rat anti-mouse Ki-67 (1:200; BioLegend, San Diego, CA, USA). TUNEL staining was performed on frozen sections using a commercially available kit (S7110, ApopTag Fluorescein In Situ Apoptosis Detection Kit; Chemicon, Billerica, MA, USA) according to the manufacturer’s instructions. Semithin sections were stained with methylene blue Azur II according to standard procedures.
Cryosectioning and immunogold labeling
Cryosectioning and immunogold labeling of ultrathin sections were performed according to the postembedding technique of Griffiths (34). Ileal biopsies from Per1/2 mice were fixed in 5% paraformaldehyde/0.2 M piperazine-N,N′-bis(2-ethanesulfonic acid) buffer, cryoprotected in 3% polyvinylpyrrolidone/1.6 M saccharose, and frozen in liquid nitrogen. Ultrathin cryosections (60–80 nm) were incubated with peptide affinity-purified rabbit anti-mouse receptor-interacting serine/threonine-protein kinase (RIP)-3 (1:200; Enzo Life Sciences) and gold-conjugated goat anti-rabbit secondary antibody for 45 min each. Labeled sections were contrasted with 4% uranyl acetate, embedded in 2% methylcellulose, and examined with a transmission electron microscope (model 1011; Jeol, Tokyo, Japan).
Small interfering RNA transfection
The mouse small intestine cell line IEC-1 (35) or the human Lenti-X 293T cell line was seeded into 6-well plates at a density of 2 × 105 cells per well in DMEM containing 10% fetal calf serum, with 1% l-glutamine and 50 μM 2-ME, in the case of IEC-1. The next day, the cells were transfected with either 25 nM of each murine small interfering RNA (siRNA) (Per1: s71484, Per2: s71485), 25 nM of each human siRNA (Per1: s10302, Per2: s16930), or 50 nM of negative control siRNA (4390844), for 48 h with the Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific, Darmstadt, Germany) according to the manufacturer’s instructions.
Cell cycle analysis
Lenti-X 293T cells were transfected with control siRNA or Per1/2-specific siRNAs for 72 h as previously described. Next, the cells were harvested and washed with ice-cold 1× PBS, fixed in absolute ethanol, and stored at −20°C. Afterward, the cells were washed with ice-cold 1× PBS, and DNA was stained with 7AAD (BD Biosciences, San Jose, CA, USA). Samples were analyzed on a flow cytometer (BD Accuri C6; BD Biosciences).
Overexpression of Wee1
Lenti-X 293T cells were seeded at a density of 2 × 105/well into a 6-well plate. On the next day, cells were transfected for 24 or 72 h with pCMV6-Entry vector (catalog no. PS100001; OriGene Technologies, Rockville, MD, USA) or pCMV6-Entry-Wee1 vector (RC209760; OriGene Technologies) by lipofection with Lipofectamine 2000, according to the manufacturer’s instructions. Expression of human Wee1 was analyzed by Western blot experiments.
Caspase-Glo assays
RNAi-treated IEC-1 cells were harvested, seeded into 96-well plates, and incubated for an additional 24 h. After incubation, the cells were stimulated with various concentrations (0–100 ng/ml) of recombinant murine TNF-α (R&D Systems, Wiesbaden, Germany), the Smac mimetic BV-6 (1 mM; Selleckchem, Munich, Germany) or combinations of both for 4 h. Measurements of caspase 3 and 7 or caspase 8 activity using commercially available kits (Caspase-Glo 3/7 Assay, Caspase-Glo 8 Assay; both Promega, Mannheim, Germany) were performed according to the manufacturer’s instructions on the Infinite M200 luminescence reader, the software i-control (v.1.9.17.0; all Tecan, Männedorf, Switzerland) and an integration time of 1000 ms.
RNA extraction and real-time quantitative PCR
RNA was extracted with the innuPREP RNA mini kit (Analytik Jena AG, Jena, Germany) and transcribed to cDNA (RevertAid H Minus reverse transcriptase; Thermo Fisher Scientific) using the T Gradient thermocycler (Whatman Biometra, Göttingen, Germany). Real-time quantitative PCR (qPCR) was performed with 10 μl of MaximaR SYBR Green qPCR Master Mix, plus 0.1 μl of each oligonucleotide, in a 96-well plate format. The amplification program consisted of: 1) preincubation at 95°C for 5 min; 2) 40 cycles of denaturation at 95°C for 45 s and annealing at 60°C for 1 min on the ABI Prism 7000 system (Thermo Fisher Scientific). Melting curve profiles were produced to ensure product specificity. Expression levels were normalized to β-actin following the ΔΔCt algorithm. The following oligonucleotides were used for murine cDNA samples β-actin: (forward) 5′-GATGCTCCCCGGGCTGTATT-3′, (reverse) 3′-GGGGTACTTCAGGGTCAGGA-5′; Muc2: (forward) 5′-GCTGACGAGTGGTTGGTGAATG-3′, (reverse) 3′-GATGAGGTGGCAGACAGGAGAC-5′; Lyz1: (forward) 5′-GCCAAGGTCTACAATCGTTGTGAGTTG-3′, (reverse) 3′-CAGTCAGCCAGCTTGACACCACG-5′; Lgr5: (forward) 5′-CGGCAACAGTGTGGACGACCT-3′, (reverse) 3′-GCGAGCACTGCACCGAGTGA-5′; ChrB: (forward) 5′-CCCGCTGGCTGAACTTTTC-3′, (reverse) 3′-GAGTTCTGACGGCGGAAGAG-5′; Wee1: (forward) 5′-GGTGTCGTGGGAGAAAGAGA-3′, (reverse) 3′- TCTGCTCATCAACAGAGCCA-5′; CIAP-2: (forward) 5′-CGAGGAGGAGGAGTCAGATG-3′, (reverse) 3′-GGAGGCAATACAGCATTGGT-5′; RIP3: (forward) 5-AGCTTTGGGATCCTCGTGTG-3′, (reverse) 3′-TGTCAGTGGAGGACGACTCT-5′; KC: (forward) 5′-GCTGGGATTCACCTCAAGAA-3′, (reverse) 3′- TGGGGACACCTTTTAGCATC-5′; IL-6: (forward) 5′- CTCCCAACAGACCTGTCTATAC-3′, (reverse) 3′-GTGCATCATCGTTGTTCATAC-5′; IFN-γ: (forward) 5′- GCAAGGCGAAAAAGGATGC-3′, (reverse) 3′-GCTTCCTGAGGCTGGATTC-5′; Per1: (forward) 5′-TGGCTCAAGTGGCAATGAGTC-3′, (reverse) 3′-GGCTCGAGCTGACTGTTCACT-5′; Per2: (forward) 5′-GCCAAGTTTGTGGAGTTCCTG-3′, (reverse) 3′-CTTGCACCTTGACCAGGTAGG-5′).
Immunoblotting
Protein levels from murine intestinal punch biopsies or Lenti-X 293T cells were determined by Western blot analysis according to standard procedures. In brief, protein lysates were separated by SDS-PAGE (Criterion TGX Precast Gel 4–15%; Bio-Rad, München, Germany), transferred to PVDF or nitrocellulose membranes using the Trans-Blot Turbo Transfer System (Bio-Rad), and incubated with antibodies against receptor-interacting serine/threonine-protein kinase (RIP)-3 (Enzo Life Sciences), cleaved Caspase 3, phosphorylated AKT, AKT, phosphorylated GSK3β, GSK3β, human Wee1 (all from Cell Signaling Technology, Danvers, MA, USA), RIP1, Lysozyme (both from Santa Cruz Biotechnologies, Santa Cruz, CA, USA), and β-actin (New England Biolabs, Ipswich, MA, USA). Horseradish peroxidase–conjugated secondary antibodies were used accordingly.
Intestinal microbiota analysis
DNA extraction, 16s rRNA PCR, and gene sequencing
DNA was extracted from frozen fecal samples with the PowerSoil DNA Isolation kit (MoBio, Vancouver, BC, Canada) (36). The hypervariable regions V1 and V2 of the 16s rRNA genes were PCR amplified with the forward primer 27F 5′-AATGATACGGCGACCACCGAGATCTACACXXXXXXXXTATGGTAATTGTAGAGTTTGATCCTGGCTCAG-3′; in italics the 5′ adaptor B (Illumina, Santa Clara, CA, USA), the 8 X’s represent unique indices; bold, the primer pad, bold and italics, the primer linker, and the underscored residues, the primer 27F. For the reverse primer 338R 5′-CAAGCAGAAGACGGCATACGAGATXXXXXXXX AGTCAGTCAGCCTGCTGCCTCCCGTAGGAGT: in italics the 3′ reverse complement sequence of the Illumina adaptor; the 8 X’s represent the unique indices; bold, the primer pad; bold and italics, the primer linker; and the underscored residues, the primer 338R. Forward and reverse primers were tagged with 16 and 24 unique indices, respectively. PCR reactions (20 µl), containing 1 µl 1:10 diluted template DNA, were performed by using Phusion Hot Start DNA Polymerase (Thermo Fisher Scientific), with the following cycling conditions: 30 s at 98°C, followed by 30 cycles of 9 s at 98°C, 60 s at 55°C, 90 s at 72°C, and a final extension for 10 min at 72°C. PCR products were quantified on a gel via the Quantum ST4 System (Vilber Lourmat, Marne La Vallée, France), and 1 subpool per gel with PCR products of equimolar ratios was prepared. Subpools were applied on a gel, extracted with the GeneJet Gel Extraction Kit (Thermo Fisher Scientific) and quantified with the Quant-iT dsDNA BR Assay Kit on a Qubit fluorometer (Thermo Fisher Scientific). Subpools were again pooled in equimolar amounts and purified by Agencourt Ampure Beads (Beckman Coulter, Krefeld, Germany). The PerfeCTa NGS Library Quantification Kit (Quanta BioSciences, Beverly, MA, USA) was used for quantification of the final library, which was sequenced with the Illumina MiSeq system (paired-end reads, 2 × 300 base pairs).
Sequence processing
Raw reads were demultiplexed with Casava v.1.8.2 and merged with fastq_mergepairs from VSearch v.2.0.2 (37), with a maximum number of mismatches of 12 and the merged reads (contigs) between 270 and 330 bp long. Subsequently, contigs were quality filtered with the fastq_filter command (VSearch package) with the expected numbers of errors set to 0.5. Finally, chimeras were identified by uchime_ref (VSearch), with the RDP Gold database (v.9) as the reference database, and were removed from the contig set. In the chimera-free data set, unique sequences were identified (dereplication; VSearch), and the resulting sequences were clustered into OTUs using the UPARSE pipeline (USearch package v.8.1.1861) with 97% sequence identity (38).
Taxonomy was assigned to the genus level for the resulting contigs, with the Silva database v.123 and Mothur with 80% bootstrap support (39). Only contigs were kept that were confirmed as bacterial in origin, and these were aligned to the 16s rRNA V1-V2 region with the Silva database as reference. For each individual 15,000 contigs were randomly chosen to normalize for the different number of contigs between the individuals.
Statistical analysis
Alpha diversity was assessed with the Shannon index (vegan package v.2.2-1 54) and Faith’s phylogenetic distance (40) [picante package version 1.6.2 (41)]. Beta diversity was investigated with Bray-Curtis dissimilarity index (vegan package) and weighted UniFrac distance [phyloseq package v.1.18.1 (42)]. Principal coordinates analysis was performed with the cmdscale command (stats package for R). All statistical analyses were performed using R v.3.2, and if necessary, P values were corrected using the Benjamini-Hochberg correction.
Data were statistically analyzed with Prism 5.0 (GraphPad, La Jolla, CA, USA). Statistical significance was determined by 1- or 2-way repeated-measures ANOVA with the Bonferroni post hoc test or with the Mann-Whitney U test. The P values were calculated, and null hypotheses were rejected when P < 0.05.
RESULTS
Per1/2 mice display loss of Paneth and goblet cells
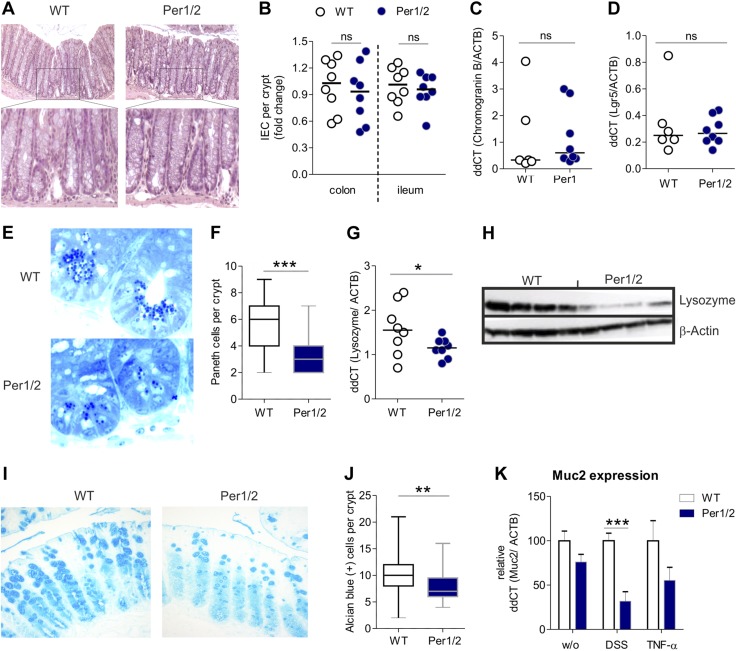
To understand the role of the circadian clock in mucosal barrier function, we analyzed the basal intestinal phenotype of circadian clock-deficient Per1/2 mice. Histologic analysis of HE-stained colonic biopsy samples revealed similar epithelial morphology in Per1/2 compared to WT mice (Fig. 1A). This observation was reflected by comparable total numbers of IECs per crypt throughout the colon and the ileum in both genotypes (Fig. 1B). Furthermore, no alterations in chromogranin B (CHGB) mRNA expression, as well as Lgr5 synthesis—as markers of enteroendocrine and intestinal stem cells, respectively—were observed in small intestinal tissue sections of Per1/2 mice (Fig. 1C, D). Detailed histologic analysis of the ileum and the colon, however, revealed decreased Paneth cell (PC) and goblet cell (GC) counts in Per1/2 compared with WT animals (Fig. 1E, F, I, J). This loss of secretory cells was accompanied by significantly decreased levels of lysozyme (LYZ) transcript and protein (Fig. 1G, H) and Muc2 mRNA levels under inflammatory conditions (DSS treatment; Fig. 1K). Together, these data indicate a selective reduction of barrier-protecting secretory epithelial cells in Per1/2 mice.
Figure 1.
Per1/2 mutant mice display loss of PCs and GCs. A) HE-stained colonic tissue samples from WT and Per1/2 mutant mice. Original magnification, ×20. B) Quantification of IECs in crypts of colonic and ileal tissues (n = 8 per strain). C, D) Chromogranin B (WT n = 6; Per1/2 n = 8) (C) and Lgr5 (WT n = 6; Per1/2 n = 8) (D) mRNA transcript levels were determined by qPCR using colonic biopsy specimens from untreated mice. E) Representative images of methylene blue Azur II–stained ileal semithin sections. F) Quantification of PCs. G, H) Ileal biopsy samples were analyzed by qPCR (n = 8 per strain) (G) or Western blot (H) to determine Lysozyme expression. I, J) Representative images of Alcian blue-stained colonic tissue (I) quantification of GCs (J). K) Muc2 mRNA transcript levels were determined by qPCR, with a colonic biopsy specimen from untreated, DSS-treated, and TNF-α-treated mice (WT, n = 8; Per1/2, n = 8; WT DSS, n = 4; Per1/2 DSS, n = 4; WT TNF-α, n = 7; and Per1/2 TNF-α, n = 7). All values are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001.
Per1/2 mice exhibit a more severe experimental chronic colitis
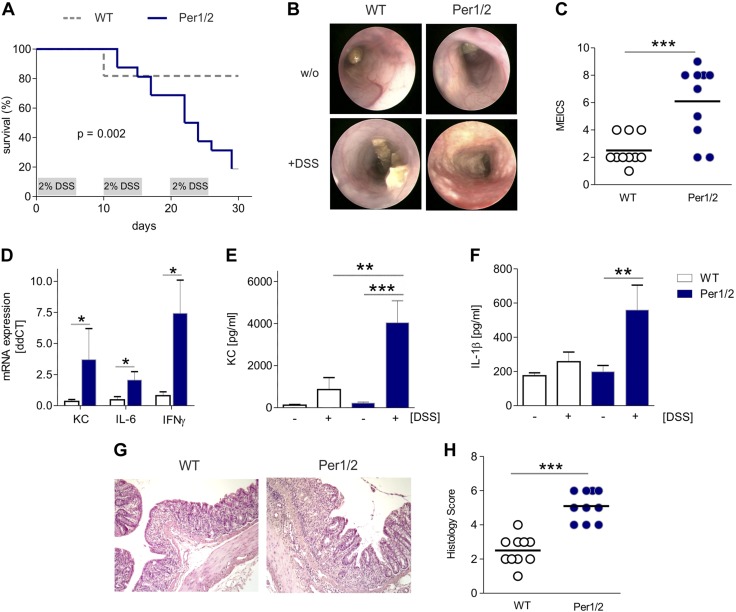
To study the impact of constituent loss of secretory epithelial cells in Per1/2 mice on severe intestinal inflammatory processes, we used an established model of experimental colitis (43, 44). Reminiscent of what was shown for period-deficient fruit flies before (45), Per1/2 mice showed an aggravated course of colitis upon DSS challenge, as reflected by augmented clinical symptoms and decreased survival rates compared with WT control animals (Fig. 2A). Endoscopic and histologic assessment at d 17 of the DSS experiment revealed increased signs of colitis severity. Specifically, Per1/2 mice exhibited higher levels of mucosal erosion, spontaneous bleeding, loose stools, and decreased colonic light transparency (Fig. 2B), resulting in a higher MEICS score (Fig. 2C), higher colonic mRNA transcript levels of keratinocyte chemoattractant (KC), proinflammatory IL-6 or IFN-γ (Fig. 2D) and increased signs of systemic inflammation as represented by serum KC (Fig. 2E) and IL-1β (Fig. 2F) levels. Histologic analysis of HE-stained colonic biopsies revealed massive intestinal infiltration of inflammatory immune cells, mucosal edema, erosions, and abscesses in Per1/2 mice, resulting in a higher overall histologic score (Fig. 2G, H).
Figure 2.
Per1/2 mutant mice display high susceptibility to experimental chronic colitis. A) Survival of WT controls (n = 11) and Per1/2 mice (n = 17) during induction of chronic DSS colitis. B, C) Representative colonoscopic images (B) and determination of MEICS scores (C; n = 10 per strain). D) KC, IL-6, or IFN-γ mRNA expression was assayed by qPCR using colonic biopsy samples from DSS-treated WT or Per1/2 mice. Data were normalized to β-actin levels. E) KC-specific ELISA with serum samples collected from DSS-treated or untreated mice (WT basal and Per1/2 basal n = 8, WT DSS n = 6, and Per1/2 DSS n = 5). F) IL-1β-specific ELISA was performed with serum samples from WT or Per1/2 mice treated with DSS or left untreated. G) Representative images of HE-stained colonic tissue from DSS-treated mice. Original magnification, ×10. H) Determination of histologic scores (n = 10 per strain). All values are means ± sem. **P ≤ 0.01, ***P ≤ 0.001.
Per1/2 deficiency impairs proliferation but increases cell death of IECs in hemicrypts
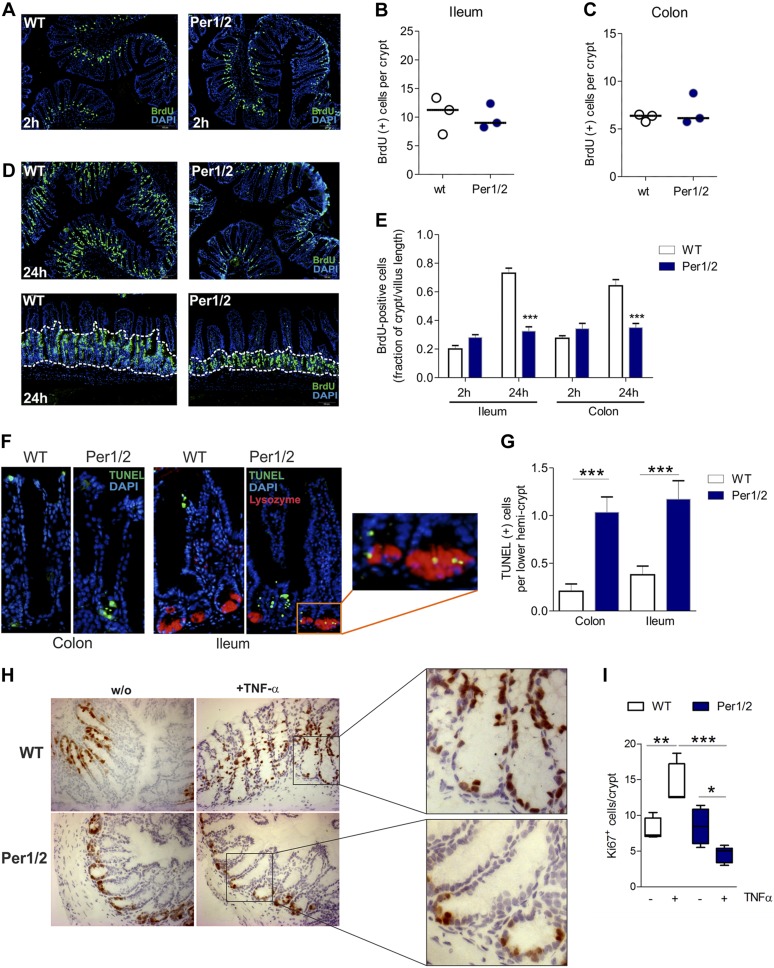
In Drosophila, period gene deficiency causes hypoproliferation of intestinal stem cells (45). A similar effect may explain the observed reduction of GCs and PCs in Per1/2 mice. To test this hypothesis in vivo, we determined cell proliferation rates by analyzing BrdU incorporation into DNA of proliferating IECs during the S phase of the cell cycle under basal conditions. However, at 2 h after BrdU injection, when BrdU was incorporated systemically, Per1/2 and WT mice displayed similar levels of BrdU incorporation into the small and large intestines (Fig. 3A–C). At 24 h after BrdU injection, though, Per1/2 mice displayed significantly lower amounts of BrdU+ cells in the transit-amplifying zones of the ileum and the colon (Fig. 3D, E), pointing to an efficient S-phase initiation, but impaired cell cycle progression in proliferating IECs.
Figure 3.
Per1/2 deficiency is accompanied by impaired proliferation and increased cell death in lower hemicrypts. A) Representative images of BrdU-labeled proliferating cells in colonic tissue after 2 h of pulsing. Original magnification, ×10. B, C) Quantification of BrdU+ epithelial cells in ileal (B) and colonic(C) crypts. Mean of 8 distinct tissue slide regions (n = 3 mice/strain). D) Representative images of BrdU-labeled proliferating cells in colonic tissue sections after 24 h of pulsing. Original magnification, ×10 (top); ×20 (bottom). E) Quantification of BrdU+ cells in ileal and colonic tissue specimens after 2 or 24 h of BrdU pulsing. Data are presented as the fraction of BrdU+ cells per crypts in relation to the villus length (n = 3 mice per strain and time point). F) Representative images of TUNEL+ cells in colonic and ileal tissue sections. Inset: colocalization of lysozyme and TUNEL in PCs. G) Quantification of positively labeled cells in colonic and ileal biopsies (n > 8 per strain). H) Representative images of Ki-67-stained cells in colonic tissues in baseline conditions or after TNF-α treatment of WT or Per1/2 mice. Original magnification, ×10, insets: lower crypts. I) Quantification of Ki67+ cells in colonic biopsies (n > 3 per strain). All values are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001.
Maintenance of intestinal tissue homeostasis is based on balanced proliferation, differentiation, and apoptosis of IECs (46). TUNEL analyses of ileal and colonic biopsies showed elevated DNA fragmentation in lower hemicrypts of Per1/2 vs. WT mice, whereas TUNEL+ cells in WT mice were exclusively located at the villus tips or crypt tops. In addition, in the small intestine, TUNEL+ cells were frequently observed in colocalization with lysozyme in mutant mice (Fig. 3F, G). These findings revealed increased cell death of IECs in lower hemicrypts of Per1/2 mice in basal conditions.
The TNF system has been demonstrated to control IEC shedding and intestinal barrier function (47). We challenged WT or Per1/2 mice with the proinflammatory cytokine TNF-α (Supplemental Fig. 1) to test for the ability of the system to respond to increased epithelial cell loss. No differences in colonic Ki-67 staining were detected between WT and Per1/2 mice in basal conditions (Fig. 3H, I). However, although TNF-α-treated WT mice displayed increased rates of migration and proliferation of IECs in colonic biopsy samples, a significant reduction in Ki67+ cells per crypt was detected in tissue sections from Per1/2 mice treated with TNF-α. Based on these findings, we hypothesized that Per1/2 deficiency in IECs may be associated with disturbed cell division rather than with impaired DNA replication in inflammatory conditions.
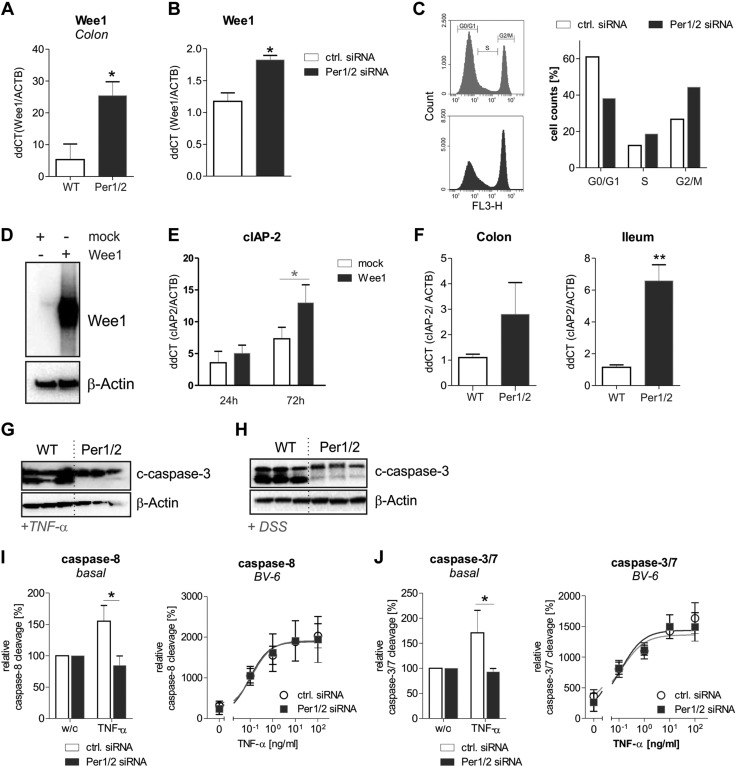
Previously, the circadian clock transcription factors CLOCK and BMAL1 were demonstrated to control the promoter activity of the cell cycle M-phase inhibitor protein kinase Wee1 (48). In line with these findings, we detected Wee1 mRNA levels to be significantly upregulated in colonic biopsies from Per1/2 compared to WT mice (Fig. 4A). Ex vivo data were verified by in vitro transfection of Lenti-X 293T cells with control or Per1/2-specific siRNAs (Supplemental Fig. 2A). A significant up-regulation of Wee1 mRNA levels was detected in cells transfected with Per1/2-specific siRNAs (Fig. 4B). Cell cycle analyses revealed a significant reduction in G0/G1 phase, an increase in the S phase and hence a G2/M-phase arrest of cells transfected with Per1/2-specific siRNAs compared to control siRNA transfected cells (Fig. 4C; Supplemental Fig. 3). Together, these results demonstrate that Per1/2 deficiency results in impaired cell division via Wee1 up-regulation.
Figure 4.
Per1/2 deficiency results in enhanced expression of the cell-cycle inhibitor Wee1 that promotes anti-apoptosis. A) Wee1 mRNA transcript levels were determined by qPCR using colonic tissue samples from WT or Per1/2 mice (n = 4 per strain). Wee1 mRNA levels were normalized to β-actin mRNA levels. B) Expression of human Wee1 mRNA was analyzed in Lenti-X 293T cells transfected for 72 h with control or human Per1/2-specific siRNAs. C) Cell cycle analysis was performed with Lenti-X 293 T cells transfected with control siRNA or Per1/2-specific siRNAs for 72 h, stained with 7AAD, and analyzed by flow cytometry. Data from 1 of 3 independent experiments are presented. D) Wee1 expression after transfection of Lenti-X 293T cells with mock or human Wee1 vectors for 72 h was assessed by Western blot experiments. E) CIAP-2 mRNA levels were quantified by qPCR after transfection of Lenti-X 293T cells with mock or human Wee1 vectors for 24 or 72 h. F) Transcript levels of antiapoptotic cIAP-2 were determined by qPCR of colonic and ileal tissue samples from WT or Per1/2 mice (n = 4 per strain). G, H) Representative Western blot for cleaved caspase-3 (c-caspase-3) of ileal (G) or colonic (H) biopsy samples from TNF-α- or DSS-treated mice, respectively. β-Actin served as a loading control. I, J) IEC-1 cells were transfected with Per1/2-specific siRNAs or a control siRNA. Afterward, the cells were stimulated with TNF-α (0.1 ng/ml under basal conditions) in the presence or absence of BV-6 or were left untreated. Activation of caspase-8 (I) and caspases 3/7 (J) was assayed. Data are presented as the means ± sem of at least 3 independent experiments. *P ≤ 0.05, **P ≤ 0.01.
Enhanced cIAP-2 expression and impaired apoptosis induction in Per1/2 mice
Based on our findings that Per1/2 mutant mice display enhanced cell death of IECs in the lower hemicrypts, and published data that show a marked up-regulation of the cellular inhibitor of apoptosis (cIAP)-2 during mitotic cell cycle arrest (49), we overexpressed Wee1 in Lenti-X 293T cells to investigate its impact on cIAP-2 mRNA expression (Fig. 4D). As expected, cIAP-2 mRNA levels were significantly up-regulated after long-term (72 h), but not after short-term (24 h) overexpression of Wee1 (Fig. 4E). In line with this result, we found increased mRNA expression of cIAP-2 in Per1/2 mutant colonic and ileal tissue sections (Fig. 4F). Increased cIAP-2 expression was accompanied by a reduced cleaved caspase 3 signal in ileal and colonic biopsies from TNF-α (Fig. 4G)- or DSS (Fig. 4H)-treated Per1/2 mice, respectively. No cleaved caspase 3 levels were observed in untreated WT or Per1/2 mice (data not shown). To more directly investigate the impact of Per1/2 deficiency on antiapoptotic processes in murine IECs, we performed siRNA-induced knockdown of Per1/2 in the IEC-1 cell line that was initially established from fetal mouse small intestinal tissue (35) (Supplemental Fig. 2B). TNF-α-triggered cleavage of caspase-8 (Fig. 4I) or caspase-3/7 (Fig. 4J) was analyzed in Per1/2 knockdown or control siRNA transfected IEC-1 cells in the presence or absence of BV-6, an inhibitor of IAP proteins. Although significant TNF-α-mediated activation of caspase-8 or -3/7 was induced in control siRNA-transfected IEC-1 cells, such effects were not detected in cells transfected with Per1/2-specific siRNAs. However, in the presence of BV-6 no differences in the activation of caspase-8 or -3/7 by TNF-α stimulation at increasing concentrations were detected between both lines. Together, cIAP-2-mediated antiapoptosis was increased in Per1/2-deficient IECs, resulting in impaired TNF-α-triggered activation of caspase-8 and downstream of caspase-3/7. Together, these data suggest that CRD triggers impaired cell division during cell proliferation via Wee1, resulting in up-regulation of antiapoptosis (via cIAP-2).
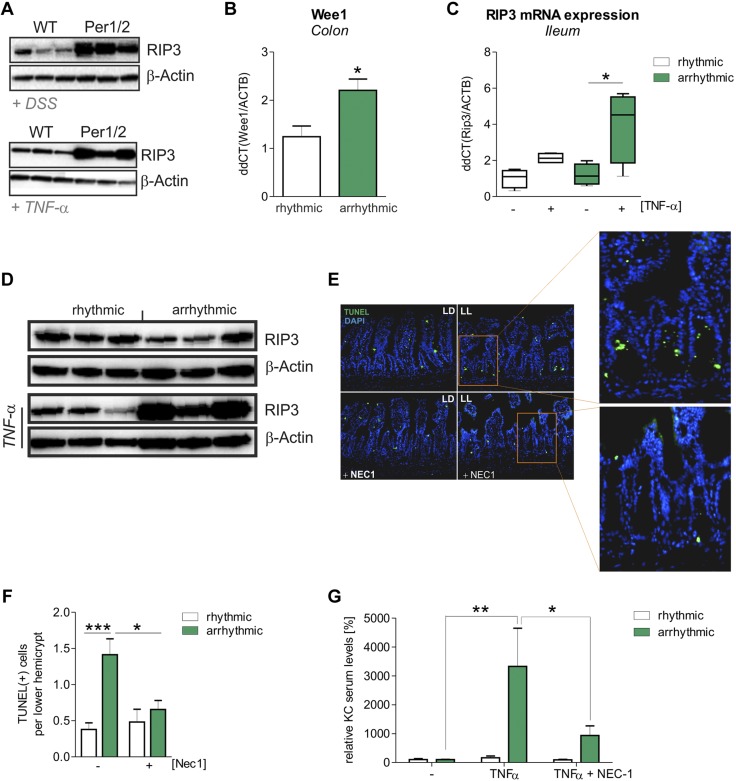
Mice with CRD display increased intestinal RIP3-dependent necroptosis
To study whether enhanced cell death of IECs at the lower hemicrypts of Per1/2 cells originates from necrosis or apoptosis, we looked for morphologic indicators of necroptotic cell death by ultrastructural analysis. In lower hemicrypts of Per1/2, but not of WT, mice we frequently observed cell morphologic characteristics of necroptosis, such as hypodense nucleo- and cytoplasm, together with enlarged mitochondria in the absence of cell membrane blebbing (50) (Supplemental Fig. 4A). In addition, slightly increased expression levels of the necroptosis mediator RIP3 were detected by Western blot analyses in colonic biopsies of Per1/2 mice in baseline conditions (Supplemental Fig. 4B), whereas strongly increased RIP3 expression was observed in colonic tissues from DSS-treated Per1/2 in comparison with WT mice. Similar results were obtained from ileal and colonic tissue samples after TNF-α treatment (Fig. 5A; Supplemental Fig. 5A). Furthermore, we found numerous amyloid-like plaques of small filamentous structure in PCs of Per1/2 mice. These plaques were not encased with double membranes and generally showed no morphologic characteristics of autophagosomes, but were positively labeled for RIP3 by immunogold staining (Supplemental Fig. 4C). RIP3+ filamentous structures are hallmarks of the so-called necrosome, an assembly of RIP1/RIP3 complexes mediating necroptosis (51). As CRD mice also displayed significantly increased Wee1 mRNA levels in the intestine when compared to WT mice, we further analyzed RIP3 expression in these mice (Fig. 5B). Results received from RIP3 expression analyses in Per1/2 mice were verified by mRNA (Fig. 5C) and protein (Fig. 5D) expression analyses of RIP3 in ileal tissues from untreated or TNF-α-treated CRD and control WT mice. In contrast to RIP3, no alterations in RIP1 protein expression levels were observed in these mice (Supplemental Fig. 4D). To verify the contribution of enhanced TNF-α-triggered necroptosis induction in ileal IECs of CRD mice, we injected TNF-α in the presence or absence of the necroptosis inhibitor Nec-1 into CRD and control WT mice. Notably, a decreased number of TUNEL+ cells were detected in ileal hemicrypts of CRD mice after coinjection of TNF-α and Nec-1, whereas no alterations were found in controls (Fig. 5E, F). Furthermore, administration of Nec-1 significantly reduced TNF-α-mediated systemic inflammation, as indicated by serum KC levels, in CRD and Per1/2 mice but not in control mice (Fig. 5G; Supplemental Fig. 5B), revealing excessive induction of necroptosis in IECs to play an important role in severe inflammatory processes.
Figure 5.
RIP3-dependent necroptotic cell death is increased in arrhythmic mice. A) Western blot analysis for RIP3 production in intestinal biopsy samples from DSS- and TNF-α-treated mice. Biopsy samples from DSS-treated mice were taken from the colon, and samples from TNF-α-treated mice were taken from the ileum. β-Actin served as the loading control. B) Wee1 mRNA transcript levels were determined by qPCR, using colonic tissue samples from rhythmic (n = 5) or arrhythmic (n = 3) mice. Wee1 mRNA levels were normalized to β-actin mRNA levels. C) Rip3 mRNA transcript levels were evaluated by qPCR, using ileal tissue samples from rhythmic or arrhythmic mice treated with TNF-α or left untreated (n = 4 per strain and treatment). Rip3 mRNA levels were normalized to β-actin mRNA levels. D) Western blot for RIP3 with ileal biopsy samples. β-Actin served as a control. E) Representative images of TUNEL+ dying cells in ileal tissue after intraperitoneal injection of TNF-α, with or without coinjection of Nec1. Insets: lower crypt of arrhythmic (LL) mice showing fewer TUNEL+ cells after Nec1 treatment. LD, rhythmic mice. F) Quantification of TUNEL+ cells in the lower crypt (n > 8 per strain). G) KC-specific ELISA with serum samples collected from rhythmic and arrhythmic mice. Mice underwent intraperitoneal injection of TNF-α, with or without coinjection of necrostatin 1 (Nec1; rhythmic, n = 3; arrhythmic, n = 6; rhythmic+TNF-α, n = 6; arrhythmic+TNF-α, n = 6; rhythmic+TNF-α+Nec1, n = 9; and arrhythmic+TNF-α+Nec1, n = 6). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
DISCUSSION
The pathogenesis of IBD is still poorly understood, but sleep disruption and chronic fatigue are among the major complaints of patients with IBD (3–7), and it has been suggested that they may be rooted in perturbations of the circadian timing system (8). In humans, a polymorphism of the clock gene Per3 has recently been associated with increased susceptibility to and disease severity of CD (27), although the underlying molecular mechanisms remain elusive. Furthermore, recent studies demonstrated clock genes to be differentially regulated in noninflamed and inflamed intestinal tissues from patients with IBD (28). Per1/2 mice are a widely used genetic mouse model of circadian clock disruption carrying loss-of-function mutations in the core clock genes Per1 and -2 (29). Ablation of clock function in these animals resulted in the present study in a severely aggravated clinical phenotype of experimental colitis. Our findings underline data published by Yu et al. (52) that demonstrate elevated intestinal TH17 cell infiltration and hence an increased susceptibility to acute colitis by environmentally triggered CRD in mice. Furthermore, they link excessive TH17 cell infiltration to the core clock gene Rev-erbα, which suppresses transcription of NFIL3, an inhibitory transcription factor in TH17 cell development. Notably, we observed decreased numbers of GCs and PCs in conjunction with low Muc2 and lysozyme gene expression in intestinal biopsies from Per1/2 mice, pointing to a prominent role of Per1/2 in intestinal barrier maintenance. In mice and humans, a diminished number or loss of function of both GCs (53, 54) and PCs (44, 55–57) has been linked to intestinal inflammation, whereas enrichment of PCs seems to be protective (58). Based on these findings and the function of secretory IECs within the intestinal barrier, one may hypothesize that decreased numbers of PCs and GCs in Per1/2 mice are accompanied by a dysbiosis in commensal microbiota. However, although some recent studies reported disturbances of CRs to result in altered intestinal microbiome composition, and hence in imbalanced homeostasis of the intestinal epithelium (14) as well as of metabolic pathways (59), we did not detect statistically significant alterations of the intestinal microbiome composition in Per1/2 mice, compared with WT mice at basal levels (Supplemental Fig. 6 and Supplemental Table 1). Therefore, we suggest the presence of compensatory mechanisms that balance the loss of secretory IECs in basal conditions in Per1/2 mice, whereas inflammatory triggers promote the breakdown of intestinal barrier integrity.
Furthermore, we hypothesized that the loss of function of secretory IECs in Per1/2 mice may constitute the main cause for the increased susceptibility to intestinal inflammation. This possibility raised the question of how CRD in these animals affects secretory epithelial cell formation and survival. Studies in fruit flies have shown that deficiency of the circadian clock component Period results in decreased proliferation of intestinal stem cells (45). In mammals, there are 3 Period homologs (Per1–3), of which Per1 and -2 are essential for circadian pacemaker function (30). In Per1/2 mice, loss of GCs and PCs was associated with decreased cell division rates but increased cell death of IECs in the lower hemicrypts. In line with previous studies indicating the activity of the promoter of the mitosis entry inhibitor Wee1 to be controlled by the clock proteins CLOCK and BMAL1 (48), we observed increased Wee1 expression in Per1/2 siRNA transfected cell lines and CRD mice. Notably, under inflammatory conditions, the increased loss of proliferating IECs in the lower hemicrypts was dramatically potentiated by RIP3-mediated necroptosis induction in Per1/2 mice. Elevated mucosal necroptosis has also been observed in patients with IBD (60). Necroptotic cell death in IECs has been shown in conjunction with mucosal Casp8 and FADD deficiency in mice (44, 61), together with increased susceptibility to intestinal inflammation. Furthermore, it is well known that the apoptosis inhibitor cIAP-2, which prevents TNF-α-induced activation of the initiator caspase 8, is up-regulated during the G2/M phase of the cell cycle, and therefore contributes to NF-κB-mediated survival during mitotic cell cycle arrest (49). Indeed, Wee1 overexpression in vitro was accompanied by increased cIAP-2 levels. Consequently, we also found cIAP-2 and RIP3 to be significantly up-regulated in CRD mice, pointing to a switch from balanced proliferation and apoptosis induction to necroptotic cell death and inflammation in the lower intestinal hemicrypts. These findings verify previously published work demonstrating cIAP-2 but not -1 to be up-regulated in regenerative IECs during active ulcerative colitis (62).
The onset and course of IBD supposedly is crucially influenced by nongenetic, environmental factors collectively called the exposome (63). Considering this concept, we attempted to reproduce the genetically determined phenotype of Per1/2 mice in WT mice with externally disrupted CR. Indeed, exposure of WT mice to constant light (64) led to a strong mucosal RIP3-dependent necroptosis and systemic inflammation. These effects were evident only upon proinflammatory challenge with TNF-α, a cytokine that is an important proinflammatory mediator contributing to intestinal inflammation in some patients with IBD (65) and could be prevented by cotreatment with the necroptosis inhibitor Nec-1 (66). These results further emphasize the role of intact CR on intestinal homeostasis. Mucosal necroptosis as a consequence of circadian disruption may trigger IBD pathogenesis, and thus, as has been shown before, alterations of the LD cycle may render mice susceptible to experimental colitis (25).
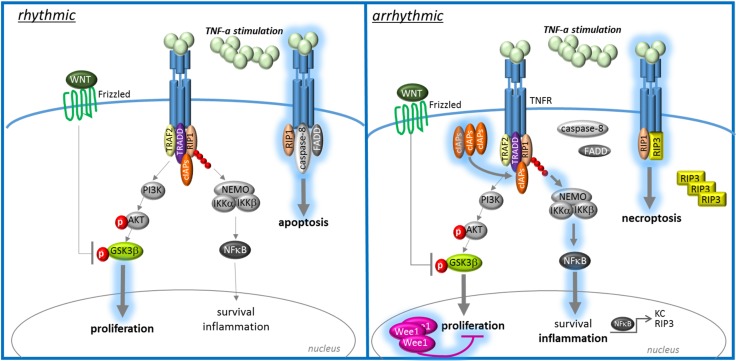
Taken together, our results indicate that severe intestinal inflammation, as observed in patients with IBD, is exacerbated by CR disturbances. This self-sustained exacerbation is mediated by a defective tissue renewal of intestinal epithelium in inflammatory conditions, as mirrored by a marked inhibition of cell division and apoptosis, finally culminating in the induction of necroptosis in proliferating IECs (Fig. 6). Hence, therapeutic strategies targeting CRDs may balance cIAP-2 and Wee1 expression, therefore resulting in maintenance of intestinal tissue homeostasis in IBD.
Figure 6.
Increased cell cycle inhibition and antiapoptosis drive chronic inflammation in patients with IBD via necroptosis. Intact CRs ensure homeostasis of intestinal epithelium by balancing apoptosis and proliferation of IECs under quiescent and inflammatory conditions. Disturbances of circadian rhythms are accompanied by impaired apoptosis and proliferation of IECs, resulting in an imbalance of intestinal tissue homeostasis, especially in inflammatory conditions. Strong apoptosis inhibition by cIAP-2 evokes NF-κB activation and thereby upregulation of the necroptosis mediator RIP3. Excessive induction of necroptosis in IECs in inflammatory conditions then leads to self-sustained chronic inflammation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Petra Langenstrassen, Ann-Kathrin Brethack, and Heidi Schlichting (all from the Institute of Nutritional Medicine, University Hospital Schleswig-Holstein), and Lidija Gutjahr, Harry Manfeldt, and Christo Örün, (all from the Institute of Anatomy, University of Lübeck) for excellent technical support. This work was supported by Grant E15-2013 from the Section of Medicine, University of Lübeck (to F.B.). H.O. is a Lichtenberg fellow of the Volkswagen Foundation. S.D. and C.S. shared senior authorship of this project. The authors declare no conflicts of interest.
Glossary
- BrdU
bromodeoxyuridine
- CD
Crohn’s disease
- cIAP
cellular inhibitor of apoptosis
- CR
circadian rhythm
- CRD
circadian rhythm disruption
- DSS
dextran sodium sulfate
- GC
goblet cell
- HE
hematoxylin-eosin
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cell
- KC
keratinocyte chemoattractant
- LD
light–dark
- MEICS
murine endoscopic index of colitis severity
- PC
Paneth cell
- qPCR
quantitative PCR
- RIP
receptor-interacting serine/threonine-protein kinase
- siRNA
small interfering RNA
- siRNA
small interfering RNA
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
R. Pagel, F. Bär, H. Oster, S. Derer, and C. Sina designed the research; R. Pagel, F. Bär, T. Schröder, and A. H. Tsang performed the experiments; K. Kalies, P. König, D. Bettenworth, K. Fellermann, and H. Oster provided material that made this study possible; R. Pagel, F. Bär, A. Sünderhauf, A. Künstner, S. M. Ibrahim, A. H. Tsang, D. Bettenworth, H. Oster, S. Derer, and C. Sina analyzed the data; R. Pagel, F. Bär, H. Oster, S. Derer, and C. Sina wrote the manuscript; and S. E. Autenrieth, P. König, S. Divanovic, H. Lehnert, and K. Fellermann critically discussed the manuscript.
REFERENCES
- 1.Khor B., Gardet A., Xavier R. J. (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogler G., Vavricka S. (2015) Exposome in IBD: recent insights in environmental factors that influence the onset and course of IBD. Inflamm. Bowel Dis. 21, 400–408 [DOI] [PubMed] [Google Scholar]
- 3.Ranjbaran Z., Keefer L., Farhadi A., Stepanski E., Sedghi S., Keshavarzian A. (2007) Impact of sleep disturbances in inflammatory bowel disease. J. Gastroenterol. Hepatol. 22, 1748–1753 [DOI] [PubMed] [Google Scholar]
- 4.Ananthakrishnan A. N., Long M. D., Martin C. F., Sandler R. S., Kappelman M. D. (2013) Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin. Gastroenterol. Hepatol. 11, 965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali T., Madhoun M. F., Orr W. C., Rubin D. T. (2013) Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm. Bowel Dis. 19, 2440–2443 [DOI] [PubMed] [Google Scholar]
- 6.Graff L. A., Vincent N., Walker J. R., Clara I., Carr R., Ediger J., Miller N., Rogala L., Rawsthorne P., Lix L., Bernstein C. N. (2011) A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm. Bowel Dis. 17, 1882–1889 [DOI] [PubMed] [Google Scholar]
- 7.Keefer L., Stepanski E. J., Ranjbaran Z., Benson L. M., Keshavarzian A. (2006) An initial report of sleep disturbance in inactive inflammatory bowel disease. J. Clin. Sleep Med. 2, 409–416 [PubMed] [Google Scholar]
- 8.Swanson G. R., Burgess H. J., Keshavarzian A. (2011) Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert Rev. Clin. Immunol. 7, 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver A. C., Arjona A., Walker W. E., Fikrig E. (2012) The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cermakian N., Boivin D. B. (2003) A molecular perspective of human circadian rhythm disorders. Brain Res. Brain Res. Rev. 42, 204–220 [DOI] [PubMed] [Google Scholar]
- 11.Pácha J., Sumová A. (2013) Circadian regulation of epithelial functions in the intestine. Acta Physiol. (Oxf.) 208, 11–24 [DOI] [PubMed] [Google Scholar]
- 12.Froy O., Chapnik N., Miskin R. (2005) Mouse intestinal cryptdins exhibit circadian oscillation. FASEB J. 19, 1920–1922 [DOI] [PubMed] [Google Scholar]
- 13.Kyoko O. O., Kono H., Ishimaru K., Miyake K., Kubota T., Ogawa H., Okumura K., Shibata S., Nakao A. (2014) Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS One 9, e98016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherji A., Kobiita A., Ye T., Chambon P. (2013) Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 153, 812–827 [DOI] [PubMed] [Google Scholar]
- 15.Scheving L. E., Burns E. R., Pauly J. E., Tsai T. H. (1978) Circadian variation in cell division of the mouse alimentary tract, bone marrow and corneal epithelium. Anat. Rec. 191, 479–486 [DOI] [PubMed] [Google Scholar]
- 16.Buchi K. N., Moore J. G., Hrushesky W. J., Sothern R. B., Rubin N. H. (1991) Circadian rhythm of cellular proliferation in the human rectal mucosa. Gastroenterology 101, 410–415 [DOI] [PubMed] [Google Scholar]
- 17.Marra G., Anti M., Percesepe A., Armelao F., Ficarelli R., Coco C., Rinelli A., Vecchio F. M., D’Arcangelo E. (1994) Circadian variations of epithelial cell proliferation in human rectal crypts. Gastroenterology 106, 982–987 [DOI] [PubMed] [Google Scholar]
- 18.Qiu J. M., Roberts S. A., Potten C. S. (1994) Cell migration in the small and large bowel shows a strong circadian rhythm. Epithelial Cell Biol. 3, 137–148 [PubMed] [Google Scholar]
- 19.Stevenson N. R., Ferrigni F., Parnicky K., Day S., Fierstein J. S. (1975) Effect of changes in feeding schedule on the diurnal rhythms and daily activity levels of intestinal brush border enzymes and transport systems. Biochim. Biophys. Acta 406, 131–145 [DOI] [PubMed] [Google Scholar]
- 20.Saito H., Terada T., Shimakura J., Katsura T., Inui K. (2008) Regulatory mechanism governing the diurnal rhythm of intestinal H+/peptide cotransporter 1 (PEPT1). Am. J. Physiol. Gastrointest. Liver Physiol. 295, G395–G402 [DOI] [PubMed] [Google Scholar]
- 21.Stevenson N. R., Sitren H. S., Furuya S. (1980) Circadian rhythmicity in several small intestinal functions is independent of use of the intestine. Am. J. Physiol. 238, G203–G207 [DOI] [PubMed] [Google Scholar]
- 22.Narducci F., Bassotti G., Gaburri M., Morelli A. (1987) Twenty four hour manometric recording of colonic motor activity in healthy man. Gut 28, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogerwerf W. A., Shahinian V. B., Cornélissen G., Halberg F., Bostwick J., Timm J., Bartell P. A., Cassone V. M. (2010) Rhythmic changes in colonic motility are regulated by period genes. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G143–G150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoogerwerf W. A. (2010) Role of clock genes in gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G549–G555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preuss F., Tang Y., Laposky A. D., Arble D., Keshavarzian A., Turek F. W. (2008) Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R2034–R2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y., Preuss F., Turek F. W., Jakate S., Keshavarzian A. (2009) Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med. 10, 597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzoccoli G., Palmieri O., Corritore G., Latiano T., Bossa F., Scimeca D., Biscaglia G., Valvano M. R., D’Incà R., Cucchiara S., Stronati L., Annese V., Andriulli A., Latiano A. (2012) Association study of a polymorphism in clock gene PERIOD3 and risk of inflammatory bowel disease. Chronobiol. Int. 29, 994–1003 [DOI] [PubMed] [Google Scholar]
- 28.Palmieri O., Mazzoccoli G., Bossa F., Maglietta R., Palumbo O., Ancona N., Corritore G., Latiano T., Martino G., Rubino R., Biscaglia G., Scimeca D., Carella M., Annese V., Andriulli A., Latiano A. (2015) Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol. Int. 32, 903–916 [DOI] [PubMed] [Google Scholar]
- 29.Zheng B., Albrecht U., Kaasik K., Sage M., Lu W., Vaishnav S., Li Q., Sun Z. S., Eichele G., Bradley A., Lee C. C. (2001) Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105, 683–694 [DOI] [PubMed] [Google Scholar]
- 30.Bae K., Jin X., Maywood E. S., Hastings M. H., Reppert S. M., Weaver D. R. (2001) Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30, 525–536 [DOI] [PubMed] [Google Scholar]
- 31.Zheng B., Larkin D. W., Albrecht U., Sun Z. S., Sage M., Eichele G., Lee C. C., Bradley A. (1999) The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400, 169–173 [DOI] [PubMed] [Google Scholar]
- 32.Becker C., Fantini M. C., Wirtz S., Nikolaev A., Kiesslich R., Lehr H. A., Galle P. R., Neurath M. F. (2005) In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 54, 950–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegmund B., Lehr H. A., Fantuzzi G., Dinarello C. A. (2001) IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl. Acad. Sci. USA 98, 13249–13254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffiths G. (1993) Fine Structure Immunocytochemistry Springer, Heidelberg, Germany [Google Scholar]
- 35.Schwerk J., Köster M., Hauser H., Rohde M., Fulde M., Hornef M. W., May T. (2013) Generation of mouse small intestinal epithelial cell lines that allow the analysis of specific innate immune functions. PLoS One 8, e72700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivas G., Möller S., Wang J., Künzel S., Zillikens D., Baines J. F., Ibrahim S. M. (2013) Genome-wide mapping of gene-microbiota interactions in susceptibility to autoimmune skin blistering. Nat. Commun. 4, 2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rognes T., Flouri T., Nichols B., Quince C., Mahé F. (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar R. C. (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998 [DOI] [PubMed] [Google Scholar]
- 39.Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., Lesniewski R. A., Oakley B. B., Parks D. H., Robinson C. J., Sahl J. W., Stres B., Thallinger G. G., Van Horn D. J., Weber C. F. (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faith D. P. (1992) Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10 [Google Scholar]
- 41.Kembel S. W., Cowan P. D., Helmus M. R., Cornwell W. K., Morlon H., Ackerly D. D., Blomberg S. P., Webb C. O. (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 [DOI] [PubMed] [Google Scholar]
- 42.McMurdie P. J., Holmes S. (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8, e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perše M., Cerar A. (2012) Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol. 2012, 718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Günther C., Martini E., Wittkopf N., Amann K., Weigmann B., Neumann H., Waldner M. J., Hedrick S. M., Tenzer S., Neurath M. F., Becker C. (2011) Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karpowicz P., Zhang Y., Hogenesch J. B., Emery P., Perrimon N. (2013) The circadian clock gates the intestinal stem cell regenerative state. Cell Reports 3, 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crosnier C., Stamataki D., Lewis J. (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 7, 349–359 [DOI] [PubMed] [Google Scholar]
- 47.Watson A. J., Hughes K. R. (2012) TNF-α-induced intestinal epithelial cell shedding: implications for intestinal barrier function. Ann. N. Y. Acad. Sci. 1258, 1–8 [DOI] [PubMed] [Google Scholar]
- 48.Matsuo T., Yamaguchi S., Mitsui S., Emi A., Shimoda F., Okamura H. (2003) Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255–259 [DOI] [PubMed] [Google Scholar]
- 49.Jin H. S., Lee T. H. (2006) Cell cycle-dependent expression of cIAP2 at G2/M phase contributes to survival during mitotic cell cycle arrest. Biochem. J. 399, 335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., El-Deiry W. S., Golstein P., Green D. R., Hengartner M., Knight R. A., Kumar S., Lipton S. A., Malorni W., Nuñez G., Peter M. E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., Melino G.; Nomenclature Committee on Cell Death 2009 (2009) Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 16, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J., McQuade T., Siemer A. B., Napetschnig J., Moriwaki K., Hsiao Y. S., Damko E., Moquin D., Walz T., McDermott A., Chan F. K., Wu H. (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu X., Rollins D., Ruhn K. A., Stubblefield J. J., Green C. B., Kashiwada M., Rothman P. B., Takahashi J. S., Hooper L. V. (2013) TH17 cell differentiation is regulated by the circadian clock. Science 342, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heazlewood C. K., Cook M. C., Eri R., Price G. R., Tauro S. B., Taupin D., Thornton D. J., Png C. W., Crockford T. L., Cornall R. J., Adams R., Kato M., Nelms K. A., Hong N. A., Florin T. H., Goodnow C. C., McGuckin M. A. (2008) Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gersemann M., Becker S., Kubler I., Koslowski M., Wang G., Herrlinger K. R., Griger J., Fritz P., Fellermann K., Schwab M., Wehkamp J., Stange E. F. (2009) Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 77, 84–94 [DOI] [PubMed] [Google Scholar]
- 55.Adolph T. E., Tomczak M. F., Niederreiter L., Ko H. J., Böck J., Martinez-Naves E., Glickman J. N., Tschurtschenthaler M., Hartwig J., Hosomi S., Flak M. B., Cusick J. L., Kohno K., Iwawaki T., Billmann-Born S., Raine T., Bharti R., Lucius R., Kweon M. N., Marciniak S. J., Choi A., Hagen S. J., Schreiber S., Rosenstiel P., Kaser A., Blumberg R. S. (2013) Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wehkamp J., Salzman N. H., Porter E., Nuding S., Weichenthal M., Petras R. E., Shen B., Schaeffeler E., Schwab M., Linzmeier R., Feathers R. W., Chu H., Lima H. Jr., Fellermann K., Ganz T., Stange E. F., Bevins C. L. (2005) Reduced paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl. Acad. Sci. USA 102, 18129–18134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaser A., Lee A. H., Franke A., Glickman J. N., Zeissig S., Tilg H., Nieuwenhuis E. E., Higgins D. E., Schreiber S., Glimcher L. H., Blumberg R. S. (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lo Sasso G., Ryu D., Mouchiroud L., Fernando S. C., Anderson C. L., Katsyuba E., Piersigilli A., Hottiger M. O., Schoonjans K., Auwerx J. (2014) Loss of Sirt1 function improves intestinal anti-bacterial defense and protects from colitis-induced colorectal cancer. PLoS One 9, e102495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thaiss C. A., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A. C., Abramson L., Katz M. N., Korem T., Zmora N., Kuperman Y., Biton I., Gilad S., Harmelin A., Shapiro H., Halpern Z., Segal E., Elinav E. (2014) Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 [DOI] [PubMed] [Google Scholar]
- 60.Pierdomenico M., Negroni A., Stronati L., Vitali R., Prete E., Bertin J., Gough P. J., Aloi M., Cucchiara S. (2014) Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am. J. Gastroenterol. 109, 279–287 [DOI] [PubMed] [Google Scholar]
- 61.Welz P. S., Wullaert A., Vlantis K., Kondylis V., Fernández-Majada V., Ermolaeva M., Kirsch P., Sterner-Kock A., van Loo G., Pasparakis M. (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 [DOI] [PubMed] [Google Scholar]
- 62.Seidelin J. B., Vainer B., Andresen L., Nielsen O. H. (2007) Upregulation of cIAP2 in regenerating colonocytes in ulcerative colitis. Virchows Arch. 451, 1031–1038 [DOI] [PubMed] [Google Scholar]
- 63.Wild C. P. (2005) Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev. 14, 1847–1850 [DOI] [PubMed] [Google Scholar]
- 64.Steinlechner S., Jacobmeier B., Scherbarth F., Dernbach H., Kruse F., Albrecht U. (2002) Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. J. Biol. Rhythms 17, 202–209 [DOI] [PubMed] [Google Scholar]
- 65.Neurath M. F. (2014) Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 [DOI] [PubMed] [Google Scholar]
- 66.Degterev A., Hitomi J., Germscheid M., Ch’en I. L., Korkina O., Teng X., Abbott D., Cuny G. D., Yuan C., Wagner G., Hedrick S. M., Gerber S. A., Lugovskoy A., Yuan J. (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.