Abstract
Endogenous fatty acid metabolism that results in elongation and desaturation lipid products is thought to play a role in the development of type 2 diabetes mellitus (T2DM). In this study, we evaluated the potential of estimated elongase and desaturase activities for use as predictive markers for T2DM remission after Roux-en-Y gastric bypass (RYGB). The results of a targeted metabolomics approach from 2 independent studies were used to calculate 24 serum FA concentration ratios (product/precursor). Gene expression data from an open public data set was also analyzed. In a longitudinal study of 38 obese diabetic patients with RYGB, we found higher baseline stearic acid/palmitic acid (S/P) ratio. This ratio reflects an elovl6-encoded elongase enzyme activity that has been found to be associated with greater possibility for diabetes remission after RYGB [odds ratio, 2.16 (95% CI 1.10–4.26)], after adjustment for age, gender, body mass index, diabetes duration, glycosylated hemoglobin A1c, and fasting C-peptide. Our results were validated by examination of postsurgical elovl6 gene expression in morbidly obese patients. The association of S/P with the metabolic status of obese individuals was further validated in a cross-sectional cohort of 381 participants. In summary, higher baseline S/P was associated with greater probability of diabetes remission after RYGB and may serve as a diagnostic marker in preoperative patient assessment. — Zhao, L., Ni, Y., Yu, H., Zhang, P., Zhao, A., Bao, Y., Liu, J., Chen, T., Xie, G., Panee, J., Chen, W., Rajani, C., Wei, R., Su, M., Jia, W., Jia, W. Serum stearic acid/palmitic acid ratio as a potential predictor of diabetes remission after Roux-en-Y gastric bypass in obesity.
Keywords: bariatric surgery, fatty acid metabolism, elovl6, UPLC/QTOFMS, targeted metabolomics
Obesity is associated with insulin resistance and type 2 diabetes mellitus (T2DM) (1). Roux-en-Y gastric bypass (RYGB) surgery is one of the most commonly used bariatric surgical procedures and is known to achieve significant and sustained weight loss and reversal of the obesity (OB)-related dysglycemia (2). Previous studies reported that 70–90% of patients who underwent RYGB had complete diabetes remission at 1 yr (3–5), and 67.5% experienced partial remission at 3 yr after surgery (6). Given the different efficacy of RYGB in obese (OB) patients with T2DM, establishing preoperative predictors of diabetes remission could be helpful for identifying patients likely to benefit from the surgery. We and others have recently demonstrated that age (7), body mass index (BMI) (7), duration of diabetes (Dur) (7, 8), glycemic control (8, 9), fasting C-peptide (CP) (10), visceral OB (4, 11), and chenodeoxycholic acid levels (a primary bile acid) (12) are reliable markers in predicting diabetes remission outcomes 1–3 yr after surgery.
Free fatty acids (FAs), released from adipocytes into the circulation through lipolysis, have been implicated as one of the most important pathogenic factors for insulin resistance and T2DM in OB patients (13). Free serum FA composition depends in part, on the endogenous metabolism of FAs via elongation (lengthened by 2 carbon atoms) and desaturation (insertion of a double bond) (14). Many previous studies have shown that FA product-to-precursor ratios can be used to estimate elongase and desaturase activities and association of their altered activity with the worsening of glycemia and incidence of T2DM (15–18). Little is known, however, about whether these estimated elongase and desaturase activities have a role in preoperative prediction of T2DM remission after RYGB.
Recently, changes in the transcriptomic profiling of subcutaneous adipose tissue (SAT) after RYGB were investigated and revealed that most of the changes produced in SAT gene expression have a prominent role for FA metabolism pathways (19). Based on this observation, we hypothesized that some important product-to-precursor FA ratios traditionally used to estimate desaturase and elongase enzyme activities would exhibit differences in OB patients after RYGB-induced weight loss and could therefore act as potential prognostic markers for the efficacy of RYGB. Using a targeted metabolomics approach, a panel of free FAs (C12–24) was measured in fasting sera using ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC/QTOFMS). Twenty-four product-to-precursor concentration ratios were then comprehensively examined in 419 individuals from 2 independent studies, including a longitudinal cohort of 38 OB diabetic patients with RYGB, with or without diabetes remission at 2-yr follow-up, and a cross-sectional cohort of 381 participants from a community-based epidemiologic survey. The final results showed that the S/P ratio, an estimate of elongase enzyme activity [elongase for very long-chain FA family member 6 (elovl6 ) gene], is a predictive biomarker for RYGB efficacy and assessment of the metabolic status of OB individuals. Our finding was consistent with the gene expression data of elovl6 in SAT of morbidly OB patients 2 yr after RYGB. This work highlighted that FA elongase activity is an important factor in the preoperative assessment of RYGB.
MATERIALS AND METHODS
Subjects
Subjects were recruited from 2 independent studies. The first was the RYGB longitudinal study (12): a total of 38 OB diabetic patients with RYGB who participated in a 2-yr follow-up examination. Medical history, age, height, weight, BMI, and current medications were recorded before and after surgery. Glucose, CP, and glycosylated hemoglobin A1c (HbA1c) were measured before surgery and at 1, 3, 6, 12, and 24 mo postsurgical intervals. The RYGB treatment excluded patients who had open abdominal surgery, insufficient heart or lung function, acute T2DM complication (that is, diabetic ketosis and diabetic ketoacidosis), severe alcohol or drug dependency, mental disorder, type 1 diabetes, secondary diabetes, or relatively high surgical risk (active ulcer). The second study was cross-sectional consisting of 381 18- to 75-yr-old subjects selected from a community-based epidemiologic survey in Shanghai, China, including 76 diabetic and 305 nondiabetic subjects who were classified as overweight/obese (OW/OB). Normal-weight (NW) diabetic patients were excluded from the present study.
All participants gave written informed consent. The study protocols were approved by the Ethics Committee of the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
Definitions
According to the Working Group on Obesity in China, normal weight (NW), OW, and OB are defined as BMI 18.5 kg/m2 ≤ 24 kg/m2, BMI 24 kg/m2 < 28 kg/m2, and BMI ≥ 28 kg/m2, for Chinese adults (20). Diabetes remission was defined as HbA1c level <6.5% and a fasting glucose concentration <7.0 mM for 1 yr or more without active pharmacological intervention (21). Glucose tolerance was categorized according to the American Diabetes Association criteria (22): 1) normal glucose tolerance (NGT) was defined as fasting plasma glucose (FPG) < 6.1 mM, along with a 2-h postprandial plasma glucose (2hPG) < 7.8 mM; 2) impaired glucose regulation (IGR); 2a) impaired glucose tolerance (IGT), FPG < 7.0 mM and 2hPG ≥7.8 and <11.1 mM; 2b) impaired fasting glycemia (IFG), FPG <7.0 and ≥ 6.1 mM, and 2hPG < 7.8 mM; and 3) T2DM, FPG ≥ 7.0 mM or 2hPG ≥ 11.1 mM, or antidiabetic treatment.
Anthropometric indices and laboratory measurements
All participants completed a questionnaire regarding present and past illness, medical therapy, and other health-related behavior. Physical examination included measurements of height, weight, waist circumference (WC), and blood pressure. BMI was calculated as weight in kilograms divided by the square of height in meters. WC was measured in the standing position, midway between the lowest rib and the iliac crest. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were calculated as the average of 3 measurements taken with a mercury sphygmomanometer at 3-min intervals.
The subjects had been instructed to fast for 10 h before presentation for the morning appointment. After a fasting venous blood sample was drawn, each participant had a 75 g oral glucose tolerance test (OGTT). Samples were collected and returned on ice to the laboratories within 2 h for blood component separation. FPG and 2hPG within 2 h of blood collection were measured by using the glucose oxidase method. Serum fasting insulin (FIns) and 2-h postload insulin (2hIns) concentrations were measured with an electrochemiluminescence immunoassay on a Cobas e411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Insulin resistance was estimated by homeostasis model assessment of insulin resistance (HOMA-IR), defined as [FPG (mM) × FIns (mU/L)] × 22.5−1. Insulin sensitivity was assessed using the Matsuda index of insulin sensitivity (ISI) as 10,000 × [Fins (mU/L) × FPG (mM) × mean Ins during OGTT (mU/L) × mean PG during OGTT (mM)]−1/2 (23). The HbA1c level was measured by HPLC (Bio-Rad, Hercules, CA, USA). All lipid profiles were analyzed with a 7600-120 Hitachi automatic analyzer (Hitachi, Tokyo, Japan), according to the manufacturer’s instructions. Serum triglyceride (TG), total cholesterol (TC), and total free FAs were measured via enzymatic procedures (Roche Diagnostics). High-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c) were measured via the direct-assay method (Sekisui Medical Co., Tokyo, Japan). Serum fasting and 2-h CP (2hCP) values were measured by radioimmunoassay (Linco Research, St. Charles, MO, USA).
RYGB intervention
The RYGB procedure was performed laparoscopically with a standardized technique (4). A 25-ml gastric pouch was divided from the distal remnant. The biliopancreatic and alimentary limbs were 100–120 cm in length.
Quantitative analysis of free FAs in sera
Collected serum samples were stored at −80°C until use. Free FA extraction was performed by using an optimized Dole’s method (24), in which endogenous contamination by possible hydrolysis of the esterified FAs during sample preparation was shown to be negligible (25). In brief, the samples were thawed at 4°C, and 10 µl of isotope-labeled internal standard (5 µg/ml of nonadecanoic-d37 acid) was added to each 40 µl of serum sample, followed by 500 µl of isopropyl/hexane/phosphoric acid (2 M) (40:10:1 by vol). Samples were then vortexed for 2 min. After incubation at room temperature for 20 min, 400 μl of n-hexane and 300 μl of water were added, vortexed, and centrifuged at 12,000 rpm for 10 min. Then 400 μl of the upper organic layer was transferred to a new tube, and 400 μl of n-hexane was added to the lower layer for further extraction. After vortexing and centrifugation, all of the upper organic phase was then combined with the first supernatant and dried in a vacuum. The residue was reconstituted with 80 μl of methanol and subjected to analysis using UPLC/QTOFMS (Waters, Milford, CT, USA) (26).
The parameters for the UPLC/QTOFMS analysis were as follows: a 100- × 2.1-mm bridged ethylsiloxane/silica hybrid (BEH) C18 column with 1.7-µm particles was used for separation with a column temperature of 40°C. The optimal mobile phase consisted of water (solvent A) and acetonitrile/isopropyl alcohol (80/20 by vol, solvent B), and the flow rate was set at 400 μl/min. The injection volume was 5 μl. A gradient elution condition was applied as follows: 70% B for 0–2 min, 70–75% B for 2–5 min, 75–80% B for 5–10 min, 80–90% B for 10–13 min, and 90–100% B for 13–16 min, maintained for 5 min, then returned to 70% B for 21–22.5 min and reequilibrated for 1.5 min. Electrospray ionization was used as the ionization source, and the analysis was performed in the negative mode. The following parameters were used: capillary voltage, 2.5 KV; sampling cone, 55 V; extraction cone, 4 V; desolvation temperature, 450°C; source temperature, 150°C; desolvation gas flow, 650 L/h; and cone gas flow, 50 L/h.
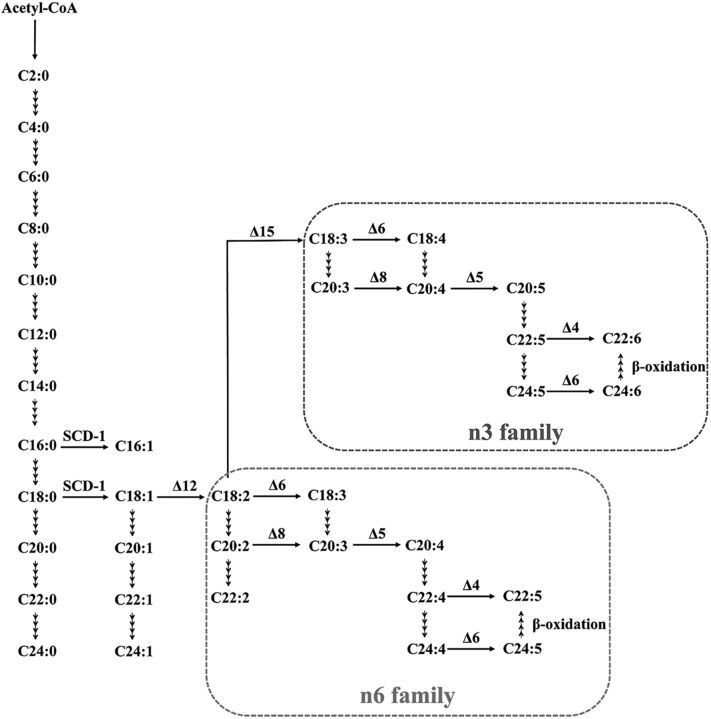
Twenty-four product-to-precursor ratios were calculated by using the absolute concentrations of individual FAs (15). For example, stearoyl-CoA desaturase 1 (SCD)-1 = C16:1 n7/C16:0 or C18:1 n9/C18:0; δ-5 desaturase (D5D) = C20:4 n6/C20:3 n6; δ-6 desaturase (D6D) = C18:3 n6/C18:2 n6; and elongase = C18:0/C16:0 or C20:1 n9/C18:1 n9. The pathway for FA metabolism is illustrated in Fig. 1 (Kyoto Encyclopedia of Genes and Genomes; map 01212 and map 01040) (27, 28).
Figure 1.
A condensed biochemical pathway related to FA metabolism.
Elovl6 gene expression analysis
Gene expression data of elovl6 were obtained from the National Center for Biotechnology Information (NCBI; Bethesda, MD, USA) Gene Expression Omnibus (GEO; series access number GSE53376), contributed by Ortega and Fernández-Real (29). In this work, wide gene expression patterns were assessed in subcutaneous adipose tissue of 16 morbidly OB women before and after surgery-induced weight loss, and 5018 different probe sets identified significant variations in gene expression after treatment. Gene expression of elovl6 and others elucidated changes in biological processes after the surgery-induced weight loss. The changes in gene expression validated our experimental results using targeted metabolomics, which were derived from product-to-precursor free FA ratio estimates of elongase and desaturase enzyme activities. All the analyses were performed with the use of R (version 2.15.0; http://www.r-project.org).
Statistical analysis
Data are presented as means ± sd or se, for variables with a skewed distribution, as median (95% CI). Group values were compared by Mann-Whitney U (or Kruskal-Wallis H) test for continuous variables or χ2 for nominal variables. Spearman correlation and partial correlation were used to assess the associations between free FA ratios and established T2DM biomarkers. Multiple logistic regression was used to predict the remission of diabetes after RYGB; odds ratios (ORs) and 95% CIs are presented. Receiver operating characteristic (ROC) area under the curve (AUC) of free FA ratios and established diabetes remission predictors were compared. Data used in heat map were log2 transformed and then Z-score normalized by a previously published method (30). Statistical analyses were performed with SPSS 16.0 software (SPSS, Chicago, IL, USA). A 2-sided value of P < 0.05 was considered significant.
RESULTS
The longitudinal RYGB study
Twenty-six of 38 (68.4%) patients who had undergone RYGB showed diabetes remission at both the 1- and 2-yr follow-up examinations. The clinical characteristics in diabetes remission and nonremission groups at baseline and at 1 and 2 yr after RYGB are shown in Table 1. In both remission and nonremission groups, significant reductions of BMI and WC were observed 2 yr after RYGB, relative to the baseline. The blood glucose levels, lipid profile, and insulin sensitivity were significantly improved 1 and 2 yr after RYGB, compared with baseline in both groups. Relative to the nonremission group, patients in the remission group had shorter Dur, lower levels of HbA1c, and higher levels of CP in the serum at baseline. Among them, as reported in our previous study (12), the baseline level of Dur was a negative predictor for diabetes remission after RYGB, independent of age, gender, and BMI.
TABLE 1.
Clinical characteristics in diabetes remission and nonremission group, before and after RYGB
| Remission, n = 26 |
No remission, n = 12 |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Baseline | 1 yr | 2 yr | Baseline | 1 yr | 2 yr |
| Female/male | 13/13 | — | — | 7/5 | — | — |
| Age (yr) | 43.7 ± 12.8 | — | — | 47.8 ± 12.2 | — | — |
| Dur (yr) | 4.7 ± 3.7 | — | — | 9.5 ± 5.1** | — | — |
| BMI (kg/m2) | 32.8 ± 3.9 | 24.4 ± 2.8‡ | 24.9 ± 2.9‡ | 31.0 ± 3.5 | 24.8 ± 2.4‡ | 24.9 ± 2.5‡ |
| SBP (mmHg) | 139.3 ± 18.9 | 118.9 ± 12.5‡ | 119.1 ± 11.3‡ | 136.8 ± 14.5 | 123.2 ± 10.1† | 127.8 ± 9.3 |
| DBP (mmHg) | 88.7 ± 12.5 | 78.0 ± 9.3‡ | 73.8 ± 9.9‡ | 86.5 ± 10.7 | 78.2 ± 5.9† | 80.6 ± 6.7† |
| WC (cm) | 108.9 ± 13.7 | 85.3 ± 8.7‡ | 86.8 ± 9.2‡ | 103.4 ± 8.7 | 87.4 ± 7.4‡ | 88.1 ± 8.3‡ |
| TC (mM) | 5.0 ± 1.0 | 4.2 ± 0.8 | 4.2 ± 0.9 | 5.4 ± 1.1 | 4.4 ± 0.7† | 4.8 ± 0.9* |
| TG (mM) | 2.0 ± 1.2 | 0.9 ± 0.3‡ | 1.0 ± 0.3‡ | 4.0 ± 5.6 | 1.2 ± 0.5‡ | 1.5 ± 0.8† |
| HDL-c (mM) | 1.0 ± 0.1 | 1.3 ± 0.3‡ | 1.2 ± 0.2‡ | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.2 ± 0.3 |
| LDL-c (mM) | 3.1 ± 0.9 | 2.4 ± 0.6† | 2.4 ± 0.7† | 3.2 ± 1.0 | 2.6 ± 0.6 | 2.8 ± 0.6 |
| FPG (mM) | 7.4 ± 2.0 | 5.3 ± 0.7‡ | 5.3 ± 0.9‡ | 9.4 ± 3.0 | 6.9 ± 1.0** | 7.5 ± 1.7** |
| 2hPG (mM) | 11.6 ± 4.1 | 5.9 ± 2.3‡ | 6.1 ± 2.1‡ | 13.9 ± 3.2 | 8.8 ± 2.3**,† | 12.1 ± 4.7** |
| CP (ng/ml) | 3.3 ± 1.3 | 1.9 ± 0.6‡ | 1.9 ± 0.6‡ | 2.2 ± 0.8* | 1.8 ± 0.6 | 1.9 ± 0.6** |
| 2hCP (ng/ml) | 9.5 ± 5.6 | 5.6 ± 3.2† | 6.5 ± 3.3 | 4.7 ± 1.8* | 5.2 ± 2.4 | 6.6 ± 3.5 |
| HbA1c (%) | 7.2 ± 1.7 | 5.5 ± 0.5‡ | 5.6 ± 0.6‡ | 8.7 ± 1.3* | 7.0 ± 1.2**,† | 7.2 ± 1.0**,‡ |
| Total free FA (µM) | 517 ± 160 | 403 ± 183† | 302 ± 114‡,‖ | 550 ± 290 | 521 ± 229 | 474 ± 139** |
| HOMA-IR | 5.5 (5.3,9.8) | 1.2 (1.3, 2.2)‡ | 1.6 (1.5, 2.1)‡ | 6.3 (1.8, 32.4) | 1.9 (1. 4, 2.8)*,‡ | 2.2 (1.6, 3.5)‡ |
| Matsuda ISI | 41.5 (33.4, 48.6) | 233.0 (206.9, 337.0)‡ | 171.8 (153.5, 268.8)‡ | 38.1 (22.7, 75.5) | 170.1 (128.7, 275.1)‡ | 120.8 (73.2, 154.0)*,‡,‖ |
| OHA [n (%)]* | 22 (84.6) | 0‡ | 0‡ | 11 (100) | 1 (9.0)‡ | 6 (54.5)**,‡,‖ |
| Insulin therapy [n (%)] | 7 (26.9) | 0‡ | 0‡ | 8 (72.7) | 1 (9.0)‡ | 3 (27.3)**,‡,‖ |
Data are means ± sd, median (95% CI), or percentages. Values were calculated by Mann-Whitney U test. *P < 0.05 between groups; **P < 0.01 between groups; †P < 0.05 within group vs. baseline; ‡P < 0.01 within group vs. baseline; ‖P < 0.05 within group vs. 1 yr after surgery.
A total of 24 product-to-precursor free FAs concentration ratios, which reflected various estimated elongase and desaturase enzyme activities, were examined at baseline and 1 yr after RYGB (Table 2). At 1 yr after RYGB compared to baseline, the ratios of eicosatrienoic acid/eicosadienoic acid (C20:3 n6/C20:2 n6) and eicosatrienoic acid/γ-linolenic acid (C20:3 n6/C18:3n6) significantly increased in both remission and nonremission groups, whereas α-linolenic acid/linoleic acid (C18:3 n3/C18:2 n6), γ-linolenic acid/linoleic acid (C18:3 n6/C18:2 n6), and nervonic acid/erucic acid (C24:1 n9/C22:1 n9) decreased. However, at baseline, only 2 ratios estimating elongase enzyme activities including stearic acid/palmitic acid (C18:0/C16:0, S/P) and eicosadienoic acid/linoleic acid (C20:2 n6/C18:2 n6, E/L), were significantly higher in the remission group than in the nonremission group. None of the ratios that estimated desaturase enzyme activity showed a significant difference between remission and nonremission subjects.
TABLE 2.
Serum product-to-precursor free FA concentration ratios, which reflect estimated desaturase and elongase enzyme activity
| Remission, n = 26 |
No remission, n = 12 |
|||
|---|---|---|---|---|
| Ratios | Baseline | 1 yr | Baseline | 1 yr |
| C16:1 n7/C16:0 a | 0.051 ± 0.004 | 0.050 ± 0.004 | 0.043 ± 0.005 | 0.043 ± 0.005 |
| C18:1 n9/C18:0a | 0.379 ± 0.033 | 0.363 ± 0.039 | 0.451 ± 0.055 | 0.492 ± 0.091 |
| C18:2 n6/C18:1 n9b | 1.083 ± 0.048 | 1.051 ± 0.053 | 1.315 ± 0.101 | 1.010 ± 0.055† |
| C18:3 n3/C18:2 n6c | 0.020 ± 0.001 | 0.017 ± 0.001† | 0.021 ± 0.002 | 0.015 ± 0.001† |
| C18:3 n6/C18:2 n6d | 0.071 ± 0.003 | 0.062 ± 0.004† | 0.066 ± 0.005 | 0.052 ± 0.003† |
| C20:3 n6/C20:2 n6e | 1.721 ± 0.177 | 7.097 ± 0.679‡ | 1.997 ± 0.184 | 8.027 ± 1.386‡ |
| C20:4 n6/C20:3 n6f | 3.848 ± 0.216 | 5.344 ± 0.505‡ | 3.183 ± 0.331 | 4.016 ± 0.620* |
| C22:5 n6/C22:4 n6g | 4.111 ± 0.244 | 4.192 ± 0.236 | 4.983 ± 0.548 | 4.593 ± 0.464 |
| C22:6 n3/C22:5 n3g | 58.050 ± 3.964 | 55.571 ± 3.490 | 65.529 ± 5.487 | 62.691 ± 8.266 |
| C14:0/C12:0h | 30.094 ± 1.389 | 25.842 ± 1.948 | 30.598 ± 5.385 | 22.856 ± 2.178 |
| C16:0/C14:0h | 6.422 ± 0.213 | 5.535 ± 0.248† | 7.237 ± 0.625 | 6.902 ± 0.4568* |
| C18:0/C16:0h | 1.206 ± 0.032 | 1.356 ± 0.064 | 1.039 ± 0.069* | 1.168 ± 0.094 |
| C20:0/C18:0h | 0.015 ± 0.000 | 0.016 ± 0.000 | 0.015 ± 0.001 | 0.015 ± 0.001 |
| C22:0/C20:0h | 0.028 ± 0.001 | 0.028 ± 0.001 | 0.030 ± 0.002 | 0.028 ± 0.002 |
| C24:0/C22:0h | 0.541 ± 0.045 | 0.577 ± 0.040 | 0.697 ± 0.079 | 0.628 ± 0.058 |
| C18:1 n9/C16:1 n7h | 9.680 ± 0.762 | 9.025 ± 0.454 | 10.806 ± 0.918 | 12.176 ± 1.078* |
| C20:1 n9/C18:1 n9h | 0.018 ± 0.001 | 0.019 ± 0.001 | 0.016 ± 0.001 | 0.018 ± 0.001 |
| C22:1 n9/C20:1 n9h | 0.209 ± 0.046 | 0.286 ± 0.050 | 0.174 ± 0.028 | 0.228 ± 0.069 |
| C24:1 n9/C22:1 n9h | 0.295 ± 0.045 | 0.142 ± 0.049‡ | 0.271 ± 0.069 | 0.100 ± 0.027‡ |
| C20:2 n6/C18:2 n6h | 0.029 ± 0.001 | 0.031 ± 0.001 | 0.024 ± 0.002* | 0.029 ± 0.002 |
| C22:2 n6/C20:2 n6h | 0.032 ± 0.001 | 0.032 ± 0.002 | 0.026 ± 0.002 | 0.029 ± 0.002 |
| C20:3 n6/C18:3 n6h | 0.774 ± 0.111 | 3.838 ± 0.464‡ | 0.761 ± 0.116 | 4.499 ± 0.855‡ |
| C22:5 n3/C20:5 n3h | 0.316 ± 0.026 | 0.317 ± 0.027 | 0.308 ± 0.040 | 0.317 ± 0.049 |
| C22:4 n6/C20:4 n6h | 0.062 ± 0.004 | 0.057 ± 0.005 | 0.066 ± 0.007 | 0.067 ± 0.009 |
a–hThe ratios reflect the estimated enzyme activities of SCD-1 (a), δ-12 desaturase (b), δ-15 desaturase (c), δ-6 desaturase (d), δ-8 desaturase (e), δ-5 desaturase (f), δ-4 desaturase (g), and elongase (h). Data are means ± se. Values were calculated by Mann-Whitney U test. *P < 0.05 between group, **P < 0.01 between group, †P < 0.05 within group, ‡P < 0.01 within group.
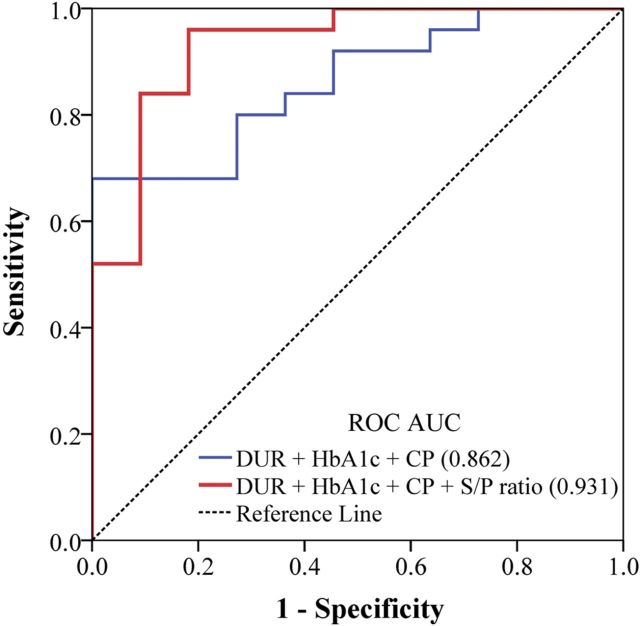
Further, multiple logistic regressions showed that patients who had higher baseline levels of S/P had a greater possibility for diabetes remission after RYGB. The ORs were 2.16 (95% CI 1.10–4.26; P-trend = 0.027), adjusted for age, gender, BMI, Dur, HbA1c, and CP. Both the univariate and multiple logistic regression effects were not significant in the E/L ratio. Finally, the ROC analysis showed that adding the S/P ratio to the model of 3 traditional clinical parameters (Dur, HbA1c, and CP) combined significantly improved the predictive potential ROC AUC (from 0.862 to 0.931) (Fig. 2).
Figure 2.
Relative contribution of the S/P ratio to the prediction of diabetes remission after RYGB. Presented are ROC AUCs comparing different multivariable-adjusted models, including the combination of 3 traditional clinical parameters (Dur, HbA1c, and CP) with or without S/P.
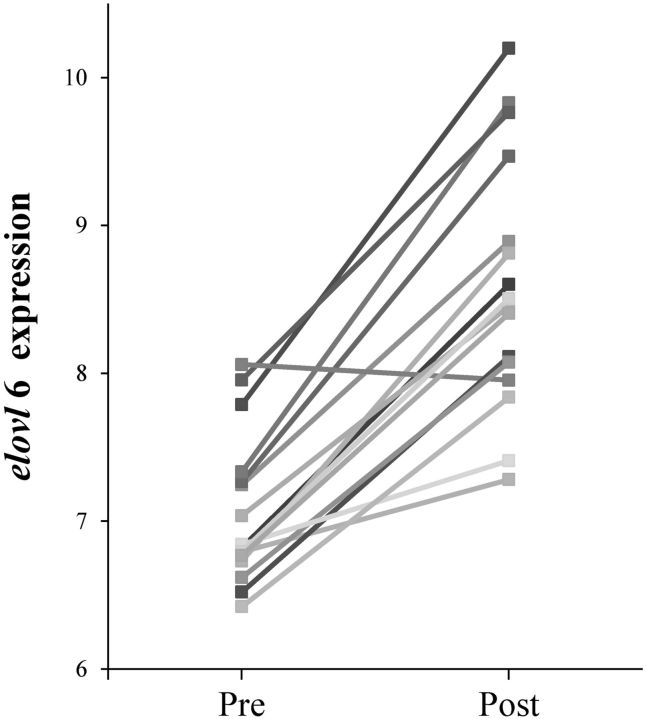
To validate the aforementioned finding about S/P, we further analyzed the gene expression of elovl6, which encodes the elongase enzyme that catalyzes the synthesis of C18:0 from C16:0 (31), in 16 morbidly OB patients before and after surgery-induced weight loss, using an open public gene expression data set (GSE53376) (29). As shown in Fig. 3, the elovl6 expression pattern in adipose tissue was significantly up-regulated after surgery (P < 0.01), which was in consistent with the result in our longitudinal RYGB cohort, estimating the elongase activity by using the S/P ratio.
Figure 3.
Elovl6 gene expression level in the subcutaneous adipose tissue from 16 morbidly OB patients before and after surgery-induced weight loss. Data obtained from public GEO data set GSE53376. Pre vs. post, P < 0.01.
The cross-sectional study
To further understand the association of estimated elongase and desaturase activities with glucose homeostasis in OB diabetics, we examined the above 24 ratios, in particular, the S/P ratio, in fasting serum in a community-based cross-sectional study. The 381 subjects (mean age 45 yr; 58.5% women) were categorized into 5 subgroups according to their metabolic phenotypes, and these subgroups were 156 normal-weight nondiabetic (NW, 155 of NGT and 1 of IGR), 71 OW nondiabetic (70 of NGT and 1 of IGR), 78 OB nondiabetic (64 of NGT and 14 of IGR), 40 OW diabetic (OW/T2DM), and 36 OB diabetic (OB/T2DM) subjects. The clinical characteristics of the subjects are shown in Table 3. The BMIs were comparable between OW and OW/T2DM and between OB and OB/T2DM, and all 4 groups had higher BMIs than the NW (P < 0.01) group. Subjects in the OW/T2DM and OB/T2DM groups were 10+ yr older than those in the OW and OB groups, respectively. As expected, FPG, 2hPG, HbA1c, TG, TC, and HOMA-IR were all significantly higher, whereas Matsuda ISI was lower in the OW/T2DM and OB/T2DM groups compared with the OW and OB groups, respectively (P < 0.01).
TABLE 3.
Clinical characteristics of NW, OW, OB, OW/T2DM, and OB/T2DM, subjects in the cross-sectional study
| Charateristic | NW, n = 156 | OW, n = 71 | OB, n = 78 | OW/T2DM, n = 40 | OB/T2DM, n = 36 | Pa |
|---|---|---|---|---|---|---|
| Female/male | 100/56 | 46/25 | 39/39 | 18/22 | 20/16 | NS |
| Age (yr) | 43 ± 11.2 | 46.2 ± 7.4 | 38.3 ± 11.9*,‡ | 56.9 ± 7.3*,‡ | 55.8 ± 8.9*,§ | <0.01 |
| BMI (kg/m2) | 20.6 ± 0.9 | 26 ± 0.7* | 30.8 ± 3.4*,‡ | 26 ± 0.6*,‡ | 30.4 ± 3.8* | <0.01 |
| SBP (mmHg) | 113.9 ± 10.8 | 115.8 ± 11.5 | 126.2 ± 17.8*,‡ | 135.1 ± 18.6*,‡ | 141.4 ± 24.8*,§ | <0.01 |
| DBP (mmHg) | 71.2 ± 8 | 75.2 ± 7.8* | 78.3 ± 11.6* | 78.6 ± 12.4* | 86.4 ± 14.4*,§ | <0.01 |
| TC (mM) | 4.2 ± 0.6 | 4.3 ± 0.5 | 4.7 ± 0.8*,‡ | 5.4 ± 0.9*,‡ | 5.5 ± 1*,§ | <0.01 |
| TG (mM) | 0.8 ± 0.3 | 1 ± 0.3* | 1.6 ± 1.3*,‡ | 2.4 ± 1.5*,‡ | 2.2 ± 1.3*,§ | <0.01 |
| HDL-c (mM) | 1.6 ± 0.3 | 1.4 ± 0.3* | 1.2 ± 0.3*,‡ | 1.3 ± 0.2*,‡ | 1.3 ± 0.3* | <0.01 |
| LDL-c (mM) | 2.4 ± 0.5 | 2.6 ± 0.4* | 2.9 ± 0.6*,‡ | 3.3 ± 0.8*,‡ | 3.3 ± 0.8* | <0.01 |
| FPG (mM) | 5 ± 0.4 | 5.1 ± 0.4* | 5.2 ± 0.5* | 8.2 ± 3*,‡ | 7.8 ± 2.1*,§ | <0.01 |
| 2hPG (mM) | 5.6 ± 1.1 | 5.8 ± 1.1 | 6.6 ± 1.5*,‡ | 15.1 ± 4.6*,‡ | 14.2 ± 4*,§ | <0.01 |
| HbA1c (%) | 5.3 ± 0.3 | 5.4 ± 0.3 | 5.4 ± 0.4 | 7.2 ± 1.5*,‡ | 7 ± 1.1*,§ | <0.01 |
| FIns (μU/ml) | 6 ± 2.7 | 8.4 ± 3.7* | 14.8 ± 14.5*,‡ | 9.7 ± 5* | 18.1 ± 25.1* | <0.01 |
| 2hIns (μU/ml) | 31.7 ± 20.2 | 36.2 ± 23.8 | 88.3 ± 73.4*,‡ | 64.2 ± 47.4*,‡ | 87.3 ± 66* | <0.01 |
| HOMA-IR | 1.3 ± 0.6 | 1.9 ± 0.9* | 3.5 ± 4.1*,‡ | 3.4 ± 1.9*,‡ | 6.5 ± 10.6*,§ | <0.01 |
| Matsuda ISI | 242.5 ± 161.3 | 178.1 ± 95.4* | 108.9 ± 76.9*,‡ | 71.3 ± 27.3*,‡ | 52.3 ± 23.3*,§ | <0.01 |
NS, not significant. aValues were calculated by Kruskal-Wallis H test. Data are means ± sd. *P < 0.01 vs. NW; ‡P < 0.01 vs. OW; §P < 0.01 vs. OB by the Mann-Whitney U test.
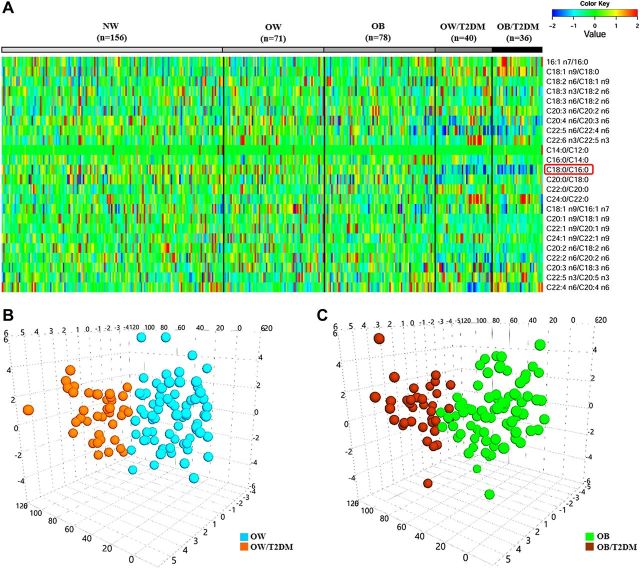
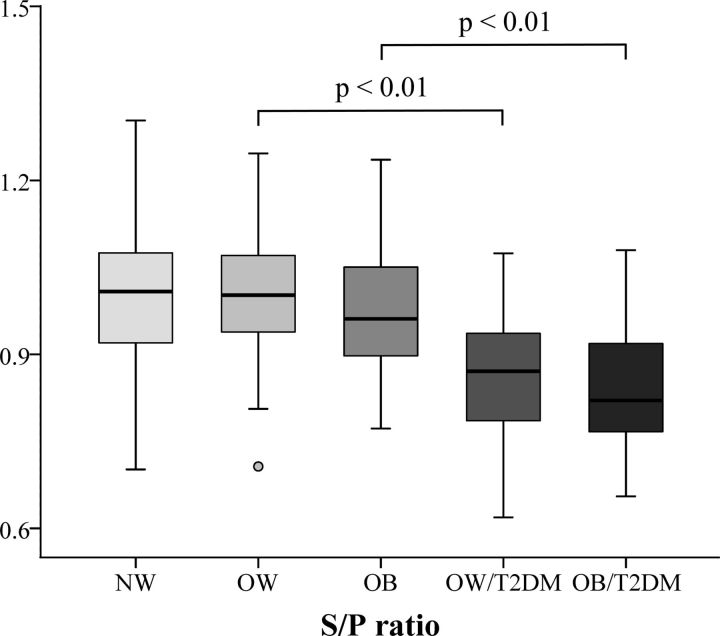
Among the 24 product-to-precursor ratios of free FAs, some were found to closely associate with the metabolic status of OW and OB individuals, as shown in the heat map of Fig. 4A. Using a multivariate analysis model of OPLS-DA, we also observed that both the OW and OB subjects with T2DM were well separated from those BMI-match subjects without T2DM based on the serum levels of 24 ratios (Fig. 4B, C). Further, Fig. 5 shows that S/P ratios from fasting serum were significantly lower in OW and OB patients with T2DM than in BMI-matched nondiabetic subjects (P < 0.01). Correlation analysis also showed that the S/P negatively correlated with FPG, 2hPG, HbA1c, TG, TC, LDL-c, and HOMA-IR and positively correlated with HDL-c and Matsuda ISI in all OW and OB subjects, with or without T2DM (n = 225). When adjustment was made for age and BMI, the S/P ratio still correlated well with most of these clinical parameters (│r│= 0.19–0.53; P < 0.01) (Table 4).
Figure 4.
A) Heat map illustrating 24 standardized product-to-precursor concentration ratios of free FAs in the 5 subgroups of the cross-sectional study. B) The 3D OPLS-DA scores plot showing metabolic status of the FAs in BMI-matched OW subjects with or without T2DM (R2X = 0.278; R2Y = 0.515; Q2 = 0.365). C) The 3-D OPLS-DA scores are plotted showing the different FA metabolic status in BMI-matched OB subjects, with or without diabetics R2X = 0.303; R2Y = 0.390; Q2 = 0.210.
Figure 5.
The fasting serum level of the S/P ratio among 5 subgroups in the cross-sectional study (n = 381).
TABLE 4.
Correlations between the S/P ratio and the clinical parameters in all OW and OB subjects, with or without T2DM in the cross-sectional study
| Variable | Spearman correlation |
Partial correlation |
||
|---|---|---|---|---|
| r | P | r | P | |
| Age | −0.18 | <0.01 | — | — |
| BMI | −0.15 | 0.02 | — | — |
| TC | −0.48 | <0.01 | −0.48 | <0.01 |
| TG | −0.57 | <0.01 | −0.53 | <0.01 |
| HDL-c | 0.23 | <0.01 | 0.22 | <0.01 |
| LDL-c | −0.18 | <0.01 | −0.19 | <0.01 |
| FPG | −0.38 | <0.01 | −0.38 | <0.01 |
| 2hPG | −0.54 | <0.01 | −0.50 | <0.01 |
| HbA1c | −0.45 | <0.01 | −0.41 | <0.01 |
| Matsuda ISI | 0.42 | <0.01 | 0.29 | <0.01 |
| HOMA-IR | −0.31 | <0.01 | 0.01 | NS |
Partial correlation coefficients have been adjusted for age and BMI. NS, not significant.
DISCUSSION
In the present targeted metabolomics study, we found that OB diabetic patients with higher baseline serum levels of S/P, commonly used to estimate elovl6 elongase activity, were more likely to achieve T2DM remission after RYGB. The result was validated by examination of elovl6 gene expression in postsurgical morbidly OB patients. Further, the association between serum S/P and diabetes incidence was corroborated by the finding from a large cross-sectional cohort. Significantly decreased S/P was observed in BMI-matched OW/T2DM subjects relative to OW subjects and in OB/T2DM subjects relative to OB subjects, suggesting an important role of S/P in the pathogenesis of T2DM. Consistent with this observation, S/P positively correlated with HDL-c and Matsuda ISI and negatively correlated with FPG, 2hPG, HbA1c, TG, TC, and LDL-c, adjusted for age and BMI.
Bariatric surgery, particularly RYGB, is an effective diabetes intervention with the potential for achieving long-term diabetes remission. Only a limited number of longitudinal observational studies, using a metabolomics approach, have investigated serum biomarkers that could predict RYGB-induced remission of diabetes. Luo et al. (32) reported a global, untargeted metabolomics strategy that used human serum samples collected before surgery and 6 and 12 mo after RYGB. Results showed that baseline levels of tryptophan, bilirubin, and indoxyl sulfate best predicted the suitability and efficacy of RYGB for patients with T2DM. Another study in which both metabolomic and lipidomic analyses were used reported that, of 14 diabetic subjects after RYGB, 7 in remission at 2-yr follow-up displayed higher presurgery levels of tricarboxylic acid cycle intermediates and TGs with long-chain FAs than did subjects who were not in remission (33). Limited by metabolic profiling analyses, these studies were unable to provide the actual values of the potential predictive markers, which greatly reduced their clinical significance. In our previous study (12), we quantitatively measured 26 serum bile acids by using a targeted metabolomics approach and found that an increased proportion of chenodeoxycholic acid in total bile acids at baseline was associated with diabetes remission after surgery and might act as the potential prognostic marker of RYGB in clinic.
Gastric bypass is associated with improvement in adipose tissue insulin sensitivity and response of glucose metabolism to insulin, which may be partially due to preferential reduction in visceral fat and decreased availability of free FAs (34). Prior studies have shown that circulating total free FAs concentrations were significantly lower after 6 and 12 mo in patients undergoing bariatric surgery, relative to presurgical values (35, 36). Our present study further correlated the changes of total free FA levels in serum with diabetes remission after RYGB. The longitudinal results showed that at 1 and 2 yr after surgery, significant decreases in serum-free FA levels (from 517 µM at baseline to 403 µM at 1 yr and 302 µM at 2 yr) was found in the remission group; however, no significant changes (from 550 µM at baseline to 521 M at 1 yr and 474 µM at 2 yr) were observed in the nonremission group (Table 1). Such differences suggest that the reduction of the total free FA level in serum is closely associated with T2DM remission after surgery. Further, the comparison between groups showed that the free FA concentration in the remission group was a little lower than that in the nonremission group at baseline, but the difference was not statistically significant. Taken together, although these results suggest a significant involvement of total free FAs in the progression and regression of T2DM, the baseline concentration of total free FAs cannot serve as a potential prognostic marker in preoperative assessment of RYGB.
To date, the role of individual free FAs or their product-to-precursor ratios in predicting before surgery the probability for T2DM remission has not been completely studied. Palmitic acid (C16:0) has long been thought to be the major culprit of inflammation, endoplasmic reticulum stress, and insulin resistance (37–39). Similarly, stearic acid (C18:0) may promote adiposity and induce insulin resistance (40). So far, only a few prospective studies have assessed the associations of the 2 saturated FAs with the incidence of T2DM. In the EPIC-InterAct case–cohort study including 340,234 people with 3.99 million person-years of follow-up (1991–2007), C16:0 and C18:0 in plasma phospholipids were positively associated with incident T2DM, with hazard ratios of 1.26 and 1.06, respectively (41). Consistent with this, a study comparing OB T2DM vs. OB nondiabetic African-American women showed that the fasting concentration of C16:0 in plasma was significantly increased (+31%), and that of C18:0 increased by only 20% (42). In another study, the serum cholesterol ester palmitate was increased significantly (P = 0.01), and stearate increased but not significantly at baseline in men who developed diabetes during follow-up compared with those who remained normoglycemic (43). Our laboratory previously reported increased fasting C16:0 levels in metabolically unhealthy OB individuals relative to control subjects and found correlations between C16:0 concentration and metabolic parameters such as glucose, insulin, and lipids profiles, whereas such correlations were not found for C18:0 (26). These studies seemed to suggest that C16:0 has a stronger association with T2DM development than does C18:0. In the present study, we further demonstrated that the ratio of free C18:0 to C16:0 was more closely associated with OB-related diabetes and remission after RYGB, than C16:0 or C18:0 alone. The finding was compatible with the results regarding esterified C16:0 and C18:0 in plasma phospholipid or cholesterol esters (41–43). To our knowledge, no one has reported that S/P may be useful for predicting diabetes remission after RYGB.
Elovl6 is ubiquitously expressed in tissues with high lipid content, such as brown and white adipose tissue, liver, and brain (44). Circulating free FA released from TG via lipolytic activity in adipose tissue may be reflective of FAs composition in SAT (14, 45). Our present study measured the ratio of free stearic acid to palmitic acid in fasting serum to estimate elovl6 activity, and the alteration of the ratio was consistent with the elovl6 gene expression in SAT in postsurgical morbidly OB patients (31). Other causes for the changes in the S/P ratio remain unclear. Emerging evidence has suggested that the excess accumulation of FAs in hepatic TG pools may differentially affect the metabolism of individual types of free FAs (46, 47). For example, when in excess, palmitic acid has a lower incorporation rate into the TG pool than unsaturated free FAs, which can lead to a higher release rate of palmitic acid into the circulation (46). OB also affects the metabolism of free FAs in the muscle; for example, muscles from extremely OB patients showed a significant decrease in palmitic acid oxidation compared with normal and OW/OB subjects (48) and therefore may slow down the clearance of this free FA from the circulation. Our findings suggest that either the increase in release of palmitic from adipose tissue or decrease in palmitate oxidation and storage, or both may be involved in the pathogenesis of T2DM in OB individuals. The current status of this field calls for further studies on the metabolism of individual types of saturated free FAs in various tissues, to understand how these processes are affected by BMI and diabetes.
Variation in FA desaturase genes can modify whole-body lipid metabolism. A few studies have provided evidence on the associations of the gene expression of desaturase enzymes with weight loss. SCD-1 is the enzyme that catalyzes the endogenous biosynthesis of monounsaturated FAs [that is, palmitoleic acid (C16:1 n7) and oleic acid (C18:1n9)] (49). Johansson et al. (50) reported that SCD-1 was down-regulated in human adipose tissue during weight loss on a low-calorie diet, but up-regulated and returned to baseline after weight maintenance. Our data showed that the estimated SCD-1 activity in serum using oleic acid/[stearic acid (C18:1 n9/C18:0) and palmitoleic acid/palmitic acid (C16:1 n7/C16:0), showed no significant differences after weight loss stabilized 1 yr after RYGB (Table 2), which was consistent with SCD-1 gene expression in adipose tissue (50). Two other desaturase enzymes, D6D and D5D, encoded by the fatty acid desaturase (FADS)-1 and FADS2 genes, respectively, are 2 main membrane-bound enzymes that catalyze the rate-limiting formation of long-chain polyunsaturated FAs (PUFAs) (51). Differential expression of the FADS genes and their isoforms have been involved in inflammation, diabetes, and coronary artery disease (52, 53). Our results demonstrated the association of surgery-induced weight loss with the desaturase enzyme activities that regulated n-6 PUFA metabolism, measured as C18:3 n6/C18:2 n6 (D6D), C20:3 n6/C20:2 n6 (D8D), and C20:4 n6/C20:3 n6 (D5D), and others. Variation of these ratios in serum after weight reduction were found in both the remission and the nonremission groups, but there was no difference between the remission and nonremission groups before surgery.
T2DM remission after RYGB has been associated with shorter Dur, younger age, higher BMI, more visceral fat, and higher ratio between chenodeoxycholic acid and total bile acid, whereas a reduced chance of T2DM remission has been associated with higher HbA1c and lower fasting CP and use of insulin (4, 7–10, 12). These preoperative clinical criteria have been used to develop effective models, such as the DiaRem (diabetes remission) score and the ABCD score (age, BMI, CP level, and Dur), to predict the probability of diabetes remission after RYGB (54–56). In this study, we found that adding the S/P ratio to the model of combination of diabetes Dur, HbA1c, and fasting CP contributed a significant increase in the predictive potential ROC AUC with 0.069. The finding suggests a strong predictive value of S/P as a preoperative measure.
The strength of this study lies in the use of concentration ratios of product-to-precursor free FAs, instead of the concentrations of individual free FAs. This method not only provided an insight to the elongase and desaturase enzyme activities in endogenous FA metabolism, but also downplayed the effects of sample storage time on the study outcome. Using the ratios of product-to-precursor may enable us to compare the results between studies performed at different times, given that error involved in sample degradation is normalized when you consider ratios instead of absolute concentrations of FAs. Further, our present study comprehensively investigated most of the upstream and downstream product-to-precursor relations from C12–C24 free FAs for the first time. Third, we examined the RYGB patients both before and after the operation, which enabled us to identify RYGB-induced changes and their correlations with T2DM remission. However, the study was limited by a medium-sized longitudinal cohort and a relatively short-term (2 yr) follow-up after RYGB. Another limitation was that elongase and desaturase enzyme activities were not measured directly but were based on product-to-precursor FA ratios, and the free FA ratios were not available for the 2-yr follow-up examination. A third limitation is that we do not have the data on the food consumption or the use of dietary supplements at baseline, and dietary factors were not accounted for during data analyses and interpretation. Even so, our present finding remains significant, presumably because fasting samples were used and dietary impact was minimized. In fact, for S/P, the interpretation is more complex because they can be synthesized endogenously through de novo lipogenesis, and thus not all of them represent dietary intake (57). Until now, the relative contribution of de novo lipogenesis vs. habitual diets to the amounts of saturated FAs circulating in the blood remains uncertain (58). In support of the significance of our findings, it is important to emphasize that what was measured was serum-free FAs (that is, nonesterified FAs), not the esterified FAs in plasma/serum lipids, and the former is less influenced by recent dietary FA intake (14, 59).
In summary, we found that S/P in fasting serum was lower in OB patients with T2DM than in those without T2DM, and that diabetic patients who had a higher baseline level of S/P were able to achieve remission after RYGB. Our result highlights the importance of S/P as a novel preoperative evaluation marker for RYGB, and its addition to the clinical panel that includes Dur before surgery, CP and HbA1c provides a powerful prediction model for the identification of patients who would benefit the most from RYGB. Further studies are needed to explain how elovl6 regulated glucose metabolism and affects the outcome of T2DM remission after bariatric surgery.
ACKNOWLEDGMENTS
This study was supported by grants from International Science and Technology Cooperation Program of China (2014DFA31870) and China Scholarship Council (201408310049). The authors thank Dr. F. J. Ortega and Dr. J. M. Fernández-Real for sharing the clinical data of microarray datasets, GEO GSE53376. The authors declare no conflicts of interest.
Glossary
- 2hCP
2-h C-peptide
- 2hIns
2-h postload insulin
- 2hPG
2-h postprandial plasma glucose
- AUC
area under the curve
- BMI
body mass index
- CP
C-peptide
- DBP
diastolic blood pressure
- D5D
δ-5 desaturase
- D6D
δ-6 desaturase
- D8D
δ-8 desaturase
- Dur
duration of diabetes
- E/L
eicosadienoic acid/linoleic acid
- FA
fatty acid
- FADS
fatty acid desaturase
- FIns
fasting insulin
- FPG
fasting plasma glucose
- HbA1c
hemoglobin A1c
- HDL-c
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment-insulin resistance
- IR
insulin resistance
- ISI
insulin sensitivity index
- LDL-c
low-density lipoprotein cholesterol
- NGT
normal glucose tolerance
- NW
normal weight
- OB
obesity or obese
- OGTT
oral glucose tolerance test
- OHA
oral hypoglycemic agents
- OPLS-DA
orthogonal projections to latent structures-discriminant analysis
- OR
odds ratio
- OW
overweight
- PUFA
polyunsaturated FA
- ROC
receiver operating characteristic
- SCD
stearoyl-CoA desaturase
- S/P
stearic acid/palmitic acid
- ROC
receiver operating characteristic area under the curve
- RYGB
Roux-en-Y gastric bypass
- SAT
subcutaneous adipose tissue
- SBP
systolic blood pressure
- T2DM
type 2 diabetes mellitus
- TC
total cholesterol
- TG
triglyceride
- UPLC/QTOFMS
ultraperformance liquid chromatography/quadruple time-of-flight mass spectrometry
- WC
waist circumference
AUTHOR CONTRIBUTIONS
W. Jia and Weip. Jia designed the research; H. Yu, P. Zhang, and Y. Bao collected the samples and performed clinical measurements; L. Zhao, A. Zhao, and J. Liu performed the serum-free FA targeted metabolomics analysis; L. Zhao, Y. Ni, T. Chen, and M. Su analyzed the data; W. Chen and R. Wei helped analyze and interpret the gene expression data; L. Zhao wrote the manuscript; H. Yu and G. Xie provided valuable suggestions in preparing the manuscript; W. Jia, J. Panee, C. Rajani, H. Yu, and Wiep. Jia revised the manuscript; and all authors approved the final version to be published.
REFERENCES
- 1.Evans D. J., Murray R., Kissebah A. H. (1984) Relationship between skeletal muscle insulin resistance, insulin-mediated glucose disposal, and insulin binding: effects of obesity and body fat topography. J. Clin. Invest. , 1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubino F. (2006) Bariatric surgery: effects on glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care , 497–507 [DOI] [PubMed] [Google Scholar]
- 3.Dixon J. B., Chuang L. M., Chong K., Chen S. C., Lambert G. W., Straznicky N. E., Lambert E. A., Lee W. J. (2013) Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care , 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H., Di J., Bao Y., Zhang P., Zhang L., Tu Y., Han X., Jia W. (2015) Visceral fat area as a new predictor of short-term diabetes remission after Roux-en-Y gastric bypass surgery in Chinese patients with a body mass index less than 35 kg/m2. Surg. Obes. Relat. Dis. , 6–11 [DOI] [PubMed] [Google Scholar]
- 5.Liang Z., Wu Q., Chen B., Yu P., Zhao H., Ouyang X. (2013) Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res. Clin. Pract. , 50–56 [DOI] [PubMed] [Google Scholar]
- 6.Longitudinal Assessment of Bariatric Surgery (LABS) Consortium (2013) Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA , 2416–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C. K., Shabbir A., Lo C. H., Tai C. M., Chen Y. S., Houng J. Y. (2011) Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25-35. Obes. Surg. , 1344–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert M., Ferrand-Gaillard C., Disse E., Espalieu P., Simon C., Laville M., Gouillat C., Thivolet C. (2013) Predictive factors of type 2 diabetes remission 1year after bariatric surgery: impact of surgical techniques. Obes. Surg. , 770–775 [DOI] [PubMed] [Google Scholar]
- 9.Schauer P. R., Burguera B., Ikramuddin S., Cottam D., Gourash W., Hamad G., Eid G. M., Mattar S., Ramanathan R., Barinas-Mitchel E., Rao R. H., Kuller L., Kelley D. (2003) Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann. Surg. , 467–484; discussion 484–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aarts E. O., Janssen J., Janssen I. M., Berends F. J., Telting D., de Boer H. (2013) Preoperative fasting plasma C-peptide level may help to predict diabetes outcome after gastric bypass surgery. Obes. Surg. , 867–873 [DOI] [PubMed] [Google Scholar]
- 11.Kim M. K., Lee H. C., Kwon H. S., Baek K. H., Kim E. K., Lee K. W., Song K. H. (2011) Visceral obesity is a negative predictor of remission of diabetes 1 year after bariatric surgery. Obesity (Silver Spring) , 1835–1839 [DOI] [PubMed] [Google Scholar]
- 12.Yu H., Ni Y., Bao Y., Zhang P., Zhao A., Chen T., Xie G., Tu Y., Zhang L., Su M., Wei L., Jia W., Jia W. (2015) Chenodeoxycholic acid as a potential prognostic marker for Roux-en-Y gastric bypass in Chinese obese patients. J. Clin. Endocrinol. Metab. , 4222–4230 [DOI] [PubMed] [Google Scholar]
- 13.Boden G. (2011) Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. , 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodson L., Skeaff C. M., Fielding B. A. (2008) Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. , 348–380 [DOI] [PubMed] [Google Scholar]
- 15.Yary T., Voutilainen S., Tuomainen T. P., Ruusunen A., Nurmi T., Virtanen J. K. (2016) Serum n-6 polyunsaturated fatty acids, Δ5- and Δ6-desaturase activities, and risk of incident type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. , 1337–1343 [DOI] [PubMed] [Google Scholar]
- 16.Lankinen M. A., Stančáková A., Uusitupa M., Ågren J., Pihlajamäki J., Kuusisto J., Schwab U., Laakso M. (2015) Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia , 2533–2544 [DOI] [PubMed] [Google Scholar]
- 17.Jacobs S., Schiller K., Jansen E. H., Boeing H., Schulze M. B., Kröger J. (2015) Evaluation of various biomarkers as potential mediators of the association between Δ5 desaturase, Δ6 desaturase, and stearoyl-CoA desaturase activity and incident type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Am. J. Clin. Nutr. , 155–164 [DOI] [PubMed] [Google Scholar]
- 18.Mahendran Y., Ågren J., Uusitupa M., Cederberg H., Vangipurapu J., Stančáková A., Schwab U., Kuusisto J., Laakso M. (2014) Association of erythrocyte membrane fatty acids with changes in glycemia and risk of type 2 diabetes. Am. J. Clin. Nutr. , 79–85 [DOI] [PubMed] [Google Scholar]
- 19.González-Plaza J. J., Gutiérrez-Repiso C., García-Serrano S., Rodriguez-Pacheco F., Garrido-Sánchez L., Santiago-Fernández C., García-Arnés J., Moreno-Ruiz F. J., Rodríguez-Cañete A., García-Fuentes E. (2016) Effect of Roux-en-Y gastric bypass-induced weight loss on the transcriptomic profiling of subcutaneous adipose tissue. Surg. Obes. Relat. Dis. , 257–263 [DOI] [PubMed] [Google Scholar]
- 20.Zhou B. F. (2002) Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases--report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed. Environ. Sci. , 245–252 [PubMed] [Google Scholar]
- 21.Buse J. B., Caprio S., Cefalu W. T., Ceriello A., Del Prato S., Inzucchi S. E., McLaughlin S., Phillips G. L. II, Robertson R. P., Rubino F., Kahn R., Kirkman M. S. (2009) How do we define cure of diabetes? Diabetes Care , 2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrannini E., Natali A., Camastra S., Nannipieri M., Mari A., Adam K. P., Milburn M. V., Kastenmuller G., Adamski J., Tuomi T., Lyssenko V., Groop L., Gall W. E. (2013) Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes , 1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda M., DeFronzo R. A. (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care , 1462–1470 [DOI] [PubMed] [Google Scholar]
- 24.Püttmann M., Krug H., von Ochsenstein E., Kattermann R. (1993) Fast HPLC determination of serum free fatty acids in the picomole range. Clin. Chem. , 825–832 [PubMed] [Google Scholar]
- 25.van der Vusse G. J., Roemen T. H., Reneman R. S. (1985) The content of non-esterified fatty acids in rat myocardial tissue: a comparison between the Dole and Folch extraction procedures. J. Mol. Cell. Cardiol. , 527–531 [DOI] [PubMed] [Google Scholar]
- 26.Ni Y., Zhao L., Yu H., Ma X., Bao Y., Rajani C., Loo L. W., Shvetsov Y. B., Yu H., Chen T., Zhang Y., Wang C., Hu C., Su M., Xie G., Zhao A., Jia W., Jia W. (2015) Circulating unsaturated fatty acids delineate the metabolic status of obese individuals. EBioMedicine , 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kegg Pathway Database. Pathway: Map 01212. Fatty acid metabolism. Accessed October 21, 2016, at http://www.kegg.jp/dbget-bin/www_bget?map01212.
- 28. Kegg Pathway Database. Biosyntheses of unsaturated fatty acids. Accessed October 21, 2016, at http://www.kegg.jp/kegg-bin/show_pathway?map01040.
- 29. Ortega, F. J., and Fernández-Real, J. M. (2014) Adipose transcriptome and microRNA profiles after surgery-induced weight loss [mRNA]. Gene Expression Omnibus, National Institutes of Biotechnology Information, Bethesda, MD, USA. Accessed August 19, 2015, at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE53376.
- 30.Chen W. L., Wang J. H., Zhao A. H., Xu X., Wang Y. H., Chen T. L., Li J. M., Mi J. Q., Zhu Y. M., Liu Y. F., Wang Y. Y., Jin J., Huang H., Wu D. P., Li Y., Yan X. J., Yan J. S., Li J. Y., Wang S., Huang X. J., Wang B. S., Chen Z., Chen S. J., Jia W. (2014) A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood , 1645–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakobsson A., Westerberg R., Jacobsson A. (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog. Lipid Res. , 237–249 [DOI] [PubMed] [Google Scholar]
- 32.Luo P., Yu H., Zhao X., Bao Y., Hong C. S., Zhang P., Tu Y., Yin P., Gao P., Wei L., Zhuang Z., Jia W., Xu G. (2016) Metabolomics study of Roux-en-Y gastric bypass surgery (RYGB) to treat type 2 diabetes patients based on ultraperformance liquid chromatography-mass spectrometry. J. Proteome Res. , 1288–1299 [DOI] [PubMed] [Google Scholar]
- 33.Arora T., Velagapudi V., Pournaras D. J., Welbourn R., le Roux C. W., Orešič M., Bäckhed F. (2015) Roux-en-Y gastric bypass surgery induces early plasma metabolomic and lipidomic alterations in humans associated with diabetes remission. PLoS One , e0126401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curry T. B., Roberts S. K., Basu R., Basu A., Schroeder D., Joyner M. J., Miles J. M. (2011) Gastric bypass surgery is associated with near-normal insulin suppression of lipolysis in nondiabetic individuals. Am. J. Physiol. Endocrinol. Metab. , E746–E751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campos G. M., Rabl C., Havel P. J., Rao M., Schwarz J. M., Schambelan M., Mulligan K. (2014) Changes in post-prandial glucose and pancreatic hormones, and steady-state insulin and free fatty acids after gastric bypass surgery. Surg. Obes. Relat. Dis. , 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mor A., Tabone L., Omotosho P., Torquati A. (2014) Improved insulin sensitivity after gastric bypass correlates with decreased total body fat, but not with changes in free fatty acids. Surg. Endosc. , 1489–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weigert C., Brodbeck K., Staiger H., Kausch C., Machicao F., Häring H. U., Schleicher E. D. (2004) Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J. Biol. Chem. , 23942–23952 [DOI] [PubMed] [Google Scholar]
- 38.Sieber J., Lindenmeyer M. T., Kampe K., Campbell K. N., Cohen C. D., Hopfer H., Mundel P., Jehle A. W. (2010) Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Renal Physiol. , F821–F829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynoso R., Salgado L. M., Calderón V. (2003) High levels of palmitic acid lead to insulin resistance due to changes in the level of phosphorylation of the insulin receptor and insulin receptor substrate-1. Mol. Cell. Biochem. , 155–162 [PubMed] [Google Scholar]
- 40.Van den Berg S. A., Guigas B., Bijland S., Ouwens M., Voshol P. J., Frants R. R., Havekes L. M., Romijn J. A., van Dijk K. W. (2010) High levels of dietary stearate promote adiposity and deteriorate hepatic insulin sensitivity. Nutr. Metab. (Lond.) , 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forouhi N. G., Koulman A., Sharp S. J., Imamura F., Kröger J., Schulze M. B., Crowe F. L., Huerta J. M., Guevara M., Beulens J. W., van Woudenbergh G. J., Wang L., Summerhill K., Griffin J. L., Feskens E. J., Amiano P., Boeing H., Clavel-Chapelon F., Dartois L., Fagherazzi G., Franks P. W., Gonzalez C., Jakobsen M. U., Kaaks R., Key T. J., Khaw K. T., Kühn T., Mattiello A., Nilsson P. M., Overvad K., Pala V., Palli D., Quirós J. R., Rolandsson O., Roswall N., Sacerdote C., Sánchez M. J., Slimani N., Spijkerman A. M., Tjonneland A., Tormo M. J., Tumino R., van der A D. L., van der Schouw Y. T., Langenberg C., Riboli E., Wareham N. J. (2014) Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. , 810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiehn O., Garvey W. T., Newman J. W., Lok K. H., Hoppel C. L., Adams S. H. (2010) Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One , e15234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vessby B., Aro A., Skarfors E., Berglund L., Salminen I., Lithell H. (1994) The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes , 1353–1357 [DOI] [PubMed] [Google Scholar]
- 44.Moon Y. A., Shah N. A., Mohapatra S., Warrington J. A., Horton J. D. (2001) Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J. Biol. Chem. , 45358–45366 [DOI] [PubMed] [Google Scholar]
- 45.Halliwell K. J., Fielding B. A., Samra J. S., Humphreys S. M., Frayn K. N. (1996) Release of individual fatty acids from human adipose tissue in vivo after an overnight fast. J. Lipid Res. , 1842–1848 [PubMed] [Google Scholar]
- 46.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V. Jr., Ory D. S., Schaffer J. E. (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA , 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaguchi K., Yang L., McCall S., Huang J., Yu X. X., Pandey S. K., Bhanot S., Monia B. P., Li Y. X., Diehl A. M. (2007) Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology , 1366–1374 [DOI] [PubMed] [Google Scholar]
- 48.Thyfault J. P., Kraus R. M., Hickner R. C., Howell A. W., Wolfe R. R., Dohm G. L. (2004) Impaired plasma fatty acid oxidation in extremely obese women. Am. J. Physiol. Endocrinol. Metab. , E1076–E1081 [DOI] [PubMed] [Google Scholar]
- 49.Flowers M. T., Ntambi J. M. (2008) Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr. Opin. Lipidol. , 248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansson L. E., Danielsson A. P., Parikh H., Klintenberg M., Norström F., Groop L., Ridderstråle M. (2012) Differential gene expression in adipose tissue from obese human subjects during weight loss and weight maintenance. Am. J. Clin. Nutr. , 196–207 [DOI] [PubMed] [Google Scholar]
- 51.De Antueno R. J., Knickle L. C., Smith H., Elliot M. L., Allen S. J., Nwaka S., Winther M. D. (2001) Activity of human Delta5 and Delta6 desaturases on multiple n-3 and n-6 polyunsaturated fatty acids. FEBS Lett. , 77–80 [DOI] [PubMed] [Google Scholar]
- 52.Vaittinen M., Walle P., Kuosmanen E., Männistö V., Käkelä P., Ågren J., Schwab U., Pihlajamäki J. (2016) FADS2 genotype regulates delta-6 desaturase activity and inflammation in human adipose tissue. J. Lipid Res. , 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S. W., Wang J., Yang Y., Liu Z. J., Cheng L., Liu H. Y., Ma P., Luo W., Liu S. M. (2016) Polymorphisms in FADS1 and FADS2 alter plasma fatty acids and desaturase levels in type 2 diabetic patients with coronary artery disease. J. Transl. Med. , 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Still C. D., Wood G. C., Benotti P., Petrick A. T., Gabrielsen J., Strodel W. E., Ibele A., Seiler J., Irving B. A., Celaya M. P., Blackstone R., Gerhard G. S., Argyropoulos G. (2014) Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol. , 38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayes M. T., Hunt L. A., Foo J., Tychinskaya Y., Stubbs R. S. (2011) A model for predicting the resolution of type 2 diabetes in severely obese subjects following Roux-en Y gastric bypass surgery. Obes. Surg. , 910–916 [DOI] [PubMed] [Google Scholar]
- 56.Lee W. J., Almulaifi A., Tsou J. J., Ser K. H., Lee Y. C., Chen S. C. (2015) Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surg. Obes. Relat. Dis. , 991–996 [DOI] [PubMed] [Google Scholar]
- 57.Hudgins L. C., Hellerstein M., Seidman C., Neese R., Diakun J., Hirsch J. (1996) Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Invest. , 2081–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hellerstein M. K., Schwarz J. M., Neese R. A. (1996) Regulation of hepatic de novo lipogenesis in humans. Annu. Rev. Nutr. , 523–557 [DOI] [PubMed] [Google Scholar]
- 59.Kröger J., Zietemann V., Enzenbach C., Weikert C., Jansen E. H., Döring F., Joost H. G., Boeing H., Schulze M. B. (2011) Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am. J. Clin. Nutr. , 127–142 [DOI] [PubMed] [Google Scholar]