Abstract
We report a successful ultrasound-guided transversus abdominis plane (TAP) block as an analgesic option for minor abdominal surgery in a 66-year-old patient with cardiac, respiratory, and renal dysfunction caused by primary systemic amyloidosis. Bilateral TAP blocks with 120 mg (1.8 mg/kg) of ropivacaine provided sufficient intra- and postoperative analgesia for insertion of a continuous ambulatory peritoneal dialysis catheter. However, the plasma concentration of ropivacaine reached a maximum of 2.5 µg/mL at 15 minutes after the TAP block, a concentration that was potentially neurotoxic. Although apparent signs of local anesthetic systemic toxicity (LAST) such as convulsion or changes in an electrocardiogram were not observed, the patient became drowsy after the TAP block, which might be one of the mild symptoms of LAST. A TAP block by itself can thus be an anesthetic option for patients undergoing minor abdominal surgery. However, cardiac and renal dysfunction might influence the pharmacokinetics of a local anesthetic used, and attention should be paid to the possibility of LAST even with a low dose of a local anesthetic for patients with cardiac and renal failure.
Keywords: transversus abdominis plane block, plasma concentration of ropivacaine, cardiac failure, renal dysfunction, central nervous toxicity
Introduction
The ultrasound-guided transversus abdominis plane (TAP) block has been widely used as a safe and useful technique for intra- and postoperative analgesia in patients undergoing lower abdominal surgery.1 The TAP block has recently been reported to be a useful anesthetic option for implantation of a continuous ambulatory peritoneal dialysis (CAPD) catheter in patients with a poor general condition or patients in whom neuraxial blocks are contraindicated.2 However, the pharmacokinetics of local anesthetics in such patients has not been reported.
We here report the intra- and postoperative analgesic efficacy of a TAP block for CAPD catheter implantation in a patient with cardiac and renal failure in whom plasma concentrations of the local anesthetic ropivacaine were measured to assess the safety of this technique.
Case report
A 66-year-old male, 66 kg in weight and 176 cm in height, with nephrotic syndrome and atrial fibrillation was diagnosed with primary systemic amyloidosis on the basis of the histopathology of kidney biopsy and detection of Bence Jones proteinuria. Written informed consent was obtained from the patient to publish this case report. Dyspnea on exertion was gradually becoming worse and oxygen delivery was needed. Persistent pericardial effusion, bilateral pleural effusions, and leg edema could not be controlled by a high dose of furosemide. Therefore, CAPD was planned to alleviate excess body water retention.
Preoperative laboratory investigations indicated chronic renal failure with an elevated serum creatinine level of 3.3 mg/dL and a blood urea nitrogen level of 75 mg/dL and no liver dysfunction. Hemoglobin level (9.3 g/dL) and platelet count (10.5×104/mm3) were low. Prothrombin time (PT) and PT-international normalized ratio were 14.9 seconds and 1.18, respectively. A chest X-ray showed a small amount of bilateral pleural effusion. Spirometry showed a mixed obstructive/restrictive pattern with forced expiratory volume in 1 second of 65% predicted and a forced vital capacity of 69% predicted. Serum brain natriuretic peptide concentration was 1,063 pg/mL, indicating ventricular dysfunction. Cardiac ultrasound showed hypertrophy of the left ventricle with moderate diastolic dysfunction, although left ventricle ejection fraction was 42%. There were also severe mitral regurgitation and pericardial effusion. His functional capacity was New York Heart Association class III.
No premedication was given. Upon arrival at the operating room, he received oxygen 2 L/min via a face mask. Preoperative arterial blood pressure was 110/70 mmHg, heart rate varied between 70 and 80 bpm, and peripheral oxygen saturation (SpO2) was 98%. The left radial artery was cannulated for continuous blood pressure monitoring and for blood collection. For the ultrasound-guided TAP block, images were obtained using an ultrasound machine (Sonosite S-Nerve®, Fujifilm Sonosite Inc., Bothell, WA, USA) with a 38 mm linear array ultrasound probe (6–13 MHz). TAP block was performed at the level of the anterior axillary line between the 12th rib and the iliac crest. After identification of the plane between the internal oblique and transversus abdominis muscles, a 20-gauge Tuohy needle (80 mm in length, Hakko Medical Co., Ltd., Nagano, Japan) was visualized entering the TAP using an in-plane technique on the right side. Following demonstration of negative aspiration, 40 mL of 0.15% ropivacaine was incrementally injected. Spread of the local anesthetic separating the fascia between the muscles was observed in the TAP. A similar procedure was performed on the left side of the abdomen, with injection of another 40 mL of 0.15% ropivacaine. The patient reported reduced sensation of pinprick stimulation in the lower abdominal wall 30 minutes after the blocks. Although tinnitus, dysgeusia, or agitation was not observed, he became drowsy 10–20 minutes after the TAP block but responded well to verbal commands. Since SpO2 was decreased to 94% after the TAP block, the patient was given oxygen (3 L/min) via a face mask, and SpO2 immediately increased to 99%. No electrocardiogram changes were observed after the block.
Twenty microgram of fentanyl was given before starting surgical insertion of a CAPD catheter. A 4 cm-long horizontal paramedian incision, 3 cm lateral to the midline and 2 cm below the umbilicus on the right side, was made. Then, the peritoneum was incised and the CAPD catheter was inserted into the abdominal cavity. The patient did not complain of any pain throughout the operation. The surgery was uneventfully completed without hemodynamic instability or complications. The duration of the surgery was 45 minutes. Loss of sensation in T10–T12 dermatomes of the abdominal wall was achieved at the end of surgery. The patient complained of moderate pain 6 hours after surgery, but he did not require analgesics. Analgesics were not needed for postoperative pain on the day of the surgery. Although the patient was slightly drowsy until several hours after surgery, he was fully awake and alert the next day. The patient felt slight abdominal pain on moving but not at rest on that day. Therefore, he did not receive any postoperative analgesics thereafter. CAPD was uneventfully initiated 7 days after catheter placement. His CAPD training took 2 weeks. His New York Heart Association classification was improved from class III to class I and he was then discharged home.
Two milliliter blood samples were obtained before the TAP blocks and at 15, 30, 45, and 60 minutes after the TAP blocks. The total plasma concentration of ropivacaine was determined by using liquid chromatography–mass spectrometry (LC–MS/MS; Shimadzu Corp., Kyoto, Japan). As shown in Figure 1, the plasma concentration of ropivacaine reached a maximum of 2.5 µg/mL at 15 minutes and was maintained at 1.7–1.8 µg/mL up to 60 minutes after the TAP blocks had been performed.
Figure 1.
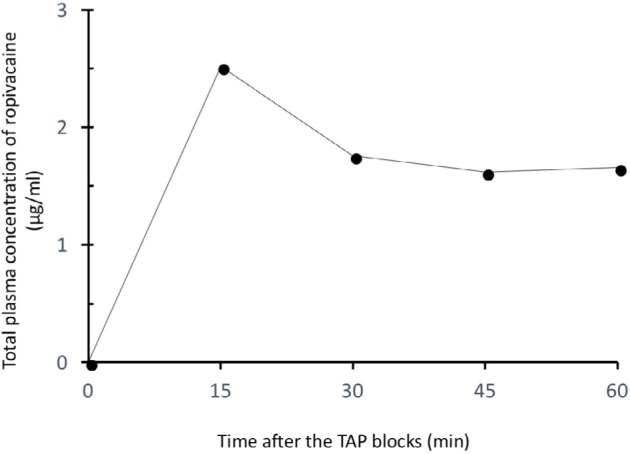
Total plasma concentration of ropivacaine after a TAP block in a patient with cardiac and renal dysfunction caused by primary systemic amyloidosis.
Notes: The TAP block was performed with 40 mL of 0.15% ropivacaine on each side. Filled circles indicate the total plasma concentrations of ropivacaine. Surgery started 30 minutes after the bilateral TAP blocks.
Abbreviation: TAP, transversus abdominis plane.
Discussion
Amyloidosis is a rare systemic disorder characterized by extracellular fibrillar protein deposits in organs and tissues, subsequently resulting in cardiac and renal failure.3 Such organ dysfunction contributes to the high mortality and poor prognosis, with a median survival time of 1–2 years from diagnosis.4 Death is commonly a result of progressive congestive cardiomyopathy or a result of sudden ventricular fibrillation.5 There are several reports describing the occurrence of fatal perioperative myocardial infarction in patients with cardiac amyloidosis.6,7 Cardiotonic agents are not useful because diastolic dysfunction, which was also seen in our patient, is the predominant feature of cardiac amyloidosis.5 We, thus, presumed that general anesthesia and central neuraxial blockade might cause severe hypotension in this patient due to sympathetic blockade, possibly increasing the risk of myocardial ischemia. Since peripheral nerve blocks would provide greater hemodynamic stability than general anesthesia and central neuraxial blockade8 and since a previous case series showed that TAP block combined with an intravenous administration of low-dose opioids provided sufficient analgesia for CAPD catheter implantation,2 we chose TAP block for CAPD catheter implantation in this patient.
The TAP block is a widely used analgesic technique involving the nerves of the anterior abdominal wall.9,10 Nerves supplying the anterior abdominal wall including the parietal peritoneum are derived from T6 to L1 and pass through this plane before supplying the anterior abdominal wall.11 Therefore, TAP block provides analgesia of the parietal peritoneum, anterior abdominal wall, and skin. However, since relatively large doses of a local anesthetic (2.5–3 mg/kg of ropivacaine) are required for a TAP block to achieve an adequate analgesic effect, the maximum plasma concentration of the local anesthetic is relatively high after a TAP block.12–14 The maximum plasma concentration of ropivacaine is relatively high (2.54±0.75 µg/mL) (mean ± SD) after 3 mg/kg of ropivacaine is administered for a TAP block in healthy patients.12 It is well known that a TAP block may result in relatively high ropivacaine plasma concentrations associated with local anesthetic systemic toxicity (LAST) even in patients without cardiac or renal failure.14–16 On the other hand, monitored anesthesia care using TAP block with ropivacaine at a dose of 100–150 mg could achieve intraoperative analgesia for abdominal wall surgery, including CAPD catheter implantation,2 implying that ropivacaine of <3 mg/kg may provide analgesia for minor abdominal surgery. The dose of ropivacaine was reduced to 120 mg (1.8 mg/kg) in consideration of the cardiac and renal failure in our patient. TAP block with 120 mg of ropivacaine could provide efficacious analgesia for CAPD catheter implantation without inducing hemodynamic instability in our patient. Although possible electrocardiogram changes due to LAST were not observed after the block, drowsiness, which might be a mild symptom of LAST, occurred. The plasma concentration of ropivacaine reached a maximum of 2.5 µg/mL at 15 minutes after the TAP block, a concentration that exceeded the widely quoted toxic concentration of 2.2 µg/mL.17 The relatively high plasma concentration of ropivacaine (1.7–1.8 µg/mL) was maintained up to 60 minutes after the TAP block, possibly being associated with the slight drowsiness seen until several hours after surgery.
The maximal concentration of ropivacaine in our patient was comparable to that in healthy patients who received a TAP block with 3 mg/kg of ropivacaine and reached a potentially neurotoxic concentration despite the fact that a considerably smaller dose of ropivacaine was used in our patient. In addition, the plasma concentrations of ropivacaine remained relatively high (1.7–1.8 µg/mL) up to 60 minutes after the TAP block. Ultrasound images clearly showed separation of the fascia between the muscles in our patient. Intramuscular injection of ropivacaine causes a rapid and pronounced rise in plasma concentration, indicating that it is unlikely that intramuscular injection of ropivacaine occurred in our patient. Cardiac and renal failure might, thus, more greatly influence the pharmacokinetics of ropivacaine than has been thought. Indeed, plasma concentrations of ropivacaine have been shown to rise rapidly in brachial plexus block in uremic patients.18 The high plasma concentration of ropivacaine at 15 minutes in our patient might also have been caused by the low clearance rate because renal and cardiac failure reduces clearance of local anesthetics.19,20 Reduced clearance of local anesthetics due to reduced blood flow to the liver and kidneys associated with advanced cardiac dysfunction is a clear indication for dose reduction. Further study is, thus, needed to determine the optimal dose of ropivacaine for a TAP block in patients with renal failure and heart failure.21
In summary, a TAP block with 1.8 mg/kg of ropivacaine provided sufficient intra- and postoperative analgesia in surgery for insertion of a CAPD catheter. However, the plasma concentration exceeded the toxic concentration, and drowsiness as a possible neurologic symptom of LAST was observed after the TAP block. Further dose reduction may be required to avoid the high initial peak of ropivacaine, especially for patients with cardiac and renal failure. In any case, special attention must be given to the toxicity of local anesthetics in patients with cardiac and renal failure when using a TAP block.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Petersen PL, Mathiesen O, Torup H, Dahl JB. The transversus abdominis plane block: a valuable option for postoperative analgesia? A topical review. Acta Anaesthesiol Scand. 2010;54(5):529–535. doi: 10.1111/j.1399-6576.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto H, Shido A, Sakura S, Saito Y. Monitored anesthesia care based on ultrasound-guided subcostal transversus abdominis plane block for continuous ambulatory peritoneal dialysis catheter surgery: case series. J Anesth. 2016;30(1):156–160. doi: 10.1007/s00540-015-2074-0. [DOI] [PubMed] [Google Scholar]
- 3.Gertz MA, Merlini G, Treon SP, Amyloidosis TSP. Macroglobulinemia Waldenström’s. Amyloidosis and Waldenström’s macroglobulinemia. Hematology Am Soc Hematol Educ Program. 2004:257–282. doi: 10.1182/asheducation-2004.1.257. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336(17):1202–1207. doi: 10.1056/NEJM199704243361702. [DOI] [PubMed] [Google Scholar]
- 5.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107(19):2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 6.Kotani N, Hashimoto H, Muraoka M, Kabara S, Okawa H, Matsuki A. Fatal perioperative myocardial infarction in four patients with cardiac amyloidosis. Anesthesiology. 2000;92(3):873–875. doi: 10.1097/00000542-200003000-00036. [DOI] [PubMed] [Google Scholar]
- 7.Massoudy P, Szabo AK, Dirsch O, et al. Amyloid of heart and lungs in a patient with low output syndrome after coronary artery bypass grafting. Herz. 2003;28(5):453–456. doi: 10.1007/s00059-003-2359-1. [DOI] [PubMed] [Google Scholar]
- 8.Borgeat A. Peripheral nerve blocks in patients with preexisting neurologic disease. Period Biol. 2011;113(2):147–150. [Google Scholar]
- 9.Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56(10):1024–1026. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 10.Mcdonnell JG, O’Donnell B, Curley G, et al. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: a prospective randomized controlled trial. Anesth Analg. 2007;104(1):193–197. doi: 10.1213/01.ane.0000250223.49963.0f. [DOI] [PubMed] [Google Scholar]
- 11.Rozen WM, Tran TM, Ashton MW, et al. Refining the course of the thoracolumbar nerves: a new understanding of the innervation of the anterior abdominal wall. Clin Anat. 2008;21(4):325–333. doi: 10.1002/ca.20621. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths JD, Barron FA, Grant S, et al. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105(6):853–856. doi: 10.1093/bja/aeq255. [DOI] [PubMed] [Google Scholar]
- 13.Ishida T, Sakamoto A, Tanaka H, et al. Transversus abdominis plane block with 0.25 % levobupivacaine: a prospective, randomized, double-blinded clinical study. J Anesth. 2015;29(4):557–561. doi: 10.1007/s00540-015-1993-0. [DOI] [PubMed] [Google Scholar]
- 14.Weiss E, Jolly C, Dumoulin JL, et al. Convulsions in 2 patients after bilateral ultrasound-guided transversus abdominis plane blocks for cesarean analgesia. Reg Anesth Pain Med. 2014;39(3):248–251. doi: 10.1097/AAP.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 15.Torup H, Mitchell AU, Breindahl T, et al. Potentially toxic concentrations in blood of total ropivacaine after bilateral transversus abdominis plane blocks; a pharmacokinetic study. Eur J Anaesthesiol. 2012;29(5):235–238. doi: 10.1097/EJA.0b013e328350b0d5. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths JD, Le NV, Grant S, Bjorksten A, Hebbard P, Royse C. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br J Anaesth. 2013;110(6):996–1000. doi: 10.1093/bja/aet015. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen K, Beckman Suurküla M, Blomberg S, Sjövall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78(5):507–514. doi: 10.1093/bja/78.5.507. [DOI] [PubMed] [Google Scholar]
- 18.Pere P, Salonen M, Jokinen M, et al. Pharmacokinetics of ropivacaine in uremic and nonuremic patients after axillary brachial plexus block. Anesth Analg. 2003;96(2):563–569. doi: 10.1097/00000539-200302000-00048. [DOI] [PubMed] [Google Scholar]
- 19.de Martin S, Orlando R, Bertoli M, Pegoraro P, Palatini P. Differential effect of chronic renal failure on the pharmacokinetics of lidocaine in patients receiving and not receiving hemodialysis. Clin Pharmacol Ther. 2006;80(6):597–606. doi: 10.1016/j.clpt.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Stenson RE, Constantino RT, Harrison DC. Interrelationships of hepatic blood flow, cardiac output, and blood levels of lidocaine in man. Circulation. 1971;43(2):205–211. doi: 10.1161/01.cir.43.2.205. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29(6):564–575. doi: 10.1016/j.rapm.2004.08.003. [DOI] [PubMed] [Google Scholar]


