Abstract
Toxoplasma gondii (T. gondii), which infects many warm-blooded animals including humans, causes toxoplasmosis, a globally widespread condition. To date, no research has explored the overall T. gondii infection seroprevalence among women in Saudi Arabia, nor have the risk factors associated with the infection been examined in the Saudi Arabian context. The present systematic review and meta-analysis therefore aimed to investigate toxoplasmosis seroprevalence based on previous study samples of Saudi women of reproductive age, and to establish the potentially risk factors in this national context. PubMed, Science Direct, and Scopus were searched for studies on T. gondii seroprevalence among women in mainland Saudi Arabia published between January 2000 and December 2017. Seroprevalence with 95% CI was presented for each study, and point estimates and their 95% CIs of pooled seroprevalence were then calculated. Twenty papers were eligible for inclusion, with samples totaling 13,597 females of childbearing age (ie, between 15 and 49 years) covering various regions of Saudi Arabia. The pooled estimation for T. gondii prevalence using a random-effect model was calculated as 27.8% (95% CI =20.6%–36.3%). A significant association was observed between age and T. gondii seroprevalence. This review represents the first comprehensive and systematic evaluation of T. gondii infection seroprevalence among Saudi Arabian women, and reports a high prevalence of Toxoplasma infection. Further research is required to support the development of more cost-effective preventive strategies.
Keywords: Toxoplasma gondii, seroprevalence, Saudi women, systematic review, meta-analysis
Introduction
Toxoplasma gondii (T. gondii), an obligate intracellular parasite belonging to phylum Apicomplexa, is a zoonotic protozoan able to infect many warm-blooded animals including humans, resulting in a globally widespread disease known as toxoplasmosis.1 This disease can potentially cause life-threatening conditions in high-risk groups, including pregnant women and immunodeficient individuals (eg, organ transplant recipients, cancer patients, and HIV-positive patients).2,3
T. gondii was first discovered a century ago by scientists investigating Leishmania in North Africa.4 Toxoplasmosis now infects 30%–50% of the world’s humans, making it a global health hazard.5 The Middle East’s overall infection rate is approximately 30%–50%, placing it among the global regions with the highest prevalence.6,7 The infection’s wide distribution is likely to be due to its complex patterns of transmission and parasite coevolution with multiple hosts.8
The global seroprevalence of T. gondii is extremely varied (from 1% to 100%), and frequently also shows significant variations within a country, and even between different regional communities.9–11 Numerous factors influence these differences, including environmental, socioeconomic, eating and drinking habits, available health care facilities, hygiene and sanitation, host susceptibility, geographical location, and soil humidity.9,10,12 However, to date, the relative effects of each of these factors have not been fully established, except for the fact that they vary from area to area. Therefore, understanding the possible sources of infection in specific communities can support the planning of cost-effective food safety and public health interventions by providing vital information.10
As far as the author is aware, no previous research study has investigated the overall seroprevalence of T. gondii infection among women in Saudi Arabia, nor has any prior author explored the overall risk factors influencing the infection in this country. The present systematic review and meta-analysis was therefore carried out to establish T. gondii seroprevalence among previously studied samples of Saudi women of reproductive age, and to evaluate the possible risk factors associated with T. gondii seroprevalence in this sector of the population.
Methods
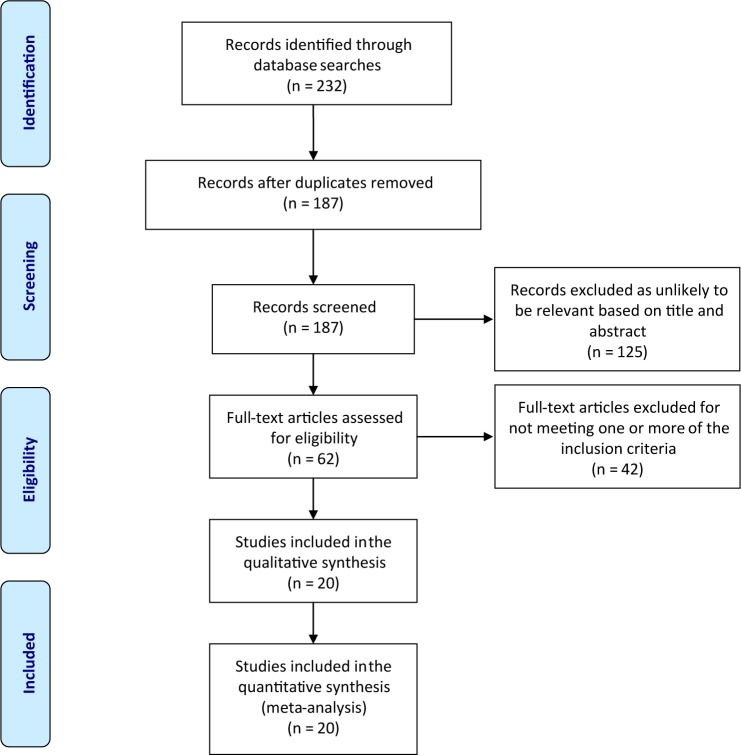
The review searched three electronic databases (PubMed, Science Direct, and Scopus) for studies published between January 2000 and December 2017. Figure 1 shows the search process and explains how the final number of articles included in the review was reached after following the various steps of the process, along with the number of articles at each stage. The searches used the key words “Toxoplasmosis” or “Toxoplasma” or “Toxoplasma gondii” or “T. gondii” and “Saudi Arabia.”
Figure 1.
Flow diagram of the selection process used in the review.
Note: Reproduced from PLoS Medicine (OPEN ACCESS) Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7):e1000097. doi:10.1371/journal.pmed1000097.45
Studies were eligible for the review if they matched the following criteria: 1) they were cross-sectional in design; 2) they had been performed in mainland Saudi Arabia; 3) their sample constituted healthy female subjects of between 15 and 49 years of age; 4) they used serological diagnostic methods; 5) they provided exact totals and positive numbers; and 6) their sample size was >25 to facilitate statistical calculations.13
All potentially eligible papers were carefully checked, and their year of publication, first author, study location, total sample size, number of subjects with positive test results (IgG+, IgM+, or both IgG+ and IgM+), and diagnostic methods for T. gondii were gathered using a preset data extraction form. The form also gathered the data from the studies on possible risk factors, including sociodemographic characteristics, behavioral factors, obstetric history, and other maternal factors.
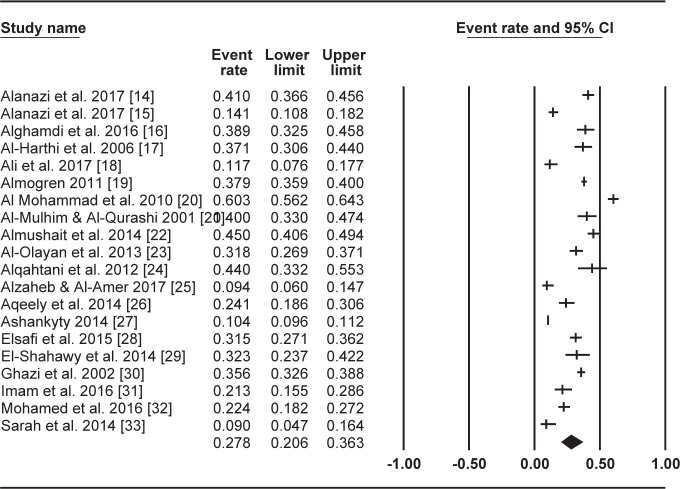
The current review presents two tables showing the extracted data, with Table 1 displaying the papers (listed in alphabetical order by the first author of each study’s surname) which reported data on T. gondii seroprevalence among Saudi women of reproductive age, and Table 2 listing the variables the papers identified as risk factors, along with the variables they found not to be associated with T. gondii infection, as well as the number of papers investigating each variable. A forest plot was also generated using the Comprehensive Meta-Analysis software version 3.0 to show the results of the meta-analyses (random-effects model) which provided estimates of prevalence, and the confidence intervals of the papers for the summary measure (Figure 2).
Table 1.
Summary of the studies included in the review
| Study authors | City (region) | Period of study | Age (years), mean (SD) | Sample size (n) | Serological method | Seroprevalence, n (%) | Total prevalence (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgG and IgM | |||||||
| Alanazi et al,14 2017 | Ad-Dawadimi (Center) | May 2014–December 2016 | 26.3 (6.4)a | 461 | ELISA | 189 (40.9) | 0 (0) | NA | 189 (40.9) |
| Alanazi et al,15 2017 | Arar (North) | January 2015–January 2016 | 20–40a,b | 340 | ELISA | 46 (13.5) | 2 (0.6) | NA | 48 (14.1) |
| Alghamdi et al,16 2016 | Riyadh (Center) | For 9 months in 2011 | 32a | 203 | ELISA | 66 (32.5) | 13 (6.4) | NA | 79 (38.9) |
| Al-Harthi et al,17 2006 | Makkah (West) | January–June 2004 | 17–45a,b | 197 | ELISA | 58 (29.4) | 11 (5.6) | 4 (2.0) | 73 (37.1) |
| Ali et al,18 2017 | Rafha (North) | NA | 16–45b | 162 | ELISA | 19 (11.7) | 0 (0) | NA | 19 (11.7) |
| Almogren,19 2011 | Riyadh (Center) | September 2009–August 2010 | 25.0 (7.3)a | 2,176 | ELISA | 825 (38.0) | 0 (0) | NA | 825 (38.0) |
| Al-Mohammad et al,20 2010 | Al Ahsa (East) | January–December 2009 | 24.1 (5.5)a | 554 | ELISA | 285 (51.4) | 49 (8.8) | 11 (2.0) | 334 (60.3) |
| Al-Mulhim and Al-Qurashi,21 2001 | Al-Khobar (East) | NA | 25 (8.0)a | 175 | MEIA | 69 (39.4) | 1 (0.6) | NA | 70 (40.0) |
| Almushait et al,22 2014 | Khamis Mushait and Abha (South) | January 2008–August 2010 | 28.6 (5.5)a | 487 | ELISA | 189 (38.8) | 30 (6.2) | 16 (3.3) | 219 (45.0) |
| Al-Olayan et al,23 2013 | Hail (North) | February–June 2011 | 20–30a,b | 318 | ELISA | 92 (28.9) | 9 (2.8) | NA | 101 (31.8) |
| Alqahtani and Hassan,24 2012 | Najran (South) | November 2011–March 2012 | NA | 75 | ELISA | 33 (44.0) | 0 (0) | NA | 33 (44.0) |
| Alzaheb and Al-Amer,25 2017 | Tabuk (North) | February–June 2016 | 19–25b | 180 | ELISA | 17 (9.4) | 0 (0) | NA | 17 (9.4) |
| Aqeely et al,26 2014 | Jazan (South) | January–June 2013 | 15–49a,b | 195 | ELISA | 39 (20.0) | 12 (6.2) | 4 (2.1) | 47 (24.1) |
| Ashankyty,27 2014 | Hail (North) | January–December 2013 | 19–43a,b | 6,076 | ELISA | 595 (9.8) | 35 (0.6) | NA | 630 (10.4) |
| Elsafi et al,28 2015 | Dhahran (East) | September 2012–October 2013 | 31.7 (8.8)a | 400 | CMIA | 114 (28.5) | 12 (3.0) | NA | 126 (31.5) |
| El-Shahawy et al,29 2014 | Najran (South) | September 2012–September 2013 | 20–40a,b | 96 | ELISA | 28 (29.2) | 3 (3.1) | NA | 31 (32.3) |
| Ghazi et al,30 2002 | Makkah (West) | N/A | NAa | 926 | ELISA | 330 (35.6) | NA | NA | 330 (35.6) |
| Imam et al,31 2016 | Almadinah (West) | January–June 2015 | 29 (4.2)a | 150 | ELISA | 32 (21.3) | 0 (0) | NA | 32 (21.3) |
| Mohamed et al,32 2016 | Makkah (West) | April–August 2014 | 30.2 (6.0)a | 326 | ELISA | 69 (21.2) | 4 (1.2) | 1 (0.3) | 73 (22.4) |
| Sarah et al,33 2014 | Hail (North) | N/A | 18–45a,b | 100 | ELISA | 9 (8.6) | NA | NA | 9 (8.6) |
Notes:
Sample was from pregnant women.
Range.
Abbreviations: CMIA, chemiluminescent microparticle immunoassay; ELISA, enzyme-linked immunosorbent assay; MEIA, microparticle enzyme-linked immunoassay; NA, not available.
Table 2.
Risk factors and their associations with seroprevalence for T. gondii in Saudi women
| Variables | Risk | Not associated | Number of studies |
|---|---|---|---|
| Sociodemographic factors | |||
| Age | (26–35 years),14,18 (>30 years),20 (increasing age),22 (increasing age),26 (25–34 years) | 17, 25, 28, 32 | 9 |
| Marital status | – | 25 | 1 |
| Years of marriage | – | 32 | 1 |
| Nationality | – | 18 | 1 |
| Race | – | 32 | 1 |
| Residence | (Urban),14,20 (rural) | 25, 26, 32 | 5 |
| Education | (Middle)28 | 14, 17, 20, 32 | 5 |
| Work status | – | 14, 20, 22, 26, 32 | 5 |
| Domestic helper | – | 17 | 1 |
| Monthly household income, SAR | (low income <6,000 SAR)20 | 25 | 2 |
| Blood groups | – | 32 | 1 |
| Height (cm) | (<120 cm)32 | – | 1 |
| Weight (kg) | – | 32 | 1 |
| Behavioral factors | |||
| Cat ownership | – | 22, 25, 28, 32 | 4 |
| Contact with cat | – | 14, 17, 18, 25, 32 | 5 |
| Raw or undercooked meat consumption | 20, 32 | 14, 17, 22, 25, 26, 28 | 8 |
| Raw or undercooked egg consumption | – | 32 | 1 |
| Raw or unwashed vegetable/fruit consumption | 25 | 20 | 2 |
| Type of meat consumed | – | 18, 32 | 2 |
| Frequency of eating meat | – | 18, 32 | 2 |
| Meat preference | – | 18 | 1 |
| Hand washing after contact with raw meat | – | 25, 32 | 2 |
| Eating outside the home | 28 | 17, 26, 32 | 4 |
| Testing food during cooking | – | 17, 28, 32 | 3 |
| Drinking untreated water | – | 17, 18, 20, 25, 32 | 5 |
| Drinking unpasteurized milk | – | 18, 22, 25, 32 | 4 |
| Contact with garden soil | 18 | 25, 28, 32 | 4 |
| Cleaning house and dusting | – | 18, 32 | 2 |
| Washing kitchen daily | – | 26, 32 | 2 |
| Recently traveled abroad | – | 28 | 1 |
| Obstetric history and other health factors | |||
| Pregnancy | – | 18 | 1 |
| Gestation age | – | 17, 22, 26, 32 | 4 |
| Abortion | 20 | 17, 18, 22, 32 | 5 |
| Stillbirth | – | 32 | 1 |
| Malformed fetus | – | 22 | 1 |
| Gravidity or parity | 22 | 14, 20, 28, 32 | 5 |
| Previous history of toxoplasmosis | 22 | – | 1 |
| Previous history of treatment with spiramycin | – | 22 | 1 |
| Previous history of blood transfusion | – | 22 | 1 |
| Intake of immunosuppressive drugs | – | 22 | 1 |
| Knowledge and awareness factors | |||
| Toxoplasmosis knowledge | 28 | 17 | 2 |
| Knowledge of the risk of soil contact and unwashed vegetables and fruits | – | 28 | 1 |
| Knowledge of the risk of handling raw meat or tasting during cooking | 28 | – | 1 |
| Knowledge of the effects of the disease on the fetus | – | 28 | 1 |
| Awareness of the risks of undercooked meat | 28 | 1 | |
| Awareness of cats as a source of infection | – | 28 | 1 |
Abbreviation: SAR, Saudi Arabian Riyal (~$0.266).
Figure 2.
Forest plot diagram of the total prevalence of T. gondii among women of reproductive age in Saudi Arabia.
Results
The initial database search resulted in 232 studies, of which 20 were identified as eligible for inclusion in the present review and meta-analysis (Figure 1). These 20 studies are outlined and summarized in Table 1.14–33 All 20 studies included in the review had cross-sectional designs, and together they tested a total of 13,597 females of childbearing age (ie, between 15 and 49 years) for toxoplasmosis in various regions across Saudi Arabia. The geographical breakdown of the 20 studies was as follows: 6 were carried out in the northern region of Saudi Arabia,15,18,23,25,27,33 4 each were performed in the southern region,22,24,26,29 and the western region,17,30–32 and 3 each were performed in the central region14,16,19 and the eastern region.20,21,28 The smallest sample among the 20 studies contained 75 participants,24 while the largest study recruited 6,076 participants.27
An analysis of the 20 studies found that they reported prevalences of T. gondii IgG and IgM antibodies among their samples of Saudi women of reproductive age ranging between 8.6% to 51.4%, and 0% to 8.8%, respectively (Table 1). The pooled seroprevalence of T. gondii infection among the sampled women based on a random-effects model meta-analysis was 27.8% (95% CI =20.6%–36.3%). Figure 2 contains a forest plot diagram of this analysis.
Table 2 displays the variables identified as risk factors alongside those which were not examined but associated with T. gondii infection and presents data on the number of studies which investigated each variable. Among the variables explored by the studies included in the present review (9/20), the following were reported to be risk factors: increasing age (5 of the 9 studies which explored this variable found it to be a risk factor),14,18,20,22,26 the consumption of raw or undercooked meat (2/8),20,32 living in an urban residence (1/5),14 living in a rural residence (1/5),20 a low level of education (1/5),28 having previously had abortion(s) (1/5),20 the number of gravida and parities (1/5),22 dining outside the home (1/4),28 having had contact with garden soil (1/4),18 earning a low income (1/2),20 consuming raw or unwashed vegetable/fruit (1/2),25 a lack of knowledge of toxoplasmosis (1/2),28 having a previous history of toxoplasmosis (1/1),22 being shorter in height (1/1),32 a lack of knowledge of the risk of handling raw meat or tasting during cooking (1/1),28 and a lack of awareness of the risks of consuming undercooked meat (1/1).28
Discussion
From its searches of the three databases, the present review identified 20 eligible articles containing data on T. gondii seroprevalence drawn from a total sample of 13,597 females of childbearing age (ie, between 15 and 49 years) living in various Saudi Arabian regions. To the best of the author’s knowledge, this review is the first to have assessed T. gondii seroprevalence in women of reproductive age at the national level in Saudi Arabia, as well as exploring the risk factors linked to the T. gondii infection in the country.
The present study reveals that the overall T. gondii seroprevalence between 2000 and 2017 among Saudi women of reproductive age was 27.8% (95% CI =20.6%–36.3%). Perhaps surprisingly, this prevalence is lower than the rates previously reported in some neighboring countries; for instance, a recent meta-analysis of 83 Iranian studies from 1994 to 2017 reported a seroprevalence of anti-T. gondii antibodies among pregnant Iranian women and women of childbearing age of 43% (95% CI =38%–48%) and 33% (95% CI =23%–43%) respectively.34 Similarly, several prior studies in other countries across the Middle Eastern region reported higher rates of T. gondii seroprevalence in pregnant women and/or those of childbearing age – including 45.7% in Kuwait,35 47.1% in Jordan,36 49.2% in Iraq,37 52.6% in Turkey,38 and 72.6% in Egypt.39
This apparently significant variation in T. gondii seroprevalence between Saudi Arabia and nearby countries may be due to the different contexts and impacts of various potential risk factors in these countries, such as the local climatic conditions, nutritional behavior, residence locations, and levels of close contact with animals, particularly cats.15,39,40 For example, none of the studies included in this paper associated T. gondii infection with participants’ level of contact with cats,14,17,18,25,32 cat ownership status,22,25,28,32 drinking of untreated water,17,18,20,25,32 or drinking of unpasteurized milk.18,22,25,32 However, these factors have previously been reported as significant by studies in countries near Saudi Arabia: a recent meta-analysis of 83 Iranian studies reported a significant association among the sampled women for T. gondii seroprevalence and cat contact.34 Similar conclusions have also been reached in other Arab and African countries.41
Toxoplasma prevalence also depends on other identified and documented risk factors (ie, residence location, education level, family income, and the consumption of undercooked meat and unwashed vegetables/fruit).34,42,43 However, the majority of the studies reviewed here did not report a significant association between T. gondii seroprevalence and these risk factors. This does not imply that none of the factors had an influence on toxoplasmosis transmission, but it may indicate that they only have a limited impact in the region, probably because of the dominant local religious beliefs and culture.17,26 Taking the example of undercooked meat as a main source of the infection,34 this eventuality is very rare in Saudi Arabia due to the country’s culture and religion.17 It should also be noted that although a single one of these factors in isolation may have little effect on the epidemiological status of Toxoplasma infection, when combined together they have the power to shape the global distribution patterns of the disease.42
The age of the participants was the variable most frequently examined by the studies in this review, having been included in nine studies and found to be significant by five of them.14,18,20,22,26 These studies reported a higher seropositivity rate among older age groups than in younger ones. A recent review of studies exploring seroepidemiology and T. gondii’s potential risk factors in various Arab and African contexts reached similar conclusions regarding the seroprevalence of T. gondii being significantly associated with a woman’s age.41 For instance, Mwambe et al44 reported that a woman’s risk of T. gondii infection increases by 7% with each additional year of age. The possible reasons for this remain unclear and require further exploration, but previous authors have suggested that it may be due to more prolonged exposure to the identified risk factors, transmission routes, and the generally low public awareness of preventive methods.34
The present review inevitably contains some limitations which should be discussed. First, only a limited number of studies exploring seroprevalence were available, and there was a notable lack of studies in certain important areas of the country. Second, the seroprevalence data included in the reviewed studies were drawn from limited samples of participants which may not necessarily have represented national seroprevalence rates. Third, the reviewed studies deployed a variety of diagnostic methods which featured a range of sensitivities and specificities, and they used different cutoff levels in defining positive results. Fourth, most of the papers omitted to evaluate at least some of the risk factors. Fifth, the information validity of many of the studies in relation to their reported variables may be called into question due to their high risk of measurement error and recall bias. Finally, all the reviewed studies deployed cross-sectional research designs, which means that it is not possible to draw conclusions on any causal relationships between the risk factors they identified and T. gondii infection.
Conclusion
The present study represents the first comprehensive systematic review and meta-analysis of T. gondii infection and its associated risk factors among samples of Saudi women of reproductive age. The review reports a pooled seroprevalence of T. gondii infection of 27.8% across the samples of women studied in the 20 prior papers. A positive association was identified between the seroprevalence of T. gondii and women’s ages. The high seroprevalence of T. gondii infection reported here reveals an urgent need to improve awareness of toxoplasmosis among the public, especially in relation to the ways in which it can be contracted, so that women understand how to protect themselves from this parasitic infection. However, further research is still required to support the development of more cost-effective preventive strategies.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12–13):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goebel WS, Conway JH, Faught P, Vakili ST, Haut PR. Disseminated toxoplasmosis resulting in graft failure in a cord blood stem cell transplant recipient. Pediatr Blood Cancer. 2007;48(2):222–226. doi: 10.1002/pbc.20537. [DOI] [PubMed] [Google Scholar]
- 3.The Lancet Infectious Diseases, Editorial Toxoplasma gondii: an unknown quantity. Lancet Infect Dis. 2012;12(10):737. doi: 10.1016/S1473-3099(12)70247-5. [DOI] [PubMed] [Google Scholar]
- 4.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Flegr J, Prandota J, Sovičková M, Israili ZH. Toxoplasmosis – a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One. 2014;9(3):e90203. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadpour E, Daryani A, Sharif M, et al. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. J Infect Dev Ctries. 2014;8(12):1503–1510. doi: 10.3855/jidc.4796. [DOI] [PubMed] [Google Scholar]
- 7.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39(12):1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Lamberton PH, Donnelly CA, Webster JP. Specificity of the Toxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk. Parasitology. 2008;135(10):1143–1150. doi: 10.1017/S0031182008004666. [DOI] [PubMed] [Google Scholar]
- 9.Iddawela D, Vithana SMP, Ratnayake C. Seroprevalence of toxoplasmosis and risk factors of Toxoplasma gondii infection among pregnant women in Sri Lanka: a cross sectional study. BMC Public Health. 2017;17(1):930. doi: 10.1186/s12889-017-4941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz-Zanzi CA, Fry P, Lesina B, Hill D. Toxoplasma gondii oocyst-specific antibodies and source of infection. Emerg Infect Dis. 2010;16(10):1591–1593. doi: 10.3201/eid1610.091674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39(12):1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava M, Abbas M. Topics in Biostatistics. Totowa, NJ: Humana Press; 2007. [Google Scholar]
- 14.Alanazi AD, Al Yousif MS, Alomar SA, et al. Seroprevalence and risk factors of Toxoplasma gondii infection among pregnant women in Ad-Dawadimi general hospital, Kingdom Of Saudi Arabia. J Egypt Soc Parasitol. 2017;47:355–362. [Google Scholar]
- 15.Alanazi FB, Hassan TM, Alanazi WF. Seroprevalence of Toxoplasma gondii among pregnant Saudi woman in Arar, Northern Borders Province, Saudi Arabia. Kasr Al Ainy Medical Journal. 2017;23(2):104–108. [Google Scholar]
- 16.Alghamdi J, Elamin MH, Alhabib S. Prevalence and genotyping of Toxoplasma gondii among Saudi pregnant women in Saudi Arabia. Saudi. Pharm J. 2015;24:645–651. doi: 10.1016/j.jsps.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Harthi SA, Jamjoom MB, Ghazi HO. Seroprevalence of Toxoplasma gondii among pregnant women in Makkah, Saudi Arabia. Umm Al-Qura Univ J Sci Med Eng. 2006;18:217–227. [Google Scholar]
- 18.Ali A, Mohamed K, Toulah F. Prevalence of Toxoplasma gondii in women population of Rafha city, Saudi Arabia. Pakistan J Zool. 2017;49:1039–1047. [Google Scholar]
- 19.Almogren A. Antenatal screening for Toxoplasma gondii infection at a tertiary care hospital in Riyadh, Saudi Arabia. Ann Saudi Med. 2011;31(6):569–572. doi: 10.4103/0256-4947.87090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Mohammad HI, Amin TT, Balaha MH, Al-Moghannum MS. Toxoplasmosis among the pregnant women attending a Saudi maternity hospital: seroprevalence and possible risk factors. Ann Trop Med Parasitol. 2010;104(6):493–504. doi: 10.1179/136485910X12786389891443. [DOI] [PubMed] [Google Scholar]
- 21.Al-Mulhim AA, Al-Qurashi AM. Seroprevalence of toxoplasmosis in pregnant mothers and new born infants in eastern province, saudi arabia. J Family Community Med. 2001;8(1):45–53. [PMC free article] [PubMed] [Google Scholar]
- 22.Almushait MA, Dajem SM, Elsherbiny NM, Eskandar MA, Al Azraqi TA, Makhlouf LM. Seroprevalence and risk factors of Toxoplasma gondii infection among pregnant women in south western, Saudi Arabia. J Parasit Dis. 2014;38(1):4–10. doi: 10.1007/s12639-012-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Olayan EM, Metwally DM, Alabooshkh F. Seroepidemiological studies of toxoplasmosis among pregnant women in Hail region Saudi Arabia. J Am Sci. 2013;9:619–625. [Google Scholar]
- 24.Alqahtani J, Hassan MM. Incidence of Toxoplasmosis gondii in Najran region, KSA. J Egypt Soc Parasitol. 2012;42(2):253–260. doi: 10.12816/0006314. [DOI] [PubMed] [Google Scholar]
- 25.Alzaheb RA, Al-Amer O. The seroprevalence and risk factors of toxoplasmosis among female undergraduate university students in Saudi Arabia. Oman Med J. 2017;32(6):486–491. doi: 10.5001/omj.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aqeely H, El-Gayar EK, Perveen Khan D, et al. Seroepidemiology of Toxoplasma gondii amongst Pregnant Women in Jazan Province, Saudi Arabia. J Trop Med. 2014;2014:1–6. doi: 10.1155/2014/913950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashankyty IM. Seroprevalence of Toxoplasma gondii among pregnant women visiting maternity hospital in Hail, KSA. Life Sci J. 2014;11:355e359. [Google Scholar]
- 28.Elsafi SH, Al-Mutairi WF, Al-Jubran KM, Abu Hassan MM, Al Zahrani EM. Toxoplasmosis seroprevalence in relation to knowledge and practice among pregnant women in Dhahran, Saudi Arabia. Pathog Glob Health. 2015;109(8):377–382. doi: 10.1080/20477724.2015.1103502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Shahawy IS, Khalil MI, Bahnass MM. Seroprevalence of Toxoplasma gondii in women in Najran City, Saudi Arabia. Saudi Med J. 2014;35(9):1143–1146. [PubMed] [Google Scholar]
- 30.Ghazi HO, Telmesani AM, Mahomed MF. TORCH agents in pregnant Saudi women. Med Princ Pract. 2002;11(4):180–182. doi: 10.1159/000065813. [DOI] [PubMed] [Google Scholar]
- 31.Imam NFA, Azzam Esra’a AA, Attia AA. Seroprevalence of Toxoplasma gondii among pregnant women in Almadinah Almunawwarah KSA. Journal of Taibah University Medical Sciences. 2016;11(3):255–259. [Google Scholar]
- 32.Mohamed K, Bahathiq A, Degnah N, et al. Detection of Toxoplasma gondii infection and associated risk factors among pregnant women in Makkah Al Mukarramah, Saudi Arabia. Asian Pac J Trop Dis. 2016;6(2):113–119. [Google Scholar]
- 33.Sarah YA, Uzma AK, Asmaa IE. Prevalence of seropositive toxoplasmosis in pregnant women in Hail region. Int J Health Sci Res. 2014;4:66–71. [Google Scholar]
- 34.Mizani A, Alipour A, Sharif M, et al. Toxoplasmosis seroprevalence in Iranian women and risk factors of the disease: a systematic review and meta-analysis. Trop Med Health. 2017;45:7. doi: 10.1186/s41182-017-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iqbal J, Hira PR, Khalid N. Toxoplasmosis in Kuwait: improved diagnosis based on quantitative immuno-assay. Clin Microbiol Infect. 2003;9(Suppl. 1):336. [Google Scholar]
- 36.Jumaian NF. Seroprevalence and risk factors for Toxoplasma infection in pregnant women in Jordan. East Mediterr Health J. 2005;11(1–2):45–51. [PubMed] [Google Scholar]
- 37.Mahdi NK, Sharief M. Risk factors for acquiring toxoplasmosis in pregnancy. J Bahrain Med Soc. 2002;14:148–151. [Google Scholar]
- 38.Ocak S, Zeteroglu S, Ozer C, Dolapcioglu K, Gungoren A. Seroprevalence of Toxoplasma gondii, rubella and cytomegalovirus among pregnant women in southern Turkey. Scand J Infect Dis. 2007;39(3):231–234. doi: 10.1080/00365540600978880. [DOI] [PubMed] [Google Scholar]
- 39.El Deeb HK, Salah-Eldin H, Khodeer S, Allah AA. Prevalence of Toxoplasma gondii infection in antenatal population in Menoufia governorate, Egypt. Acta Trop. 2012;124(3):185–191. doi: 10.1016/j.actatropica.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8(10):634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 41.Alsammani MA. Seroepidemiology and risk factors for Toxoplasma gondii among pregnant women in Arab and African countries. J Parasit Dis. 2016;40(3):569–579. doi: 10.1007/s12639-014-0558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daryani A, Sarvi S, Aarabi M, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. 2014;137:185–194. doi: 10.1016/j.actatropica.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Foroutan-Rad M, Khademvatan S, Majidiani H, Aryamand S, Rahim F, Malehi AS. Seroprevalence of Toxoplasma gondii in the Iranian pregnant women: A systematic review and meta-analysis. Acta Trop. 2016;158:160–169. doi: 10.1016/j.actatropica.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Mwambe B, Mshana SE, Kidenya BR, et al. Seroprevalence and factors associated with Toxoplasma gondii infection among pregnant women attending antenatal care in Mwanza, Tanzania. Parasit Vectors. 2013;6:222. doi: 10.1186/1756-3305-6-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]