Abstract
Purpose
This study aimed to investigate the effect of ethylacetate extract from Tetrastigma hemsleyanum (EET) on the proliferation and apoptosis of HepG2 and SMMC-7721 cells and determine the underlying mechanisms.
Materials and methods
HepG2 and SMMC-7721 cells were cultured in vitro until the exponential growth phase and then treated with different concentrations of EET for 24 h. We performed a colony forming assay to determine colony forming ability, CCK8 assay to detect cell proliferation, Annexin V–FITC/PI double staining to analyze cell apoptosis, and Western blot to measure the protein expression of Caspase-3, Bcl-2, and Bax.
Results
EET significantly inhibited the proliferation of HepG2 and SMMC-7721 cells in a concentration- and time-dependent manner (P<0.05). After treatment with 0, 50, 100, 150, 200, and 250 μg/mL EET for 24 h, HepG2 the proliferation rates were 100.00%±0.00%, 90.33%±1.76%, 67.67%±0.88%, 47.33%±0.88%, 37.00%±0.00%, and 30.33%±0.67%, respectively, and 100.00%±0.00%, 18.25%±1.05%, 19.99%±0.59%, 23.42%±0.46%, 29.70%±0.79%, and 29.8%±0.41% for SMMC-7721 cells, respectively. After treatment with 0, 50, 100, 150, 200, and 250 μg/mL EET for 24 h, the apoptotic rates were 11.08%±0.72%, 27.44%±0.51%, 32.92%±0.41%, 26.20%±0.47%, 22.92%±0.24%, and 55.60%±0.08%, for HepG2 cells, respectively, and 59.18%±0.17%, 41.24%±2.05%, 52.54%±0.39%, 50.54%±1.08%, and 57.44%±1.93% for SMMC-7721 cells, respectively. Compared with the treatment groups, the control group showed a significantly lower apoptotic rate (47.91%±1.09%, P<0.05). EET at the different concentrations downregulated the protein expression of Caspase-3 in HepG2 cells and upregulated it in SMMC-7721 cells; it also downregulated the protein expression of Bcl-2 in HepG2 and SMMC-7721 cells and upregulated the protein expression of Bax in HepG2 and SMMC-7721 cells.
Conclusion
These findings suggest that EET exerts antiproliferative and proapoptotic effects against HepG2 and SMMC-7721 cells mediated by downregulation or upregulation of Caspase-3 expression. Our study may help to develop EET for the pharmacological treatment of hepatoblastoma or hepatocellular carcinoma.
Keywords: Tetrastigma hemsleyanum Diels et Gilg, EET, cell proliferation, apoptosis, HepG2 cells, SMMC-7721 cells, Caspase-3, Bcl-2
Introduction
Hepatoblastoma (HB), the most common solid tumor, is the third leading cause of cancer-related deaths worldwide.1 HB is the most common liver malignancy in children (75%), and its incidence is second only to renal cell tumor and neuroblastoma, ranking no. 3 among the abdominal malignant tumors.2 The search for safe and low-toxicity chemotherapy drugs is currently eliciting considerable research attention. The HepG2 cell line was originally established in 1979 by Aden et al3 and was mistakenly reported as a hepatocellular carcinoma (HCC). In 2009, López-Terrada et al4 suggested that HepG2 is a HB-derived cell line, but researchers in recent years have continued to call the HepG2 cell a HCC cell.5
At present, HCC is the third leading cause of cancer deaths worldwide.6 China possesses the highest rate of HCC and accounts for 55% of all new cases of HCC worldwide. Early surgical resection is considered the only potential curative treatment for HCC. Monomers or precursor compounds from natural sources that regulate cell proliferation and apoptosis hold great promise for antitumor drug discovery.
Tetrastigma hemsleyanum Diels et Gilg (Family: Vitaceae), a traditional Chinese medicinal plant, is native to southwest China. The plant is suited to grow in shady and moist hillsides and valleys. The roots of T. hemsleyanum are widely used for the treatment of infantile fever, high fever convulsion, pneumonia, asthma, hepatitis, rheumatism, menstrual disorders, and pharynx pain because of its anti-inflammatory, analgesic, and antipyretic properties.7,8 T. hemsleyanum, which demonstrates potential antitumor activities, has become a focus of attention in recent years.9 Ethylacetate extract from T. hemsleyanum (EET) inhibits cell growth by arresting the cell cycle, increasing the protein expression of Bax and P53, and significantly decreasing the protein expression of Bcl2.10
Although many studies have shown that EET can induce apoptosis in various cancer cells, the exact link between the apoptotic pathway and their activation has yet not been clarified. Moreover, many studies have shown the antitumor activity of T. hemsleyanum.11 EET exhibits antitumor and immune regulatory effects.12,13 Although much attention has been directed toward the study of the chemical components of T. hemsleyanum, its antitumor effects remain unclear.14,15 In the present study, we investigated the effect of EET on the proliferation and apoptosis of HepG2 and SMMC-7721 cells cultured in vitro. These results could serve as a basis for the later study on the anticancer mechanism and the medicinal value development of T. hemsleyanum.
Materials and methods
Plant material and extraction
T. hemsleyanum was purchased from the Shanghai Farm, Hetang, Gutian, China. The freshly collected plant tuber was washed, dried, and then milled to powder. The powdered plant material (20 g) was immersed in double distilled water (200 mL) for 3 days. After reflux at 60°C for 1 day, the mixture was filtered with gauze to remove residuals. The supernatant was treated with ethyl acetate, plated, and then evaporated in a rotary evaporator to obtain the crude extract.
Cells and antibodies
HepG2 HB cells and SMMC-7721 HCC cells lines were purchased from BeNa Culture Collection (Kunshan, China). Antibodies to Bax and Bcl-2 were purchased from Abcam (Cambridge, UK) and Caspase-3 was purchased from Cell Signaling (Danvers, MA, USA).
Chemical and reagents
High-quality fetal bovine serum was obtained from Pan Biotech (Wimborne, UK). Roswell Park Memorial Institute 1640 and Dulbecco’s Modified Eagle Medium were obtained from Gibco (Grand Island, NY, USA). CCK8 was obtained from Tongren (Tokyo, Japan). A cell cycle assay kit was purchased from Xinbosheng (Shenzhen, China). An annexin V–FITC/PI apoptosis assay kit was purchased from Haitian Biotechnology Co., Ltd. (Fuzhou, People’s Republic of China).
Instruments
The JS-1070 chemiluminescence imaging system was obtained from Shanghai Peiqing Technology Co., Ltd. (Shanghai, China). The DYY-7C DNA electrophoresis tank and system were obtained from Beijing Liuyi Biotech Co., Ltd. (Beijing, China). The HF90/HF240 CO2 cell culture system was obtained from Heal Force Co., Ltd. (Shanghai, China). The FACSCalibur/Calibur BD flow cytometer was purchased from BD (Franklin Lakes, NJ, USA). The DF-101S constant-temperature heating magnetic stirrer was purchased from Zhengzhou Changcheng Science and Industry Co., Ltd. (Zhengzhou, China). The YRE-52A rotary evaporator was purchased from Gongyi Yuhua Instrument Co., Ltd. (Gongyi, China).
Cell culture
HepG2 HB cells and SMMC-7721 HCC cells were grown in Roswell park Memorial Institute 1640 supplemented with 10% fetal bovine serum, 1% penicillin streptomycin, and Dulbecco’s Modified Eagle Medium. The cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air. When the cells were 100% confluent, they were digested with trypsin and then passaged to obtain cells at the exponential phase.
CCK8 for cell proliferation detection
Cells at the exponential phase were seeded into a 96-well plate at a density of 1×105/mL (100 μL/well). EET (100 μL) at different concentrations (50, 100, 150, 200, and 250 μg/mL) was added to adherent cells with 6 wells for each group, and repeated 3 times. After 24 h the fluid was removed, and EET (100 μL) at different concentrations was added. For the untreated cells (control), the vehicle medium was added. The cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air for 24 h. CCK8 was added to each well and cultured for 1 h. Enzyme-linked immunoabsorbent assay was performed to detect optical density (OD).
%Cell proliferation = [mean OD of treated cells/mean OD of untreated cells] ×100
Colony formation assay to determine colony forming efficiency
At the exponential phase, cell suspensions were seeded into a 12-well plate at a density of 50, 100, and 200 cells/culture dish (1 mL/well). The plate was gently shaken in a cross- shaped manner. The cells were incubated at 37°C in 5% CO2 in air. EET at different concentrations (50, 100, and 150 μg/mL) was added to the adherent cells. After 8 days, when colonies were observed, the culture fluid was discarded. The cells were washed twice with PBS and dried in air, methanol fixed for 15 min, dried in air, Giemsa stained for 10 min, and then washed and dried for microscopic examination. The colony formation ability was assessed as follows when colony forming units were >50:
%Relative colony forming efficiency = [colony forming efficiency of treated cells/colony forming efficiency of untreated cells] ×100
Apoptosis analysis by flow cytometry
HepG2 and SMMC-7721 cells were separately treated at a density of 3×105/mL. After discarding the medium, the cells were washed with pre-cooled PBS and centrifuged with 10,000 rpm at 4°C for 3 min. The washing and centrifugation steps were repeated. After discarding the medium, the cells were treated with buffer liquid (1×) at a density of 2×105/mL. Annexin V–FITC (5 μL) was added to cell suspensions (195 μL), gently mixed, and incubated at room temperature for 10 min away from light. The cells were washed with buffer liquid (200 μL), suspended with buffer liquid (195 μL), treated with PI (20 μg/mL, 10 μL), and gently mixed for fluorescent activated cell sorting.
Western blot to detect the expression of apoptosis-related proteins
HepG2 and SMMC-7721 cells were separately treated at a density of 5×105/mL. Protein in the cell extracts was fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes. The membranes were blocked in 5% bovine serum albumin on a rotary shaker at room temperature for 1.5 h and probed using Bcl-2, Bax, and Caspase-3 monoclonal primary antibodies at 4°C overnight. After washing 3 times with 1× TBST for 10 min, the membranes were incubated with secondary antibodies on a rotary shaker for 1.5 h, and then washed 3 times with 1× TBST for 10 min for final chemiluminescence detection.
Statistical analysis
All statistical analyses were performed by using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± SD. One-way analysis of variance and Student’s t-test were used. A P-value <0.05 was considered statistically significant.
Results
Cell proliferation and relative colony forming efficiency
As shown in Figure 1, EET significantly inhibited the proliferation of HepG2 cells after 24 h (Figure 1A). As the concentration of EET was increased, cell proliferation rates gradually decreased by 10%, 32%, 53%, 63%, and 70%. Compared with the control group, the treatment groups showed significantly lower cell proliferation rates (P<0.05). EET significantly inhibited the proliferation of SMMC-7721 cells after 24 h (Figure 1B). Cell proliferation rates decreased by 82%, 80%, 77%, 70%, and 70% and were not concentration dependent. Compared with the controls, each group had significantly lower cell proliferation rates (P<0.05).
Figure 1.
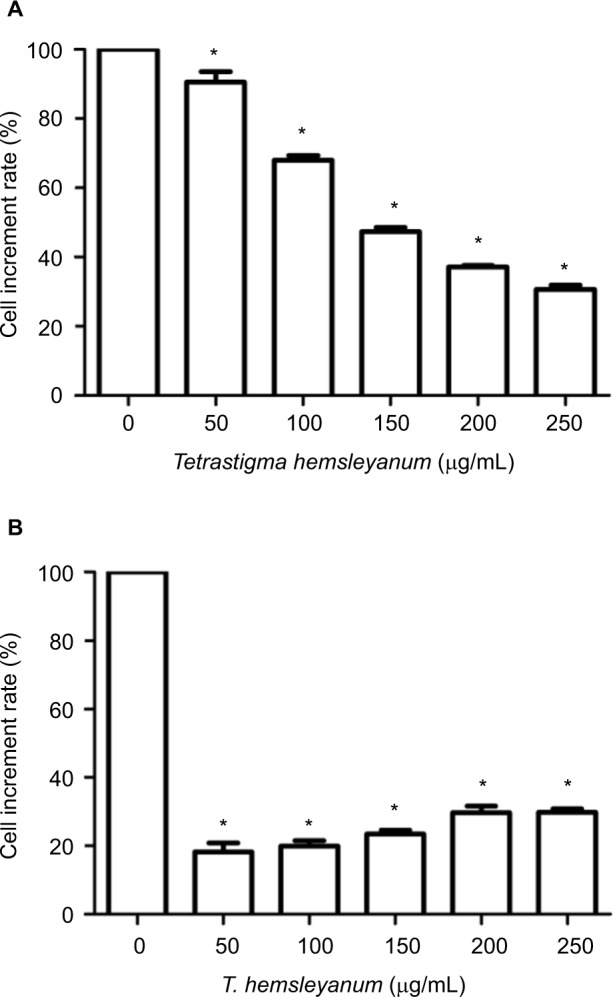
The inhibitory effects of EET on the proliferation rate of HepG2 cells (A) and SMMC-7721 cells (B).
Note: *P<0.05 compared with the control group.
Abbreviation: EET, ethylacetate extract from T. hemsleyanum.
As shown in Figure 2, the colony forming efficiency of HepG2 cells decreased to 55% or less 24 h after EET treatment (Figure 2A). The colony forming efficiency became 0% when few seeded cells were present. The colony forming ability gradually decreased as the concentration of EET was increased. Compared with the controls, each group showed significantly lower colony forming efficiency (P<0.05). The colony forming efficiency of SMMC-7721 cells decreased to 0% (Figure 2B). When 200 seeded cells or more were present, the colony forming efficiency was 16%–17% and was not concentration dependent. Compared with the control group, each treatment group showed a significantly lower colony forming efficiency (P<0.05).
Figure 2.
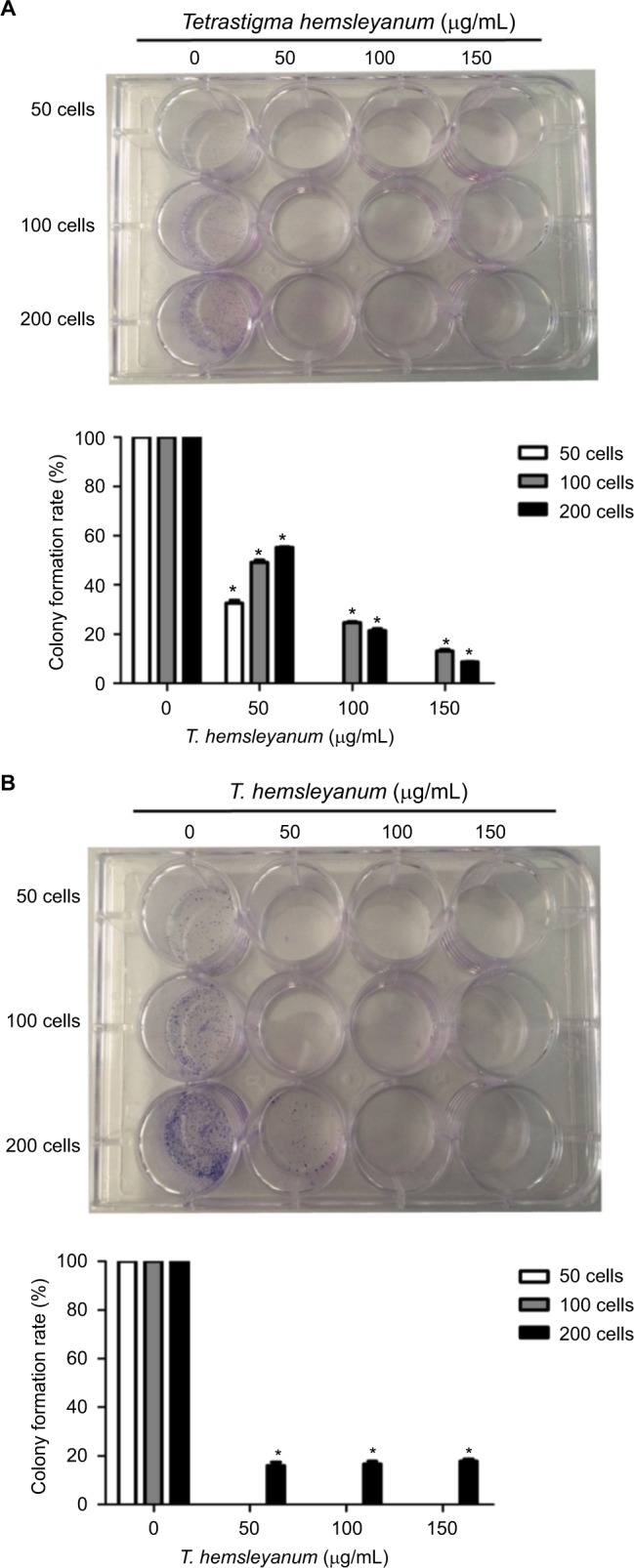
The inhibitory effects of EET on the colony forming efficiency of HepG2 cells (A) and SMMC-7721 cells (B).
Note: *P<0.05 compared with the control group.
Abbreviation: EET, ethylacetate extract from T. hemsleyanum.
Apoptosis
As shown in Figure 3, EET significantly increased the apoptotic rates of HepG2 cells after 24 h (Figure 3A). As the concentration of EET was increased, the apoptotic rates significantly increased by 16%, 21%, 15%, 11%, and 44% when compared to the controls (P<0.05). EET significantly increased the apoptotic rates of SMMC-7721 cells after 24 h (Figure 3B). Except for the concentration of 100 μg/mL, cell apoptotic rates significantly increased 12%, 5%, 3%, and 10% as the concentration of EET was increased. Compared with the control group, each treatment group indicated significantly higher apoptotic rates except for the concentration of 200 μg/mL (P<0.05).
Figure 3.
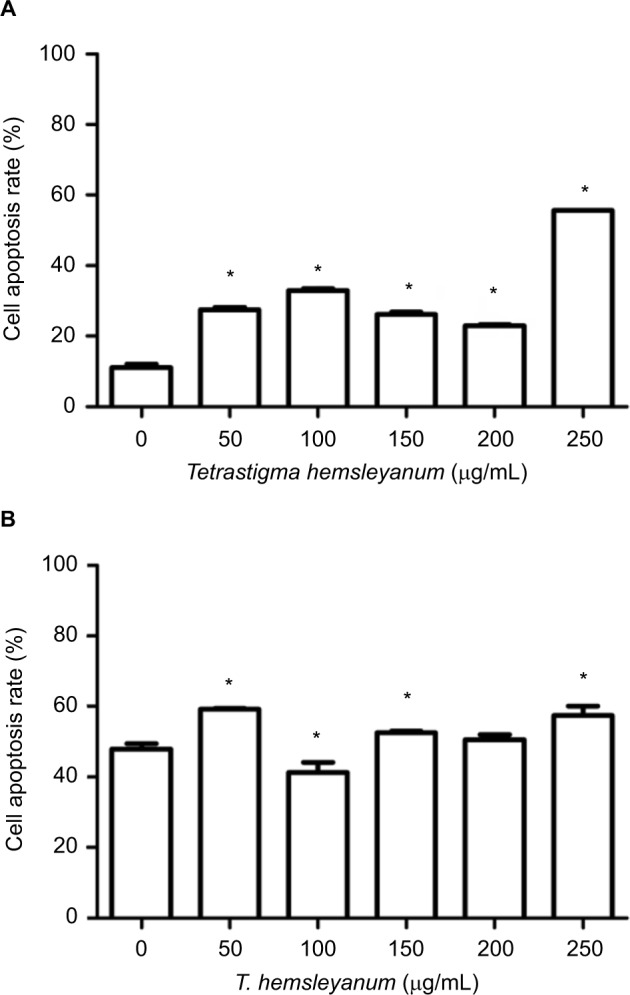
The effects of EET on cell apoptotic rates of HepG2 cells (A) and SMMC- 7721 cells (B).
Note: *P<0.05 compared with the control group.
Abbreviation: EET, ethylacetate extract from T. hemsleyanum.
Expression of apoptosis-related proteins
As shown in Figure 4, EET increased the protein expression of Bax in HepG2 cells after 24 h (Figure 4A). When the concentration of EET was 250 μg/mL, the protein expression levels of Bcl-2and Caspase-3 decreased. As shown in Figure 4B, the protein expression of Bax and Bcl-2 significantly decreased, whereas the expression of Caspase-3 increased in SMMC-7721 cells.
Figure 4.
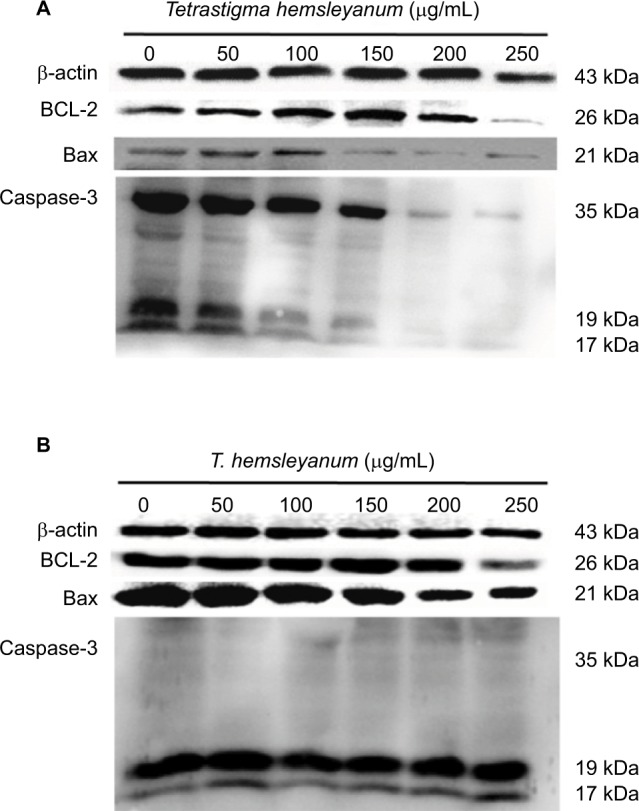
The effects of EET treatment for 24 h on protein expressions of Caspase-3, Bcl-2, and Bax of HepG2 cells (A) and SMMC-7721 cells (B).
Abbreviation: EET, ethylacetate extract from T. hemsleyanum.
Discussion
Tumor therapy has elicited considerable medical research, and traditional Chinese medicine and related nutritional support therapy have become a promising method of cancer treatment. Recently, numerous studies have investigated the antitumor mechanism of T. hemsleyanum. EET exerts a definitive inhibitory effect and toxicity on HepG2 cells. Our study found that EET significantly inhibited the proliferation rates of HepG2 and SMMC-7721 cells; this result is consistent with those of other studies.16,17 These findings suggest that EET inhibits the proliferation of HepG2 and SMMC- 7721 cells. Additionally, the colony forming efficiency was decreased, indicating that EET affects the adaptability and independent survival of HB or HCC cells.
Apoptosis is a process of programmed cell death that leads to morphological, genetic, and intracellular structural changes in the cells. Apoptosis is essential for the normal development and maintenance of the organism by removing physiologically unwanted cells to maintain homeostasis without harming the entire organism. The programmed cell deformation and death are regulated by apoptotic signals and transduction pathways. Cell overproliferation and insufficient apoptosis or normal cell proliferation with definitive weak apoptosis might cause tumors.18 Our results showed that EET significantly increased the apoptotic rates of HepG2 and SMMC-7721 cells compared with the controls. Other studies revealed that T. hemsleyanum induces tumor cell apoptosis.19–21 Therefore, we considered that EET induces the apoptosis of HepG2 and SMMC-7721 cells.
Many factors influence the cell apoptosis. The Caspase family, Bcl-2 gene family, and mitochondria are key points in signal transduction. As the most important protease in apoptosis, the Caspase family uses 2 types of inactive pro- enzymes, including a regulator and an effector. Caspase-3, which is an effector molecule of apoptosis, participates in the activation of Caspase by 2 different pathways, induces the downstream cascade amplification reaction, causes chromosome condensation, and leads to apoptosis-specific morphological changes.22,23
As the key element to regulate apoptosis, the Bcl-2 protein family consists of 2 groups, apoptosis-inducing proteins Bax, Bcl-xs, Bad, and Bak, and antiapoptotic proteins Bcl-2 and Bcl-xl. When cells are stimulated by death signals, apoptosis-inducing proteins undergo conformational changes under the action of certain proteases, translocate from the cytoplasm to organelles especially the outer membrane of the mitochondria, and interact with antiapoptotic proteins on the membrane and the intramembrane. The functional failure of antiapoptotic proteins causes the loss of cell function and the release of apoptosis-inducing factors and eventual cell apoptosis.24,25 In our study, expression of active components Caspase-3 increased and Bax decreased in SMMC-7721 cells, expression of active components Caspase-3 decreased and Bax increased in HepG2 cells, whereas the expression of Bcl-2 decreased in HepG2 and SMMC-7721 cells with an increase in EET concentration. Our findings indicate that EET regulates apoptosis via the apoptotic signaling pathway; this result is consistent with other studies.26,27
Conclusion
EET inhibits the proliferation and colony formation of HepG2 and SMMC-7721 cells and regulates apoptosis via the Caspase family and Bcl-2 gene family signaling pathways. EET exhibits a significant inhibitory effect on tumor cell proliferation and induces apoptosis. Our results provide a basis for the development of EET as a potential anticancer drug. In the future, we will continue to study the anticancer effect of T. hemsleyanum by the immune system to improve human immunity.
Acknowledgments
This work was supported by the Department of the Project of Fujian Province, China (No. K8114007A) and the Medicinal Plant Cultivation and Utilization of Fujian Agriculture and Forestry University Discipline Development Fund of Fujian Province, China (No. 61201400804).
Footnotes
Author contributions
Meixiu Luo, Liang Ma, and Wenjun Lin collected the raw data, and Meixiu Luo and Shipin Chen performed the statistical analysis and drafted the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Kasper HU, Longerich T, Stippel DL, Kern MA, Drebber U, Schirmacher P. Mixed hepatoblastoma in an adult. Arch Pathol Lab Med. 2005;129(1):234–237. doi: 10.5858/2005-129-234-MHIAA. [DOI] [PubMed] [Google Scholar]
- 3.Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB. Controlled synthesis of HbsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979;282(5739):615–617. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- 4.López-Terrada D, Cheung SW, Finegold MJ, Knowles BB. Hep G2 is a hepatoblastoma-derived cell line. Hum Pathol. 2009;40(10):1512–1515. doi: 10.1016/j.humpath.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Kumar B, Smita K, Seqqat R, Benalcazar K, Grijalva M, Cumbal L. In vitro evaluation of silver nanoparticles cytotoxicity on hepatic cancer (Hep-G2) cell line and their antioxidant activity: green approach for fabrication and application. J Photochem Photobiol B. 2016;159:8–13. doi: 10.1016/j.jphotobiol.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(201):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Ju JH, Lin G, Xv X, Yang J, Tu G. New C-glycosylflavones from Tetrastigma hemsleyanum (Vitaceae) Acta Bot Sin. 2002;44(2):227–229. [Google Scholar]
- 8.Huang Z, Mao QQ, Wei JP. Evaluation of anti-inflammatory, analgesic and antipyretic actions for the extracts from Radix Tetrastigmae. Chin New Drugs J. 2005;14:861–864. [Google Scholar]
- 9.Huang SW, Liang JY. Advances in studies on Tamarix plants. Strait Pharm. 2007;19(3):5. [Google Scholar]
- 10.Peng X, Zhuang DD, Guo QS. Induction of S phase arrest and apoptosis by ethylacetate extract from in human hepatoma HepG2 cells. Tumor Biol. 2015;36(4):2541–2550. doi: 10.1007/s13277-014-2869-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao YW, Li YF. Identification of the pharmacognosy of Tetrastigma hemsleyanum Diels et Gilg. J Basic Chin Med. 1998;12(3):7–8. [Google Scholar]
- 12.Zhang XY, Ling LQ, Mao QM. Studies on chemical constituents of Chinese tamarisk twing. Herbal Med. 1989;20(8):4. [Google Scholar]
- 13.Nawwar MA, Souleman AMA, Buddrus J, et al. Flavonoids of the flowers of Tamarix nilotica. Phytochemisty. 1984;23(10):2347. [Google Scholar]
- 14.Liu D, Yang JS. Studies on the chemical constituents of Tetrastigma hemsleyanum Diels et Gilg. Chin J Tradit Chin Med. 1999;24(10):611–612. [PubMed] [Google Scholar]
- 15.Li YQ, Lu WC, Yu ZG. Studies on the chemical constituents of Tetrastigma hemsleyanum Diels et Gilg. Herbal Med. 2003;34(11):982–983. [Google Scholar]
- 16.Li Y, Xiao YL, Chen CH, et al. The impact of curcumin of proliferation and metastasis on Hepatoblastoma cell lines HepG2. Chin J Gen Surg. 2014;23(1):117–120. [Google Scholar]
- 17.Fan X, Du H, Sun Y, et al. Suppression of the Wnt signaling pathway may contribute to the inhibition of proliferation of human hepatocellular carcinoma SMMC-7721 cells by esculetin. Oncol Lett. 2017;14(2):1731–1736. doi: 10.3892/ol.2017.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin HM, Wang JZ. Pathophysiology. Beijing: People’s Health Press; 2008. p. 125. [Google Scholar]
- 19.Peng X, Zhuang DD, Guo QS. Induction of S phase arrest and apoptosis by ethyl acetate extract from Tetrastigma hemsleyanum in human hepatoma HepG2 cells. Tumor Biol. 2015;36(4):2541–2550. doi: 10.1007/s13277-014-2869-x. [DOI] [PubMed] [Google Scholar]
- 20.Peng X, Zhang YY, Wang J, Ji Q. Ethylacetate extract from Tetrastigma hemsleyanum induces apoptosis via the mitochondrial caspase-dependent intrinsic pathway in HepG2 cells. Tumor Biol. 2016;37(1):865–876. doi: 10.1007/s13277-015-3579-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang LM, Fan RJ, Yang FQ. Experimental study of apoptosis of SMMC-7221 hepatoma cells induced by Tetrastigma hemsleyanum Diels et Gilg flavone. Shizhen Tradit Med. 2010;11(21):2850–2851. [Google Scholar]
- 22.Cheng EH, Kirsch DG, Clem RJ, et al. Conversion of Bcl-2 to a Bax- like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 23.Xiong SQ, Zhu XH. Caspase family and signal transduction of apoptosis mediated by Fas/APO-1. Mianyixue Zazhi. 1999;15:212–216. [Google Scholar]
- 24.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8(5):615–625. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 25.Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26(6):390–397. doi: 10.1016/s0968-0004(01)01844-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P, Huang CR, Wang W, et al. Harmine hydrochloride triggers G2 phase arrest and apoptosis in MGC-803 cells and SMMC-7721 cells by upregulating p21, activating caspase-8/Bid, and downregulating ERK/Bad pathway. Phytother Res. 2016;30(1):31–40. doi: 10.1002/ptr.5497. [DOI] [PubMed] [Google Scholar]
- 27.Liang XL, Li M, Li J, et al. WangEquol induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells through the intrinsic pathway and the endoplasmic reticulum stress pathway. Preclinical Rep. 2014;6(25):633–640. doi: 10.1097/CAD.0000000000000085. [DOI] [PubMed] [Google Scholar]


