Abstract
Background
In the past few years, new drugs made their appearance in the first-line setting of treatment for metastatic renal cell carcinoma (mRCC), and cabozantinib is one among them. The present systematic review aims to point out any evidence published to date about first-line treatment with cabozantinib for mRCC patients, describing their outcome in all end points explored by the literature.
Methods
PRISMA guidelines were followed. A systematic assessment of literature and peer- reviewed presentations was performed by searching PubMed and major oncology meeting resources, from the database inception until June 25, 2018. The following keywords were used: “cabozantinib or cabozantinib-s-malate or XL184” and “renal cell carcinoma or kidney cancer or clear cell renal carcinoma or renal cancer” and “first-line or untreated or treatment-naïve or primary treatment”. All types of original clinical studies were included, evaluating either cabozantinib monotherapy or any systemic drug combination containing cabozantinib for previously untreated patients with mRCC.
Results
From potential 75 titles and abstracts, seven publications were selected. One was the main report of a randomized clinical trial (the CABOSUN study); four papers reported updated results, secondary or subgroup analyses from the same study population; and further two reports consisted of network meta-analyses. From the additional search for ongoing clinical trials, six studies currently in progress were reported.
Conclusion
According to the reported evidence, cabozantinib may be a viable first-line option in mRCC patients with intermediate or poor risk according to International Metastatic Renal Cell Carcinoma Database Consortium model. It offers an undoubtful advantage in terms of progression-free survival, despite quite high rates of G3–4 toxicity, modest objective response rate, and no survival advantage. Nevertheless, given the availability of an immunotherapy combination that significantly improved overall survival for the same population in a Phase III trial and the indisputable efficacy of cabozantinib as second-line treatment, this drug may be devoted as a rescue option in patients progressive to primary therapy.
Keywords: cabozantinib, renal cell carcinoma, first-line treatment, CABOSUN
Introduction
Kidney cancer is the 12th most common cancer in the world.1 The European regions with the highest incidence reported rates from 13.5/100,000 to 31.4/100,000 person- years in men, with approximately half rates among women.2 The American Cancer Society estimates that about 63,340 new cases of kidney cancer will occur and 14,970 people will die from this disease in the US in 2018.3 These numbers include all types of kidney and renal pelvis cancers. Renal cell carcinoma (RCC) with clear cell histology is the most common type of kidney cancer in adults.4
If detected in its early stages, the 5-year survival rate for RCC is high; for patients with advanced or late-stage metastatic RCC (mRCC), however, the 5-year survival rate falls to 12%, with no identified definitive cure for the disease.5
The majority of clear-cell RCC tumors have lower than normal levels of a protein called von Hippel–Lindau, which leads to higher levels of hepatocyte growth factor receptor (MET), anexelekto (AXL), and vascular endothelial growth factor (VEFG).6–8 These proteins promote tumor angiogenesis, growth, invasiveness, and metastasis.7–9
The landscape of primary systemic treatment for mRCC, previously exclusively constituted by vascular endothelial growth factor receptor (VEGFR) inhibition, was recently enriched by new options. In the past few years, new drugs with new mechanisms of action made their appearance in this setting, introducing new possibilities in the range of choice for mRCC primary therapy, at least for certain subgroups. Immune checkpoint inhibitors (CKIs), namely, the combination of nivolumab and ipilimumab,10 and the novel multitarget tyrosine kinase inhibitor (TKI) cabozantinib11 were recently approved by US FDA as frontline treatment options, since they both demonstrated superiority compared to the current standard of therapy, represented by the VEGFR TKI sunitinib.10,12
In US, cabozantinib is approved for all settings of mRCC, while nivolumab combined with ipilimumab is approved for first-line treatment of poor- and intermediate-risk mRCC patients. In Europe, the Committee for Medicinal Products for Human Use (CHMP), after prior approval for second (and subsequent) treatment line, approved cabozantinib for the first-line treatment of adults with intermediate- or poor- risk advanced RCC, based on the results from the randomized Phase II CABOSUN trial in patients with previously untreated mRCC.12 On the other hand, following publication of the results of the CheckMate-214 Phase III study,10 the CHMP adopted a negative opinion about the combination of nivolumab and ipilimumab in the same setting, due to alleged doubts about the contribution of ipilimumab and the risk–benefit profile.10
Both cabozantinib and CKIs demonstrated their efficacy and superiority over the first-line comparator in the sub- population of patients with intermediate- or poor-risk per International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria, unexpectedly transforming this prognostic score into a possible predictive model.10,12
Cabozantinib is an oral small molecule inhibitor of multiple receptor tyrosine kinase, with activity toward VEGFR-2, MET, RET (rearranged during transfection), KIT (mast/stem cell growth factor receptor), AXL, TIE2 (angiopoietins receptor), and FLT3 (Fms-like tyrosine kinase), important mediators of tumor cell survival, metastasis, and tumor angiogenesis.11 The activation of AXL and MET signaling potentially represents a mechanism of primary or secondary resistance to traditional antiangiogenic agents, used today as first-line treatment for mRCC. This suggests that the added value of cabozantinib beyond VEGFR inhibition could be crucial to improve its clinical efficacy.13
As long as CKIs demonstrated their superiority in this setting in a Phase III randomized trial, cabozantinib was approved on the basis of a Phase II randomized study (namely the CABOSUN trial), after previous demonstration of efficacy in pretreated mRCC patients from a Phase III trial.14 Despite some concerns about the strength of evidence for the use of cabozantinib as first-line therapy,15 its regular approval also for second (and subsequent)-line setting did not discourage its potential employment in the early phase of the metastatic disease, reserving immunotherapy for a later time in selected patients.
The aim of the present systematic review was to point out any evidence published to date about first-line treatment with cabozantinib for mRCC patients, describing their outcome in all end points explored by the literature. The use of cabozantinib as first-line therapy in currently ongoing clinical trials was also explored, with the intent to offer an overview about the future development of the drug in this setting.
Methods
PRISMA guidelines were followed for the present systematic review.16 A systematic assessment of literature and peer-reviewed presentations was performed by searching PubMed (MEDLINE) and major oncology meeting (ASCO.org and ESMO.org) resources, from the database inception until June 25, 2018. The references of the included article were also reviewed for any further potential publication. The search was then implemented, with explorative intent, by the systematic check of the ongoing clinical trials from clinicaltrials.gov and from the “trial in progress” section of American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) meeting libraries (performed on June 25, 2018). All authors independently performed the search, to increase accuracy and to minimize the subjectivity-related bias.
The following keywords were used: “cabozantinib or cabozantinib-s-malate or XL184” and “renal cell carcinoma or kidney cancer or clear cell renal carcinoma or renal cancer” and “first-line or untreated or treatment-naïve or primary treatment”. The first study selection was made in consensus by all authors. Publications not primarily published in English were excluded. All types of original clinical studies were included, namely retrospective studies, prospective trials of any phase, meta-analyses, and network meta-analyses (NMA). Preclinical studies, case reports, reviews without meta-analysis, letters, editorials, and any other narrative paper were excluded. Automatic filters were avoided: a manual selection of publications was performed after reading all titles and abstracts. Full text of the selected publications was obtained, and the content was then tabulated and summarized by one author for the final selection [MB]). All discrepancies were resolved by consensus by two reviewers (MB and SB). Studies evaluating cabozantinib only in pretreated patients (with any other systemic agent for RCC) were excluded; in case of heterogeneous study populations, the results for the subgroups of interest were included if the primary end point of the study was available for this subpopulation. All studies evaluating either cabozantinib monotherapy or any systemic drug combination containing cabozantinib for previously untreated patients with advanced renal cancer were included in the review. All types of end points about efficacy and safety of the drug were considered; ancillary studies with exploratory end points were also reported in the review. In case of multiple publications about the same clinical trial, only duplicates were excluded, while reports about different end points or aspects of the same study (eg, post hoc analyses) were considered separately.
Different study types were grouped and reported together according to their methodological similarities. Due to the broad inclusion criteria, which allowed for the inclusion of extremely different studies in terms of methodology and strength of evidence, we expected high heterogeneity among the included studies. Thus, no meta-analysis was scheduled, and a narrative synthesis of the results is provided.
Results
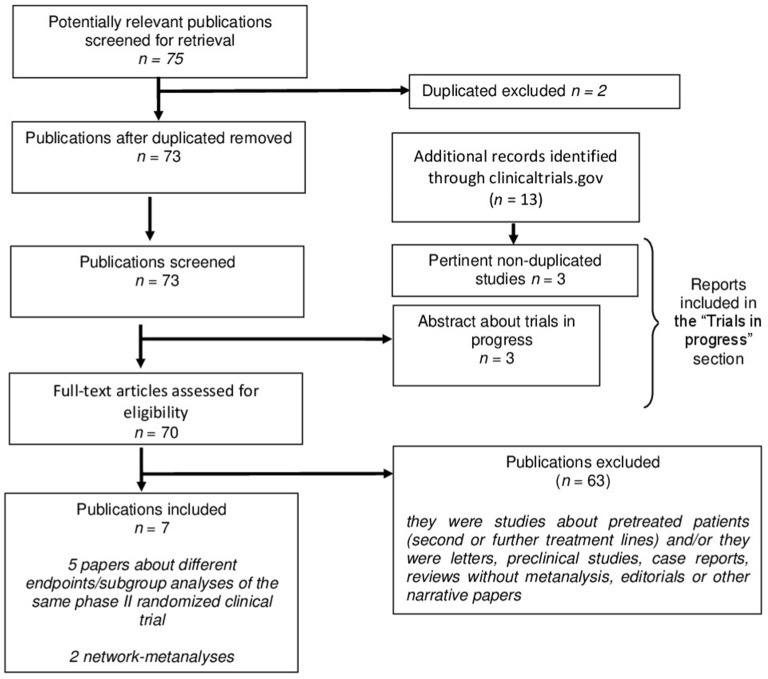
The literature search initially identified 75 titles and abstracts of potential studies to be included. Using the pre-specified criteria, seven publications were selected for the present review (Figure 1 shows the study selection protocol). Of them, only one was the main report of a randomized clinical trial, namely the CABOSUN study.12 Further four papers reported updated results, secondary or subgroup analyses from the same study population.17–20 The other two reports included NMA of cabozantinib and its comparators in first- line setting for RCC patients.21,22 Publications included in the review are presented in Table 1.
Figure 1.
Flow diagram of the study selection.
Note: Duplicated excluded n=2.
Table 1.
Publications of completed clinical studies about treatment with cabozantinib in first-line setting for advanced renal cell carcinoma patients
| Author and year | Study type | Type of patients | Phase | Comparators | Number of patients and/or studies | Primary end point | Secondary end points | Outcomes in favor of cabozantiniba |
|---|---|---|---|---|---|---|---|---|
| Choueiri et al, 201712 | Randomized multicenter trial | Naïve, intermediate, and poor risk | II | Sunitinib | 157 | PFS | ORR, OS, safety | PFS, ORR |
| Choueiri et al, 201817 | Update and IRC assessment of a randomized trial | Naïve, intermediate, and poor risk | II | Sunitinib | 156b | PFS | ORR, OS, safety | PFS, ORR |
| George et al, 201818 | Subgroup analyses of a randomized trial | Naïve, intermediate, and poor risk | II | Sunitinib | 157 | PFS basing on IMDC risk group, bone metastases, age, sex, ECOG PS, MET status | ORR basing on IMDC risk group, bone metastases, age, sex, ECOG PS, MET status | PFS and ORR in all subgroups |
| Feldman et al, 201819 | HRQoL post hoc analysis of a randomized trial | Naïve, intermediate, and poor risk | II | Sunitinib | 157 | ECOG PS score pre- and post- PD | ECOG score deterioration | – |
| Chen et al, 201820 | Q-TWiST post hoc analysis of a randomized trial | Naïve, intermediate, and poor risk | II | Sunitinib | 157 | Q-TWiST | – | NAc |
| Schmidt et al, 201821 | Network meta-analysis | Favorable, intermediate, and poor risk | NA | Sunitinib, sorafenib, IFN, bevacizumab plus IFN, temsirolimus | 13 | PFS | OS | PFS |
| Wallis et al, 201822 | Network meta-analysis | Favorable, intermediate, and poor risk | NA | NA Nivolumab plus ipilimumab, axitinib, sorafenib, sunitinib, atezolizumab plus bevacizumab | 10 (4,819 patients) | PFS | OS, AEs | PFS |
Notes:
Only statistically significant differences were considered;
one patient of 157 was not assessed by IRC;
statistical significance not assessed (descriptive data favored cabozantinib for Q-TWiST).
Abbreviations: PFS, progression-free survival; ORR, objective response rate; OS, overall survival; IRC, independent radiologic review committee; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; HRQoL, health-related quality of life; PD, progression of disease; Q-TWiST, quality-adjusted time without symptoms of disease or toxicity of treatment; NA, not applicable; AEs, adverse events; ECOG, Eastern Cooperative Oncology Group; PS, performance status; IFN, interferon.
Following an additional search for ongoing clinical trials, six studies which are currently in progress were selected, the details of which are reported in the section “Trials in progress” (Table 2).23–28
Table 2.
Trials in progress with the use of cabozantinib for previously untreated patients with advanced renal cancer
| Trial number and/or name | Study type | Phase | Disease | Setting | Treatment arms | Estimated enrollment (No of patients) | Primary end point | Start date | Estimated completion date |
|---|---|---|---|---|---|---|---|---|---|
| NCT0317096023 | Single-arm, dose-escalation | Ib-II | UC ccRCC NSCLC CRPC | First-line for ccRCC (expansion of cohort 1) | Cabozantinib plus atezolizumab | 360 (total) | ORR (expansion of cohorts) | 2017 | 2020 |
| NCT03141177 CheckMate 9ER24 | Multicenter, randomized, open-label | III | RCC with a clear- cell component | First-line | Nivolumab plus cabozantinib vs sunitinib | 630 | PFS | 2017 | 2023 |
| CABOPRE25 | Multicenter, single-arm | II | RCC with a clear- cell component, suitable for CN | First-line | Cabozantinib for 12 weeks before CN and then until PD | 50 | ORR at 12 weeks | NA | NA |
| NCT02761057 PAPMET26 | Multicenter, randomized | II | Papillary RCC | First- and second-line | Sunitinib vs cabozantinib vs crizotinib vs volitinib | 180 | PFS | 2016 | 2020 |
| NCT03354884 BONSAI27 | Single-center, single-arm | II | Collecting ducts carcinoma | First-line | Cabozantinib | 23 | ORR | 2018 | 2020 |
| NCT03541902 CABOSUN II28 | Multicenter, randomized | II | Variant histology RCC (papillary, chromophobe, Xp.11 translocation, undifferentiated, unclassified) | First-line | Cabozantinib vs sunitinib | 84 | PFS | 2018 | 2020 |
Abbreviations: No, number; UC, urothelial carcinoma; ccRCC, clear-cell renal cell carcinoma; NSCLC, non-small-cell lung cancer; CRPC, castration-resistant prostate cancer; ORR, objective response rate; RCC, renal cell carcinoma; PFS, progression-free survival; CN, cytoreductive nephrectomy; PD, progression of disease; NA, not available.
Clinical trials
To date, the only clinical trial that electively used cabozantinib in the first-line setting for RCC patients is represented by the CABOSUN study.12 It was a randomized, Phase II clinical trial enrolling untreated mRCC patients whose tumors had a clear cell component, classified as intermediate or poor risk by IMDC criteria. Patients with a performance status (PS) of 0–2 were eligible. Patients were stratified by IMDC risk category and presence of bone metastases and randomly assigned to receive cabozantinib (administered orally, 60 mg once per day) or sunitinib (administered orally, 50 mg once per day with the classical schedule of 4 weeks on, 2 weeks off). The primary end point was duration of progression-free survival (PFS); secondary end points were overall survival (OS), objective response rate (ORR), and safety. Overall, 157 patients were enrolled, with balanced treatment groups. According to the first report of the study, from the investigators’ assessment, the median PFS was 8.2 months (95% CI 6.2–8.8) with cabozantinib and 5.6 months (95% CI 3.4–8.1) with sunitinib.12 Hazard ratio (HR) was 0.66 (95% CI 0.46–0.95, P=0.012), demonstrating that cabozantinib reduced the rate of disease progression or death by 34% compared with sunitinib. The ORR was 33% with cabozantinib and 12% with sunitinib.12
These results were subsequently updated and adjusted per independent radiology review committee (IRC) assessment, retrospectively performed, confirming the superiority of cabozantinib over sunitinib with respective PFS of 8.6 months (95% CI 6.8–14) vs 5.3 months (95% CI 3.0–8.2), HR=0.48 (95% CI 0.31–0.74), P=0.0008. Confirmed objective responses per IRC were observed in 20% of patients in the cabozantinib group vs 9% in the sunitinib group (all were partial responses).17
The difference between groups in terms of OS was not statistically significant: median OS was 26.6 months (95% CI 14.6–not reached) with cabozantinib and 21.2 months (95% CI 16.3–27.4) with sunitinib, HR=0.80 (95% CI 0.53–1.21).17
The incidence of adverse events (AEs) was similar in both groups: regardless of causality, 96% of patients experienced AEs of any grade with cabozantinib and 99% with sunitinib; the incidence of grade 3 or 4 AEs was 68% with cabozantinib and 65% with sunitinib. The most common grade 3–4 AEs with cabozantinib were hypertension (28%), diarrhea (10%), palmar-plantar erythrodysesthesia (8%), and fatigue (6%). Dose reductions occurred for 46% of cabozantinib-treated subjects and 35% of sunitinib-treated patients. Median duration of exposure was 6.5 months for cabozantinib-treated patients and 3.1 months for sunitinib-treated patients.17
In the same updated report of the study, subgroup analyses of PFS per IRC assessment based on MET expression level were reported: for MET-positive patients, median PFS was 13.8 months (95% CI 5.7–22.1) with cabozantinib and 3.0 months (95% CI 2.5–5.4) with sunitinib, HR =0.32 (95% CI 0.16–0.63). For MET-negative patients, median PFS was 6.9 moths (95% CI 5.4–14.6) with cabozantinib and 6.1 months (95% CI 3.6–9.6) with sunitinib, not reaching a statistically significant difference (HR =0.67 [95% CI 0.37–1.23]).17
Subgroup analyses based on stratification factors have been subsequently presented at 2018 ASCO Annual Meeting, pending full publication, as well as post hoc analyses on ECOG score as a proxy for health-related quality of life assessment and on quality-adjusted time without symptoms or toxicity (Q-TWiST).18,20
According to subgroup analyses based on stratification factors (age, sex, baseline ECOG PS, and MET tumor expression by immunohistochemistry), the HR for PFS per IRC favored cabozantinib over sunitinib across all subgroups analyzed. Similarly, odds ratios for ORR favored cabozantinib, with the highest response rate in the MET-positive subgroup (34% vs 10% with sunitinib).18
The post hoc analysis with Q-TWiST methodology revealed that cabozantinib was associated with longer Q-TWiST compared with sunitinib (73 days in favor of cabozantinib), primarily due to longer time without symptoms of disease (121 days longer with cabozantinib) and to shorter time after progression or relapse (104 days longer for sunitinib) than to time spent with grade 3–4 toxicities (8 days longer for cabozantinib).20
Based on the results of a post hoc analysis that preliminarily reported about ECOG PS, the authors pointed out that the pre-progression period was associated with better PS compared to that achieved in the post-progression period, regardless of the treatment arm. The risk of deterioration to poor PS was reduced by cabozantinib, but this result was not statistically significant (HR =0.44, 95% CI 0.16–1.26).19
NMA
In the last year, two NMA have been published regarding first-line treatment of mRCC, none of them citing each other.
The first, by Schmidt et al, directly compares cabozantinib with other first-line standard-of-care drugs, such as sunitinib, sorafenib, temsirolimus, interferon (IFN), or bevacizumab plus IFN, respectively.21 The NMA included 13 trials; considering that the unique clinical trial with cabozantinib in this setting enrolled only poor- and intermediate-risk patients, while other studies used in this NMA also included favorable-risk cases, both the overall study populations and the subgroup of interest were explored. In either case, cabozantinib provided longer PFS than all alternative therapies, while the improvement in OS was not statistically significant. In intermediate-risk patients, HRs (95% CI) for PFS were respectively 0.52 (0.33–0.82), 0.46 (0.26–0.8), 0.20 (0.12–0.36), and 0.37 (0.20–0.68) when cabozantinib was compared with sunitinib, sorafenib, IFN, or bevacizumab plus IFN. In poor-risk patients, the NMA also demonstrated better PFS for cabozantinib; HRs were 0.31 (0.11–0.90), 0.22 (0.06–0.87), 0.16 (0.04–0.64), and 0.2 (0.05–0.88) when it was compared with sunitinib, temsirolimus, IFN, or bevacizumab plus IFN. Although overall populations included favorable-risk patients in some studies, the overall results were consistent with the subgroup analyses. In particular, the HR for PFS also significantly favored cabozantinib over both pazopanib and sunitinib (HR =0.48, 95% CI 0.3–0.75 and HR =0.48, 95% CI 0.31–0.74, respectively). The authors concluded that cabozantinib is a promising first-line treatment for mRCC compared to available standard-of-care options, with clinical implications for intermediate- and poor-risk patients. 21
A little more recently, Wallis et al similarly performed NMA on the same topic, although including different clinical trials in first-line setting for mRCC.22 They selected studies using “clinically relevant” comparisons, based on input of content experts. Moreover, they included newer trials testing immunotherapy with CKI in the same setting. This NMA compared nivolumab plus ipilimumab with cabozantinib, axitinib, sorafenib, sunitinib, and atezolizumab plus bevacizumab. According to their results, based on ten trials with 4,819 patients, PFS differed in a significant manner only for those patients who received cabozantinib (HR =0.48, 95% CI 0.31–0.74). When compared to cabozantinib, all other treatments had significantly worse PFS except axitinib, tivozanib, and pazopanib alternating with everolimus. Basing on the surface under the cumulative ranking curves (SUCRA method), there was a 91% probability that cabozantinib had the greatest PFS. Nevertheless, on indirect comparison, there was no difference in OS between nivolumab plus ipilimumab and cabozantinib (HR =1.2, 95% CI 0.84–1.6), and based on the analysis of SUCRA, there was a 48% probability that nivolumab plus ipilimumab was the preferred option with respect to OS (whilst sunitinib was likely to be the lowest choice). Since inclusion criteria basing on patients’ risk differed between studies, also in this case the authors performed a further sensitivity analysis for PFS and OS assessing only patients with intermediate- and poor-risk disease. This included two studies that compared nivolumab plus ipilimumab and cabozantinib, respectively, with sunitinib. These results recapitulated the primary analysis: there was a 99% probability that cabozantinib was the preferred choice for PFS and an 85% probability that nivolumab plus ipilimumab was the preferred choice for OS.22
Trials in progress
Currently, six clinical trials are in progress with the use of cabozantinib for untreated mRCC patients.23–28 Two of these studies use cabozantinib as monotherapy. Four of them investigate its combination to other drugs with different mechanism of action. Three studies enroll patients whose tumors had at least a clear-cell component, while three other studies are tailored for non-clear-cell histologies. The characteristics of these trials are summarized in Table 2.
Discussion
The drug profile that emerges from the present review is undoubtedly quite interesting, configuring cabozantinib as a viable first-line option for mRCC patients with intermediate or poor risk according to IMDC model. Indeed, it offers an undoubtful advantage in terms of PFS, despite quite high rates of G3–4 toxicity, modest ORR, and no survival advantage.12,17 The NMA comparing this drug to other TKIs used today for untreated mRCC patients, as well as to the new treatment options with CKIs, confirmed that cabozantinib could represent the best choice for PFS, with non-significant penalty in terms of OS.21,22 Finally, the subgroup analyses of the treated population suggest that a biomarker-driven approach, using MET expression to select patients for treatment, may potentially strengthen the outcomes of treatment with this drug.18
Nevertheless, summarizing the findings from the present systematic review, it is clear that all the evidence for the use of cabozantinib for untreated mRCC patients comes from a single Phase II study. The CABOSUN trial represented the first break of the primacy of sunitinib as first-line treatment for mRCC, demonstrating cabozantinib superiority in terms of PFS. The merit of testing a new drug against the probably stronger comparator in Phase II has been widely disappointing by the lack of continuation in a Phase III study. This non- negligible flaw entails the permanence of legitimate doubts about the strength of evidence required to definitely change clinical practice. Indeed, the good results of the CABOSUN study can be interpreted as controversial, due to the relatively small sample size (157 patients overall), relatively poor efficacy of the control arm compared to that would be expected (both in terms of ORR and OS), low absolute rate of objective response (20%), and not statistically significant result in terms of OS (probably due to high rates of crossover and to the fact that the study was neither designed nor powered to test such secondary end point).15 Despite these concerns, the trial was well-designed in a comparative fashion, with a good primary end point (PFS) for a Phase II study, and above all it was randomized, thus increasing the likelihood of a successful middle-phase clinical trial. An additional merit of the CABOSUN study was represented by the enrollment of patients with ECOG PS =2, often excluded by Phase III trials, with the consequent advantage for cabozantinib of being the only drug with tangible data in this subgroup. However, it was just a middle-phase study, and despite good results, the refrain that good Phase II results do not predict Phase III success does not constitute an empty affirmation. The percentage of “confirmatory” Phase III trials that fail is unexpectedly high, about 50%.29,30 In a recent PAREXEL analysis, data on 38 Phase III failures were collected and evaluated from mid-2012 through 2015 from a variety of publicly available sources. All these trials, that collectively enrolled nearly 150,000 patients, failed to meet primary or secondary efficacy end points.31
On the other hand, the combination of nivolumab and ipilimumab was investigated in the same setting in a Phase III trial enrolling more than a thousand patients. At a median follow-up of 25.2 months, the 18-month OS rate was 75% with nivolumab plus ipilimumab and 60% with sunitinib; the median OS was not reached at 26 months (HR for death, 0.63; P<0.001). Furthermore, the experimental arm reached the high ORR of 42%, including 9% of complete responses.10
Conclusion
Given the availability (at least in the US countries) of an immunotherapy combination that demonstrated to significantly improve OS for untreated intermediate- and poor-risk mRCC patients, meeting its primary end point in a high-quality Phase III trial, cabozantinib may be considered as a first-line alternative to nivolumab plus ipilimumab only in selected cases for which they are contraindicated. On the other hand, the outstanding results obtained by cabozantinib as second- line treatment, in terms of ORR, PFS, and OS,14 undoubtedly devote this drug as a rescue option in patients progressive to primary therapy. However, the results of Phase III clinical trials testing its combination with immunotherapy are still pending, potentially offering cabozantinib a second chance to be included in the first-line treatment of mRCC patients.
Footnotes
Disclosure
All authors received honoraria as consultants and as speakers at scientific events by Bristol-Myers Squibb (BMS), IPSEN, Pfizer and Novartis. Also, they all received grants for participation at scientific events by BMS, IPSEN, Pfizer and Novartis. The authors report no other conflicts of interest in this work.
References
- 1. Wcrf.org [webpage on the Internet] Kidney cancer statistics. [Accessed July 21, 2018]. [cited July 20, 2018]. Available from: https://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/kidney-cancer-statistics.
- 2.Li P, Znaor A, Holcatova I, et al. Regional geographic variations in kidney cancer incidence rates in European countries. Eur Urol. 2015;67(6):1134–1141. doi: 10.1016/j.eururo.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 3. Cancer.org [webpage on the Internet]. American Cancer Society Key Statistics about kidney cancer. [Accessed July 21, 2018]. [cited July 20, 2018]. Available from: https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html.
- 4.Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society . Cancer Facts & Figures. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 6.Harshman LC, Choueiri TK. Targeting the hepatocyte growth factor/c-Met signaling pathway in renal cell carcinoma. Cancer J. 2013;19(4):316–323. doi: 10.1097/PPO.0b013e31829e3c9a. [DOI] [PubMed] [Google Scholar]
- 7.Rankin EB, Fuh KC, Castellini L, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111(37):13373–13378. doi: 10.1073/pnas.1404848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koochekpour S, Jeffers M, Wang PH, et al. The von Hippel-Lindau tumor suppressor gene inhibits hepatocyte growth factor/scatter factor- induced invasion and branching morphogenesis in renal carcinoma cells. Mol Cell Biol. 1999;19(9):5902–5912. doi: 10.1128/mcb.19.9.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa M, Emoto A, Hanada T, Nasu N, Nomura Y. Tubulogenesis by microvascular endothelial cells is mediated by vascular endothelial growth factor (VEGF) in renal cell carcinoma. Br J Urol. 1997;79(5):681–687. doi: 10.1046/j.1464-410x.1997.00140.x. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Tannir NM, McDermott DF, et al. CheckMate 214 Investigators Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacy SA, Miles DR, Nguyen LT. Clinical Pharmacokinetics and Pharmacodynamics of Cabozantinib. Clin Pharmacokinet. 2017;56(5):477–491. doi: 10.1007/s40262-016-0461-9. [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35(6):591–597. doi: 10.1200/JCO.2016.70.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687–2697. doi: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choueiri TK, Escudier B, Powles T, et al. METEOR investigators Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 15.Buti S, Bersanelli M. Is Cabozantinib Really Better Than Sunitinib As First-Line Treatment of Metastatic Renal Cell Carcinoma? J Clin Oncol. 2017;35(16):1858–1859. doi: 10.1200/JCO.2016.71.6506. [DOI] [PubMed] [Google Scholar]
- 16. Prisma-statement.org [webpage on the Internet] Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. [Accessed July 21, 2018]. [cited July 20, 2018]. Available from: http://prisma-statement.org/PRISMAStatement/
- 17.Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression- free survival by independent review and overall survival update. Eur J Cancer. 2018;94:e125. doi: 10.1016/j.ejca.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George DJ, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib for previously untreated patients with advanced renal cell carcinoma (RCC) of intermediate or poor risk: Subgroup analysis of progression- free survival (PFS) and objective response rate (ORR) in the Alliance A031203 CABOSUN trial. J Clin Oncol. 2018;36(Suppl 6):582. [Google Scholar]
- 19.Feldman DR, Feully M, Meng J, et al. ECOG score analysis as a proxy for health-related quality of life assessment in patients with poor or intermediate risk metastatic renal cell carcinoma from the CABOSUN trial (Alliance A031203) J Clin Oncol. 2018;36(15 Suppl) [Google Scholar]
- 20.Chen RC, Feully M, Meng J, et al. Quality-adjusted time without symptoms or toxicity (Q-TWiST): Analysis of cabozantinib (Cabo) vs sunitinib (Sun) in patients with advanced renal cell carcinoma (aRCC) of intermediate or poor risk (Alliance A031203) J Clin Oncol. 2018;36(Suppl) abstr 4556. [Google Scholar]
- 21.Schmidt E, Lister J, Neumann M, et al. Cabozantinib Versus Standard- of-Care Comparators in the Treatment of Advanced/Metastatic Renal Cell Carcinoma in Treatment-naïve Patients: a Systematic Review and Network Meta-Analysis. Target Oncol. 2018;13:205–216. doi: 10.1007/s11523-018-0559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallis CJD, Klaassen Z, Bhindi B, et al. First-line Systemic Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis. Eur Urol Oncol. 2018;74(3):309–321. doi: 10.1016/j.eururo.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 23. Clinicaltrials.gov Study of cabozantinib in combination with atezolizumab to subjects with locally advanced or metastatic solid tumors. [Accessed July 20, 2018]. [updated June 4, 2018; cited July 20, 2018]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03170960?term=NCT03170960&rank=1. NLM identifier: NCT03170960.
- 24.Choueiri TK, Apolo A, Powles T, Escudier B, Aren OR, Shah A. A phase 3, randomized, open-label study of nivolumab combined with cabozantinib vs sunitinib in patients with previously untreated advanced or metastatic renal cell carcinoma (RCC; CheckMate 9ER) J Clin Oncol. 2018;36(Suppl) abstr TPS4598. [Google Scholar]
- 25.de Velasco G, González B, Alonso T, et al. CABOPRE: Phase II study of cabozantinib prior to cytoreductive nephrectomy (CN) in locally advanced and/or metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2018;36(Suppl) abstr TPS4603. [Google Scholar]
- 26. Clinicaltrials.gov Cabozantinib S-malate, crizotinib, volitinib, or sunitinib malate in treating patients with locally advanced or metastatic kidney cancer. [Accessed July 20, 2018]. [cited July 20, 2018]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02761057?term=NCT02761057&rank=1. NLM identifier: NCT02761057.
- 27.Procopio G, Ratta R, Fuc G, et al. A phase 2 study of cabozantinib as first-line treatment in collecting ducts renal cell carcinoma: The BONSAI trial. J Clin Oncol. 2018;36(6 Suppl):TPS709. [Google Scholar]
- 28. Clinicaltrials.gov Cabozantinib Versus sunitinib for metastatic variant histology renal cell carcinoma. 1902. [Accessed July 20, 2018]. [updated July 16, 2018; cited July 20, 2018]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03541902?term=NCT03541902&rank=1. NLM identifier: NCT0354.
- 29.Sacks LV, Shamsuddin HH, Yasinskaya YI, et al. Scientific and regulatory reasons for delay and denial of FDA approval of initial applications for new drugs, 2000–2012. JAMA. 2014;311(4):378–384. doi: 10.1001/jama.2013.282542. [DOI] [PubMed] [Google Scholar]
- 30.Grignolo A, Pretorius S. Phase III Trial Failures: Costly, But Preventable. Appl Clin Trial. 2016;25(8):36–42. [Google Scholar]
- 31.Grignolo A, Pretorius S. Phase III trial failures: costly, but preventable. Applied Clinical Trials. 2016;25:8. [Google Scholar]