Abstract
Introduction
Hemodialysis treatment requires anticoagulation to prevent thrombosis of the dialyzer. The Hydrolink® (NV series; Toray) has been designed to reduce thrombotic complications by increasing membrane hydrophilic properties. Previous studies have confirmed reduced platelet activation, improved removal of β2-microglobulin and excellent small-solute removal.
Methods
We designed a prospective, multi-centered, randomized clinical study to compare the antithrombogenic effects (platelet count) of NV dialyzers versus conventional treatment. To compare the possibility of performing heparin-free dialysis, we carried out progressive heparin reduction tests. Patients with an average platelet count lower than 170,000 cells/mm3 using standard high flux membranes in the 6 months prior to the study were enrolled and randomized. Patients were either dialyzed for 6 months without changing the previous membrane (control group) or treated with the Hydrolink® membrane (NV group). After the third week, the heparin reduction test was conducted for 5 weeks in order to assess the minimum amount of anticoagulant needed to safely perform a 4-hour dialysis treatment. Performance and safety were evaluated measuring platelet count and activation, middle-molecule removal rate and nutritional status.
Results
We found no significant difference in platelet count, platelet activation factors β-thromboglobulin and platelet factor 4 (PF-4), between the groups. More patients in the study group reached heparin-free dialysis without clotting events during the heparin reduction test. The NV dialyzers displayed anti-thrombogenic effects as compared to conventional dialyzers.
Conclusions
The NV dialyzer series is safe with no adverse events reported. Further studies are required to understand the mechanisms of anti-thrombogenic effects.
Keywords: Anticoagulation, Antithrombogenic surface, Dialysis membrane, Hemocompatibility, Heparin, Platelet count
Introduction
Biocompatibility and solute clearance are the mainstays in the design of the artificial kidney. Membrane composition and structure are important determinants of these aspects. Current hemodialysis membranes are composed of synthetic polymers such as polysulfone (PS), polyethersulfone (PES), polymethylmetacrylate (PMMA) and others. In dialysis therapy, blood cells including white blood cells (WBCs) and platelets, are activated by physical contact with the dialysis membrane, and then activated blood cells produce reactive oxygen species (ROS) and inflammatory cytokines. ROS and cytokines trigger inflammatory response in dialysis patients and result in various complications such as cardiovascular disease and anemia. Despite improvements in membrane biocompatibility, important aspects of the blood membrane interaction still remain to be optimized and many attempts have been made in this area. In particular, hydrophobic-based polymers tend to be less friendly to the blood component and therefore are hydrophilized with polyvinylpyrrolidone (PVP) to avoid platelet and leucocyte adhesion to the membrane. Nevertheless, given the high water solubility of PVP, its release to patient blood can occur, depending on the amount of PVP and the technology used to fix it to the membrane surface. Elution of PVP can cause inflammation and may contribute to reducing the tolerability of hemodialysis treatment.
Despite all efforts to improve membrane biocompatibility, hemodialysis treatment still requires anticoagulation therapy, usually with unfractionated or low- molecular-weight heparin to prevent thrombosis of the dialyzer and of the extracorporeal circuit. Side effects of heparin include thrombocytopenia, hyperlipidemia and hyperkalemia.
Heparin-introduced thrombocytopenia in patients undergoing anticoagulation therapy with heparin in 5% to 10% of the cases being characterized by a drop in platelet count and altered clotting profile. The disorder is typically discovered 5 to 10 days following exposure to unfractionated heparin. The drop in platelet count is typically 30% to 50% from baseline, rarely reaching the very low values seen in other drug-induced thrombocytopenias (1, 2). Heparin has been implicated in the dyslipidemia of end-stage kidney disease (ESKD) (3). Heparin may cause hyperkalemia by an effect on aldosterone, although in hemodialysis patients, its intermittent use rarely results in significant effects on the potassium balance. Heparin administration may cause hypersensitivity reactions. The hypersensitivity that develops to standard heparin and cross-reactivity with low-molecular-weight heparin can pose a serious clinical problem.
Heparin anticoagulation is contraindicated in patients with active bleeding or increased bleeding risk. In these cases the use of direct thrombin inhibitors, regional citrate anticoagulation, citrate dialysate, and heparin-free dialysis may be considered. Heparin-free dialysis using intermittent saline flushes is commonly used, although there is limited evidence of the safety and efficacy of this method. Saline infusion is also used, but it may lead to an increased volume load, which must subsequently be removed by dialysis. For all these reasons, the search for hemocompatible nonthrombogenic materials and membranes has been one of the main topics of recent years in the area of extracorporeal therapies.
The hemocompatibility of a polymeric biomaterial is strongly influenced by the layer of water at the blood-membrane interface. The quantity of water molecules linked to the membrane surface describes the polymer hydrophilic characteristics and its capacity to become “wet” (4).
Based on this concept, Toray Medical (Tokyo, Japan) has developed a new dialysis membrane based on a specific hydrophilic polymer (Hydrolink™ NV) in the attempt to completely suppress platelet adhesion even in the absence of heparin (5).
The Hydrolink™ NV hydrophilic polymer was designed with a focus on the mobility of adsorbed water at the blood membrane interface, specifically aiming at antithrombogenic and antifouling effects.
The early internal data of the manufacturer showed that adhesion of blood components, the adhered platelet count and the amount of adhered fibrinogen in Hydrolink™ NV drastically decreased to 1/100th and to 1/4th, respectively, compared with conventional polysulfone dialyzers. Furthermore, the membrane biocompatibility in NV was better than that of conventional polysulfone dialyzers (5–7).
Preliminary studies carried out in Japan have indicated the potential benefits of the Hydrolink™ NV membrane in terms of anti-thrombus activity, low stimulation of platelets and leukocytes and improved removal of β2-microglobulin, without compromising the removal performance of regular solutes during dialysis (5–7).
Based on these results, we performed a pilot, prospective, multi-center, randomized clinical trial study on the Hydrolink™ NV membrane to determine the efficacy and safety of Hydrolink™ NV membrane at different anticoagulation regimes compared to polysulfone based conventional membranes.
Methods
Study Design
The study was a pilot, prospective, multi-centered, randomized clinical trial to determine the efficacy and safety of Hydrolink™ NV membrane based on a hydrophilic polymer compared to different polysulfone membranes. The objective of this study was to study the effect of the Hydrolink™ NV dialyzer on platelet count in chronic hemodialysis patients and to evaluate the optimization of anti-coagulation therapy by progressive heparin reduction policy (heparin reduction test), and the safety and efficiency of long-term dialysis using the Hydrolink™ NV dialyzer.
Patient Characteristics and Enrollment Criteria
Patients were included in the study if they were 20–85 years of age, had been undergoing chronic hemodialysis (4-hour sessions, 3 times weekly) with high-flux dialyzers and anti-coagulation therapy for at least 6 months prior to enrollment, with a platelet count before the dialysis session less than 170,000 cells/mm3.
Patients were excluded if they were undergoing other renal insufficiency therapies than hemodialysis, were enrolled in other clinical studies, were pregnant or intent to become pregnant within 1 year, or if they had comorbidities that were likely to influence the platelet count.
Patients who met all inclusion/exclusion criteria and had given informed consent were randomized to either conventional or Hydrolink™ NV dialysis. The 2 groups were balanced based on dialysis vintage, platelet count and diabetes. The study flow chart is described in Figure 1. The sample size was calculated based on the assumption that Hydrolink™ NV reduces the heparin requirement by at least 50% compared to routine treatment.
Fig. 1.
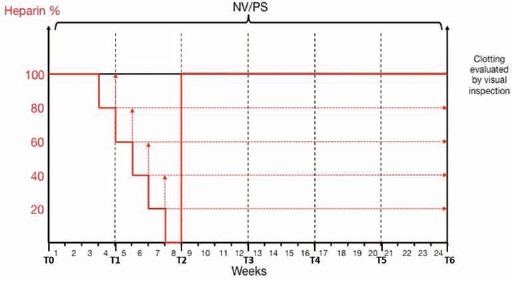
Heparin reduction scheme. A heparin reduction test was carried out from week 4 until week 8, starting at 100% heparin and gradually reducing until reaching 0% heparin at the beginning of week 8.
A t-test for unpaired samples was performed. The statistical power was set to 80% and the level of significance to 5%. A 50%-reduction heparin with Hydrolink™ was assumed, compared to the control. A sample size of 20 patients was calculated to be sufficient for this pilot study.
Dialysis Procedure
Dialysis was performed as per standard protocols of the individual units, ensuring that patients continued with the same level of support as prior to enrollment in the study. Patients underwent dialysis 3 times per week for a period of 4 hours each session throughout the entire 6-month study period. Anticoagulant therapy was continued for each patient as received prior to study enrollment, unless altered for clinical motives.
Clinical and laboratory parameters
Standard blood analysis was performed at each time point from T0–T6, where TO represents the starting point, and T1, T2, T3, T4, T5 and T6 represent months 1, 2, 3, 4, 5, and 6 after the starting point. Platelet activation was assessed by alteration in beta-thromboglobulin (β-TG), platelet factor 4 (PF-4) and fibrinogen in whole blood. Blood samples were collected at each time point just before the dialysis session. Lipid metabolism was evaluated by measuring total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides. Atherosclerotic index (Al) was calculated using the formula: Al = (total cholesterol - HDL)/HDL. Arterial blood samples were collected before and after the hemodialysis session to measure the concentrations of urea and β2-microglobulin (β2M). Since KT/V was always greater than 1.0 in our study population, we decided to use the urea reduction ratio to better describe the patient conditions.
Heparin reduction test
Three weeks after initiation of the study, both groups underwent a step-wise reduction in heparin anticoagulation dose to determine the minimal required dose. The heparin dose required for hemodialysis before the test was defined as 100%. At week 4 of the study, all patients had the dose of anti-coagulant decreased to 80% of the initial dose, without any other changes to the hemodialysis parameters. At week 5 the anticoagulant dose was decreased to 60% and so forth until reaching 0% at week 8. It is important to note that we performed heparin-free dialysis for the entire eighth week (or a total of 3 dialysis sessions) and then at the start of the ninth week, the dose was returned to the original value at the initiation of the study. In case of moderate clotting, no further reduction of the anti-coagulant dose was made. In the event of severe clotting, the initial anticoagulant dose was used for the next session.
The degree of coagulation was assessed in the dialyzer filters following treatment. Grading was evaluated by visual inspection following drainage of the system at the end of the dialysis session, using the following index scores: 1) clean filter; 2) a few blood stripes (less than 5% of the fibers at the surface of dialyzer); 3) many blood stripes (more than 5% of the fibers at the surface of dialyzer); 4) coagulated filter (Fig. 2). A cumulative clotting score adjusted for heparin dosage was calculated based on the formula: ∑((clotting score-clotting score at baseline)*heparin percentage).
Fig. 2.

Visual inspection of dialyzers and blood lines. Grading was evaluated by visual inspection following drainage of the system at the end of the dialysis session, using the following index scores: Score 1) clean filter; Score 2) a few blood stripes (less than 5% of the fibers at the surface of dialyzer); Score 3) many blood stripes (more than 5% of the fibers at the surface of dialyzer); Score 4) coagulated filter.
Statistical Analysis
Normality tests were performed using the Kolmogorov-Smirnov test. Quantitative normally distributed variables are presented as mean ± standard deviation (SD) and non-normally distributed variables as median and the 25th–75th quartile range. Univariate comparison was performed to compare variables between the 2 groups. In all comparisons p<0.05 was considered statistically significant.
Student's t-test, Wilcoxon test and Mann-Whitney test were used to compare means in case of normal paired or unpaired samples, non-normal paired samples and non-normal unpaired samples, respectively. Chi square test was used to assess the independence of observed and expected data between the two groups. P was considered significant when <0.05.
Results
Nineteen patients fulfilled the inclusion/exclusion criteria and were enrolled in the study; 11 patients were randomized to dialysis with Hydrolink™ NV membrane and 8 patients were dialyzed with a conventional dialyzer.
We found that the Hydrolink™ NV membrane dialyzer was safe to use and no adverse events were reported. Two patients did not complete the study, 1 patient due to thrombosis of the AVF and another patient due to hospital admission and subsequent shift to a different dialyzer. With the sample size used in this study, we did not find any significant differences in the biochemical parameters measured throughout the 6-month study period (Tab. I). There was no significant change in platelet count throughout the study period. Platelet counts remained within the range of 100,000/mm3 to 200,000/mm3 for all patients, both in the control group and in the Hydrolink™ NV group. Platelet activation markers PF4 and βTG were measured for the assessment of platelet adhesion, but no significant changes were found between the 2 groups. The Hydrolink™ NV membrane showed a more efficient removal of β2-microglobulin as measured before and after each dialysis session, compared to the control (Tab. I).
Table I.
Biochemical and Clinical Parameters
| Parameters | Membrane | TO (Month 0) | T1 (Month 1) | T2 (Month 2) | T4 (Month 4) | T6 (Month 6) | P (T0 vs. T6) |
|---|---|---|---|---|---|---|---|
| Platelets (x103 cells/mm3) | Hydrolink | 144.3 ± 22.4 | 134.1 ± 25.8 | 138.7 ± 30.9 | 145.6 ± 30.0 | 150.3 ± 47.1 | NS |
| Control | 155.6 ± 20.2 | 161.8 ± 41.1 | 168.5 ± 36.1 | 155.9 ± 31.3 | 169.0 ± 35.7 | NS | |
| β-TG (pg/mL) | Hydrolink | 901 (869–1292) | 912 (869–1115) | 976 (916–1146) | 965(933–1120) | 976 (927–1183) | NS |
| Control | 1053 (906–1806) | 1090 (958–1760) | 1017 (890–2488) | 1109 (967–2353) | 1119 (973–2258) | NS | |
| PF-4(ng/mL) | Hydrolink | 36 (29–73) | 32 (29–57) | 29 (28–44) | 29 (27–46) | 35 (27-44) | NS |
| Control | 37 (30–124) | 36 (31–130) | 74 (33–109) | 34 (30–86) | 35 (30-62) | NS | |
| Albumin (g/dl) | Hydrolink | 3.45 ± 0.41 | 3.43 ± 0.46 | 3.27 ± 0.33 | 3.27 ± 0.33 | 3.22 ± 0.36 | NS |
| Control | 3.31 ± 0.35 | 3.27 ± 0.30 | 3.10 ± 0.19 | 3.23 ± 0.19 | 3.30 ± 0.24 | NS | |
| Triglycerides (mg/dL) | Hydrolink | 169 ± 147 | 131 ± 73 | 147 ± 98 | 145 ± 105 | 155 ± 129 | NS |
| Control | 183 ± 75 | 165 ± 68 | 159 ± 71 | 199 ± 72 | 175 ± 82 | NS | |
| Total cholesterol (mg/dL) | Hydrolink | 119 ± 27 | 123 ± 32 | 127 ± 33 | 129 ± 34 | 137 ± 40 | NS |
| Control | 130 ± 8 | 124 ± 7 | 122 ± 11 | 130 ± 18 | 120 ± 19 | NS | |
| HDL cholesterol (mg/dL) | Hydrolink | 47.6 ± 22.2 | 51.5 ± 20.8 | 50.2 ± 20.2 | 52.7 ± 25.1 | 53.0 ± 22.1 | NS |
| Control | 37.0 ± 6.1 | 37.3 ± 7.0 | 40.2 ± 4.1 | 37.8 ± 5.3 | 38.7 ± 6.6 | NS | |
| LDL cholesterol (mg/dL) | Hydrolink | 46.5 ± 12.2 | 48.4 ± 20.5 | 55.8 ± 20.8 | 55.3 ± 15.7 | 54.8 ± 22.1 | NS |
| Control | 59.8 ± 6.9 | 54.1 ± 11.4 | 52.4 ± 16.6 | 51.8 ± 19.5 | 50.4 ± 24.6 | NS | |
| Al (ratio) | Hydrolink | 2.73 ± 3.02 | 1.69 ± 3.02 | 1.97 ± 2.06 | 1.79 ± 2.67 | 1.07 ± 1.35 | NS |
| Control | 4.34 ± 3.04 | 3.83 ± 2.66 | 3.09 ± 2.03 | 4.54 ± 2.63 | 3.92 ± 3.11 | NS | |
| Red blood cells (x1012/L) | Hydrolink | 3.95 ± 0.47 | 3.89 ± 0.45 | 3.71 ± 0.46 | 3.60 ± 0.35 | 3.60 ± 0.14 | NS |
| Control | 3.58 ± 0.43 | 3.55 ± 0.50 | 3.11 ± 0.37 | 3.52 ± 0.31 | 3.94 ± 0.28 | NS | |
| Hematocrit (%) | Hydrolink | 37.3 ± 3.6 | 36.7 ± 3.7 | 35.3 ± 4.0 | 34.0 ± 2.9 | 34.9 ± 2.0 | NS |
| Control | 35.6 ± 3.5 | 35.7 ± 3.9 | 31.6 ± 2.9 | 34.8 ± 2.1 | 39.2 ± 2.3 | NS | |
| Hemoglobin (g/dL) | Hydrolink | 12.0 ± 1.1 | 11.9 ± 1.1 | 11.3 ± 1.8 | 11.0 ± 0.8 | 11.1 ± 0.5 | NS |
| Control | 11.4 ± 0.8 | 11.4 ± 1.4 | 10.0 ± 1.2 | 11.2 ± 0.7 | 12.4 ± 0.5 | NS | |
| Transferrin (mg/dL) | Hydrolink | 189 ± 27 | 184 ± 56 | 176 ± 54 | 177 ± 45 | 176 ± 62 | NS |
| Control | – | 194 ± 48 | 186 ± 59 | 221 ± 63 | 209 ± 35 | NS | |
| Fibrinogen (mg/dL) | Hydrolink | 319 ± 69 | 323 ± 81 | 345 ± 97 | 361 ± 103 | 388 ± 159 | NS |
| Control | 315 ± 49 | 361 ± 40 | 432 ± 44 | 3523 ± 62 | 370 ± 73 | NS | |
| Leukocytes (x109/L) | Hydrolink | 5.37 ± 1.46 | 5.44 ± 1.43 | 5.61 ± 1.95 | 5.25 ± 1.63 | 5.42 ± 2.05 | NS |
| Control | 6.18 ± 1.46 | 5.54 ± 1.35 | 5.30 ± 1.63 | 5.68 ± 0.59 | 6.30 ± 1.13 | NS | |
| APC (ratio) | Hydrolink | 1.01 ± 0.79 | 1.17 ± 0.88 | 1.42 ± 1.16 | 2.28 ± 2.34 | 1.47 ± 2.64 | NS |
| Control | 0.75 ± 0.68 | 2.15 ± 2.44 | 1.63 ± 1.44 | 0.65 ± 0.52 | 0.90 ± 0.61 | NS | |
| PTH (pg/mL) | Hydrolink | 94 (63–388) | 126 (75–382) | 152 (52–354) | 193 (87–447) | 201 (44–467) | NS |
| Control | 155 (75–196) | 193 (114–273) | 94 (33–206) | 117 (100–231) | 198 (95–361) | NS | |
| Sodium (mmol/L) | Hydrolink | 137 ± 3 | 138 ± 3 | 138 ± 4 | 138 ± 3 | 139 ± 3 | NS |
| Control | 139 ± 2 | 139 ± 2 | 139 ± 3 | 139 ± 1 | 139 ± 3 | NS | |
| Chloride (mmol/L) | Hydrolink | 102 ± 4 | 103 ± 4 | 104 ± 6 | 103 ± 4 | 104 ± 3 | NS |
| Control | 104 ± 2 | 105 ± 4 | 105 ± 3 | 104 ± 4 | 106 ± 3 | NS | |
| Potassium (mmol/L) | Hydrolink | 5.0 ± 0.6 | 5.1 ± 0.5 | 5.1 ± 0.5 | 5.2 ± 0.6 | 4.8 ± 0.5 | NS |
| Control | 5.6 ± 0.7 | 5.7 ± 0.7 | 5.6 ± 1.0 | 5.5 ± 0.6 | 5.1 ± 0.8 | NS | |
| Urea, -pre (mg/dL) | Hydrolink | 124 ± 35 | 117 ± 32 | 118 ± 46 | 117 ± 50 | 98 ± 39 | NS |
| Control | 172 ± 26 | 162 ± 28 | 150 ± 9 | 149 ± 32 | 128 ± 24 | NS | |
| Urea, -post (mg/dL) | Hydrolink | 39 ± 17* | 41 ± 16* | 41 ± 23* | 38 ± 22* | 33 ± 20* | NS |
| Control | 48 ± 10* | 46 ± 12* | 51 ± 14* | 46 ± 7* | 41 ± 11* | NS | |
| β2M,-pre (mg/dL) | Hydrolink | 27.1 ± 7.1 | 27.4 ± 7.3 | 26.0 ± 7.4 | 27.1 ± 10.7 | 25.2 ± 5.2 | NS |
| Control | 29.6 ± 8.0 | 31.8 ± 8.4 | 31.6 ± 10.7 | 30.7 ± 11.4 | 31.5 ± 8.9 | NS | |
| β2M, -post (mg/dL) | Hydrolink | 9.0 ± 3.6* | 9.8 ± 2.4* | 9.9 ± 2.9* | 9.0 ± 2.9* | 8.7 ± 2.6* | NS |
| Control | 19.7 ± 13.4 | 27.5 ± 23.8 | 32.8 ± 19.4 | 17.1 ± 10.7† | 17.4 ± 10.2† | NS | |
| Dialysis duration (hours) | Hydrolink | 3.98 ± 0.26 | 4.05 ± 0.22 | 4.05 ± 0.33 | 3.98 ± 0.25 | 3.92 ± 0.22 | NS |
| Control | 4.00 ± 0.14 | 3.92 ± 0.26 | 4.00 ± 0.20 | 4.00 ± 0.35 | 3.92 ± 0.41 | NS | |
| UFR (ml/min) | Hydrolink | 11.4 ± 2.3 | 11.3 ± 3.2 | 12.5 ± 3.6 | 12.6 ± 3.3 | 10.2 ± 4.1 | NS |
| Control | 14.0 ± 1.1 | 11.2 ± 3.3 | 12.9 ± 3.6 | 12.7 ± 2.9 | 12.4 ± 4.0 | NS |
Al = atherosclerotic index; APC = activated protein C; β2M = β2-microglobulin; PTH = parathyroid hormone; UFR = ultrafiltration rate. Normally distributed samples are described as mean ± SD = non-normally distributed samples are described as median (interquartile range). Urea and β2-microgobulin were measured before and after each hemodialysis session;
p < 0.001 and
p < 0.05 for pre- versus post-measurements; NS = non-significant.
A heparin test was carried out as illustrated in Figure 1. The heparin reduction tests showed that more patients in the study group reached a reduction in heparin dialysis without clotting events than was the case in the control group. This was consistent with the cumulative clotting score adjusted for the heparin percentage used in each patient, which showed a clear tendency towards less clotting in the Hydrolink™ NV membrane group (Fig. 3). The degree of coagulation was assessed in the dialyzer filters following each treatment and grading was evaluated by visual inspection following drainage of the system at the end of the dialysis session. Figure 2 shows representative examples of the scores given from 1 to 4.
Fig. 3.

Cumulative coagulation score adjusted for heparin percentage. A cumulative clotting score adjusted for heparin dosage was calculated based on the formula: ∑([clotting score – clotting score at baseline] * heparin percentage).
Five out of 10 patients (50%) in the Hydrolink™ NV group reached 0% heparin, whereas 2 out of 8 (25.0%) in the control group reached 0% heparin (p = 0.007). Six out of 10 in the study group and 3 out of 8 in the control group reached 20% heparin (p = 0.035). Seven out of 10 in the Hydrolink™ NV group and 3 out of 8 in the control group reached ≥60% reduction in heparin dosage (p = 0.007).
Discussion
Our pilot study was designed to study the coagulation characteristics and the feasibility of using a new antithrombogenic, hydrophilic dialysis membrane. It is encouraging to know that preliminary clinical evaluations of the Hydrolink™ NV membrane dialyzer conducted in Japan have been able to demonstrate a recovery of platelet count (5, 6). We know that platelet adhesion is an important factor in the determination of biocompatibility. In our study, we measured platelet activation markers PF4 and βTG, which are easy to analyze, but unfortunately are sensitive to aspirin, heparin and some laboratory techniques. βTG has a plasma half-life of 100 minutes and PF4 can be upregulated up to 20-fold in the presence of heparin. It is important to note that our study cohort included patients with higher platelet counts that those in the Japanese cohort, and none of our patients presented with thrombocytopenia at baseline. Even though our study included a limited number of patients in both groups and was not designed to capture the long-term effects of heparin-free dialysis, we were able to make an interesting observation that more patients in the study group reached heparin-free dialysis without any clotting events than those in the control group. This is evident from our heparin reduction tests.
Even considering the limitations of our study, it is not unreasonable to say that the Hydrolink™ NV membrane dialyzer might demonstrate superior anticoagulant effects when compared with conventional filters. We hope that further trials involving larger patient groups are conducted in the near future to strengthen this conclusion. The further development of these dialyzers can potentially reduce complications and difficulties associated with anticoagulation therapy in dialysis patients and hence should be supported.
Conclusions
The quest for blood-sparing hemodialysis
We found that the Hydrolink™ NV membrane dialyzer was safe to use and no adverse events were reported. Further studies are warranted to understand the mechanism by which this dialyzer exerts its effects. Based on the preliminary results obtained in this study, we propose that future studies with the Hydrolink™ NV dialyzer focus on the potential beneficial effects of heparin-free dialysis and the improved biocompatibility, testing the effect on the various factors known to be involved in cardiovascular disease and inflammation.
Disclosures
Conflict of interest: None of the authors has financial interest related to this study to disclose.
References
- 1.Daugirdas J.T., Bernardo A.A.. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int. 2012; 82(2): 147–157. [DOI] [PubMed] [Google Scholar]
- 2.Hakim R.M., Schafer Al. Hemodialysis-associated platelet activation and thrombocytopenia. Am J Med. 1985; 78(4): 575–580. [DOI] [PubMed] [Google Scholar]
- 3.Barbagallo C.M., Noto D., Cefalu A.B. et al. Heparin induces an accumulation of atherogenic lipoproteins during hemodialysis in normolipidemic end-stage renal disease patients. Hemodial Int. 2015; 19(3): 360–367. [DOI] [PubMed] [Google Scholar]
- 4.Tsuruta T.. On the role of water molecules in the interface between biological systems and polymers. J Biomater Sci Polym Ed. 2010; 21(14): 1831–1848. [DOI] [PubMed] [Google Scholar]
- 5.Yamaka T., Ichikawa K., Saito M. et al. Biocompatibility of the new anticoagulant dialyzer Toraylight NV. Science Postprint. 2014; 1(1): e00020. [Google Scholar]
- 6.Hidaka S., Kobayashi S., Maesato K. et al. Hydrophilic polymer-coated polysulfone membrane improves endothelial function of hemodialysis patients: a pilot study. Journal of Clinical Nephrology and Research. 2015; 2(2): 1020. [Google Scholar]
- 7.Kakuta T., Komaba H., Takagi N. et al. A Prospective Multicenter Randomized Controlled Study on lnterleukin-6 Removal and Induction by a new Hemodialyzer With Improved Biocompatibility in Hemodialysis Patients: A Pilot Study. Ther Apher Dial. 2016; 20(6): 569–578. [DOI] [PubMed] [Google Scholar]


