Abstract
In the current era of human life, we have been facing an increased consumption of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Nevertheless, NSAIDs are not completely metabolized by humans and are further excreted into domestical effluents. Several studies have been showing that a wide variety of pharmaceuticals are present in water effluents and are thus a matter of serious concern in the public health. Although treatment plants use sophisticated technologies for pollutants/contaminants removal, none of these processes was particularly designed for NSAIDs. In this perspective, this work addresses the use of a liquid-liquid extraction approach, employing ionic liquids (ILs), for the removal of NSAIDs from aqueous media. In particular, aqueous biphasic systems (ABS) composed of ILs and aluminium-based salts, which are already used in water treatment plants, were tested for the removal of diclofenac, ibuprofen, naproxen and ketoprofen. With these systems, extraction efficiencies of NSAIDs up to 100% were obtained in a single-step. The recovery of NSAIDs from the IL medium and the recyclability of the IL-rich phase were then ascertained to guarantee the development of a more sustainable and cost-effective strategy. Based on the remarkable increase in the solubility of NSAIDs in the IL-rich phase (from a 300- to a 4100-fold when compared with pure water), water was then studied as an effective anti-solvent, and where single-step recovery percentages of NSAIDs from the IL-rich phase up to 91% were obtained. After the “cleaning” of the IL-rich phase by the induced precipitation of NSAIDs, the phase-forming components were recovered and reused in four consecutive cycles, with no detected losses on both the extraction efficiency and recovery of NSAIDs by induced precipitation. Finally, an integrated process is here proposed, which comprises the (i) removal of NSAIDs from aqueous media, (ii) the cleaning of the IL-rich phase by the recovery of NSAIDs by induced precipitation, and (iii) the phase-forming components recycling and reuse, aiming at unlocking new doors for alternative treatment strategies of aqueous environments.
Keywords: Active Pharmaceutical Ingredients, Removal, Wastewater Treatment Plants, Aqueous Biphasic Systems, Ionic Liquids, Extraction Efficiency, Recovery
Introduction
In the 21st century, the detection of forthcoming pollutants, such as pseudo-persistent compounds, in different environmental matrices has gained a crucial attention. The classification of pharmaceuticals and personal care products (PPCPs) as major pollutants was firstly recommended by Daughton and Ternes,1 being afterwards accepted as a rising class of potentially harmful environmental pollutants. Advances on analytical techniques have allowed their identification in an increasing number of environmental matrices.2, 3 Within the PPCPs class are active pharmaceutical ingredients (APIs), which have raised serious concerns in recent years after their identification in non-negligible levels in sewage treatment plants (STPs), wastewater treatment plants (WWTPs) and surface water effluents.4–11 APIs are known as mutagenic, carcinogenic, and endocrine disruptors, and have been detected in concentrations up to µg.L-1 in worldwide effluents of STPs and WWTPs.4, 7, 10, 12, 13 A global occurrence and perspective of pharmaceuticals in the environment has been summarized by aus de Beek et al.14 APIs found in the environment include prescription drugs, drugs used in hospital by humans and veterinary drugs.7, 15–17 Variable quantities (10–70%) of the taken doses are metabolized by organisms whereas the rest is excreted (in either metabolized or unchanged forms).6, 7, 18–24 Furthermore, unnecessary or expired medications are recurrently straightly disposed into wastewaters.7, 16, 17 However, even at low concentrations, the continuous contact with APIs can result in the intoxication of living organisms.7 Based on extensive criteria, the Global Water Research Coalition (GWRC) selected ten priority APIs.25 This list comprises antibiotics, anti-epileptics, anti-inflammatory drugs, β-blockers and lipid regulators.7, 16, 17 Although WWTPs use advanced processes for water purification, such as membrane filtration, ozonation, chlorination, flocculation/sedimentation and adsorption, none of these processes was specifically designed to remove APIs,6, 7, 9–11, 24 and some of these contaminants were already detected in drinking water.26
Within APIs, the non-steroidal anti-inflammatory drugs (NSAIDs) diclofenac, ibuprofen and naproxen are included in the list of the top 10 persistent pollutants.15 These compounds display a high-octanol partition coefficient, and thus a high ability to passively diffuse across biological membranes, low pKa values and high persistence in aquatic environments.27 Some classic methods have already been tested for APIs removal. In particular, the addition of several salts to promote coagulation of ibuprofen, naproxen, diclofenac, carbamazepine and diazepam was used, whereas the best results were obtained for diclofenac with 50% of removal efficiency.28 Ozonation29 and chloride oxidation30 have also been studied for NSAIDs degradation, where ozone was found to be the most effective oxidizer. Kahn et al.31 compared several techniques, such as lime clarification, dissolved air flotation, dual media filtration, ozonation, activated carbon, combined reverse-osmosis/nanofiltration and UV disinfection units for the removal of APIs. The authors31 concluded that reverse osmosis is an effective process for removing a wide range of pharmaceuticals, yet it is highly energy-intensive. Therefore, the development of a cost-efficient removal technique for NSAIDs from aqueous media is an urgent requirement of modern society.
Aqueous biphasic systems (ABS) fit within the liquid-liquid extraction techniques, and are constituted by two aqueous-rich phases formed by the addition of two water-soluble phase-forming components. In general, two polymers, a salt and a polymer or two salts lead to the creation of two-phase aqueous systems above given concentrations.32, 33 ABS are formed by non-volatile solvents in a water-rich environment, and thus they can be seen as a more environmentally friendly approach. The partition of the target compounds occurs between the coexisting phases, where the chemical nature and physical properties of both the phase-forming components and solute play a pivotal role. Even so, the limited polarity differences between the two phases and the restricted type of interactions between the solute and the phase-forming components, aiming at tailoring the extraction and selectivity, are the major drawbacks found in the more conventional polymer-based ABS. In the past few years, the polymers functionalization and addition of ligands have been investigated to overcome this constraint.34, 35
In 2003, Rogers and co-workers36 demonstrated that the addition of an inorganic salt to an aqueous solution of a given ionic liquid (IL) leads to phase separation. After this proof of concept, in the following years it was demonstrated that these systems can be created with a large number of salts, amino acids, carbohydrates and polymers, offering a new plethora of extraction/separation systems.37 Although most ILs display some outstanding properties, namely a negligible vapor pressure, non-flammability, high thermal and chemical stabilities, and a large liquid temperature range,38–41 the most important feature conveys on their tailoring ability (by an adequate choice of their ions), which is transferrable to IL-based ABS.42 In fact, IL-based ABS already proved a superior performance on extraction efficiencies and selectivities for a wide range compounds, comprising proteins, alkaloids, phenolic compounds, dyes, among others.37 In particular, IL-based ABS have also been investigated for the extraction of pharmaceuticals,43–48 mainly to evaluate their performance as purification and concentration techniques,43–46 as well as to recover value-added compounds from pharmaceutical wastes.47, 48
From a different perspective of previous works regarding the use of IL-based ABS for the concentration and purification of pharmaceuticals,43–48 herein, we propose a new integrated and highly efficient ABS-based strategy to remove and recover NSAIDs (diclofenac, ibuprofen, naproxen, and ketoprofen), as current persistent pollutants, from aqueous environments. Since STPs and WWTPs currently use Al2(SO4)3 as a flocculating agent in the purification of drinking water, this salt was chosen to create the IL-based ABS under study. In a simplified version of a WTTP, three different stages (mechanical, biological and disinfection treatments) are combined.49 Therefore, the ABS strategy designed here for the NSAIDs removal should be introduced in the final stage of a WTTP. Finally, and aiming at developing a more sustainable technique for the removal of persistent pollutants from aqueous environments, the recovery of the investigated NSAIDs from the IL-rich phase and the IL recycling were also addressed, allowing us to propose an integrated and highly efficient process which comprises the removal and recovery of NSAIDs and the phase-forming components recovery and reuse.
Experimental Section
Materials
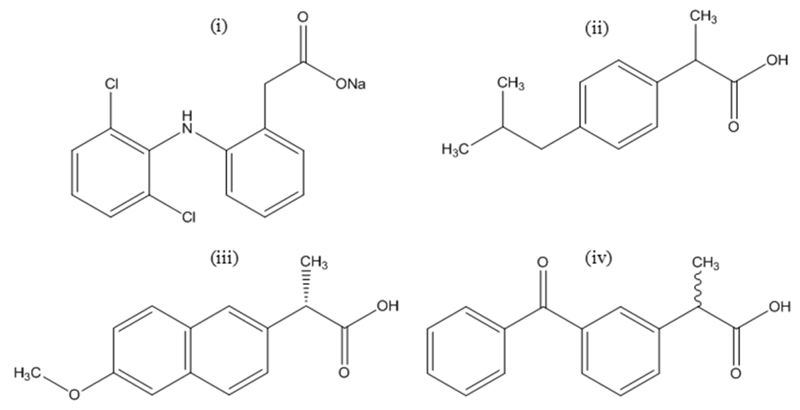
The non-steroidal anti-inflammatory drugs investigated were diclofenac sodium salt (2-[(2,6-dichlorophenyl)amino]benzene acetic acid sodium salt, CAS# 15307-79-6), ibuprofen ((±)-2-(4-Isobutylphenyl)propanoic acid, CAS# 15687-27-1), naproxen ((S)-(+)-2-(6-Methoxy-2-naphthyl)propionic acid, CAS# 22204-53-1) and ketoprofen ((RS)-2-(3-Benzoylphenyl)propionic acid, CAS# 22071-15-4), with a purity level ≥ 99 % for diclofenac, and ≥ 98 % for ibuprofen, naproxen and ketoprofen. All NSAIDs were acquired from Sigma-Aldrich, and used as received. The chemical structures of the NSAIDs investigated are depicted in Figure 1.
Figure 1.
Chemical structures of the NSAIDs investigated: diclofenac sodium salt (i), ibuprofen (ii), naproxen (iii), and ketoprofen (iv).
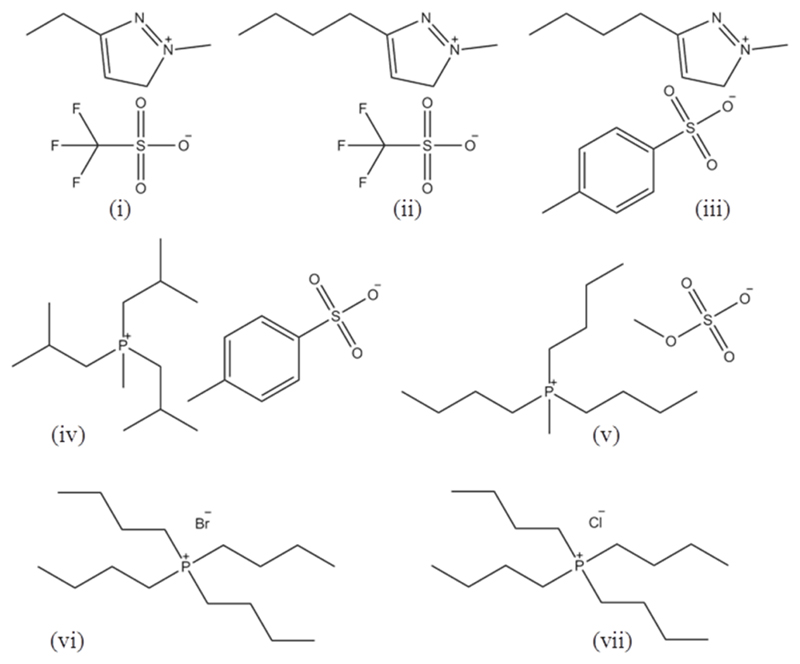
The ILs used were 1-ethyl-3-methylimidazolium trifluoromethanesulfonate (triflate) ([C2C1im][CF3SO3], purity 99 wt%, CAS# 145022-44-2); 1-butyl-3-methylimidazolium trifluoromethanesulfonate (triflate) ([C4C1im][CF3SO3], purity 99 wt%, CAS# 174899-66-2); 1-butyl-3-methylimidazolium tosylate ([C4C1im][Tos], purity 99 wt%, CAS# 410522-18-8); tri(isobutyl)methylphosphonium tosylate ([Pi(444)1][Tos], purity 98 wt%, CAS# 374683-35-9); tributylmethylphosphonium methylsulfate ([P4441][CH3SO4], purity 96-98 wt%, CAS# 69056-62-8); tetrabutylphosphonium bromide ([P4444]Br, purity 95 wt%, CAS# 3115-68-2); and tetrabutylphosphonium chloride ([P4444]Cl, purity 97 wt%, CAS# 2304-30-5). All imidazolium-based ILs were purchased from Iolitec, while the phosphonium-based fluids were gently supplied by Cytec Industries Inc. To decrease the volatile impurities and water content, individual samples of ILs were purified under constant stirring under vacuum for a minimum of 24h at 50 ºC, with the exception of [P4444]Br and [P4444]Cl that were purified at a higher temperature (100 ºC), under vacuum, and for a minimum of 72h, due their higher amount of water. The purity of each IL was checked by 1H and 13C NMR spectra and found to be in agreement with the purities given by the suppliers. The chemical structures of the ILs investigated are shown in Figure 2.
Figure 2.
Chemical structures of the ILs used to form ABS: [C2C1im][CF3SO3] (i), [C4C1im][CF3SO3] (ii), [C4C1im][Tos] (iii), [Pi(444)1][Tos] (iv), [P4441][CH3SO4] (v), [P4444]Br (vi), and [P4444]Cl (vii).
The inorganic salt Al2(SO4)3 (CAS# 17927-65-0) was acquired from José Manuel Gomes dos Santos, LDA (purity ≥ 98.0 wt%). The water applied was doubled distilled, passed across a reverse osmosis system and further treated with Milli-Q plus 185 water purification equipment. Buffers solutions with pH of 4.00 and 7.00, acquired from Panreac, were used for the pH meter equipment calibration.
Phase diagrams and tie-lines
The liquid-liquid ternary phase diagrams used in the current work were previously reported by Neves et al.50 However, additional tie-lines (TLs), which describe the compositions of the phases in equilibrium for given mixture compositions, were determined in this work. Each TL was determined according to the lever-arm rule originally proposed by Merchuk et al.51 Further details on the TLs determination and respective length (tie-line length, TLL) can be found in the Supporting Information.
Removal of NSAIDs using IL-based ABS
IL-based ABS investigated for the removal of NSAIDs from aqueous media require the use of ternary mixtures (ionic liquid + salt + aqueous solutions containing the target NSAID) within the biphasic region of each system. The concentration of NSAIDs in the aqueous solutions was of 0.060 g.L-1, 0.049 g.L-1 and 0.046 g.L-1 for diclofenac sodium salt, naproxen and ketoprofen, respectively. These concentrations are significantly higher than those found in STPs and WWTPs, thus guaranteeing that there is no saturation of each NSAID in the coexisting phases when envisaging the use of the proposed technology in real water samples. The ternary mixtures were prepared gravimetrically within ± 10-4 g, using a Mettler Toledo Excellence XS205 DualRange analytical balance. All mixtures were vigorously stirred and left to equilibrate for 24 h at (25 ± 1) ºC, to allow the complete separation of both liquid phases and consequent NSAIDs partitioning. The two phases were then separated, and both IL- and salt-rich phases were weighted and each NSAID quantified through UV-spectroscopy, using a Shimadzu UV-1700, Pharma-Spec UV-Vis Spectrophotometer, at a wavelength of 276, 221, 230 and 258 nm for diclofenac sodium salt, ibuprofen, naproxen and ketoprofen, respectively, using calibrations curves previously established. To eliminate interferences of the IL and salt in the quantification of each NSAID, ternary mixtures at the same weight fraction composition were prepared, using pure water instead of the aqueous solutions containing NSAIDs. However, in the extractions of ibuprofen and ketoprofen using the [C4C1im][CF3SO3]- and [C4C1im][Tos]-based ABS, a large interference of the ILs on the UV-spectroscopy quantification method was observed. Since the extraction efficiencies could not be accurately determined for these two particular systems, they are not presented.
The percentage extraction efficiencies (%EE) of each system for NSAIDs are defined according to:
| (1) |
where wIL and wsalt are the total weight of the IL-rich phase and salt-rich phase, respectively, and [NSAID]IL and [NSAID]salt are the concentration of each NSAID in the IL-rich phase and salt-rich phase, respectively.
At least three individual systems were prepared for each ABS and each NSAID, allowing the determination of the average %EE value and respective standard deviation. The possible loss of each NSAID (e.g. by precipitation and/or saturation of the phases) was evaluated by comparing the amount of each NSAID adedd and that quantified in each phase, showing that no losses of NSAIDs occurred in the systems investigated.
pH determination
The pH values (± 0.02) of the IL-rich phase were measured at 25 (± 1) ºC, using a Mettler Toledo S47 SevenMulti™ dual meter pH/conductivity. The calibration of the pH meter was beforehand performed with two buffers solutions with pH values of 4.00 and 7.00.
Solubility of NSAIDs in the IL-rich phase
To infer on the possible saturation of the systems investigated with NSAIDs, the solubility of each pharmaceutical in the IL-rich phase of the system composed of 58.5 wt% of [P4441][CH3SO4] + 2.2 wt% of Al2(SO4)3 + 39.5 wt% of H2O was determined at (25 ± 1) ºC. At least three individual systems were prepared for each NSAID, allowing the determination of the average solubility value and respective standard deviations. To a total weight of 1 g of the IL-rich phase, small amounts of each NSAID were added (0.002 – 0.005 g) and stirred under controlled temperature (25 ± 1) ºC using an Eppendorf Thermomixer® comfort equipment. The samples were left to equilibrate and NSAIDs were continuously added until the detection of a cloud point (visual identification of the first solid in solution). After the identification of the cloud point, the samples were left under stirring for at least 24 h at (25 ± 1) ºC to guarantee that the no further NSAID is dissolved and that the saturation of the IL-rich phase was achieved.
Recovery of NSAIDs and IL Recycling
To ascertain on the recycling ability of the studied ABS, the recovery of the NSAIDs from the IL-rich phase was first addressed followed by the IL reuse in a new cycle of NSAIDs removal. After the extraction step and NSAIDs enrichment in the IL-rich phase, water was added to this phase as an anti-solvent, in different amounts, and the mixture was vigorously stirred. Since NSAIDs have a low water solubility,52 and considering the recently demonstrated ILs hydrotropic effect,53 the precipitation of NSAIDs is easily achieved, in a single-step, by the simple addition of water. The precipitated NSAIDs were recovered by filtration under vacuum, using a Sartorius Stedim Biotech Cellulose Nitrate filter, with a pore size of 0.45 µm. The obtained precipitate was further washed with 10 mL of deionized water, dried at 70 ºC and weighted until constant weight.
The percentage of recovered NSAIDs (%Recovery) was determined according to:
| (2) |
where (wNSAID)recovered and (wNSAID)IL-rich phase are the total weight of each NSAID after the filtration and drying step and the total weight of each NSAID present in the IL-rich phase, respectively.
In order to explore the viability of the ABS reuse, it is necessary to know the exact composition of the IL-rich phase, so that the necessary amount of Al2(SO4)3 and aqueous solutions containing NSAIDs for the formation of a new ABS can be directly added. This information was obtained by the phase’s compositions and TLs data given in detail in the Supporting Information. After the recovery step of NSAIDs, the IL aqueous solution was placed in a rotary evaporator at 70 ºC for the removal of excess water. The water content of the IL-rich phase was further determined by Karl-Fischer titration, using a Metrohm 831 Karl Fischer coulometer. The reagent employed was Hydranal - Coulomat AG from Riedel-de Haën. Then, the concentrated IL aqueous solution was recovered and different amounts of Al2(SO4)3 and aqueous solutions of each NSAID were added to proceed to a new extraction cycle. The removal of NSAIDs and recycling of the IL-rich was repeated for 4 consecutive cycles.
Results and Discussion
Removal of NSAIDs using IL-based ABS
The compositions of each ABS used in the removal of NSAIDs from aqueous media ranged between 29.97 and 42.03 wt% for the IL, while a fixed composition, 15 wt%, was chosen for Al2(SO4)3, in order to carry out the extraction studies at a fixed TLL (≈70). This fixed TLL was chosen to maintain the difference between the compositions of the two phases, thus allowing a better evaluation of the IL nature effect. Furthermore, the use of a long TLL usually leads to an increase in the extraction efficiency47 as well as to a lower cross-contamination of each phase by the component enriched in the opposite layer.50 The liquid–liquid ternary phase diagrams used in this work were previously reported by Neves et al.50 However, additional TLs (composition of each phase for a given mixture) were determined in this work for the mixtures compositions used in the extraction/removal studies of NSAIDs. The detailed initial mixture compositions and respective TLs used in the extraction studies of each NSAID are presented in Tables S.I.1 to S.I.4, in the Supporting Information. The values of the extraction efficiencies and pH of the IL-rich phase, as well as the respective standard deviations are also provided in the Supporting Information (Tables S.I.1 to S.I.4).
The pH values of the IL-rich phases of the ABS prepared ranged between 1.48 and 3.17 - a consequence of the Al2(SO4)3 acidic nature in aqueous media. Therefore, in the studied ABS, the NSAIDs investigated are preferentially in a non-charged form (pKa values > 3.88)),54 meaning that electrostatic interactions do not play a major role in the investigated ABS extraction performance. The only exception occurs for diclofenac that is a sodium salt. However, no major differences in the diclofenac partition behavior are observed, as discussed below, confirming the negligible effect of electrostatic interactions. The respective dissociation curves and pKa values of each NSAID are shown in the Supporting Information (Figures S.I.1 to S.I.4).
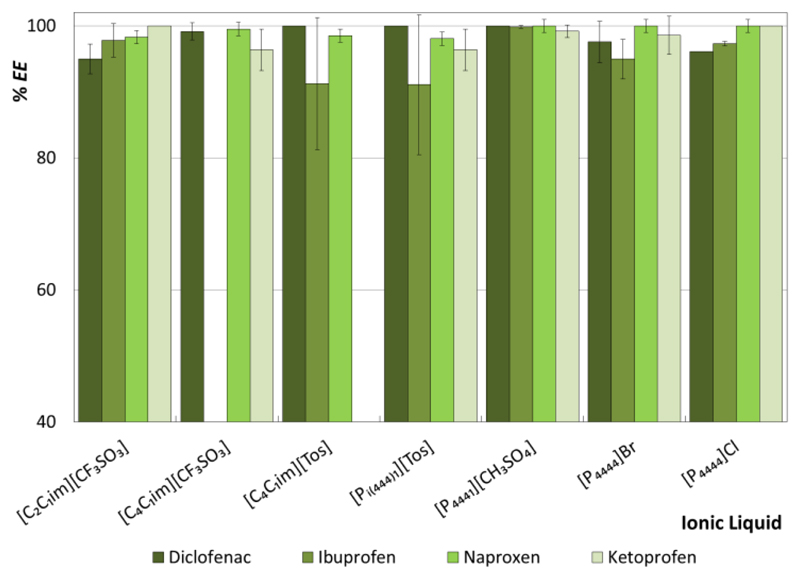
Figure 3 depicts the extraction efficiencies (%EE) of the investigated ABS for NSAIDs. In general, all studied ABS display a remarkable one-step performance to extract NSAIDs to the IL-rich phase from aqueous media, with %EE varying from 91 to 100%. NSAIDs are highly hydrophobic molecules (logKow values ranging between 3.1255 and 4.5156 – Supporting Information, Table S.I.5) and thus preferentially partition to the less hydrophilic and of lower ionic strength IL-rich phase. The preferential partition of NSAIDs to the IL-phase is also a consequence of the strong salting-out effect of the salt used, according to the Hofmeister series.57
Figure 3.
Extraction efficiencies (%EE) of ABS composed of IL + Al2(SO4)3+ H2O (at 25 ºC) for non-steroidal anti-inflammatory drugs.
In general, the differences on the %EE are dependent on both the IL employed and NSAID used. An increase in the cation alkyl side chain length (from [C2C1im][CF3SO3] to [C4C1im][CF3SO3]), leads to an increase in the %EE for diclofenac and naproxen, and to an opposite behavior for ketoprofen. Regarding the IL anion effect, the [C4C1im][CF3SO3]-based ABS leads to higher %EE for diclofenac than [C4C1im][Tos]-based ones, while the opposite trend is observed for naproxen. On the other hand, small differences are observed in the %EE of all NSAIDs with the [P4444]Br- and [P4444]Cl-based systems, with the exception of ketoprofen where the last ABS seems to be more promising.
Although imidazolium-based ILs are amongst the most investigated ILs for ABS creation and further use in extraction/purification processes,37 it is here shown that phosphonium-based ILs display a higher ability to extract NSAIDs from aqueous media. It was already demonstrated that phosphonium-based ILs are more efficient to form ABS,50, 58, 59 i.e., require lower amounts of IL and salt to undergo phase separation, in agreement with their higher hydrophobic nature. This phenomenon is independent of the salt used and aqueous media pH.50, 58, 59 This higher hydrophobic nature of phosphonium-based salts mainly derives from the butyl chains at the quaternary cation, which seems to be promising for the extraction of highly hydrophobic compounds, such as NSAIDs. Furthermore, there is a lower loss of IL for the salt-rich phase (or cross-contamination) when phosphonium-based ILs are used. For instance, for the mixtures investigated, the amount of all phosphonium-based ILs in the Al2(SO4)3-rich phase is ca. or below 1 wt% – Supporting Information with detailed TL data. Phosphonium-based ILs also are less toxic, thermally more stable, commercially produced in larger scales, and less expensive than imidazolium-based fluids,60, 61 which can be seen as further advantages in large-scale operations.
The NSAIDs diclofenac, ibuprofen and naproxen are included in the top 10 persistent pollutants.15 As mentioned before, several methods have already been tested for APIs removal, such as the addition of salts28 and reverse osmosis,31 and APIs degradation, such as ozonation29 and chloride oxidation.30 However, the low extraction efficiencies provided by these techniques as well as their high energy requirements clearly indicate that the development of a cost-efficient removal technique for NAIDs from aqueous media is a crucial requirement. In this work, and amongst all the ABS investigated, the [P4441][CH3SO4]-based one led to %EE of 100% of all NSAIDs to the IL-rich phase, achieved in a single-step and at room temperature, thus representing a promising alternative strategy for the treatment of aqueous environments. Taking into account these results and the advantages associated to phosphonium-based ILs discussed above, this IL was chosen for the next steps of NSAIDs recovery and IL regeneration and reuse.
Recovery of NSAIDs and IL Recycling
The solubility of all NSAIDs in the [P4441][CH3SO4]-rich phase of the respective ABS was determined at 25 ºC in order to better understand the high extraction ability of IL-based ABS and to design more sustainable NSAIDs removal techniques. Table 1 presents the solubility (saturation point) of each NSAID in the [P4441][CH3SO4]-rich phase and in pure water for comparison purposes.
Table 1.
Solubility of NSAIDs in water52 and in the [P4441][CH3SO4]-rich phase at 25 ºC.
| Solubility of NSAIDs / mg.L-1 |
||
|---|---|---|
| Water52 | [P4441][CH3SO4]-rich phase | |
| Diclofenac | 2.37 | 9720 ± 142 |
| Ibuprofen | 21.0 | 23024 ± 257 |
| Naproxen | 15.9 | 22594 ± 210 |
| Ketoprofen | 51.0 | 16780 ± 130 |
NSAIDs are highly hydrophobic compounds, and thus present a low solubility in pure water.52 However, from the data shown in Table 1, it is clearly shown that the solubility of NSAIDs in the [P4441][CH3SO4]-rich phase is significantly higher. The solubility of NSAIDs in the IL-rich phase increases from a 300- to a 4100-fold (≈4100-fold for diclofenac, ≈1100-fold for ibuprofen, ≈1400-fold for naproxen and ≈300-fold for ketoprofen) when compared with pure water. This increase in solubility closely follows the logKow values of the investigated NSAIDs, meaning that the higher the hydrophobic nature of the drug (logKow values ranging between 3.1255 and 4.5156 – Supporting Information, Table S.I.5), the higher is the increase in the solubility observed in the IL-rich phase. This remarkable increase in the solubility of NSAIDs in aqueous media is a consequence of the ILs hydrotropic ability recently proposed.53 Cláudio et al.53 reported a maximum in the solubility of antioxidants in aqueous solutions of imidazolium-based ILs of 40-fold. In this work, a significantly higher increase in the solubility of NSAIDs was observed further suggesting that phosphonium-based ILs are a promising class of hydrotropes, and that ILs act as excellent hydrotropes of very hydrophobic substances.
The boosted solvation ability of ILs for drugs (e.g. analgesic, non-steroidal anti-inflammatory drugs and antibiotics) has been studied by other authors,62–64 where a significant dependence on both the IL and drug hydrophobicity-hydrophilicity character was observed. Nevertheless, in all of these studies, pure and non-water miscible ILs were investigated. Although out of the scope of this work, the remarkable ability shown here of phosphonium-based ILs to perform as hydrotopes leading to an exceptional increase on the solubility of highly hydrophobic drugs in aqueous media should be stressed. Aqueous solutions of water-soluble ILs can thus be seen as promising alternatives to increase the bioavailability of relevant pharmaceuticals.
The significantly high solubility values of NSAIDs in the IL-rich phase support the possibility of using the same system to recover large amounts of NSAIDs from aqueous media or to be used in continuous processes before reaching the system saturation. For instance, and amongst the studied NSAIDs, diclofenac presents the lowest solubility in the [P4441][CH3SO4]-rich phase (9720 mg.L-1). According to Pal et al.,12 diclofenac is found in WWTP/STP effluents at a concentration ca. 0.0033 mg.L-1. Thus, working at the composition studied in this work for the [P4441][CH3SO4]-based ABS, ideally it would be possible to treat 3319 L of water with 1 g of [P4441][CH3SO4], i.e., up to the saturation of diclofenac in the IL-rich phase.
After the IL-rich phase saturation with each NSAID, the drugs recovery was carried out followed by the reuse of the IL, aiming at developing cost-efficient and more sustainable removal technologies. As clearly demonstrated in this work as well as in the literature,37 the use of ILs as phase-forming components in ABS leads to outstanding extraction performances compared to more traditional routes. Nevertheless, the IL regeneration, recycling and reuse lagged behind and still remain a challenging assignment. Due to the negligible volatility of ILs, the recovery of the compounds extracted and the ILs reutilization are still major obstacles towards the development of more sustainable IL-based techniques. Taking into account the ILs hydrotropic nature and the low solubility of NSAIDs in pure water, the recovery of NSAIDs was herein addressed by induced precipitation from the IL-rich phase through the addition of water (the greenest solvent overall) as an anti-solvent. Several volume ratios of the IL-rich-phase:water were investigated. Table 2 presents de percentage recovery of each NSAID (%Recovery) from the IL-rich phase by the addition of different amounts of water.
Table 2.
Recovery of NSAIDs from the IL-rich phase (%Recovery) by the addition of different volumes of water as anti-solvent.
| Volume ratio of the IL-rich-phase:water |
|||
|---|---|---|---|
| 1:1 | 1:3 | 1:5 | |
| %(Recovery ± σ) | |||
| Diclofenac | 53 ± 3 | 68 ± 6 | 69 ± 3 |
| Ibuprofen | 76 ± 2 | 80 ± 3 | 83 ± 3 |
| Naproxen | 79 ± 4 | 86 ± 5 | 91 ± 2 |
| Ketoprofen | 40 ± 3 | 46 ± 4 | 48 ± 3 |
As expected, an increase in the volume of water added (as anti-solvent) leads to an increase of the NSAIDs precipitation, although non-significant differences are seen between the 1:3 and 1:5 volume ratios. The NSAIDs recovery from the IL-rich phase by induced precipitation ranged between 40 and 91%, obtained in a single-step. The NSAIDs recovery efficiency follows the order: naproxen > ibuprofen > diclofenac > ketoprofen. With the exception of the diclofenac sodium salt, the recovery of NSAIDs closely follows their hydrophobic nature, i.e., the higher the logKow value the higher the recovery of each NSAID by the addition of water (Table S.I.5 in the Supporting Information). It seems thus that the induced precipitation of a NSAID in a salt form is more difficult to achieve by the addition of water as anti-solvent – an expected trend since salts display a higher solubility in water than their non-charged forms.
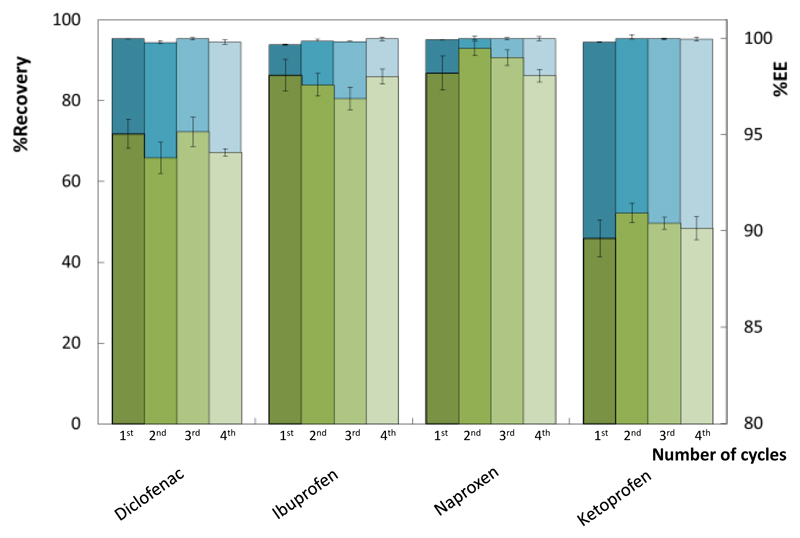
Based on the possibility of saturating the IL-rich phase and its further “cleaning”, the IL-rich phase was recovered and reused in the formation of new ABS to explore their viability as continuous removal platforms for NSAIDs. At least in four sequential cycles, a decrease on the ABS ability to extract NSAIDs from aqueous media was not observed nor a decrease on the NSAIDs recovery by induced precipitation from the IL-rich phase – Figure 4 (detailed data in Table S.I.6 in the Supporting Information). The %EE of the ABS is maintained at 100%, in a single-step, along the four cycles. Thus, the [P4441][CH3SO4]-based system does not lose its ability to completely remove NSAIDs from aqueous media after recovery and reuse. In the 4 cycles, more than 94 wt% of the IL was recovered and reused. This remarkable recovery of the IL is a main result of the strong salting-out ability of the salt used, Al2(SO4)3, as previously discussed, with the additional advantage of being currently used as a flocculant agent in the treatment of drinking water.65 Furthermore, the NSAIDs recovery efficiencies in the four cycles are similar to those previously presented (Table 2), with %Recovery values ranging between 46 and 93%.
Figure 4.
Recovery of non-steroidal anti-inflammatory drugs (%Recovery) from the IL-rich phase (green bars) and extraction efficiencies of non-steroidal anti-inflammatory drugs (%EE) (blue bars), in four consecutive cycles.
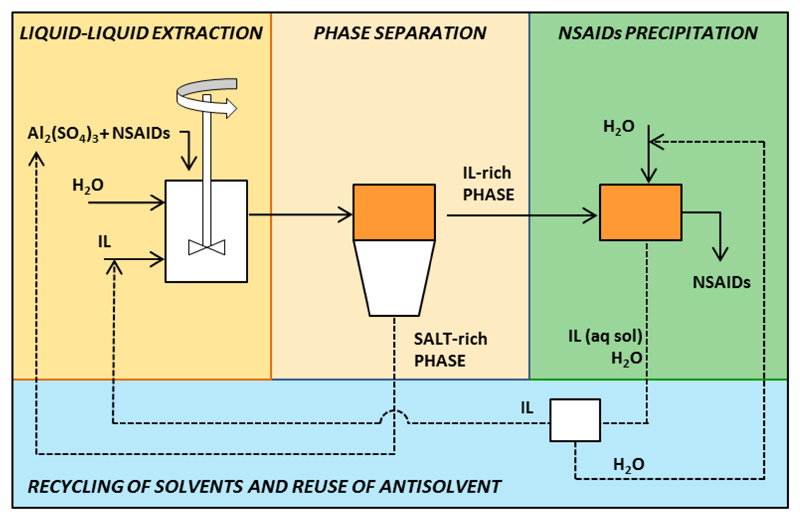
In summary, the use of ABS composed of [P4441][CH3SO4] + Al2(SO4)3 allows the complete removal of NSAIDs from aqueous media in a single-step, the further cleaning of the IL-rich phase and NSAIDs recovery by the addition of water as anti-solvent, and further IL reuse in the creation of new ABS. Figure 5 depicts the developed integrated process for NSAIDs removal from aqueous media, comprising the step of removal of NSAIDs followed by the recyclability of the IL-rich phase, thus guaranteeing the sustainability of the proposed process.
Figure 5.
Representative scheme of the overall process for NSAIDs removal, comprising the NSAIDs recovery and IL recycling.
Conclusions
A novel method to remove NSAIDs, such as diclofenac, ibuprofen, naproxen and ketoprofen, from aqueous media was here proposed. ABS composed of Al2(SO4)3 and imidazolium- or phosphonium-based ILs lead to extraction efficiencies of NSAIDs up to 100% in a single-step. Amongst the ILs investigated, phosphonium-based fluids display the best performance.
In addition to the high ability of IL-based ABS to extract a wide variety of compounds, the IL recycling and reuse remains a challenging issue towards the development of greener and more sustainable and cost-effective processes. An integrated process was proposed here and comprises: (i) the NSAIDs removal from the aqueous media; (ii) the NSAIDs recovery from the IL-rich phase by induced precipitation; and (iii) the IL recovery and reuse. Based on the high hydrophobic nature of NSAIDs, a proper choice of an anti-solvent, namely water which stands amongst the greener solvents overall, was used in order to precipitate NSAIDs and to “clean” the IL-rich phase, in which recovery percentages of NSAIDs up to 93% were obtained in a single-step. The IL was then recovered (more than 94 wt%) and reused in 4 consecutive cycles, ensuring the sustainability of the proposed process and without losses on the ABS extraction ability.
The proposed integrated process represents an improvement towards the use of IL-based ABS with a lower environmental footmark and economic impact, while demonstrating the potential of these systems to remove pharmaceutical drugs from aqueous media, unlocking thus new doors to the treatment of aqueous streams/effluents.
Supplementary Material
Supporting Information. Initial composition and weight fraction percentages (wt%) of ionic liquid ([IL]) + aluminium sulfate ([salt]) + water at the coexisting phases of each ABS are presented; Extraction efficiencies for the diclofenac sodium salt, ibuprofen, naproxen and ketoprofen, pH values of the IL-rich phases, and speciation curves and logKow values of all NSAIDs are also presented.
Acknowledgment
This work was developed in the scope of the project CICECO-Aveiro Institute of Materials (Ref. FCT UID/CTM/50011/2013), financed by national funds through the FCT/MEC and co-financed by FEDER under the PT2020 Partnership Agreement. Hugo F. D. Almeida and Isabel M. Marrucho acknowledge FCT for doctoral grant SFRH/BD/88369/2012 and the 2012 FCT Investigator Program, respectively.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Daughton CG, Ternes TA. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect. 1999;107:907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd GR, Grimm DA. Occurrence of Pharmaceutical Contaminants and Screening of Treatment Alternatives for Southeastern Louisiana. Ann N Y Acad Sci. 2001;948:80–89. doi: 10.1111/j.1749-6632.2001.tb03989.x. [DOI] [PubMed] [Google Scholar]
- 3.Drewes JE, Heberer T, Rauch T, Reddersen K. Fate of Pharmaceuticals During Ground Water Recharge. Ground Water Monit R. 2003;23:64–72. [Google Scholar]
- 4.Zhang Y, Habteselassie MY, Resurreccion EP, Mantripragada V, Peng S, Bauer S, Colosi LM. Evaluating Removal of Steroid Estrogens by a Model Alga as a Possible Sustainability Benefit of Hypothetical Integrated Algae Cultivation and Wastewater Treatment Systems. ACS Sustain Chem Eng. 2014;2:2544–2553. [Google Scholar]
- 5.Halling-Sørensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lützhøft HC, Jørgensen SE. Occurrence, fate and effects of pharmaceutical substances in the environment- A review. Chemosphere. 1998;36:357–393. doi: 10.1016/s0045-6535(97)00354-8. [DOI] [PubMed] [Google Scholar]
- 6.Salgado R, Noronha JP, Oehmen A, Carvalho G, Reis MaM. Analysis of 65 pharmaceuticals and personal care products in 5 wastewater treatment plants in Portugal using a simplified analytical methodology. Water Sci Technol. 2010;62:2862–2871. doi: 10.2166/wst.2010.985. [DOI] [PubMed] [Google Scholar]
- 7.Maja D, Alg-Göran D, Elzbieta P. Low concentrations of high priority' : Pharmaceutical and personal care products (PPCPs); Occurrence and removal at wastewater treatment plants. Vatten. 2006;62:139–148. [Google Scholar]
- 8.Lindberg RH, Östman M, Olofsson U, Grabic R, Fick J. Occurrence and behaviour of 105 active pharmaceutical ingredients in sewage waters of a municipal sewer collection system. Water Res. 2014;58:221–229. doi: 10.1016/j.watres.2014.03.076. [DOI] [PubMed] [Google Scholar]
- 9.Murray KE, Thomas SM, Bodour AA. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environmental Pollut. 2010;158:3462–3471. doi: 10.1016/j.envpol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009;43:363–380. doi: 10.1016/j.watres.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 11.Okuda T, Kobayashi Y, Nagao R, Yamashita N, Tanaka H, Tanaka S, Fujii S, Konishi C, Houwa I. Removal efficiency of 66 pharmaceuticals during wastewater treatment process in Japan. Water Sci Technol. 2008;57:65–71. doi: 10.2166/wst.2008.822. [DOI] [PubMed] [Google Scholar]
- 12.Pal A, Gin KY-H, Lin AY-C, Reinhard M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci Total Environ. 2010;408:6062–6069. doi: 10.1016/j.scitotenv.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Chan A, Salsali H, Mcbean E. Heavy Metal Removal (Copper and Zinc) in Secondary Effluent from Wastewater Treatment Plants by Microalgae. ACS Sustain Chem Eng. 2014;2:130–137. [Google Scholar]
- 14.Aus Der Beek T, Weber F-A, Bergmann A, Hickmann S, Ebert I, Hein A, Küster A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ Toxicol Chem. 2016;35:823–835. doi: 10.1002/etc.3339. [DOI] [PubMed] [Google Scholar]
- 15.De Voogt P, Janex-Habibi M-L, Sacher F, Puijker L, Mons M. Development of a common priority list of pharmaceuticals relevant for the water cycle. Water Sci Technol. 2009;59:39–46. doi: 10.2166/wst.2009.764. [DOI] [PubMed] [Google Scholar]
- 16.Rahman MF, Yanful EK, Jasim SY. Endocrine disrupting compounds (EDCs) and pharmaceuticals and personal care products (PPCPs) in the aquatic environment: implications for the drinking water industry and global environmental health. J Water Health. 2009;7:224–243. doi: 10.2166/wh.2009.021. [DOI] [PubMed] [Google Scholar]
- 17.Castiglioni S, Bagnati R, Fanelli R, Pomati F, Calamari D, Zuccato E. Removal of Pharmaceuticals in Sewage Treatment Plants in Italy. Environ Sci Technol. 2005;40:357–363. doi: 10.1021/es050991m. [DOI] [PubMed] [Google Scholar]
- 18.Almeida B, Oehmen A, Marques R, Brito D, Carvalho G, Barreto Crespo MT. Modelling the biodegradation of non-steroidal anti-inflammatory drugs (NSAIDs) by activated sludge and a pure culture. Bioresource Technol. 2013;133:31–37. doi: 10.1016/j.biortech.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Tran NH, Urase T, Ta TT. A Preliminary Study on the Occurrence of Pharmaceutically Active Compounds in Hospital Wastewater and Surface Water in Hanoi, Vietnam. CLEAN – Soil, Air,Water. 2014;42:267–275. [Google Scholar]
- 20.Kosma CI, Lambropoulou DA, Albanis TA. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci Total Environ. 2014;466–467:421–438. doi: 10.1016/j.scitotenv.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 21.Yargeau V, Lopata A, Metcalfe C. Pharmaceuticals in the Yamaska River, Quebec, Canada. Water Qual Res J Can. 2007;42:231–239. [Google Scholar]
- 22.Ortiz De García S, Pinto Pinto G, García Encina P, Irusta Mata R. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci Total Environ. 2013;444:451–465. doi: 10.1016/j.scitotenv.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 23.Santos JL, Aparicio I, Callejón M, Alonso E. Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain) J Hazard Mater. 2009;164:1509–1516. doi: 10.1016/j.jhazmat.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 24.Rosal R, Rodríguez A, Perdigón-Melón JA, Petre A, García-Calvo E, Gómez MJ, Agüera A, Fernández-Alba AR. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010;44:578–588. doi: 10.1016/j.watres.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Gwrc. Pharmaceuticals and personal care products in the water cycle. An international review. London, UK: 2004. [Google Scholar]
- 26.Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett. 2002;131:5–17. doi: 10.1016/s0378-4274(02)00041-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhou JL, Zhang ZL, Banks E, Grover D, Jiang JQ. Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J Hazard Mater. 2009;166:655–661. doi: 10.1016/j.jhazmat.2008.11.070. [DOI] [PubMed] [Google Scholar]
- 28.Suarez S, Lema JM, Omil F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresource Technol. 2009;100:2138–2146. doi: 10.1016/j.biortech.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Ternes TA, Stüber J, Herrmann N, Mcdowell D, Ried A, Kampmann M, Teiser B. Ozonation: a tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003;37:1976–1982. doi: 10.1016/S0043-1354(02)00570-5. [DOI] [PubMed] [Google Scholar]
- 30.Westerhoff P, Yoon Y, Snyder S, Wert E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ Sci Technol. 2005;39:6649–6663. doi: 10.1021/es0484799. [DOI] [PubMed] [Google Scholar]
- 31.Khan S, Wintgens T, Sherman P, Zaricky J, Schafer A. Removal of hormones and pharmaceuticals in the advanced water recycling demonstration plant in Queensland, Australia. Water Sci Technol. 2004;50:15–22. [PubMed] [Google Scholar]
- 32.Albertsson PA. Partition of Cell Particles and Macromolecules. 3rd ed. Wiley; New York: 1986. [Google Scholar]
- 33.Zaslavsky BY. Aqueous Two-Phase Partitioning. Marcel Dekker, Inc.; New York: 1994. [Google Scholar]
- 34.Pereira JFB, Ventura SPM, E Silva FA, Shahriari S, Freire MG, Coutinho JaP. Aqueous biphasic systems composed of ionic liquids and polymers: A platform for the purification of biomolecules. Sep Purif Technol. 2013;113:83–89. [Google Scholar]
- 35.Li F-W, Xu L-W, Xia C-G. Polymer-supported palladium–nickel bimetallic hydroxycarbonylation of styrene under aqueous–organic two-phase system. App Cat A. 2003;253:509–514. [Google Scholar]
- 36.Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD. Controlling the Aqueous Miscibility of Ionic Liquids: Aqueous Biphasic Systems of Water-Miscible Ionic Liquids and Water-Structuring Salts for Recycle, Metathesis, and Separations. J Am Chem Soc. 2003;125:6632–6633. doi: 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- 37.Freire MG, Claudio AFM, Araujo JMM, Coutinho JaP, Marrucho IM, Lopes JNC, Rebelo LPN. Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem Soc Rev. 2012:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- 38.Welton T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem Rev. 1999;99:2071–2084. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- 39.Gaune-Escard M, Seddon KR. Molten Salts and Ionic Liquids: Never the Twain? John Wiley; Hoboken, N.J.: 2010. [Google Scholar]
- 40.Rogers RD, Seddon KR. Ionic Liquids--Solvents of the Future? Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- 41.Conceicao LJA, Bogel-Lukasik E, Bogel-Lukasik R. A new outlook on solubility of carbohydrates and sugar alcohols in ionic liquids. RSC Adv. 2012;2:1846–1855. [Google Scholar]
- 42.Pereira JFB, Lima AS, Freire MG, Coutinho JaP. Ionic liquids as adjuvants for the tailored extraction of biomolecules in aqueous biphasic systems. Green Chem. 2010;12:1661–1669. [Google Scholar]
- 43.Domínguez-Pérez M, Tomé LIN, Freire MG, Marrucho IM, Cabeza O, Coutinho JaP. (Extraction of biomolecules using) aqueous biphasic systems formed by ionic liquids and aminoacids. Sep Purif Technol. 2010;72:85–91. [Google Scholar]
- 44.Han J, Wang Y, Kang W, Li C, Yan Y, Pan J, Xie X. Phase equilibrium and macrolide antibiotics partitioning in real water samples using a two-phase system composed of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and an aqueous solution of an inorganic salt. Microchim Acta. 2010;169:15–22. [Google Scholar]
- 45.Han J, Wang Y, Yu C-L, Yan Y-S, Xie X-Q. Extraction and determination of chloramphenicol in feed water, milk, and honey samples using an ionic liquid/sodium citrate aqueous two-phase system coupled with high-performance liquid chromatography. Anal Bioanal Chem. 2011;399:1295–1304. doi: 10.1007/s00216-010-4376-2. [DOI] [PubMed] [Google Scholar]
- 46.Jiang Y, Xia H, Yu J, Guo C, Liu H. Hydrophobic ionic liquids-assisted polymer recovery during penicillin extraction in aqueous two-phase system. Chem Eng J. 2009;147:22–26. [Google Scholar]
- 47.Silva FAE, Sintra T, Ventura SPM, Coutinho JaP. Recovery of paracetamol from pharmaceutical wastes. Sep Purif Technol. 2014;122:315–322. [Google Scholar]
- 48.Silva FAE, Caban M, Stepnowski P, Coutinho JaP, Ventura SPM. Recovery of ibuprofen from pharmaceutical wastes using ionic liquids. Green Chem. 2016;18:3749–3757. [Google Scholar]
- 49.Khopkar SM. Environmental Pollution Monitoring and Control. New Age International Ltd; 2004. [Google Scholar]
- 50.Neves CMSS, Freire MG, Coutinho JaP. Improved recovery of ionic liquids from contaminated aqueous streams using aluminium-based salts. RSC Adv. 2012;2:10882–10890. [Google Scholar]
- 51.Merchuk JC, Andrews BA, Asenjo JA. Aqueous two-phase systems for protein separation: Studies on phase inversion. J Chromatogr B. 1998;711:285–293. doi: 10.1016/s0378-4347(97)00594-x. [DOI] [PubMed] [Google Scholar]
- 52.Drug Bank - Open Data Drug & Drug Target Database. [Accessed on October 2015]; at http://www.drugbank.ca.
- 53.Claudio AFM, Neves MC, Shimizu K, CanongiaLopes JN, Freire MG, Coutinho JaP. The magic of aqueous solutions of ionic liquids: ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015;17:3948–3963. doi: 10.1039/C5GC00712G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.ChemSpider - The free chemical database. [Accessed on October 2015]; at www.chemspider.com.
- 55.Sangster J. logKow - a databank of evaluated octanol-water partition coefficients. Sangster Research Laboratories; Montreal: 1993. [Google Scholar]
- 56.Avdeef A, Box KJ, Comer JEA, Hibbert C, Tam KY. pH-Metric logP 10. Determination of Liposomal Membrane-Water Partition Coefficients of lonizable Drugs. Pharm Res. 1998;15:209–215. doi: 10.1023/a:1011954332221. [DOI] [PubMed] [Google Scholar]
- 57.Shahriari S, Neves CMSS, Freire MG, Coutinho JaP. Role of the Hofmeister Series in the Formation of Ionic-Liquid-Based Aqueous Biphasic Systems. J Phys Chem B. 2012;116:7252–7258. doi: 10.1021/jp300874u. [DOI] [PubMed] [Google Scholar]
- 58.Bridges NJ, Gutowski KE, Rogers RD. Investigation of aqueous biphasic systems formed from solutions of chaotropic salts with kosmotropic salts (salt-salt ABS) Green Chem. 2007;9:177–183. [Google Scholar]
- 59.Louros CLS, Cláudio AFM, Neves CMSS, Freire MG, Marrucho IM, Pauly J, Coutinho JaP. Extraction of Biomolecules Using Phosphonium-Based Ionic Liquids + K3PO4 Aqueous Biphasic Systems. Int J Mol Sci. 2010;11:1777–1797. doi: 10.3390/ijms11041777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atefi F, Garcia MT, Singer RD, Scammells PJ. Phosphonium ionic liquids: design, synthesis and evaluation of biodegradability. Green Chem. 2009;11:1595–1604. [Google Scholar]
- 61.Bradaric CJ, Downard A, Kennedy C, Robertson AJ, Zhou Y. Industrial preparation of phosphonium ionic liquids. Green Chem. 2003;5:143–152. [Google Scholar]
- 62.Smith KB, Bridson RH, Leeke GA. Solubilities of Pharmaceutical Compounds in Ionic Liquids. J Chem Eng Data. 2011;56:2039–2043. [Google Scholar]
- 63.Faria RA, Bogel-Łukasik E. Solubilities of pharmaceutical and bioactive compounds in trihexyl(tetradecyl)phosphonium chloride ionic liquid. Fluid Phase Equilibr. 2015;397:18–25. [Google Scholar]
- 64.Forte A, Melo CI, Bogel-Łukasik R, Bogel-Łukasik E. A favourable solubility of isoniazid, an antitubercular antibiotic drug, in alternative solvents. Fluid Phase Equilibr. 2012;318:89–95. [Google Scholar]
- 65.Wilms DA, VanHaute AA. In: Studies in Environmental Science. Pawlowski AJVL, Lacy WJ, editors. Vol. 23. Elsevier; 1984. pp. 213–220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.