PDK1 has gained interest in the field as a potential target for antithrombotic therapies [1]; however, the role of PDK1 downstream of the P2Y1/12 receptors remained unknown. We therefore read with great interest a recent report published in the Journal of Thrombosis and Haemostasis, by Manne et al. [2] describing the role of PDK1 in regulating platelet activation and TxA2 generation downstream of the P2Y1/12 receptors. In their study, Manne et al. used both pharmacological and genetic methods to conclusively show that targeting PDK1 can significantly attenuate pulmonary thromboembolism-induced mortality in an in vivo murine model. They further show that PDK1 regulates activation of Raf1 in the MAPK pathway induced by 2MeSADP.
Cytoplasmic phospholipase A2 (cPLA2) is recognized as one of the main PLA2 that is responsible for the release of AA from membrane phospholipids and subsequent TxA2 generation in platelets. Phosphorylation of cPLA2 at Ser505 increases its activity [3]. While initial studies of cPLA2 in cell free kinase assays showed that cPLA2 could be phosphorylated directly by either ERK or p38 [4]; follow-up studies using p38 inhibitors suggested that it was p38 and not ERK1/2, which is responsible for phosphorylating cPLA2-Ser505 in platelets [5]. Unfortunately, the identity of the kinase directly responsible for phosphorylation of cPLA2-Ser505 remained in limbo for over a decade. However, recent reports using knockout mice may have solved this conundrum. Recently, we found that Ask1 null platelets showed a loss of p38 phosphorylation, but enhanced activation of ERK1/2 when stimulated with thrombin, collagen, or ADP. Interestingly, we observed a complete absence of cPLA2 phosphorylation despite augmented ERK1/2 activity [6]. These data suggested that it was in fact p38, and not ERK, which is responsible for cPLA2-Ser505 phosphorylation. This result was further supported by a contemporary report by Shi et al., who found that p38α null platelets also showed an absence of cPLA2-Ser505 phosphorylation despite ERK1/2 being hyperphosphorylated [7].
In their report, Manne et al. described a mechanism of cPLA2 phosphorylation in platelets induced by 2MeSADP. Manne et al. report that: (1) the P2Y1/12 agonist 2MeSADP induces cPLA2-Ser505 phosphorylation in an ERK1/2 and p38 dependent manner, as the PDK1 inhibitor BX-795 was able to block ERK1/2 and cPLA2 phosphorylation, while the p38 inhibitor SB203580 also blocked cPLA2 phosphorylation. (2) p38 can be activated downstream of P2Y1/12 independently of ASK1 as the ASK1 inhibitor MSC2032964A failed to block 2MeSADP-induced p38 activation [2]. However, previously published data from our group and others suggests that ERK1/2 is not the main kinase responsible for directly phosphorylating cPLA2 in platelets [6, 7], and that agonist-induced p38 phosphorylation is exclusively dependent on ASK1 signaling in platelets [6]. The difference in these studies is that we and Shi et al., used ADP and not 2MeSADP as used by Manne et al.
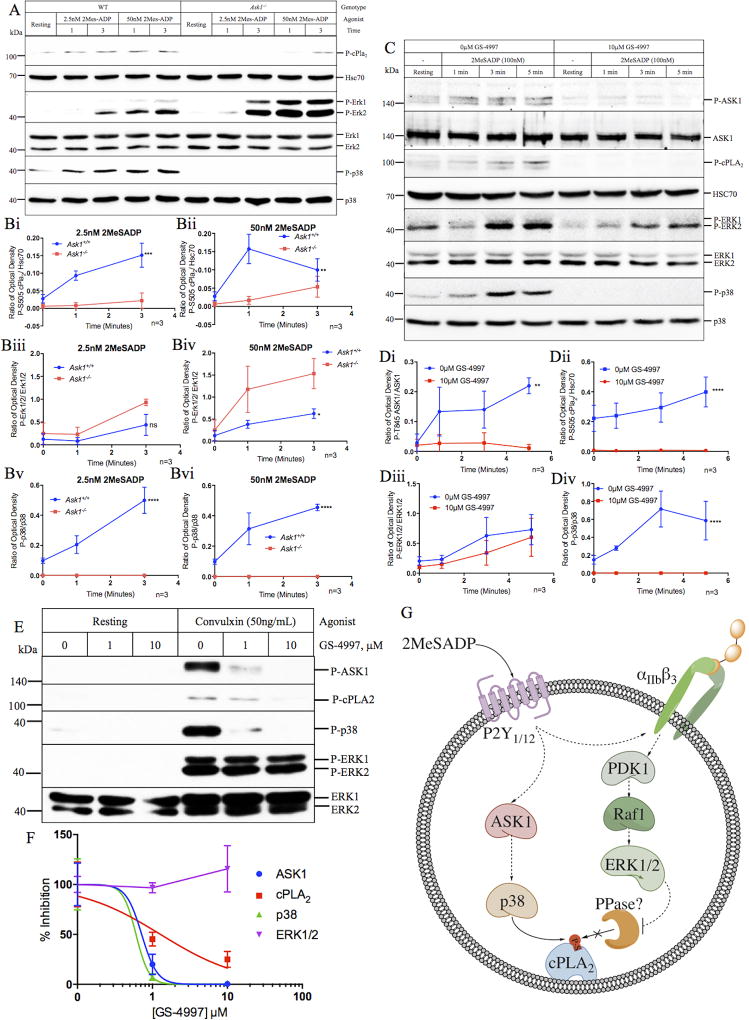
To resolve this discrepancy, we performed a series of experiments using platelets isolated from Ask1−/− mice. In agreement with our previous report, when Ask1 null platelets were stimulated with 2MeSADP, we found that p38 activation was completely ablated while Erk1/2 activation was augmented (Fig. 1A–B). At a low dose, 2MeSADP (2.5nM) rapidly (within 1min) phosphorylated cPla2 in WT platelets but failed to cause any cPla2 phosphorylation in Ask1 null platelets, even after 3 minutes. However, when the dose was increased to 50nM we found that cPla2 was phosphorylated, albeit at a later time point (3 minutes post stimulation) (Fig. 1A–B). While our data indicates that cPla2 can be phosphorylated in the absence of p38 activity, this phosphorylation appears to occur downstream of outside-in signaling. In fact, cPLA2 has been shown to be associated with αIIbβ3 and is activated downstream of outside-in signaling [8]. Additionally, it has been shown that ADP-induced TxA2 generation requires integrin outside-in signaling [9]. In addition, this data suggests, unlike Manne et al., that p38 is exclusively activated downstream of ASK1 in platelets.
Figure 1. PDK1 and ASK1 regulate ADP-induced cPLA2 phosphorylation and TxA2 generation through two distinct mechanisms.
(A) Western blot of lysates of washed murine platelets (2×108 platelets/mL) isolated from WT or Ask1−/− mice and stimulated with 2MeSADP (2.5 or 50nM). Blots were probed with phospho-specific antibodies against cPla2-Ser505, Erk1/2, and p38 as indicated. Blots were reprobed with anti-Hsc70, anti-p38, and anti-Erk1/2 to ensure equal loading. (B) Quantitation of optical density of bands from A for cPla2-Ser505 (Bi–ii), Erk1/2 (Biii–iv), and p38 (Bv–vi) when stimulated with either 2.5nM (Bi, iii, v) or 50nM (Bii, iv, vi) 2MeSADP. (C) Western blot of lysates of washed human platelets (4×108 platelets/mL) pretreated with GS-4997 for 20 minutes and stimulated with 2MeSADP (100nM) for 1, 3, and 5 minutes. Blots were processed as in A. (D) Quantitation of optical density of bands from C for ASK1-Thr845 (Di), cPLA2-Ser505 (Dii), ERK1/2 (Diii), and p38 (Div). (E) Western blot of human platelet lysate (4×108 platelets/mL) pretreated with GS-4997 and stimulated with convulxin (50ng/mL) for 3 minutes. (F) Quantitation of band intensity was expressed as percent inhibition from E. (G) Schematic representation of the signaling mechanism (based on our observations and those of Manne et al.,) describing the relationship between ASK1/p38 and PDK1/ERK1/2 as it relates to cPLA2-Ser505 phosphorylation. Two-way ANOVA (B & D) and liner regression analysis (F) were performed using Graphpad Prism Software. Representative blots are shown in panel (A, C, and E). Results are from 3 independent experiments.
In addition to murine platelets, when we stimulated human platelets with 100nM 2MeSADP we found that ASK1 and p38 were activated, albeit weekly. We found that pretreating platelets with an ASK1 specific inhibitor, GS-4997, significantly attenuated ASK1, p38, and cPLA2 phosphorylation while having minimal effect on ERK1/2 activation (Fig. 1C–D), suggesting again that p38 is solely activated downstream of ASK1 in human and murine platelets and that ASK1/p38 signaling regulates cPLA2 phosphorylation.
To determine if our findings are specific to P2Y1/12 signaling, or can be attributed to a more generalized signaling mechanism, we examined if ASK1 regulated p38 activation and cPLA2 phosphorylation downstream of convulxin. When washed human platelets stimulated with 50ng/mL of convulxin, we observed robust activation of ASK1, p38, cPLA2, and ERK1/2. Interestingly, except for ERK1/2, phosphorylation of ASK1, p38, and cPLA2 was dose dependently attenuated by GS-4997 (Fig. 1E–F), suggesting that it is p38, and not ERK1/2, which is responsible for phosphorylating cPLA2 in platelets.
Taken together, our data suggests that: (1) p38 is exclusively phosphorylated downstream of ASK1 in platelets and (2) that phosphorylation of cPLA2 at Ser505 is primarily mediated by p38 in platelets. Given that both Pdk1 null murine platelets and human platelets pretreated with BX-795 show a dramatic reduction in the level of cPLA2 phosphorylation and TxA2 generation [2], it is reasonable to conclude that PDK1 is involved in the regulation of cPLA2 in platelets. However, Manne et al.’s findings showing that both SB203580 and BX-795 can completely abolish cPLA2 phosphorylation, suggests that both p38 and PDK1 regulate cPLA2 phosphorylation albeit through distinct mechanisms as depicted in Figure 1G. It is possible that, in platelets, p38 is the kinase that phosphorylates cPLA2 and PDK1 regulates cPLA2 phosphorylation via ERK1/2 by negatively regulating a cPLA2-Ser505 phosphatase [10].
Acknowledgments
This study was supported in part by R01 HL119374-01.
Footnotes
Addendum
P. Patel designed the research, performed experiments, analyzed results and wrote the manuscript. K. Golla performed experiments. U. Naik designed the research, analyzed results and wrote the manuscript.
Disclosure of Conflicts of Interest
The authors state that they have no conflict of interest.
References
- 1.Chen X, Zhang Y, Wang Y, Li D, Zhang L, Wang K, Luo X, Yang Z, Wu Y, Liu J. PDK1 regulates platelet activation and arterial thrombosis. Blood. 2013;121:3718–26. doi: 10.1182/blood-2012-10-461897. [DOI] [PubMed] [Google Scholar]
- 2.Manne BK, Munzer P, Badolia R, Allgaier BW, Campbell RA, Middleton E, Weyrich AS, Kunapuli SP, Borst O, Rondina MT. PDK1 governs thromboxane generation and thrombosis in platelets by regulating activation of Raf1 in the MAPK pathway. Journal of thrombosis and haemostasis : JTH. 2018 doi: 10.1111/jth.14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–78. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 4.Borsch-Haubold AG, Ghomashchi F, Pasquet S, Goedert M, Cohen P, Gelb MH, Watson SP. Phosphorylation of cytosolic phospholipase A2 in platelets is mediated by multiple stress-activated protein kinase pathways. European journal of biochemistry. 1999;265:195–203. doi: 10.1046/j.1432-1327.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- 5.Borst O, Walker B, Munzer P, Russo A, Schmid E, Faggio C, Bigalke B, Laufer S, Gawaz M, Lang F. Skepinone-L, a novel potent and highly selective inhibitor of p38 MAP kinase, effectively impairs platelet activation and thrombus formation. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31:914–24. doi: 10.1159/000350110. [DOI] [PubMed] [Google Scholar]
- 6.Naik MU, Patel P, Derstine R, Turaga R, Chen X, Golla K, Neeves KB, Ichijo H, Naik UP. Ask1 regulates murine platelet granule secretion, thromboxane A2 generation, and thrombus formation. Blood. 2017;129:1197–209. doi: 10.1182/blood-2016-07-729780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi P, Zhang L, Zhang M, Yang W, Wang K, Zhang J, Otsu K, Huang G, Fan X, Liu J. Platelet-Specific p38alpha Deficiency Improved Cardiac Function After Myocardial Infarction in Mice. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:e185–e96. doi: 10.1161/atvbaha.117.309856. [DOI] [PubMed] [Google Scholar]
- 8.Prevost N, Mitsios JV, Kato H, Burke JE, Dennis EA, Shimizu T, Shattil SJ. Group IVA cytosolic phospholipase A2 (cPLA2alpha) and integrin alphaIIbbeta3 reinforce each other's functions during alphaIIbbeta3 signaling in platelets. Blood. 2009;113:447–57. doi: 10.1182/blood-2008-06-162032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin J, Quinton TM, Zhang J, Rittenhouse SE, Kunapuli SP. Adenosine diphosphate (ADP)-induced thromboxane A(2) generation in human platelets requires coordinated signaling through integrin alpha(IIb)beta(3) and ADP receptors. Blood. 2002;99:193–8. doi: 10.1182/blood.v99.1.193. [DOI] [PubMed] [Google Scholar]
- 10.Moscardo A, Valles J, Pinon M, Aznar J, Martinez-Sales V, Santos MT. Regulation of cytosolic PlA2 activity by PP1/PP2A serine/threonine phosphatases in human platelets. Platelets. 2006;17:405–15. doi: 10.1080/09537100600757869. [DOI] [PubMed] [Google Scholar]