Cellular responsiveness to retinoic acid and its metabolites is conferred through two structurally and pharmacologically distinct1 families of receptors: the retinoic acid receptors (RAR)2·3 and the retinoid X receptors (RXR)1. Here we report that the transcriptional activity of RAR and RXR can be reciprocally modulated by direct interactions between the two proteins. RAR and RXR have a high degree of cooperativity in binding to target DNA, consistent with previous reports indicating that the binding of either RAR or RXR to their cognate response elements is enhanced by factors present in nuclear extracts4,5. RXR also interacts directly with and enhances the binding of nuclear receptors conferring responsiveness to vitamin D3 and thyroid hormone T3; the DNA- binding activities of these receptors are also stimulated by the presence of nuclear extracts6–9. Together these data indicate that RXR has a central role in multiple hormonal signalling pathways.
RXR, but not RAR, can activate gene expression through the RXR response element found in the promoter of the cellular retinol binding protein type II (CRBPII-RXRE)10. Unexpectedly, RAR represses RXR-mediated activation through the CRBPII-RXRE (ref. 10 and Fig. la). By contrast, transfection of expression plasmids for either RAR or RXR resulted in an induction of expression from a reporter construct driven by two copies of an RAR response element (RARE)11 (Fig. 1b, RAR or RXR); cotransfection of expression plasmids for both receptors together yielded an enhanced level of expression relative to transfection of either receptor expression plasmid alone (Fig. 1b, RAR+RXR). Together, these data provide evidence for a functional interaction between the two retinoid responsive
FIG. 1.
a, the C terminus of RAR is required for suppression of RXR-dependent transactivation through the CRBPII-RXRE. CV-1 cells were cotransfected in duplicate with the reporter construct ΔSV-CRBPII-CAT, expression plasmid RS-hRXRa, and the control expression vector RS-LUC (no competitor) or the expression plasmids RS-hRARa, RS-Δ81–153, RS-185* and RS-ΔA203–360. Cells (right-hand figure) were then exposed to either ethanol (cross-hatched) or 10 μΜ retinoic acid (filled bars). CAT (chloramphenicol acetyltransferase) activity Is presented as per cent conversion where retinoic acid-induced activation in the presence of RXR is arbitrarily set at 100%. b, RXR enhances RAR-dependent transactivation through an RARE. CV-1 cells were cotransfected in duplicate with the reporter construct tk-DR5.2-LUC, containing two copies of the DR-5 RARE upstream of the thymidine kinase promoter, and expression plasmids RS-CAT (−), RS-RARα, and/or RS-RXRα as Indicated. Cells were then exposed to either ethanol or 10 μΜ retinoic acid (cross-hatched and filled bars respectively). Luciferase activity is presented as per cent normalized response where retinoic acid-induced activation in the presence of RAR is arbitrarily set at 100%.
METHODS. CV-1 monkey kidney cell culture, transfections, CAT and luciferase assays were done as previously described10,11,20. Transfection was on 10-cm plates and, for experiments using CAT, included 1 μg of RS-receptor or RS-LUC, 0.5 μg RS-hRXRα, 1 μg ΔSV-CRBPII-CAT reporter, 5 μg RAS-β- galactosidase reporter and 7.5 μg pGEM4 carrier DNA. Transfections using luciferase included 50 ng RS-RARα and/or 100 ng RXRα (the total amount of RS-expression plasmid was maintained constant in each transfection through the addition of RS-CAT), 0.5 μg tk-DR5.2-LUC reporter, 5 μg RAS-/3- galactosidase reporter and 8.5 μg pGEM4 carrier DNA.
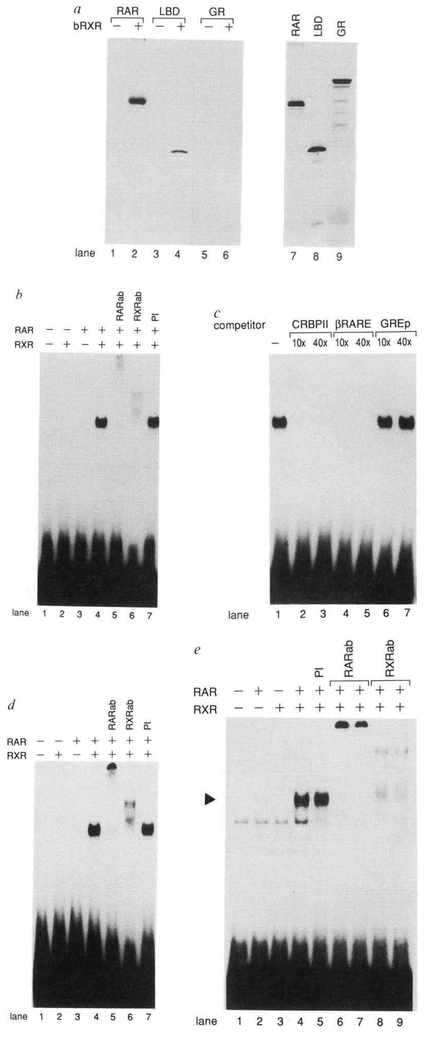
Similar experiments in which RAR was replaced with radiolabelled glucocorticoid receptor (GR) failed to reveal RXR-GR interactions, demonstrating the specificity of the RAR-RXR interaction (Fig. 2a, lanes 5 and 6). Consistent with our transfection data indicating the importance of the C terminus of RAR in mediating RAR-RXR interactions, a truncated RAR protein (amino acids 155–462), lacking the amino terminus and DNA binding domain of the protein, was also efficiently coprecipitated with RXR (Fig. 2a, lanes 3 and 4). Thus, the C terminus of RAR, containing the dimerization domain, is sufficient for forming a stable solution complex with RXR.
FIG. 2.
Direct Interactions between RAR and RXR in the absence or presence of DNA. a, RAR and RXR form a complex In solution. Immunoprecipltation reactions were done using in vitro synthesized, 35S-methionlne-labelled RARα (lanes 1 and 2), the ligand binding domain of RARα (LBD) (amino acids 155–462) (lanes 3 and 4), or the GR (glucocorticoid receptor) (lanes 5 and 6) In either the absence (lanes 1,3,5) or presence (lanes 2,4,6) of bacterlally expressed RXR (bRXR). Polyclonal antisera prepared against RXRa was used, in vitro synthesized RAR, LBD, and GR proteins not subjected to Immunoprecipitatlon are shown in lanes 7–9. b, RAR and RXR bind cooperatively to the CRBPII-RXRE. Gel mobility shift assays were done using in vitro synthesized RAR and/or RXR as indicated and 32P-labelled CRBPII-RXRE oligonucleotide10. Polyclonal antisera prepared against either RAR (RARab) (lane 5) or RXR (RXRab) (lane 6) or prelmmune serum (PI) (lane 7) were included in the reactions as indicated, c, Binding specificity of the RAR-RXR complex. Gel mobility shift competition reactions were done using 32P-labelled CRBPII-RXRE oligonucleotide and either a 10-fold (10 ×) or 40-fold (40 ×) excess of unlabelled competitor oligonucleotide encoding either the CRBPII-RXRE (lanes 2 and 3), the βRARE (lanes 4 and 5), or a palindromic GRE20 (lanes 6 and 7). d, RAR and RXR bind cooperatively to the βRARE. Gel mobility shift assays were done using in vitro synthesized RAR and/or RXR as indicated and 32P-labelled βRARE oligonucleotide. Polyclonal antisera prepared against either RAR (RARab) (lane 5) or RXR (RXRab) (lane 6) or preimmune serum (PI) (lane 7) were Included in the reactions as Indicated, e, RAR and RXR overexpressed in COS cells bind cooperatively to the βRARE. Gel mobility shift assays were done using 32P-labelled βRARE and whole cell extracts prepared from COS cells in which RAR (lane 2), RXR (lane 3) or RAR and RXR (lanes 4–9) were overexpressed. Prelmmune serum (3 μl) (lane 5) or 0.2 μl or 1 μl RAR-specific antiserum (RARab) (lanes 6 and 7, respectively) or 1 μl or 3 μl RXR-specific antiserum (RXRab) (lanes 8 and 9, respectively) were included in the reactions as Indicated. The position of the RAR-RXR-βRARE complex is Indicted by an arrowhead.
METHODS. The LBD expression vector was generated through insertion of an Xhol-BamHI fragment of Δ81–153, including amino acids 155–462 of RARα, Into the pCMX expression vector containing a synthetic translation start site sequence11,21. RARα, LBD, and GR RNA was prepared and subsequently translated in rabbit reticulocyte lysates as directed by the supplier (Promega). RXR was expressed in bacteria as a fusion with glutathlone-S-transferase using the pGEX-2T expression vector (Pharmacia) and purified as previously described10. Immunoprecipltation reactions (20 μl) included 5 μl 35S-methionine-labelled receptor protein and 150 ng of either purified GST-RXR or GST alone in 20 mM Tris, pH 8.0. Proteins were incubated 20 min on Ice before the addition of 5 μl polyclonal antisera prepared against an RXRα peptide (amino acids 214–229). Antigen-antibody complexes were collected by the addition of Protein A-Sepharose (Pharmacia) and the Immunocomplexes washed three times with 400 μl RIPA buffer (10 mM Tris (pH 8.0), 150 mM NaCI, 1% Triton X-100,1% sodium deoxycholate). Immunopreclpl- tated complexes were resolved by SDS-PAGE on 10% gels which were then fixed In 30% methanol, 10% acetic acid, dried and autoradiographed. Gel mobility shift assays (20 μl) contained 10 mM Tris (pH 8.0), 40 mM KCI, 0.05% Nonidet P-40,6% glycerol, 1 mM DTT, 0.2 pg of poly(dl-dC) and 2.5 μl each of in vitro synthesized RAR and RXR proteins. The total amount of reticulocyte lysate was maintained constant in each reaction (5 μl) through the addition of unprogrammed lysate. Where indicated, prelmmune serum or polyclonal rabbit antisera prepared against bacterlally expressed RARa or an RXRa peptide (amino acids 214–229) were included. After a 10 min Incubation on ice 1 ng 32P-labelled oligonucleotide was added and the incubation continued for an additional 10 min. DNA-protein complexes were resolved on a 4% polyacrylamide gel in 0.5× TBE (lx TBE is 90mM Tris, 90mM boric acid, 2 mM EDTA). Gels were dried and autoradiographed at −70°. Gel mobility shift assays using COS cell-expressed receptors were as described11, using 5 μg whole cell extracts prepared from COS cells transfected with 10 μg of either pCMX-hRARα, pCMX-hRXRα, or both expression plasmids.
These results led us to examine the properties of the RAR-RXR complex when associated with DNA. Gel mobility shift experiments were done using in vitro synthesized RAR and RXR and a radiolabelled oligonucleotide encoding the CRBPII-RXRE. RAR synthesized in vitro bound with very low affinity to the CRBPII-RXRE (Fig. 2b, lane 3). But the affinity of binding of RAR to CRBPII-RXRE was greatly stimulated by the addition of in vitro synthesized RXR (Fig. 2b, lane 4). In vitro synthesized RXR alone had no detectable binding activity (Fig. 2b, lane 2). Inclusion of polyclonal antisera prepared against either RAR or RXR in the reaction resulted in complexes with reduced mobility (Fig. 2b, lanes 5 and 6), indicating that both RAR and RXR were present in the complex. Thus, the RAR-RXR complex is capable of interacting with the CRBPII- RXRE with an affinity much higher than either receptor alone.
The specificity of the RAR-RXR interaction with DNA was next examined using unlabelled oligonucleotides as competitor. Oligonucleotides containing the CRBPII-RXRE competed efficiently for RAR-RXR complex binding at a 10-fold molar excess (Fig. 2c, lane 2), whereas oligonucleotides containing an unrelated glucocorticoid response element (GRE) failed to compete when used at a 40-fold molar excess relative to the radiolabelled CRBPII-RXRE (Fig. 2c, lane 7). Oligonucleotides containing the RARE of the RARβ promoter (βRARE)15,16 also competed efficiently for RAR-RXR binding to the CRBPII- RXRE (Fig. 2c, lanes 4 and 5).
To investigate further this interaction of the RAR-RXR complex with the βRARE, oligonucleotides containing the βRARE were labelled and used as probe in a gel mobility shift assay. As in the case of the CRBPII-RXRE, both in vitro synthesized RAR and RXR were required for high-affinity DNA-protein interactions with the βRARE (Fig. 2d, lanes 2–4). Similar results indicating a requirement for the presence of both RAR and RXR for formation of a high-affinity DNA-protein complex on the βRARE were obtained using whole-cell extracts prepared from COS cells which had been transfected with either RAR, RXR or RAR and RXR (Fig. 2e). Taken together, these results demonstrate that RXR dramatically stimulates the binding affinity of RAR to a strong RARE and that the RAR-RXR complex is likely to be present in vivo.
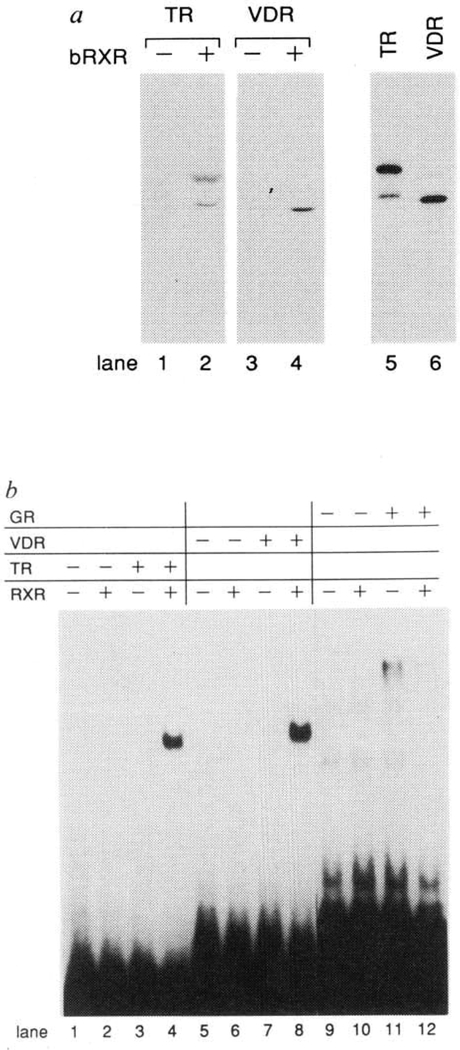
A functional relationship among the vitamin D receptor (VDR), thyroid hormone receptor (TR) and RAR has recently been described in which these receptors bind and activate through tandem repeats of consensus AGGTCA spaced by 3, 4 and 5 nucleotides, respectively (3–4-5 rule)11. Like the RAR, accessory factors present in nuclear extracts are necessary for high-affinity binding of the TR and VDR to their cognate response elements6–9. The relatively high degree of amino-acid conservation in the C termini of these nuclear receptors suggested that RXR might functionally interact with TR and VDR. Indeed, in immunoprecipitation experiments, in vitro synthesized TR and VDR coprecipitate with bacterially expressed RXR (Fig. 3a, lanes 1–4). The interactions of these receptors with RXR were also manifest at the level of DNA binding: in vitro synthesized RXR greatly stimulates TR and VDR binding to the Moloney leukaemia virus long terminal repeat thyroid hormone response element (MLV-LTRTRE) and osteopontin vitamin D response element (VDRE), respectively (Fig. 3b, lanes 1–8). The ability of RXR to stimulate the binding of nuclear receptors was not a general phenomenon, however, as RXR failed to increase GR binding to a GRE (Fig. 3b, compare lanes 11 and 12). Taken together, these data strongly suggest a central role for RXR in modulating the hormonal responses conferred through the RAR, TR and VDR.
FIG. 3.
Direct interactions between RXR and TR or VDR in the absence or presence of DNA. a, RXR forms complexes with either TR or VDR in solution. Immunoprecipitation reactions were done with RXR-specific antiserum and in vitro synthesized, 35S-methionine-labelled TRβ (lanes 1 and 2) or VDR (lanes 3 and 4) in either the absence (lanes 1, 3) or presence (lanes 2, 4) of bacterially expressed RXR (bRXR). Vitamin D3 (1 ×10−7M) was included in reactions containing the VDR. In vitro synthesized TR and VDR proteins not subjected to immunoprecipitation are shown in lanes 5 and 6. b, RXR interacts cooperatively with TR and VDR in DNA binding. Gel mobility shift assays were done using in vitro synthesized RXR, TR, VDR and GR as indicated and 32P-labelled oligonucleotides encoding Moloney leukaemia virus LTR TRE11 (lanes 1–4), the mouse osteopontin VDRE11 (lanes 5–8), or the palindromic GRE20 (lanes 9–12).
METHODS. TRβ and VDR RNA was prepared and subsequently translated in rabbit reticulocyte lysates as directed by the supplier (Promega). Immunoprecipitation and gel mobility shift assays are described in Fig. 2 legend.
The formation of RXR complexes with RAR, VDR and TR in which the complex displays new DNA-binding properties relative to the individual homodimers is reminiscent of interactions reported between the Jun and Fos families of proteins17 as well as between members of the HLH family of transcription factors such as MyoD and E12/47 (ref. 18). Through the formation of heterodimers, small families of structurally related proteins can yield large numbers of transcription factors with distinct functional properties. Two additional isoforms of RXR (RXRβ and RXRγ) have been recently identified (ref. 19, and D.J.M. and R.E., unpublished observations). Thus, the interaction of multiple RXR isoforms with additional nuclear receptors responsive to a diverse array of ligands is likely to have a critical role creating the high degree of diversity and specificity necessary to regulate the battery of hormone responsive genes.
Why the VDR, TR and RAR interact with a common partner is not yet clear, particularly as vitamin D, and thyroid hormone actions are not apparently retinoic acid-dependent. Further adding to the puzzle is the observation that RXR can activate through the CRBPII-RXRE in the absence of VDR, TR and RAR10, suggesting a role for other nuclear factors in this process. It is clear that characterization of the RXR family, its patterns of expression, and the nature of the RXR ligand will be essential to better understanding the complex molecular nature of hormonal signalling.
ACKNOWLEDGEMENTS.
We thank P. Rangarajan for RAR mutant expression constructs RS-Δ81–153 and RS-Δ203–360, R. Heyman for RAR mutant expression construct RS-185*, J. Dyck for antisera prepared against RARa and RXRa and H. Sucov, M. McKeown and T. Perlmann for discussions and critically reading the manuscript and E. Stevens for manuscript preparation. S.A.K. is a Fellow of the Jane Coffin Childs Memorial Fund for Medical Research. K.U. and D.J.M. are Research Associates and R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute for Biological Studies. This work was supported by the NIH and the Mathers Foundation.
References
- 1.Mangelsdorf D,J„ Ong ES„ Dyck JA & Evans RM Nature 345, 224–229 (1990). [DOI] [PubMed] [Google Scholar]
- 2.Giguere V, Ong ES„ Segui P & Evans RM Nature 330, 624–629 (1987). [DOI] [PubMed] [Google Scholar]
- 3.Petkovich M„ Brand N,J„ Krust A & Chambon P, Nature 330, 444–450 (1987). [DOI] [PubMed] [Google Scholar]
- 4.Glass CK„ Devary OV & Rosenfeld M,G Cell 63, 729–738 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Rottman JN et al. Molec. cell. Biol 11, 3814–3820 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao J et al. Proc. natn. Acad. Sci. U.S.A 87, 9751–9755 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray MB & Towle HC Molec. Endocr 3, 1434–1442 (1989). [DOI] [PubMed] [Google Scholar]
- 8.Burnside J, Darling DS & Chin WW J. biol. Chem 265, 2500–2504 (1990). [PubMed] [Google Scholar]
- 9.Lazar MA & Berrodin TJ Molec. Endocr 4, 1627–1635 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Mangelsdorf DJ et al. Cell 66, 555–561 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Umesono K„ Murakami KK, Thompson CC & Evans RM Cell 65, 1255–1266 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fawell S,E„ Lees JA, White R & Parker MG Cell 60, 953–963 (1990). [DOI] [PubMed] [Google Scholar]
- 13.Forman MB & Samuels ΗH Molec. Endocr 4, 1293–1301 (1990). [DOI] [PubMed] [Google Scholar]
- 14.Glass CK„ Lipkln SM, Devary OV & Rosenfeld MG Cell 59, 697–708 (1989). [DOI] [PubMed] [Google Scholar]
- 15.Sucov HM„ Murakami KK & Evans RM Proc. natn. Acad. Sei. U.S.A. 87, 5392–5398 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de The H et al. Nature 343, 177–180 (1990). [DOI] [PubMed] [Google Scholar]
- 17.Ransone L,J & Verma IMA Rev. Cell Biol. 6, 539–557 (1990). [DOI] [PubMed] [Google Scholar]
- 18.Murre C et al. Cell 58, 537–544 (1989). [DOI] [PubMed] [Google Scholar]
- 19.Hamada K et al. Proc. natn. Acad. Sei. U.StA 86, 8289–8293 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umesono K & Evans RM Cell 57, 1139–1146 (1989). [DOI] [PubMed] [Google Scholar]
- 21.Schule et al. Proc. natn. Acad. Sei. U.S.A 88, 6092–6096 (1991). [Google Scholar]