Abstract
Rationale
The cytosolic protease calpain has been recently implicated in the vascular remodeling of angiotensin II (AngII) type-1 receptor (AT1r) signaling. The role of AngII/AT1r/calpain signaling on endothelial function, an important and early determinant of vascular pathology, remains though totally unknown. Accordingly, we investigated the role of calpain in the endothelial dysfunction of AngII.
Objective
To demonstrate a mechanistic role for calpain in the endothelial dysfunction induced by AngII/AT1r signaling. To establish endothelial-expressed calpains as an important target of AT1r signaling.
Methods and Results
Subchronic administration of nonpressor doses of AngII to rats and mice significantly increased vascular calpain activity via AT1r signaling. Intravital microscopy studies revealed that activation of vascular expressed calpains causes endothelial dysfunction with increased leukocyte-endothelium interactions and albumin permeability in the microcirculation. Western blot and immunohistochemistry studies confirmed that AngII/AT1r signaling preferentially activates the constitutively expressed μ-calpain isoform and demonstrated a calpain-dependent degradation of IκBα, along with upregulation of NF-κB-regulated endothelial cell adhesion molecules (eCAMs). These physiological and biochemical parameters were nearly normalized following inhibition of AT1r or calpain in vivo. Antisense depletion studies in microvascular endothelial cells along with knockout and transgenic mouse studies further confirmed the role of μ-calpain in the endothelial adhesiveness induced by AngII.
Conclusions
This study uncovers a novel role for calpain in the endothelial dysfunction of AngII/AT1r signaling and establishes the calpain system as a novel molecular target of the vascular protective action of RAS inhibition. Our results may have significant clinical implications in vascular disease.
Keywords: Adhesion Molecules, Nuclear factor-kappa-B, Protease, Polymorphonuclear leukocyte adhesion
Background
Calpain is a ubiquitously expressed, cytosolic, Ca2+ activated, neutral cysteine protease that consists of a large 80 kDa catalytic subunit and a small 30 kDa regulatory subunit. Two calpain isoforms, μ and m, are ubiquitously expressed and, thus, found in the vascular wall where they regulate important functions of endothelial1 and smooth muscle cells2. Two recent studies have implicated calpain in the vascular pathology of AngII by demonstrating a mechanistic role for calpain in smooth muscle cell homeostasis2 and vascular remodeling3. Surprisingly, no study has addressed the role of calpain in the endothelial dysfunction of AngII, albeit recent work has emphasized a key mechanistic role for the vascular endothelium in AngII-induced pathology that affects the entire vascular wall4. Indeed, because of its strategic location between the blood compartment, the vascular wall, and the parenchyma of organ tissues the endothelium plays a very early mechanistic role in the initiation and progression of vascular disease, and related organ damage.
Several lines of research have now extensively demonstrated that upregulation of AngII signaling causes endothelial dysfunction mainly via activation of the AT1r, even in the absence of significant changes in systemic blood pressure. The dysfunctional endothelium initiates and maintains vascular pathology by becoming abnormally adhesive to circulating leukocytes and leaky to plasma proteins, two processes responsible for vascular and organ tissue damage. Thus, activation of the AngII/AT1r signaling pathway has been shown to: a) upregulate endothelial cell adhesion molecules (eCAMs) in vitro5 as well as and in microcirculation of experimental animal models6; b) induce leukocyte-endothelium interactions6; and c) increase vascular permeability7. Preclinical and clinical data demonstrate that pharmacological inhibition of the renin angiotensin system (RAS) attenuates endothelial dysfunction and cardiovascular events independently of blood pressure lowering effects8. Of note, AT1r blockers (ARBs) attenuate expression of endothelial cell adhesion molecules9, leukocyte-endothelium interactions10, and vascular permeability11. Despite intensive research, the precise molecular mechanisms by which AngII/AT1r disrupts critical components of endothelial function remain largely unknown, which obstructs full therapeutic exploration of RAS inhibition in vascular disease.
We hypothesized that stimulation of AngII/AT1r signaling induces endothelial dysfunction in part via activation of the endothelial calpain system. Accordingly we studied the role of μ- and m-calpain in the leukocyte-endothelium interaction and vascular permeability induced by AngII/AT1 signaling. Using pharmacological and genetic approaches, we demonstrate that AngII upregulates endothelial calpain activity via AT1r signaling thus causing a distinct endothelial inflammatory phenotype characterized by increased leukocyte-endothelium interactions and albumin permeability. We also demonstrate that inhibition of calpain activity is an important molecular mechanism of the vascular protective action of ARBs.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
AngII infusion Protocols
All experimental procedures were approved by Temple University IACUC and performed according to the NIH guidelines for the use of experimental animals. Subpressor concentrations of AngII were systemically delivered to randomly selected groups of rats or mice using Alzet osmotic minipumps implanted subcutaneously (Model 2002; ALZA Scientific Products, Mountain View, CA, USA). Pumps were filled with either saline or with AngII at the concentration of 20 ng/kg/min or 500 ng/kg/min, for rat or mice respectively. All pumps delivered saline or AngII for 14 days. Previous studies in the species rat have demonstrated that infusion of 20 ng/kg/min induces endothelial dysfunction with inflammation in the absence of significant changes in systemic blood pressure12. Since considerably higher doses of AngII are required to elicit vascular responses in C57BL/6J mice13, we used the higher dose of 500 ng/kg/min AngII.
In vivo endothelial function
We used quantitative intravital microscopy of the mesenteric microcirculation to measure important indices of endothelial function such as vessel diameter, venular wall shear rates, leukocyte rolling, adhesion, and extravasation, as well as vascular permeability. The microcirculation of the rat mesentery was used for these studies because it is highly amenable to intravital microscopy and because it expresses a typical inflammatory phenotype in response to very low, subpressor concentration of AngII II14.
Data Analysis
Data are presented as mean±SEM, and compared by ANOVA with post hoc analysis by Fisher’s corrected t test. Probabilities of 0.05 or less were considered statistically significant.
Results
AngII infusion increases calpain in the vasculature via AT1r signaling
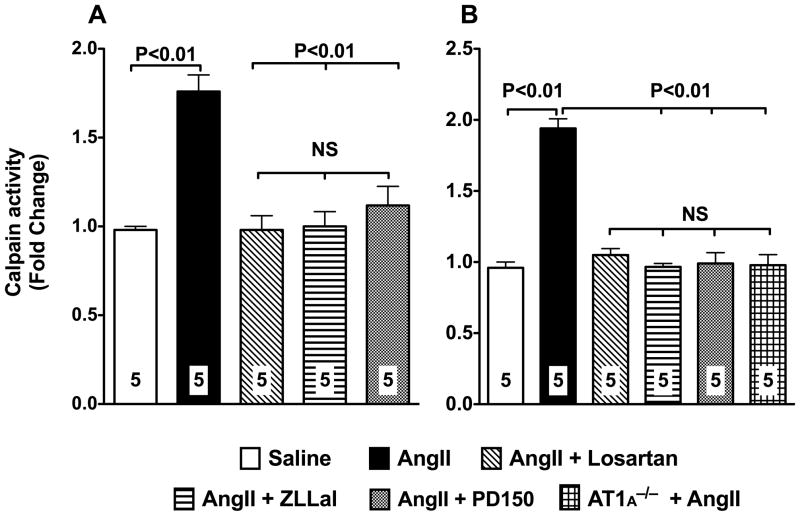
To study the relationship between AngII and calpain, we first measured calpain activity in extracts of vascularized sections of mesenteries isolated from rat and mice infused with subpressor doses of AngII for 14 consecutive days. Calpain activity in tissue extracts was measured using the fluorogenic calpain substrate Succ-LLVY-AMC. We found evidence of 2-fold increase in vascular calpain activity under these experimental conditions (Figure 1). Treatment of AngII infused animals with 10 mg/kg/day of the AT1r blocker losartan attenuated calpain activity to values found in control animals (Figure 1). Moreover, infusion of AngII to mice deficient in AT1A receptor, the major mouse AT1r isoform and the closest murine homolog to the single human AT115, failed to increase calpain activity in the vasculature thus demonstrating the obligatory role of the AT1r in the process of calpain activation by AngII (Figure 1, panel B).
Figure 1. Activation of the AngII/AT1r signaling pathway increases vascular calpain activity.
Calpain activity in vascular mesenteric extracts was measured using a fluorescent based assay with the use of the fluorescent probe T- Succ-LLVY-AMC. Two-week infusion of subpressor doses of AngII to rats (20 ng/Kg/min; left panel) or mice (500 ng/Kg/min; right panel) significantly increases calpain activity. A 5-day treatment of rat and mice with 10 mg/kg/day of the AT1r blocker losartan prevents upregulation of calpain activity in response to AngII. Similar results were obtained following treatment of AngII infused rats with two different calpain inhibitors, 140 μg/kg/day ZLLal or 1 mg/kg PD105. AngII failed to increase calpain activity in the vasculature of mice deficient in the AT1r. Data are expressed as the mean±SEM. Numbers at the base of the bars indicate the number of animals studied in each group.
Comparable results were obtained following pharmacological inhibition of calpain in AngII infused animals (Figure 1). Thus, a five-day treatment with either 140 μg/kg/day ZLLal or 1 mg/kg/day PD150, two selective calpain inhibitors, each with different molecular structure and mechanism of action, normalized calpain activity in the vasculature.
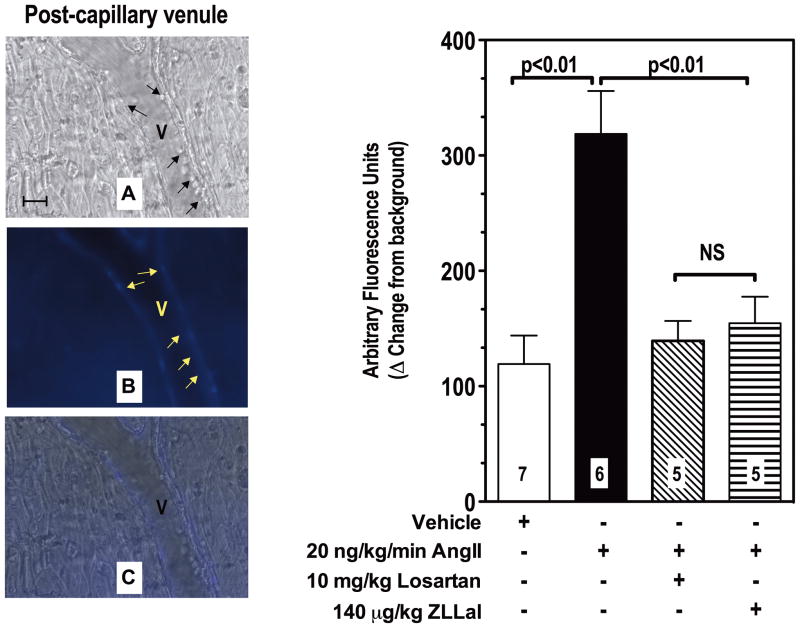
We next measured calpain activity in the intact microcirculation of live rats infused with AngII. For these studies we coupled the fluorescent based assay of calpain activity described in Figure 1 with in vivo microscopy of mesenteric post-capillary venules, which are microvessels comprised only by a layer of endothelial cells, a basement membrane, and pericytes16. The advantage of this approach over the biochemical one described above is that it allows for simultaneous quantification and spatial localization of active calpains within the vascular endothelium of the observed microcirculation in vivo. In fact, the results obtained with the mesenteric tissue homogenates described in Figures 1 could in theory have been contaminated with vascular smooth muscle cells which also express calpain2. Fluorescent staining clearly demonstrated that following AngII infusion the wall of post-capillary venules experiences increased calpain activity (Figure 2, Panel B). Quantification of the fluorescent staining revealed a 3-fold increase from control in calpain activity in post-capillary venules of AngII-infused rats, which was attenuated to control levels following pharmacological inhibition of either AT1r or calpain (P<0.05, Figure 2, bar graph to the left). These results are consistent with the biochemical analysis for calpain activity reported in Figure 1 and they further confirm that upregulation of AngII/AT1r signaling increases calpain activity in the endothelium of post-capillary venules.
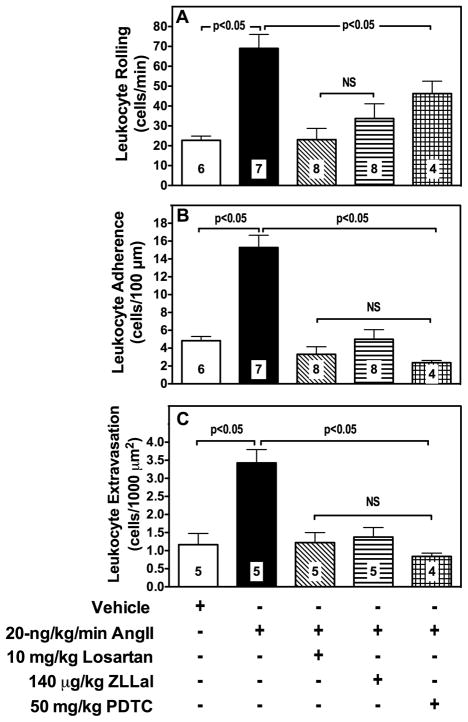
Figure 2. Ang II Infusion increases leukocyte adhesion and calpain activity in post-capillary of the rat microcirculation.
Post-capillary venules (V) were first viewed under brightfield microscopy to measure leukocyte rolling, adhesion and extravasation (panel A, black arrows). Subsequently they were superfused with the fluorogenic calpain substrate t-BOC-Leu-Met-CMAC to visualize and measure by computerized densitometry endothelial calpain activity in live rats (panel B, yellow arrows). Calpain activity was increased by AngII and nearly normalized by a five-day treatment with either the AT1r blocker losartan or the calpain inhibitor ZLLal. Noteworthy, increased leukocyte-endothelium interactions were observed in post-capillary venules experiencing increased calpain activity (overlay Panel C). Right graph shows quantitative analyses of calpain activity in all experimental groups of rat. Bars represent mean SEM, and numbers at the base of the bars represent the number of rats studied in each group.
Identification of the calpain isoform implicated in the endothelial dysfunction of AngII/AT1r signaling
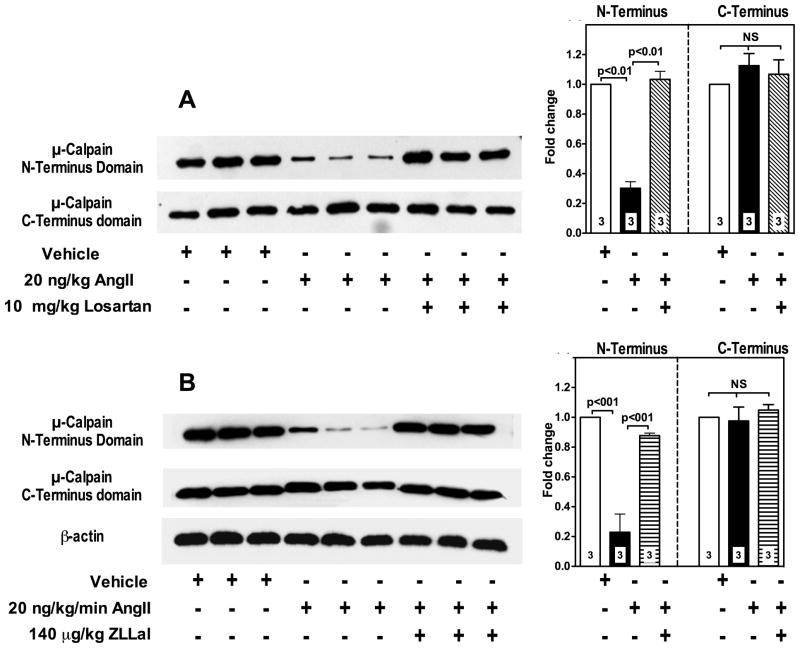
Two calpain isoforms, namely μ-calpain and m-calpain, are constitutively expressed in endothelial cells1. To investigate the effect of AngII/AT1r signaling on μ- and m-calpain activity in vivo, we measured expression levels of the N-terminus domain of the calpain large 80 kDa subunit. Upon activation, calpains undergo autoproteolysis with removal of the 9–14 amino acids from the N-terminus domain of the large 80 kDa subunit17. Accordingly, m- and μ-calpain autoproteolysis/activation levels in freshly isolated vascular segments of mesenteric tissue from all groups of rats were assessed by immunoblot analysis using primary antibodies to their respective N-terminus domains. We found increased proteolytic activity of μ-calpain only in AngII-infused rats, as demonstrated by an average 70% loss in N-terminus domain antibody recognition (Figure 3, upper lanes of panels A and B and bar graphs). A five-day treatment of AngII infused rats with either losartan or ZLLal significantly blocked μ-calpain activation (Figure 3, upper lanes of panels A and B, respectively). Total μ-calpain content was quantified using a primary antibody against the stable domain IV of the large subunit, which recognizes both unautolyzed and autolyzed (i.e., active) μ-calpain. No significant differences in total μ-calpain expression were found in all experimental groups rats (Figure 3, middle lanes of panels A and B and bar graphs), which indicates that AngII did not change significantly the transcriptional regulation of calpain, at least in the timeframe of our experimental conditions. In contrast, the proteolytic activity of m-calpain was not increased by AngII infusion as demonstrated by lack of changes in m-calpain N-terminus domain expression level (Online Figure I, panels A and B).
Figure 3. AngII/AT1r signaling upregulates the μ-calpain isoform in mesenteric vascular extracts.
μ-Calpain activity was assessed by immunoblot analysis using a primary antibody that recognizes the μ-calpain large subunit N-terminus domain, which is autolyzed in active calpains (panels A and B, upper lanes). Thus, calpain activation is demonstrated by loss of antibody recognition. A primary antibody against μ-calpain large subunit C-Terminus domain, which is stable and therefore recognizes both inactive and active μ-calpain, was used to quantify total μ-calpain content in the tissue extracts (panels A and B, middle lanes). Equal loading was also confirmed by β-actin staining (panel B, bottom lane). Bar graphs summarize densitometric analysis. AngII causes activation of μ-calpain as demonstrated by reduced N-Terminus domain expression (panels A and B, upper lanes). No significant changes were observed in the overall expression levels of μ-calpain in all groups of rats as demonstrated by lack of difference in C-Terminus domain expression (panels A and B, middle lanes). The effect of AngII on μ-calpain activation is reversed by a 5-day treatment with either the AT1r blocker losartan (panel A and upper graph) or the calpain inhibitor ZLLal (panel B and lower graph). Numbers at the base of the bars indicate the number of rats studied in each group.
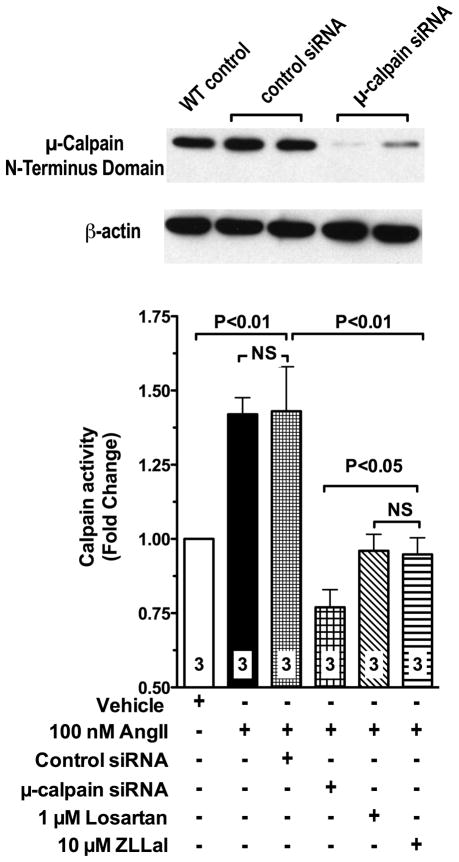
Parallel genetic studies in vitro were undertaken to further confirm μ-calpain as the target of AngII/AT1r signaling in the vascular endothelium. Thus, we used siRNA technology to knockdown μ-calpain in primary cultures of mesenteric microvascular endothelial cells (MMEC), freshly isolated from donor control rats. The siRNA used in this study effectively reduced μ-calpain expression levels (Figure 4, immunoblot) without affecting expression levels of the m-calpain isoform (data not shown). Calpain activity in attached MEC was then measured using the fluorescent assay described above. Data shown in Figure 4 demonstrate that MMEC exposed to 10 nM AngII for 6 hours experience a 1.5 fold increase in calpain activity. The calpain activating actions of AngII were abolished by μ-calpain siRNA treatment, as well as following inhibition of either the AT1r or calpain with losartan and ZLLal, respectively (Figure 4). Taken together the in vivo and in vitro data reported in Figures 1–4 strongly demonstrate that AngII/AT1r signaling increases μ-calpain activity in the vascular endothelium. They also suggest that μ-calpain may represent an important target of the pleiotropic actions of AT1r blockers in cardiovascular disease.
Figure 4. Depletion of μ-calpain with siRNA prevents upregulation of calpain activity in MMEC endothelial cells exposed to AngII.
Treatment of MMEC with siRNA to μ-calpain effectively reduces calpain expression levels (immunoblot). AngII fails to upregulates calpain activity in μ-calpain deficient MMEC (bar graph), thus demonstrating that the endothelial expressed μ-calpain isoform is the specific molecular target of AngII/AT1r signaling under our experimental conditions. AngII increases calpain activity via activation of the AT1r, as demonstrated by loss of increased calpain activity in MMEC treated with losartan. The calpain inhibitor ZLLal also prevented calpain activation in response to AngII. Calpain activity in attached MMEC was measured using a standard fluorescent assay and the calpain selective substrate T- Succ-LLVY-AMC. Numbers at the base of the bars indicate the number of independent experiments.
Ang II-induced calpain activation causes endothelial dysfunction with increased leukocyte-endothelium interactions and disruption of the endothelial cell barrier
To study the functional implications of calpain activation induced by the AngII/AT1r, we measured leukocyte-endothelium interactions and albumin permeability by intravital microscopy. As shown in panel A of Figure 2 and quantified in Figure 5, the venular endothelium of AngII infused rats experienced increased adhesiveness to circulating leukocytes. Specifically, we found evidence of a 3-fold increase in leukocyte rolling and a 4-fold increase in both leukocyte adhesion and extravasation in mesenteric post-capillary venules of AngII-infused rats compared to control rats receiving saline (Figure 5, Panels A, B and C). Leukocyte rolling, adhesion and extravasation were all reduced following treatment with the either the AT1r blocker losartan, the calpain inhibitor ZLLal, or the NF-κB inhibitor PDTC (Figure 5). Furthermore AngII failed to increase leukocyte adhesion in the mesenteric microcirculation of μ-calpain deficient mice (Online Figure II).
Figure 5. Leukocyte-endothelium interactions in mesenteric post-capillary venules.
Leukocyte rolling (panel A), adhesion (panel B), and extravasation (panel C) were studied in all experimental groups of rats by brightfield intravital microscopy and expressed as the number of leukocytes/min, leukocytes/100-μm vessel length, and leukocytes/1000 μm2 extravascular space, respectively. AngII increases leukocyte endothelium interactions in the microcirculation, which were reduced similarly by losartan, ZLLal or PDTC treatment. Bars represent mean ± SEM, and numbers at the base of the bars represent the number of rats studied in each group.
Parallel ex vivo experiments with aortas and leukocytes isolated from wild-type C57BLK mice demonstrated an essential role for the vascular endothelium in the pro-adhesive action of AngII (Online Figure III, panel A). Specifically, when AngII-stimulated aortic segments were incubated with unstimulated leukocytes a significantly high number of leukocytes adhered to the aortic endothelium (p<0.05 versus control unstimulated aortas/unstimulated leukocytes). In contrast, no difference from control was observed following incubation of unstimulated aortas with AngII-stimulated leukocytes (p>0.05), even though we found evidence of calpain-dependent increase in CD11b expression in circulating leukocytes of AngII infused rats (Online Figure IV). The increased adhesiveness of AngII-stimulated aortas was also prevented by incubation of the aortic tissue with losartan or ZLLal (Online Figure III, panel A).
Additional mechanistic studies were undertaken to dissect the contribution of endothelial-expressed and leukocyte-expressed calpains in these studies. The results obtained demonstrated that endothelial-expressed calpain and not leukocyte-expressed calpain was the target of AngII under our experimental conditions. Specifically, AngII failed to increase the adhesion of leukocytes isolated from wild-type mice to the aortic endothelium of μ-calpain deficient mice as well as of mice overexpressing the endogenous inhibitor of calpain, calpastatin (Online Figure III, panel B). In contrast, increased leukocyte adhesion to AngII was observed when aortas isolated from wild-type mice were incubated with leukocytes isolated from μ-calpain deficient mice or calpastatin overexpressing mice (Online Figure III, panel B). These data are in agreement with data on leukocyte adhesion obtained by intravital microscopy and they demonstrate that in the setting of increased AngII/AT1r signaling, the vascular endothelium develops a widespread pro-adhesive phenotype affecting both large conduit vessels and the microcirculation.
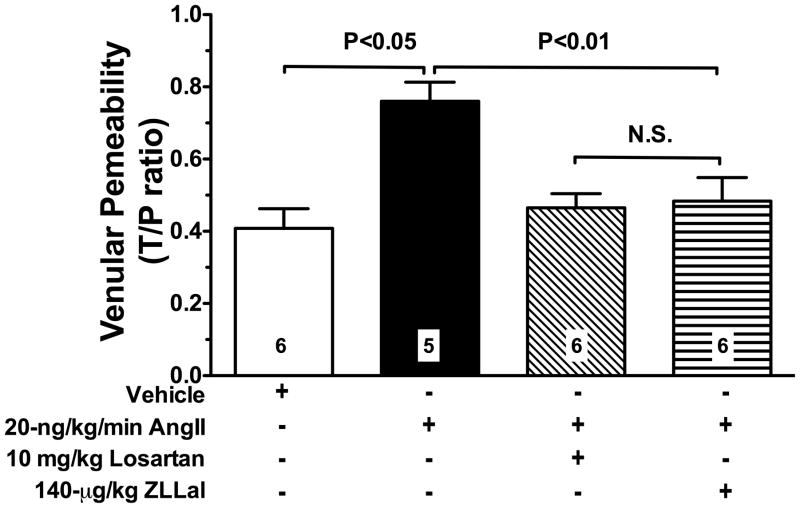
We also used fluorescent intravital microscopy of post-capillary venules to measure albumin leakage, another established marker of endothelial function. Data reported in Figure 6 demonstrate that AngII disrupts the physiological endothelial cell barrier as demonstrated by the increased albumin leakage found in AngII-infused Infused rats. This permissive action of AngII on albumin permeability was abolished by treatment of AngII infused rats with either losartan or ZLLal (Figure 6).
Figure 6. Elevated AngII/AT1r signaling increases albumin permeability.
The average permeability index to TR-labeled albumin was calculated during intravital microscopy in single mesenteric post-capillary venules of all experimental groups of rats. A two-week exposure of the mesenteric microcirculation to a low, subpressor dose of AngII increases albumin permeability via AT1r signaling and in a calpain-dependent manner. Thus, both losartan and the calpain inhibitor ZLLal attenuated albumin permeability in AngII injected rats. Data are expressed as the mean±SEM, and numbers at the base of the bars represent the number of rats studied in each group.
These functional results were observed in the absence of significant changes in mean arterial blood pressure, vessel diameter, venular shear rates, and total leukocyte blood counts (Online Table I).
Taken together, these data first demonstrate that endothelial expressed calpains are, at least in part, responsible for the endothelial inflammatory actions AngII/AT1r signaling in the cardiovascular system.
AngII/calpain signaling upregulates endothelial eCAMs via downregulation of IκBα
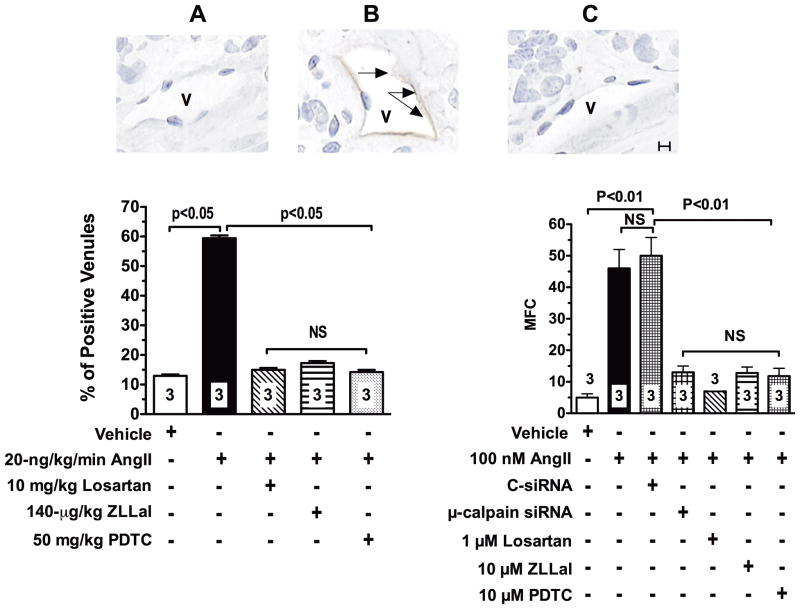
To investigate the molecular mechanism(s) responsible for the dysfunctional endothelial phenotype induced by AngII-mediated μ-calpain activation we studied expression levels of the cell adhesion molecules ICAM-1, VCAM-1 and E-selectin both in vivo and in vitro. Figure 7 illustrates ICAM-1 expression levels in all experimental groups of rats studied by immunohistochemistry. Thus, we found a 5-fold increase in ICAM-1 expression in the microcirculation of AngII infused rats compared to control rats given saline. Consistent with the functional data reported above, ICAM-1 expression was attenuated by treatment with losartan, ZLLal or the NF-κB inhibitor PDTC (Figure 7, left bar graph). Similar results were observed for VCAM-1 and E-selectin (Online Figure V)
Figure 7. AngII upregulates expression levels of ICAM-1 in the vascular endothelium.
In vivo. ICAM-1 expression in microvessels (V) of the mesentery was studied by immunohistochemistry in all experimental groups of rats. Representative photomicrographs (A, B and C) illustrate the effect of calpain inhibition on AngII-induced ICAM-1 expression in rat ileal venules (V). Brown immunoperoxidase reaction product (black arrows) indicates positive staining. Percentage of venules staining positive for ICAM-1 in all experimental groups of rats are illustrated in the bar graph to the left. Control microvessels had a very low level of ICAM-1 expression (Panel A and bar graph). ICAM-1 expression levels were markedly increased in AngII infused rats (Panel B, black arrows and bar graph). Losartan, ZLLal, and PDTC treatment returned ICAM-1 expression to control levels (Panel C and bar graph). In vitro. Right bar graph shows summary of ICAM-1 expression levels in mesenteric microvascular endothelial cells (MMEC) measured by flow cytometry. Upregulation of ICAM-1 in MMEC by AngII also occurs via activation of the AT1r and it is mediated by calpain activity as demonstrated by reduced ICAM-1 expression levels in MMEC treated with losartan, ZLLal, and PDTC respectively. Loss of AngII-induced upregulation of ICAM-1 following depletion of μ-calpain with siRNA confirms the role of the μ-calpain isoform in the process of ICAM-1 upregulation by AngII. MFC indicates mean fluorescence channels values for ICAM-1 staining measured by flow cytometry. Bars represent mean ± SEM, and numbers at the base of the bars represent either the number of rats studied in each group (left graph) or the number of independent experiments (right graph). Twenty tissue sections were studied in each rat to evaluate percentage of positive venules. Black line in Panel C represents a scale of 10 μm.
Flow cytometry studies in MMEC further confirmed the increased ICAM-1 expression levels in MEC exposed to 10 nM AngII for 6 hours, a phenomenon that was prevented by either inhibition of AT1r, calpain, or NF-κB with losartan, ZLLal, and PDTC, respectively (Figure 7, right bar graph). Furthermore, The effect of AngII on eCAMs expression were also found to be largely μ-calpain dependent as demonstrated by lack of ICAM-1 upregulation following siRNA depletion of μ-calpain (Figure 7, right bar graph). These data correlate with the anti-adhesive effect of AT1r blockade and calpain inhibition on leukocyte-endothelium interactions reported above.
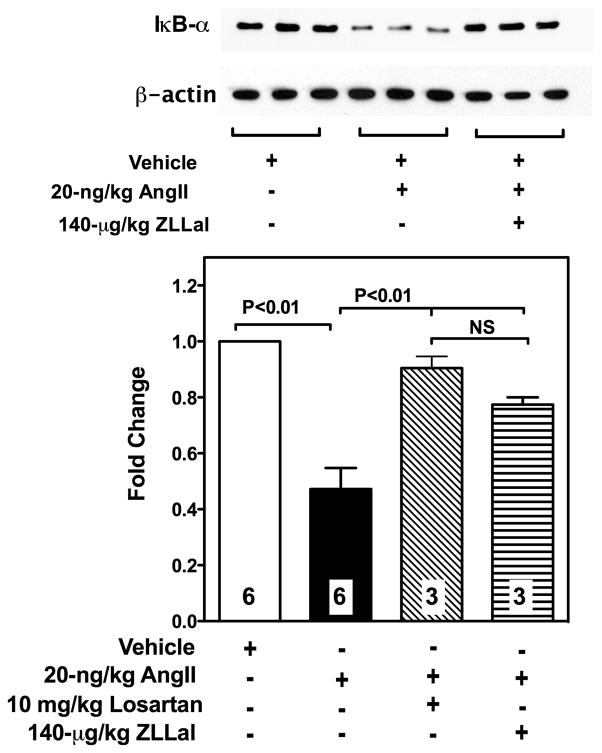
In the vascular endothelium, ICAM-1, VCAM-1 and E-selectin are largely regulated by NF-κB, an inflammatory nuclear transcription factor that it sequestered inactive in the cytoplasm by the inhibitor protein IκBα. Accordingly, we measured IκBα expression levels in vascular sections of the mesentery from all experimental groups of rats. Data reported in Figure 8 demonstrate that the microcirculation of AngII infused rats experiences loss of IκBα, which can be prevented by pharmacological inhibition of either AT1r or calpain with losartan and ZLLal, respectively. Taken together, these data demonstrate that AngII/AT1r signaling causes IκBα degradation in part via increased calpain activity and they provide a novel mechanism by which AngII/AT1r signaling induces transcription of inflammatory mediators in the dysfunctional endothelium.
Figure 8. IκBα expression in the vasculature of AngII infused rats.
IκBα levels in all experimental groups of rats were studied by western blot analysis in the cytosolic fractions of vascularized mesenteric tissue lysates (immunoblot). Bar graph shows densitometric analysis of IκBα expression levels in all experimental groups of rats. Reduced IκBα expression levels were found in the mesenteric vasculature of AngII-infused rats. Treatment of AngII-infused rats with either losartan or the calpain inhibitor ZLLal significantly restored IκB-α expression. Equal loading was confirmed by β-actin staining. Bars represent mean ± SEM, and numbers at the base of the bars represent the number of rats studied in each group.
Discussion
The current work to our knowledge is the first to uncover the role of the cytosolic protease calpain in the endothelial dysfunction associated with elevated AngII signaling. We show that subpressor doses of AngII selectively activate the endothelial expressed μ-calpain isoform, a process that induces a distinct endothelial inflammatory phenotype. Importantly, we show that μ-calpain is a molecular target of AT1r signaling, which may also help uncover new mechanism(s) of the pleiotropic actions of ARBs.
Disruption of the regulatory functions of the vascular endothelium results in multiple maladaptive modifications, including making the endothelium hyper adhesive to circulating leukocytes and leaky to macromolecules. Studies in research animals and humans have clearly linked AngII/AT1r signaling to endothelial dysfunction, independently of its vasopressor actions. In agreement with our results, others have demonstrated that nanomolar concentrations of AngII acutely increase leukocyte-endothelium interactions18 and vascular permeability19 in the rat microcirculation without affecting local or systemic hemodynamic. Leukocyte-endothelium interactions and vascular permeability are two fundamental functions of the endothelium that are also crucial to the homeostasis of organ tissues, including the vascular wall itself. In fact, leukocyte trafficking is mechanistically important in vascular pathology. Extravasation of proteins into the adjacent interstitial compartment modifies the composition of the interstitium, which disrupts traffic of fluid and vital substrates to the cellular mass, and the removal of waste products20. Accordingly, full understanding of the molecular mechanisms by which AngII increases leukocyte-endothelium interaction and vascular permeability becomes crucial for the management of vascular disease and related organ damage. With most data indirectly derived from studies using pharmacological inhibitors of the RAS, our understanding of the primary cellular pathways(s) responsible for the endothelial inflammatory action of AngII remains largely elusive, which hinders development of new therapeutic strategies. Our current data are the first to identify in the calpain system a direct molecular effector of the endothelial AngII/AT1r signaling pathway. This novel working hypothesis is supported by data in the scientific literature. Calpains are cytosolic proteases that require calcium for their activation and function17. Of interest, published data have demonstrated that in cultured endothelial cells AngII increases intracellular calcium concentrations via activation of AT1r 21. The detailed signaling pathway(s) by which activation of the AT1r leads to increased endothelial calpain activity with subsequent leukocyte adhesion remains unknown. Data in the literature have highlighted a role for the calcitonin gene-related peptide (CGRP) in the leukocyte adhesion associated with activation of the AT1r22, which may suggest a role for CGRP in these studies. We found that AngII selectively activates the μ-calpain isoform, but not the m-calpain isoform. This result is in agreement with recent studies demonstrating a role for μ-calpain in the homeostasis of the vascular wall2, 3, 23 and it compatible with the knowledge that only the Ca2+ concentrations required by autolyzed μ-calpain are in the physiological range, while the Ca2+ concentrations required by m-calpain are much higher than those found in living cells17. Nonetheless, the possibility that with chronic disease other vascular expressed calpain isoform may also become deregulated should not be entirely excluded based on in vitro studies demonstrating a role for m-calpain in the microvascular endothelial toxicity induced by inflammatory mediator24. Furthermore, evidence in the literature suggests the possibility of sequential activation of calpain isoforms17
Specific adhesion molecules expressed on the vascular endothelium and circulating leukocytes regulate leukocyte-endothelium interactions in the cardiovascular system. In this study, we focused on the role of NF-κB regulated cell adhesion molecules such as ICAM-1, VCAM-1 and E-selectin. Despite intensive research, the molecular mechanisms by which AngII/AT1r signaling upregulates NF-κB controlled eCAMs is not fully understood. Our in vitro and in vivo results first demonstrate a mechanistic role for μ-calpain in the process of eCAMs upregulation by AngII. Interestingly, others have implicated calpains in the upregulation of eCAMs expression via NF-κB25. We also found evidence of increased CD11b expression levels on the cell surface of blood resident leukocytes of AngII infused rats, although isolated leukocytes stimulated with AngII failed to adhere the endothelium of control aortas in vitro. This result indicates that activation of endothelial expressed CAMs is necessary for the vascular inflammatory action of AngII, which is consistent with published information demonstrating that AngII-induced leukocyte-endothelium interactions can be largely abrogated by eCAMs blockade alone6. Albeit AngII-induced upregulation of CD11b was also attenuated by calpain inhibition in our study, the overall impact of calpain inhibition on leukocyte function remains still controversial. Studies have reported increased leukocyte adhesion, polarization, and chemotaxis following inhibition of basal calpain activity in resting leukocytes26. In contrast, more recent reports demonstrate that inhibition of calpain activity reduces the adhesiveness and migratory properties of leukocytes primed by clinically relevant inflammatory stimuli23, 27. A recent study also supports a role for μ-calpain in neutrophil-induced ICAM clustering, a process important in leukocyte extravasation28. Overall, emerging views in the field consider activation of leukocyte-expressed calpains necessary for leukocyte expansion, a process central in leukocyte extravasation29. Relevant to the physiology of leukocyte-endothelium interactions, endothelial expressed calpains have been shown to influence the adhesive and migratory behavior of circulating leukocytes30. Conversely adhesion of leukocytes initiates calcium signaling events that lead to activation of the endothelial calpain system, which in terms favors leukocyte transmigration in inflammation31. Further studies are clearly needed to understand how inhibition of the calpain system impacts the adhesive behavior of resting and primed leukocytes, both in the presence and absence of the vascular endothelium.
Leukocytes interact with the endothelium in a process that allows blood resident leukocytes to cross the barrier created by endothelial cells. This cellular migration can result in modifications of the vascular permeability that permit the non-physiological transfer of macromolecules from the blood compartment to the vascular wall and peripheral tissue. Sumagin et al. 32 have recently shown that vascular permeability alterations can be induced by endothelial expressed CAMs, such as ICAM-1, following ligation by rolling or adhering leukocytes. Interestingly, we also found that inhibition of μ-calpain prevents albumin permeability in microvascular networks inflamed by AngII, which suggest a key role for the endothelial calpain system in this process. In the present paper, we did not investigate whether the hyperpermeability of AngII was secondary to adhering leukocytes or primarily driven by eCAMs-induced signaling. Since studies in this direction can provide additional therapeutic targets, further work should be undertaken to understand the primary contribution to vascular permeability of calpain-mediated eCAMs upregulation in disease states associated with elevated AngII signaling.
The molecular mechanism by which AngII upregulates eCAMs appears to be related to activation of the nuclear transcription factor NF-κB 33. In vitro studies have demonstrated increased activation of NF-κB in response to Ang II in several cell types, including VSMCs and endothelial, glomerular, tubular, and mononuclear cells34. In vivo, increased NF-κB activity and expression have been shown in the vasculature, heart, and kidneys of double transgenic rats and Ang II-infused wild-type rats35, 36. Nonetheless, the precise signaling pathway by which AngII upregulates NF-κB remains largely unknown. Interestingly, calpain is known to degrade the NF-κB inhibitory subunit IκBα, thus leading to NF-κB activation37. Here we report evidence of calpain-dependent degradation of IκBα in response to activation of AngII/AT1r signaling in the rat vasculature, which uncovers a novel molecular mechanism by which AngII upregulates NF-κB activity and actions in the vascular wall.
Data in the literature have consistently shown that Ang II-induced vascular inflammation is largely mediated via stimulation of the AT1r. Relevant to the present study, the AT1r blockers, such as losartan, attenuate a) leukocyte-endothelium interactions in AngII inflamed microvascular networks38; b) vascular permeability in experimental animal models 11; and c) albuminuria in patients with cardiovascular disease39. However the precise cellular targets of the endothelial pleiotropic actions of ARBs remains largely unknown. Our results strongly demonstrate a role for the endothelial calpain system in the beneficial vascular actions of ARBs. In fact, losartan prevented calpain activation both in vivo and in vitro, a result that was also confirmed in AT1r deficient mice. Furthermore, pharmacological blockade of calpain achieved a degree of endothelial protection which was comparable to that observed with losartan treatment in vivo. Thus, both losartan and the calpain inhibitor ZLLal stabilized IκBα levels, and prevented leukocyte-endothelium interactions and albumin permeability in the face of increased AngII levels.
In summary, the present studies demonstrate that μ-calpain contributes to the development of Ang II–induced endothelial dysfunction. Specifically, inhibition of calpain activation attenuates leukocyte-endothelium interactions, albumin permeability, endothelial CAMs expression, and IκBα degradation in AngII infused rats. Finally, these protective actions of calpain inhibition are related to inhibition of AT1r signaling, which demonstrate a mechanistic role for calpain in the pleiotropic actions of ARBs. Our studies may have important clinical implications in that they provide evidence for a novel molecular target in the treatment of AngII-mediated vascular disease.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. Andrew C. Issekutz, Dalhousie University, Halifax, NS. Canada, for generously providing the RME-1 antibody to rat E-selectin.
Sources of Funding
This work was supported by NIDDK Grant DK064344 to R.S.
Non-standard Abbreviations and Acronyms
- MEC
mesenteric microvascular endothelial cells
- T-Succ-LLVY-AMC
N-succinyl-l-leucyl-l-valyl-l-tyrosine-7-amino-4-methylcoumarin
- t-BOC-Leu-Met-CMAC
7-amino-4-chloromethylcoumarin, t-BOC-L-leucyl-L-methionine amide
- ZLLal
benzyloxycarbonyl-leucyl-leucinal
Footnotes
Disclosure: None
References
- 1.Fujitani K, Kambayashi J, Sakon M, Ohmi SI, Kawashima S, Yukawa M, Yano Y, Miyoshi H, Ikeda M, Shinoki N, Monden M. Identification of mu-, m-calpains and calpastatin and capture of mu-calpain activation in endothelial cells. Journal of cellular biochemistry. 1997;66:197–209. doi: 10.1002/(sici)1097-4644(19970801)66:2<197::aid-jcb7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin ii-induced hypertension. Circulation research. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 3.Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, Lakatta EG. Increased aortic calpain-1 activity mediates age-associated angiotensin ii signaling of vascular smooth muscle cells. PLoS ONE. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of ang ii type 1a receptors attenuates ang ii-induced ascending aortic aneurysms in ldl receptor−/− mice. Circulation research. 2011 doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, Ferri C, Desideri G, Gulino A, Santucci A. Angiotensin ii stimulates intercellular adhesion molecule-1 (icam-1) expression by human vascular endothelial cells and increases soluble icam-1 release in vivo. Circulation. 1999;100:1646–1652. doi: 10.1161/01.cir.100.15.1646. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez A, Cerda-Nicolas M, Naim Abu Nabah Y, Mata M, Issekutz AC, Panes J, Lobb RR, Sanz MJ. Direct evidence of leukocyte adhesion in arterioles by angiotensin ii. Blood. 2004;104:402–408. doi: 10.1182/blood-2003-08-2974. [DOI] [PubMed] [Google Scholar]
- 7.Newton CR, Curran B, Victorino GP. Angiotensin ii type 1 receptor activation increases microvascular permeability via a calcium dependent process. J Surg Res. 2005;123:33–39. doi: 10.1016/j.jss.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Montecucco F, Pende A, Mach F. The renin-angiotensin system modulates inflammatory processes in atherosclerosis: Evidence from basic research and clinical studies. Mediators Inflamm. 2009;2009:752406. doi: 10.1155/2009/752406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cianchetti S, Del Fiorentino A, Colognato R, Di Stefano R, Franzoni F, Pedrinelli R. Anti-inflammatory and anti-oxidant properties of telmisartan in cultured human umbilical vein endothelial cells. Atherosclerosis. 2008;198:22–28. doi: 10.1016/j.atherosclerosis.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Petnehazy T, Stokes KY, Wood KC, Russell J, Granger DN. Role of blood cell-associated at1 receptors in the microvascular responses to hypercholesterolemia. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:313–318. doi: 10.1161/01.ATV.0000193625.32499.71. [DOI] [PubMed] [Google Scholar]
- 11.Akiyoshi K, Akimitsu T, Hara M, Saikawa T, Yoshimatsu H. At1 receptor blockade prevents microvascular dysfunction induced by ischemia/reperfusion injury. J Atheroscler Thromb. 2006;13:231–239. doi: 10.5551/jat.13.231. [DOI] [PubMed] [Google Scholar]
- 12.Bataller R, Gabele E, Schoonhoven R, Morris T, Lehnert M, Yang L, Brenner DA, Rippe RA. Prolonged infusion of angiotensin ii into normal rats induces stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol. 2003;285:G642–651. doi: 10.1152/ajpgi.00037.2003. [DOI] [PubMed] [Google Scholar]
- 13.Cassis LA, Huang J, Gong MC, Daugherty A. Role of metabolism and receptor responsiveness in the attenuated responses to angiotensin ii in mice compared to rats. Regul Pept. 2004;117:107–116. doi: 10.1016/j.regpep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Nabah YN, Mateo T, Estelles R, Mata M, Zagorski J, Sarau H, Cortijo J, Morcillo EJ, Jose PJ, Sanz MJ. Angiotensin ii induces neutrophil accumulation in vivo through generation and release of cxc chemokines. Circulation. 2004;110:3581–3586. doi: 10.1161/01.CIR.0000148824.93600.F3. [DOI] [PubMed] [Google Scholar]
- 15.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (at2) angiotensin receptor-mediated blood pressure regulation in mice lacking both at1a and at1b receptors for angiotensin ii. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voisin MB, Probstl D, Nourshargh S. Venular basement membranes ubiquitously express matrix protein low-expression regions: Characterization in multiple tissues and remodeling during inflammation. The American journal of pathology. 2010;176:482–495. doi: 10.2353/ajpath.2010.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiological reviews. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 18.Piqueras L, Kubes P, Alvarez A, O’Connor E, Issekutz AC, Esplugues JV, Sanz MJ. Angiotensin ii induces leukocyte-endothelial cell interactions in vivo via at(1) and at(2) receptor-mediated p-selectin upregulation [in process citation] Circulation. 2000;102:2118–2123. doi: 10.1161/01.cir.102.17.2118. [DOI] [PubMed] [Google Scholar]
- 19.Victorino GP, Newton CR, Curran B. Effect of angiotensin ii on microvascular permeability. J Surg Res. 2002;104:77–81. doi: 10.1006/jsre.2002.6412. [DOI] [PubMed] [Google Scholar]
- 20.Plante GE, Chakir M, Lehoux S, Lortie M. Disorders of body fluid balance: A new look into the mechanisms of disease. Can J Cardiol. 1995;11:788–802. [PubMed] [Google Scholar]
- 21.Pueyo ME, Arnal J-F, Rami J, Michel J-B. Angiotensin ii stimulates the production of no and peroxynitrite in endothelial cells. Am J Physiol Cell Physiol. 1998;274:C214–220. doi: 10.1152/ajpcell.1998.274.1.C214. [DOI] [PubMed] [Google Scholar]
- 22.Yusof M, Kamada K, Gaskin FS, Korthuis RJ. Angiotensin ii mediates postischemic leukocyte-endothelial interactions: Role of calcitonin gene-related peptide. American journal of physiology. Heart and circulatory physiology. 2007;292:H3032–3037. doi: 10.1152/ajpheart.01210.2006. [DOI] [PubMed] [Google Scholar]
- 23.Scalia R, Gong Y, Berzins B, Zhao LJ, Sharma K. Hyperglycemia is a major determinant of albumin permeability in diabetic microcirculation: The role of mu-calpain. Diabetes. 2007;56:1842–1849. doi: 10.2337/db06-1198. [DOI] [PubMed] [Google Scholar]
- 24.Quiniou C, Sennlaub F, Beauchamp MH, Checchin D, Lahaie I, Brault S, Gobeil F, Jr, Sirinyan M, Kooli A, Hardy P, Pshezhetsky A, Chemtob S. Dominant role for calpain in thromboxane-induced neuromicrovascular endothelial cytotoxicity. The Journal of pharmacology and experimental therapeutics. 2006;316:618–627. doi: 10.1124/jpet.105.093898. [DOI] [PubMed] [Google Scholar]
- 25.Read MA, Neish AS, Luscinskas FW, Palombella VJ, Maniatis T, Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995;2:493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 26.Lokuta MA, Nuzzi PA, Huttenlocher A. Calpain regulates neutrophil chemotaxis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4006–4011. doi: 10.1073/pnas.0636533100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiemer AJ, Lokuta MA, Surfus JC, Wernimont SA, Huttenlocher A. Calpain inhibition impairs tnf-alpha-mediated neutrophil adhesion, arrest and oxidative burst. Mol Immunol. 2010;47:894–902. doi: 10.1016/j.molimm.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams SL, Milne IR, Bagley CJ, Gamble JR, Vadas MA, Pitson SM, Khew-Goodall Y. A proinflammatory role for proteolytically cleaved annexin a1 in neutrophil transendothelial migration. Journal of immunology. 2010;185:3057–3063. doi: 10.4049/jimmunol.1000119. [DOI] [PubMed] [Google Scholar]
- 29.Dewitt S, Hallett M. Leukocyte membrane “expansion”: A central mechanism for leukocyte extravasation. Journal of Leukocyte Biology. 2007;81:1160–1164. doi: 10.1189/jlb.1106710. [DOI] [PubMed] [Google Scholar]
- 30.Hussain AM, Zhang QX, Murray AG. Endothelial cell calpain activity facilitates lymphocyte diapedesis. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5:2640–2648. doi: 10.1111/j.1600-6143.2005.01077.x. [DOI] [PubMed] [Google Scholar]
- 31.Cuvelier SL, Paul S, Shariat N, Colarusso P, Patel KD. Eosinophil adhesion under flow conditions activates mechanosensitive signaling pathways in human endothelial cells. The Journal of experimental medicine. 2005;202:865–876. doi: 10.1084/jem.20041315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via icam-1-mediated signaling. Am J Physiol Heart Circ Physiol. 2008;295:H969–977. doi: 10.1152/ajpheart.00400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tham DM, Martin-McNulty B, Wang YX, Wilson DW, Vergona R, Sullivan ME, Dole W, Rutledge JC. Angiotensin ii is associated with activation of nf-kappab-mediated genes and downregulation of ppars. Physiological genomics. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases: Expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin ii into normal rats activates nuclear factor-kappab and ap-1 in the kidney: Role of at(1) and at(2) receptors. Am J Pathol. 2001;158:1743–1756. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller DN, Heissmeyer V, Dechend R, Hampich F, Park JK, Fiebeler A, Shagdarsuren E, Theuer J, Elger M, Pilz B, Breu V, Schroer K, Ganten D, Dietz R, Haller H, Scheidereit C, Luft FC. Aspirin inhibits nf-kappab and protects from angiotensin ii-induced organ damage. Faseb J. 2001;15:1822–1824. doi: 10.1096/fj.00-0843fje. [DOI] [PubMed] [Google Scholar]
- 37.Han Y, Weinman S, Boldogh I, Walker RK, Brasier AR. Tumor necrosis factor-alpha-inducible ikappabalpha proteolysis mediated by cytosolic m-calpain. A mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-kappab activation. The Journal of biological chemistry. 1999;274:787–794. doi: 10.1074/jbc.274.2.787. [DOI] [PubMed] [Google Scholar]
- 38.Petnehazy T, Stokes KY, Russell JM, Granger DN. Angiotensin ii type-1 receptor antagonism attenuates the inflammatory and thrombogenic responses to hypercholesterolemia in venules. Hypertension. 2005;45:209–215. doi: 10.1161/01.HYP.0000154085.27868.93. [DOI] [PubMed] [Google Scholar]
- 39.Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlof B, Devereux RB, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wan Y. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.