Abstract
Pregnancy is accompanied by complex biological adaptations, including extreme hormonal fluctuations. Moreover, changes on the endocrine level are accompanied by changes in cerebral anatomy, such as reductions in brain or gray matter volume. Since declining brain and tissue volumes are characteristic for normal aging, the question arises of whether such pregnancy-induced anatomical effects are permanent or transient. To answer this question, we acquired high-resolution brain image data of 14 healthy women in their mid-twenties to late thirties at two time points: within 1–2 days of childbirth (early postpartum) and at 4–6 weeks after childbirth (late postpartum). At both time points, we estimated the brain ages for each woman using a well-validated machine learning approach based on pattern recognition. Ultimately, this algorithm - designed to identify anatomical correlates of age across the entire brain - reveals a single score for each individual: the BrainAGE index. Comparing the BrainAGE indices between both time points, female brains at late postpartum were estimated to be considerably younger than at early postpartum. On average, that difference was about five years (mean±SD: 5.4±2.4 years). These findings suggest a substantial restoration / rejuvenation effect after giving birth, which is evident already within the first couple of months.
Keywords: aging, brain, estradiol, postpartum, pregnancy, progesterone
Introduction
During pregnancy and the postpartum period, the maternal body undergoes tremendous adaptations, including extreme changes in hormone levels (Brunton and Russell, 2008). Perhaps surprising, being pregnant also seems to affect the gross anatomy of the brain, albeit existing research is extremely sparse - most likely due to the restrictions imposed on magnetic resonance imaging (MRI) during pregnancy. Nevertheless, at least two independent studies concluded that pregnancy is accompanied by significant decreases in brain and gray matter volumes (Oatridge et al., 2002; Hoekzema et al., 2017). As dwindling brain sizes and declining brain tissue in otherwise healthy subjects are common trademarks of brain aging (Raz et al., 2010; Pfefferbaum et al., 2013), the question arises as to whether any pregnancy-induced brain loss is permanent or transient. While the two aforementioned studies (Oatridge, et al., 2002; Hoekzema, et al., 2017) closely agree on various aspects, there seems to be some discrepancy on the endurance of the effect. More specifically, Oatridge and colleagues reported that brain size decreased during pregnancy, but then increased again after giving birth, with a relative restoration within the first few months postpartum (2002). Hoekzema and colleagues also reported gray matter reductions during pregnancy, but observed that most of the incurred loss actually persisted until at least two years after pregnancy (2017). A third study (Kim et al., 2010) compared gray matter between two time points after giving birth, more specifically between 2–4 weeks postpartum and 3–4 months postpartum, and revealed gray matter increases at the later time point. These latter findings (Kim, et al., 2010) appear in line with the outcomes of the first study which examined brain and ventricle size (Oatridge, et al., 2002), although the morphological substrate measured by Kim and colleagues (voxel-wise gray matter) is more similar to the second study (Hoekzema, et al., 2017).
To shed further light on the nature of the effect (transient vs. persistent) - without tying our observations to a specific morphometric measure (e.g., voxel-wise tissue volume) - we applied a well-validated image analysis framework trained to identify anatomical correlates of aging in the brain and translating those into one single score: the BrainAGE index (Franke et al., 2010; Franke et al., 2012). The BrainAGE index is negative if a brain is estimated younger than its chronological age; it is positive if a brain is estimated older than its chronological age. The absolute BrainAGE index indicates the magnitude of the deviation (in years) from the true chronological age. Of note, a number of variations of this approach have been developed, refined, and/or tested by other groups yielding good prediction accuracies (e.g., Valizadeh et al., 2017). For a recent review on estimating brain age using neuroimaging data, please refer to Cole and Franke (2017).
Since declining brains (decrease in overall size, increase in ventricular volume, loss of gray matter tissue, etc.) are a hallmark of aging, the reversal of such pregnancy-induced changes, even if only partly, will manifest as altered BrainAGE indices during compared to after pregnancy. However, given the aforementioned concerns regarding MRI during pregnancy, the current study focused on the postpartum period altogether, similar as in Kim et al. (2010), discriminating between early and late states. In contrast to Kim and colleagues who acquired their initial brain scan at 2–4 weeks, we focused on a time even closer to giving birth, namely within 1–2 days postpartum, hereafter referred to as “early postpartum”. Importantly, although pregnancy-related hormones have already started to decline at this point, the full extent of the dramatic postpartum endocrine changes manifests only a few days later. Thus, we have a unique opportunity to study the maternal brain during this early postpartum period as an approximation of the pregnant brain. Our follow-up scan was obtained at 4–6 weeks postpartum, hereafter referred to as “late postpartum”. In addition to determining whether there is a significant change in the individual BrainAGE indices between early and late postpartum, we set out to test whether there is a significant correlation between hormonal levels and BrainAGE indices.
Methods
Participants
Our study sample included 14 right-handed, healthy postpartum women between 25 and 38 years of age. For sample characteristics, please refer to Table 1. All women had normal pregnancies, uncomplicated deliveries (vaginal: n=9; Caesarean: n=5) and at least one night of sleep following delivery. Moreover, all women were breastfeeding at the time of the follow-up (late postpartum) brain scan. Exclusion criteria were post pregnancy complications, admission of infants to the neonatal intensive care unit, ongoing depression or anxiety disorders, treatment with hormonal compounds and/or psychotropic drugs within three months prior to the study, as well as contraindications to MRI. All procedures were approved by the Regional Ethical Review Board, Uppsala (Sweden), and all participants provided written informed consent.
Table 1.
Sample characteristics.
| Age: mean±SD years (range) | 32.8 ± 4.0 (25–38) |
| Pre-pregnancy BMI: mean±SD kg/m2 (range) | 23.9 ± 2.8 (20.2 – 31.2) |
| Nordic origin: n (%) | 13 (92.9) |
| Married or cohabiting: n (%) | 13 (92.9) |
| University education: n (%) | 11 (78.6) |
| Smokers: n (%) | 0 (0) |
| Non-pregnancy light-to-moderate alcohol use: n (%) | 10 (71.4) |
| First delivery: n (%) | 7 (50.0) |
| Singleton pregnancy: n (%) | 14 (100) |
BMI = body mass index, SD = standard deviation
Brain Image Acquisition and Processing
High-resolution T1-weighted brain images were acquired at 27±10 hours (early postpartum) and at 34±5 days (late postpartum) after delivery. For this purpose, we used a whole-body scanner (Achieva 3T X; Philips Medical Systems, Best, The Netherlands) equipped with an eight-channel head coil applying the following parameters: 5,700 ms repetition time, 15 ms echo time, 400 ms inversion time, 90° flip angle, and 0.45 × 0.45 × 2.0 mm3 voxel size. As described elsewhere (Luders et al., 2016), the acquired brain images were processed in Matlab (http://www.mathworks.com/products/matlab/), using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html), resulting in spatially normalized and smoothed gray matter segments. Using these gray matter segments, the individual brain ages were estimated, as further described in the next paragraph, ultimately revealing a so-called BrainAGE index.
The BrainAGE Index
The BrainAGE framework utilizes relevance vector regression, a machine learning approach based on pattern recognition (Franke, et al., 2010; Franke et al., 2012). It has been initially trained using brain scans and ageing information of more than 650 subjects, ranging between 19 and 86 years of age. Importantly, those subjects are not part of the current sample. When applied to new brain scans - specifically the processed gray matter segments - of the current sample, the trained algorithm generates an estimated brain age. The difference between estimated age and true chronological age yields the so-called brain [A]ge [G]ap [E]stimate (BrainAGE). For example, if the algorithm computes +5 for the brain of a 32- year-old, this individual shows the typical aging pattern of a 37-year-old. Conversely, if the algorithm computes −5 for the brain of a 32-year-old, this individual shows the typical aging pattern of a 27-year-old. In the current study, a BrainAGE index was calculated at early postpartum as well as at late postpartum for each of the 14 women.
Hormonal Analysis
Blood samples for the hormonal analyses were drawn approximately twenty minutes prior to each brain scanning session. As previously described (Gingnell et al., 2015), serum progesterone and estradiol levels were analyzed by competitive immunometric electrochemical luminescence at the Department of Clinical Chemistry, Medical Sciences using a Cobas e601 analyzer and Cobas Elecsys estradiol and progesterone reagent kits (Roche Diagnostics, Bromma, Sweden). The measurement intervals for progesterone and estradiol were 0.1–191 nmol/l and 18.4–15,781 pmol/l, respectively. The intra-assay coefficients of variation were 2.2% at 2.4 nmol/l and 2.8% at 31.6 nmol/l for progesterone, and 6.8% at 85.5 pmol/l and 2.8% at 1640 pmol/l for estradiol.
Statistical Analysis
Paired t-tests were applied to test for significant changes (early postpartum versus late postpartum) in BrainAGE as well as in serum concentrations of estradiol and progesterone. Moreover, Pearson correlation coefficients were calculated to test for significant relationships between BrainAGE and serum concentrations at each time point. In addition, we used two linear mixed models (i.e., one for each serum measure) - with BrainAGE as the dependent variable, the serum concentrations as fixed effects, and subject as random effect - to test for significant relationships between BrainAGE and serum concentrations across both time points. For this analysis, we used log10-scaled values for the serum measures in order to account for the large differences in values and variance between the two time points. Finally, Pearson correlation coefficients were calculated to test for significant relationships between changes in BrainAGE and changes in serum concentrations, again using the log10-scaled values. For all analyses, alpha was set at 0.05 (two-tailed). Importantly, for all analyses, the assumptions for parametric testing (i.e., normal distribution of the residuals; equal variance between groups, if applicable) were assessed using Lilliefors tests for normality and two-sample F-tests for equal variance. The aforementioned assumptions were violated in one instance: when assessing changes in serum concentrations of estradiol (early postpartum versus late postpartum). Thus, for this specific analysis, a non- parametric Monte-Carlo simulation with 10,000 permutations was conducted to derive the final p-value.
Results
BrainAGE
The BrainAGE (mean ± SD) at early postpartum (i.e., within 1–2 days of delivery) was 1.35 ± 3.61 years. In contrast, the BrainAGE at late postpartum (i.e., at 4–6 weeks after delivery) was −4.02 ± 3.09 years, indicating considerably younger brains during the late compared to the early phase of postpartum. As shown in Figure 1, the magnitude of the change in BrainAGE ranged between 1.7 and 8.3 years (median: 5.64 years). The mean difference between the two time points was more than five years (5.36 ± 2.4 years) constituting a robust effect (T = −8.37, p < 0.001, d = −4.64).
Figure 1.
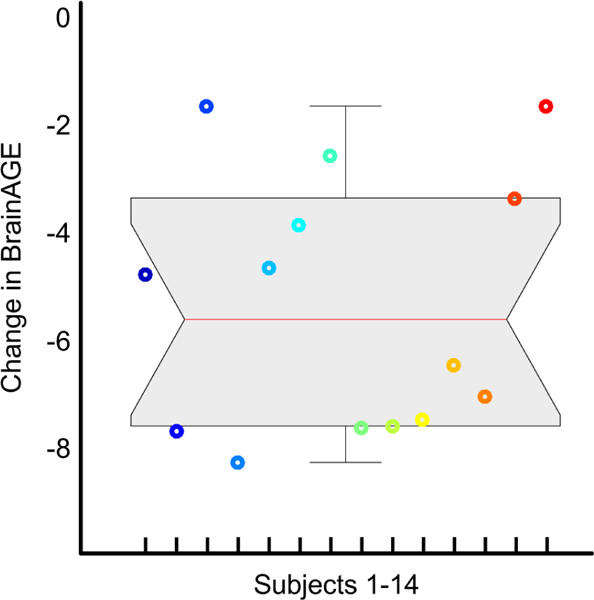
Change in BrainAGE (in years) between Early and Late Postpartum. The data are displayed as a boxplot, with the gray shaded area containing the values between the 25th and 75th percentiles of the sample (the red line indicates the median; the two short black lines the 1.5 interquartile ranges). Negative numbers show that brains were estimated younger at late postpartum than at early postpartum. The 14 different colors refer to the 14 individuals.
Hormone Levels and Links to BrainAGE
As shown in Table 2, serum concentrations of estradiol as well as of progesterone were significantly lower at late postpartum compared to early postpartum (estradiol: T = −10.51, p< 0.001, d = −7.01; progesterone: T = −8.97, p< 0.001, d = −5.98). While there was no significant correlation between serum concentrations and BrainAGE at either time point, the link was significant across both time points for both hormones (estradiol: T = 5.77, p < 0.001, r = 0.78; progesterone: T = 5.01, p < 0.001, r = 0.74), with lower values for all measures at late compared to early postpartum. There were no significant correlations between changes in BrainAGE and changes in serum concentrations. Individual measures for BrainAGE, log10- estradiol, and log10-progesterone are depicted in Figure 2.
Table 2.
Levels of estradiol and progesterone.
| Estradiol (pmol/l) | Progesterone (nmol/l) | |
|---|---|---|
| Early postpartum* | 1,533 ± 694 | 41.9 ± 37.4 |
| Late postpartum | 118 ± 55 | 0.8 ± 0.5 |
Serum levels were missing for 4 individuals at early postpartum.
pmol/l = picomoles per liter
nmol/l = nanomoles per liter
Figure 2.
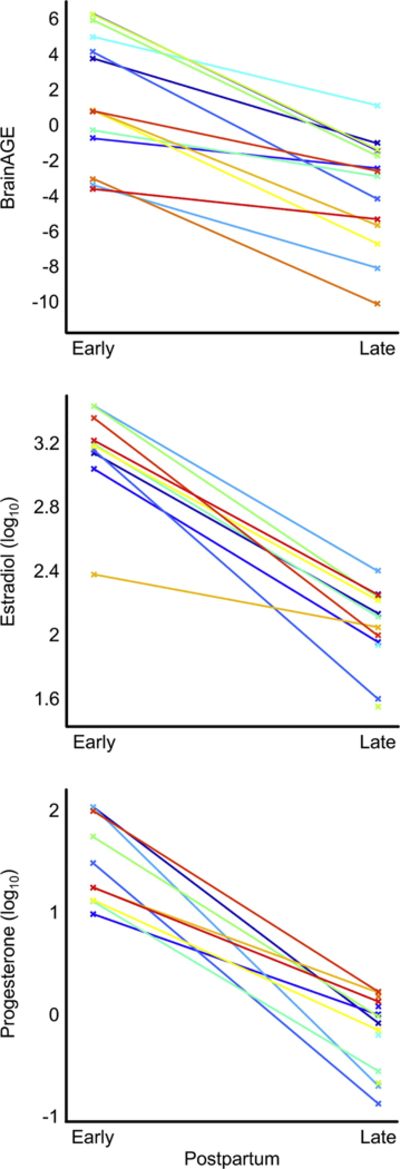
Individual Measures at Early Postpartum and at Late Postpartum. BrainAGE is indicated in years, estradiol in pmol/l, and progesterone in nmol/l. For the latter two measures log10-scaled values were used. The 14 different colors refer to the 14 individuals. At early postpartum, serum measures were missing for four individuals.
Discussion
A significant change in estrogen / progesterone levels (i.e., from a manifold increase during pregnancy to almost non-measurable levels after birth) is one of the characteristics of the maternal body. However, the full effect of the postpartum endocrine changes manifests only a few days after giving birth. This is not only evident in the actual hormonal measures but also reveals itself, for example, as an adjustment period during which performance on hormone-sensitive tasks is successively normalized (Kask et al., 2008). Thus, even though serum hormone concentrations have already started to decline, there is a small window of opportunity to study the maternal brain during the very early postpartum period as an approximation of the pregnant brain. Contrasting such very early postpartum measures (i.e., obtained within 1–2 days of giving birth) with later postpartum measures (i.e., obtained at 4– 6 weeks after giving birth), our findings extend existing work in this understudied field of research (Oatridge, et al., 2002; Kim, et al., 2010; Hoekzema, et al., 2017). The current analyses revealed significantly lower brain ages (i.e., seemingly younger brains) at the follow-up time point compared to the initial time point.
Correspondence with Previous Research Outcomes
Altogether, these findings seem to suggest a substantial restoration / rejuvenation effect after giving birth, which is evident already within 4–6 weeks postpartum. Prior research suggested that brain and tissue volumes - albeit initially decreasing during pregnancy - are restored within the first few months after giving birth (Oatridge, et al., 2002; Kim, et al., 2010). Our findings are consistent with those reports in that restored brain and tissue volumes may be reflections of seemingly younger brains. In other words, the calculated time- and subject-specific BrainAGE index is based on the tissue concentrations in specific brain regions (i.e., those deemed as age-relevant when training the algorithm). Since ageing is accompanied by dwindling brain tissue, increased volumes at late postpartum as compared to early postpartum (as observed by the two aforementioned studies) translate to lower brain ages at late postpartum versus early postpartum (as observed in the current study). In contrast, another study suggested that pregnancy-induced gray matter reductions endured for at least a few years (Hoekzema, et al., 2017). However, even in that latter study it was observed that there was a partial volume recovery in the hippocampus, a brain region known to be extremely plastic and amenable to structural changes due to synaptogenesis, angiogenesis, dendritogenesis - and perhaps even neurogenesis (Eriksson et al., 1998), although the latter is not unequivocally supported (Sorrells et al., 2018). Moreover, the hippocampus is also one of the key structures implicated in brain aging (Fraser et al., 2015; Kurth et al., 2017). Thus, the hippocampus-specific tissue regain, as reported by Hoekzema and colleagues (2017) even if only evident after two years, appears somewhat in line with the direction of the current outcomes measuring BrainAGE, a composite index capturing the complex and multidimensional ageing pattern across the brain, including the hippocampus.
Links to Hormone Measures
During pregnancy, dramatic changes occur in the levels of sex hormones, where marked increases in estradiol and progesterone levels during pregnancy are followed by a rapid decrease and suppression of those hormones postpartum. The findings of the current study confirm this with significantly lower hormonal concentrations at late postpartum compared to early postpartum. The seemingly missing link between estradiol and BrainAGE at either time point (or their change over time), might have come as a surprise. However, hormone levels differed considerably across individuals in the current study (up to 10-fold) and so did the individual changes in hormone levels between early and late postpartum. In fact, when assessing the link between BrainAGE and serum levels across both time points, effect were highly significant. Since all measures decreased from early to late postpartum, our study seems to suggest that decreases in estradiol as well as in progesterone are associated with a reduced BrainAGE (although the magnitude of change in BrainAGE is not determined by the magnitude of change in hormone levels). Interestingly, the direction of this effect is in contrast to what one might expect based on studies relating hormonal and brain measures across the menstrual cycle. More specifically, it was reported that increases in estradiol are accompanied by increases in hippocampal tissue volume and fractional anisotropy (Lisofsky et al., 2015; Barth et al., 2016). Similarly, increases in estradiol during the menstrual cycle were found to be associated with a reduced BrainAGE (Franke et al., 2015).
It is important to realize, however, that the outcomes of the aforementioned studies focused on the menstrual cycle may not be directly comparable to the current study. That is, during pregnancy, the brain is exposed to simultaneous, extreme, and long-term elevated estradiol and progesterone levels, rather than to regularly occurring, swift, and comparatively subtle changes, such as the increase in estradiol in the follicular phase (or the increase in progesterone during the luteal phase) of the menstrual cycle. Thus, changes in the gross anatomy of the brain during the physiologically exceptional state of pregnancy (Oatridge, et al., 2002; Hoekzema, et al., 2017) are likely to differ from the normally existing fluctuations in brain tissue (estimated BrainAGE, respectively). The link between hormones and brain anatomy after pregnancy may be even further complicated by the abrupt and massive plunge in estradiol and progesterone after giving birth. In the present study, hormone levels were still significantly higher at the initial compared to the follow-up time point, but the early postpartum levels most likely already differed from existing prepartum levels.
Strengths, Limitations and Outlook for Future Studies
Relative strengths of the study are its longitudinal design, the very narrow time frames within which all subjects were scanned at early / late postpartum, the combination of relevant hormonal data with high-resolution neuroimaging data, as well as a well-validated state-of-the-art approach estimating, automatically and objectively, the age of individual brains. Limitations of the current study are the small sample size as well as the lack of any pre-pregnancy hormonal and/or imaging data. In addition to addressing these limitations, it would be desirable in future studies to obtain additional post-pregnancy data (e.g., a third brain scan) after more than only 4–6 weeks as well as data from a control group, possibly of women who spent an equal amount of time in clinical care. Follow-up research might consider collecting alternative endocrine and other measures known to change during pregnancy and the postpartum period, such as related to cortisol, oxytocin, or monoamine oxidase activity, just to name a few (Nissen et al., 1995; Meinlschmidt et al., 2010; Sacher et al., 2010). In addition to biological factors, the cognitive and behavioral demands of motherhood (or parenthood in general) are likely to shape and remodel the brain of the caregiver (Anderson and Rutherford, 2012; Abraham et al., 2014). Thus, future studies might further advance this field of research by obtaining relevant non-biological information (e.g., measures of affective processing, attachment, mother-infant interactions). Last but not least, as reviewed and discussed elsewhere (Cole and Franke, 2017), the field of brain age prediction is rapidly evolving. Thus, rather than relying on T1-weighted data alone, future studies might benefit from using a combination of multiple neuroimaging modalities (e.g., T1-weighted, T2*-weighted, and diffusion-weighted data) to further enhance the prediction performance of the machine learning approach.
Highlights.
Brains at late postpartum were estimated to be younger than at early postpartum.
On average, that difference was about five years.
These findings suggest a substantial restoration / rejuvenation effect after giving birth.
The effect seems to be already evident within 4–6 weeks postpartum.
Acknowledgments
This study was supported by a research grant from the Swedish Research Council to I.S.P. (K2014–54X-20642–07-4). In addition, E.L. is funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R01HD081720).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham E, Hendler T, Shapira-Lichter I, Kanat-Maymon Y, Zagoory-Sharon O, Feldman R (2014), Father’s brain is sensitive to childcare experiences. Proc Natl Acad Sci U S A 111:9792–9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MV, Rutherford MD (2012), Cognitive reorganization during pregnancy and the postpartum period: an evolutionary perspective. Evol Psychol 10:659–687. [PubMed] [Google Scholar]
- Barth C, Steele CJ, Mueller K, Rekkas VP, Arelin K, Pampel A, Burmann I, Kratzsch J, et al. (2016), In- vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Scientific reports 6:32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA (2008), The expectant brain: adapting for motherhood. Nature review s Neuroscience 9:11–25. [DOI] [PubMed] [Google Scholar]
- Cole JH, Franke K (2017), Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci 40:681–690. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998), Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317. [DOI] [PubMed] [Google Scholar]
- Franke K, Hagemann G, Schleussner E, Gaser C (2015), Changes of individual BrainAGE during the course of the menstrual cycle. Neuroimage 115:1–6. [DOI] [PubMed] [Google Scholar]
- Franke K, Luders E, May A, Wilke M, Gaser C (2012), Brain maturation: predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage 63:1305–1312. [DOI] [PubMed] [Google Scholar]
- Franke K, Luders E, May A, Wilke M, Gaser C (2012), Brain maturation: Predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage 63:1305–1312. [DOI] [PubMed] [Google Scholar]
- Franke K, Ziegler G, Kloppel S, Gaser C (2010), Estimating the age of healthy subjects from T1- weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage 50:883–892. [DOI] [PubMed] [Google Scholar]
- Fraser MA, Shaw ME, Cherbuin N (2015), A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. NeuroImage. [DOI] [PubMed] [Google Scholar]
- Gingnell M, Bannbers E, Moes H, Engman J, Sylven S, Skalkidou A, Kask K, Wikstrom J, et al. (2015), Emotion Reactivity Is Increased 4–6 Weeks Postpartum in Healthy Women: A Longitudinal fMRI Study. PLoS One 10:e0128964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Barba-Muller E, Pozzobon C, Picado M, Lucco F, Garcia-Garcia D, Soliva JC, Tobena A, et al. (2017), Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci 20:287–296. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE (2010), The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci 124:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Cherbuin N, Luders E (2017), The impact of aging on subregions of the hippocampal complex in healthy adults. NeuroImage 163:296–300. [DOI] [PubMed] [Google Scholar]
- Lisofsky N, Martensson J, Eckert A, Lindenberger U, Gallinat J, Kuhn S (2015), Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage 118:154–162. [DOI] [PubMed] [Google Scholar]
- Luders E, Cherbuin N, Gaser C (2016), Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. NeuroImage 134:508–513. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Martin C, Neumann ID, Heinrichs M (2010), Maternal cortisol in late pregnancy and hypothalamic-pituitary-adrenal reactivity to psychosocial stress postpartum in women. Stress 13:163–171. [DOI] [PubMed] [Google Scholar]
- Nissen E, Lilja G, Widstrom AM, Uvnas-Moberg K (1995), Elevation of oxytocin levels early post partum in women. Acta Obstet Gynecol Scand 74:530–533. [DOI] [PubMed] [Google Scholar]
- Oatridge A, Holdcroft A, Saeed N, Hajnal JV, Puri BK, Fusi L, Bydder GM (2002), Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. AJNR Am J Neuroradiol 23:19–26. [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV (2013), Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage 65:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U (2010), Trajectories of brain aging in middle-aged and older adults: regional and individual differences. NeuroImage 51:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM, Stewart DE, Meyer JH (2010), Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry 67:468–474. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, et al. (2018), Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valizadeh SA, Hanggi J, Merillat S, Jancke L (2017), Age prediction on the basis of brain anatomical measures. Hum Brain Mapp 38:997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]


