Abstract
Exogenous nitric oxide (NO) represents an attractive antibacterial agent because of its ability to both disperse and directly kill bacterial biofilms while avoiding resistance. Due to the challenges associated with administering gaseous NO, NO-releasing macromolecular scaffolds have been developed to facilitate NO delivery. This review describes the rational design and application of NO-releasing macromolecular scaffolds as antibacterial therapeutics. Special consideration is given to the role of the physiochemical properties of the NO storage vehicles on antibacterial or antibiofilm activity.
Keywords: antibacterial, biofilm, nitric oxide, macromolecular scaffolds, rational design
1. Introduction
Infections caused by pathogenic bacteria pose a tremendous threat to society. While the use of antibiotics is still the gold standard in the treatment of bacterial infections, bacteria continue to grow, adapt, and develop mechanisms that render these treatments ineffective.[1] The World Health Organization and the U.S. Centers for Disease Control and Prevention consider infections caused by multi-drug-resistant (MDR) bacteria to be a major global health problem.[1b] In addition, over 80% of bacterial infections are associated with biofilm formation. Biofilms are co-operative communities of bacteria protected by an extracellular polysaccharide (EPS) matrix that both impede the immune response and greatly diminish antibiotic efficacy.[2] Compared to planktonic bacteria, biofilms are significantly more resistant to antibiotic intervention.[3] Often, the EPS matrix interacts with the antibiotic, and compromises the diffusion, penetration, and activity of the drug. Bacteria embedded in biofilms exhibit altered metabolic activity with some bacteria expressing biofilm-specific resistance genes. As a result, biofilm-based bacteria may require up to 1000 times greater antibiotic doses to elicit inhibition or killing.[4] The threats of MDR bacterial and biofilm infections have resulted in an urgent need to expand research into the design of novel non-conventional or alternative antimicrobial therapies.
2. Nitric Oxide as an Antibacterial and Antibiofilm Agent
Nitric oxide (NO) is an endogenously (i.e., within the body) produced gaseous molecule involved in multiple physiological processes.[5] In the body, NO is produced enzymatically from l-arginine by one of three NO synthase (NOS) isoforms: endothelial (eNOS), neuronal (nNOS), and inducible (iNOS).[6] At low concentrations (pM to nM), NO produced by eNOS and nNOS regulates processes such as vasodilation, angiogenesis, and neurotransmission.[6b] At larger concentrations (μM), NO produced by iNOS in macrophages and neutrophils acts as an antimicrobial agent as part of the body’s innate immune response to foreign pathogens.[7]
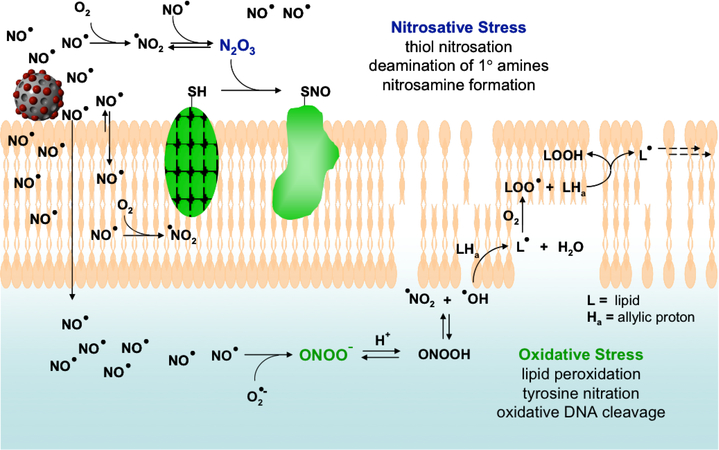
As an antibacterial agent, NO possesses broad-spectrum activity that may be attributed to its reactivity and formation of equally reactive byproducts that cause oxidative and nitrosative damage to microbial proteins, DNA, metabolic enzymes, and exterior membrane structure (Figure 1).[8] Nitrosative species such as dinitrogen trioxide (N2O3) cause DNA deamination and S-nitrosation of thiols on both cell surface and intracellular proteins, altering vital protein functions and inducing bacterial cell death.[5b, 9] Nitric oxide may also react with superoxide, endogenously derived from the bacterial respiration process, to form peroxynitrite (ONOO−) that elicits oxidative DNA damage and lipid peroxidation.[10] Based on NO’s size and multi-mechanistic antibacterial activity, bacterial resistance to NO has not been observed or reported.[11]
Figure 1.
Proposed mechanisms by which NO acts as an antibacterial agent. NO’s antibacterial properties are attributed to both nitrosative and oxidative stress exerted by reactive byproducts such as N2O3 and ONOO− (peroxynitrite). Nitrosative stress leads in part to nitrosation of thiols on proteins and DNA deamination, while oxidative stress is responsible for membrane destruction via lipid peroxidation. Of note, increased NO and O2 concentrations in lipid membranes leads to enhanced production of both nitrosative and oxidative species such as N2O3 and NO2 in the membrane. Reproduced with permission.[8] Copyright 2008, American Chemical Society.
Recent studies have focused on the utility of exogenous NO for biomedical applications, including treating infections and reducing bacterial adhesion.[5, 12] The efficacy of NO gas as a bactericidal agent has been demonstrated against a wide range of both Gram-negative (e.g., Pseudomonas aeruginosa and Escherichia coli) and Gram-positive (e.g., Staphylococcus aureus and Staphylococcus epidermidis) bacterial strains, as well as drug-resistant bacteria (e.g., methicillin-resistant S. aureus (MRSA)). For example, Miller et al. reported the use of gaseous NO to eradicate drug-resistant strains of S. aureus and E. coli without exhibiting significant toxicity against mammalian cells via intermittent NO exposure (160–200 ppm for 30 min every 4 h for 24 h) in vitro.[13] Using the same exposure protocol, they also demonstrated the antibacterial efficacy of gaseous NO in vivo with a P. aeruginosa pneumonia model in rats.[14] The exogenous gaseous NO significantly reduced the bacterial viability of P. aeruginosa in rat lungs after 24 h of treatment, but did not elicit adverse lung inflammation and toxic nitrite/nitrate levels. Although the long term (>24 h) cytotoxicity of NO exposure at bactericidal levels remains unknown, these prior studies highlight the unique advantage of NO as a broad-spectrum antibacterial agent.
In addition to showing antibacterial activity against planktonic species, NO has proven effective against biofilms in a dose-dependent manner.[12b, 15] At low NO concentrations (typically pM to nM), NO has been reported to regulate biofilm formation and dispersal as a signaling molecule.[15a] Biofilm dispersal represents the final stage of biofilm development that is essential for bacterial survival and allows for further colonization and disease transmission.[16] The three main phases of biofilm dispersion include: (i) the detachment of cells from mature biofilms; (ii) translocation of planktonic cells; and, (iii) attachment of planktonic cells to a substrate.[16] Although the mechanisms or pathways of NO-regulated biofilm dispersal are still not clear, one popular hypothesis is that NO mediates the biofilm structure via cyclic-di-GMP (c-di-GMP), a signaling molecule involved in the production and maintenance of the biofilm EPS matrix.[15a, 17] Recent experimental evidence indicates the similarity of heme-NO/oxygen domains (H-NOX) and the genes coding for c-di-GMP cyclases or phosphodiesterases in bacteria, suggesting that the ligation of H-NOX with NO results in NO-mediated regulation of biofilms through a c-di-GMP signaling.[17]
Barraud and Kjelleberg reported the benefit of low levels of exogenous NO on biofilm dispersal using low molecular weight NO donors. In an early study, NO was shown to disperse mature P. aeruginosa biofilms at ~0.025–0.50 nM over 24 h, ultimately initiating detachment of bacteria without compromising the viability of planktonic bacteria. [18] In a follow-on study, the behavior of the same NO donors was investigated against a wider range of pathogenic bacterial biofilms, including Gram-negative E. coli, Serratia marcescens, and Fusobacterium nucleatum, and Gram-positive S. epidermidis and Bacillus licheniformis.[15b] Low levels of NO treatment (24 h at 0.025 to 10 nM) consistently resulted in the dispersal of 30 to 90% of the biofilms.
For clinical applications, triggering biofilm dispersal and transitioning bacteria to a planktonic state via treatment with NO has been shown to increase bacterial susceptibility to common antibacterial agents.[12c, 18] For example, Barruad et al. tested a number of antimicrobial compounds (e.g., tobramycin and hydrogen peroxide) against P. aeruginosa biofilms as a function of NO administration using low molecular weight NO donors.[18] Although NO treatment (~0.5 nM for 24 h) did not affect biofilm viability, it significantly increased the susceptibility of the biofilms to the antimicrobials. Indeed, antimicrobial treatments (both tobramycin and hydrogen peroxide) following the same NO exposure resulted in an additional 2-log reduction in biofilm viability compared to antimicrobial treatments alone.
Complete biofilm eradication has been reported using greater concentrations of NO (e.g., μM-mM).[12b, 19] While the exact mechanism for NO-mediated eradication is still under study, physical destruction of the biofilm is likely the initiator via NO’s ability to alter the EPS matrix.[20] At the same concentration range, NO is exerting antibacterial activity via nitrosative and oxidative stresses.[21] Indeed, a recent study by Neufeld and Reynolds reported that a minimum concentration of ~1.5 mM NO delivered by low molecular weight NO donors was needed for reducing the bacterial viability of P. aeruginosa biofilms that were attached to a medical-grade polyurethane fiber by >90 %.[19]
3. Low Molecular Weight Nitric Oxide Donors
While NO is effective at killing bacteria and disrupting biofilms, delivery of gaseous NO to infection sites is often therapeutically intractable. The highly reactive nature of exogenously delivered NO (i.e., biological lifetime of seconds) impedes treatment as significantly larger doses may be required to counteract the loss of NO on its way to the target.[22] Additionally, the use of gaseous NO requires specialized gas cylinders and oversight. In this manner, the necessity of frequent hospital visits may hinder patient compliance.[9, 23] In order to ameliorate the problems associated with gaseous NO, low molecular weight molecules capable of storing NO through covalent bonds or coordination complexes have been developed as NO donors.[24] Through unique decomposition mechanisms, chemical NO donors may more effectively deliver NO to sites of infection. While many NO donors have been reported, we will focus on NO donors that have shown promise as antibacterial agents (Table 1).
Table 1.
Common nitric oxide donors for antibacterial applications
| NO Donor | Chemical Formula | Common Examples | Notable NO-Release Triggers |
|---|---|---|---|
| Organic nitrate | RONO2 | Nitroglycerin Isosorbide dinitrate | Enzymatic decomposition Thiol-initiated reduction |
| Metal nitrosyl | Varies | Sodium nitroprusside Roussin’s Black Salt | Photolysis |
| N-Diazeniumdiolate | R2NN(O)NO | PROLI/NO DETA/NO | Proton-initiated decomposition |
| S-Nitrosothiol | RSNO | S-nitroso-N-acetylpenicillamine (SNAP) | Photolysis Thermal decomposition Cu(I)-catalyzed reduction |
3.1. Organic Nitrates
Organic nitrates are among the oldest and most prevalent classes of NO donors used clinically.[24] Structurally, organic nitrates take the form of ester-bound nitrate groups tethered to an organic backbone (RONO2, Figure 2A). Synthesis of these compounds involves either nitric acid esterification of alcohols or reaction of alkyl halides with silver nitrate. Nitroglycerin, pentaerythrityl tetranitrate, isosorbide dinitrate, and isosorbide mononitrate have been utilized as NO donors in several cardiovascular applications.[24] Nitric oxide generation from organic nitrates is triggered by enzymatic breakdown of the donor (e.g., by mitochondrial aldehyde dehydrogenase), although some non-enzymatic mechanisms (e.g., thiol reactions) have also been proposed to play a minor role.[25] The production of NO from these compounds is slow in the absence of enzymes, thus limiting their effectiveness outside of enzymatic tissue.
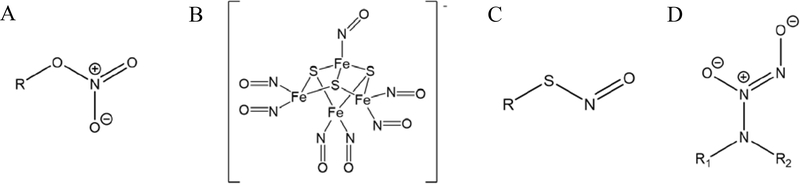
Figure 2.
Representative structures of nitric oxide donors including A: organic nitrate, B: metal nitrosyl (Roussin’s Black Salt anion shown), C: S-nitrosothiols, and D: N-diazeniumdiolate zwitterion.
3.2. Metal Nitrosyls
Metal nitrosyls are a family of coordination complexes containing NO ligands. As NO often binds to metal ions, the binding energy and bond character are determinants for the extent of NO release. Indeed, the literature has shown that certain metals (e.g., iron, ruthenium, manganese, and chromium) are more suitable as NO-releasing complexes because of the resulting metal-NO bond lability, facilitating NO release under certain triggers such as photolysis or chemical reduction.[26]
Iron-containing nitrosyl complexes are widely used as metal-based nitrosyl NO donors. For example, sodium nitroprusside (SNP; Na2[Fe(CN)5NO]), is used clinically to alleviate hypertension.[22] Iron-sulfur complexes such as Roussin’s Black Salt (RBS, Figure 2B), Roussin’s Red Salt (RRS), and Roussin’s Red Esters (RREs) have also shown utility as NO donors. In general, these compounds mimic complexes found in nature, such as those in enzymes and other metalloproteins. The syntheses of iron-sulfur NO complexes are based on reactions between iron nitrosyls and sulfide salts or thiols (e.g., glutathione and l-cysteine). Although enzymes, and/or chemical reactions with thiols, proteins, or ascorbic acid in biological media have been reported to initiate NO release from iron-sulfur NO complexes, light is the most common trigger.[24] The NO-release properties can be altered by the intensity, wavelength, and duration of the incident light.[27]
Like some of the above iron-sulfur NO complexes, manganese metal compounds are capable of achieving tunable NO release.[28] These NO donors are highly light sensitive and thus provide enhanced spatial and temporal control of their NO release even under low intensity irradiation of visible light.[28b, 29] The ability to use a single, benign trigger to initiate a burst of NO makes these donors relatively unique among NO donors.
3.3. S-Nitrosothiols
S-Nitrosothiols, also referred to as RSNOs based on their chemical structure (Figure 2C), are a family of NO donors derived from thiols. RSNOs are easily prepared via nitrosation of thiols with nitrite (NO2−), nitrogen oxides (NO2, N2O3, N2O4), or alkyl nitrites.[30] The most common method for nitrosation involves nitrite, for which the presence of acid is necessary to facilitate the reaction. Conversely, alkyl nitrites form S-nitrosothiols under more basic conditions (up to pH 10).[24] S-nitrosothiols derived from cysteine and glutathione have been measured in blood and are generally hypothesized to act as endogenous NO carriers for NO-mediated processes.[30–31]
Nitric oxide release from S-nitrosothiols is initiated via multiple pathways, each involving breaking the S-NO bond to liberate NO. Thermal decomposition and photolysis (primarily UV) are proposed to induce direct homolytic cleavage of the S-NO bond, generating NO and a thiyl radical. The thiyl radical may then react with another radical to form a disulfide, or with the liberated NO to regenerate the S-nitrosothiol. Some metal ions, in particular copper (I), are capable of catalyzing NO release from S-nitrosothiols. The variety of mechanisms by which S-nitrosothiol breakdown to NO allows for exquisite NO-release versatility for potential therapeutic indications.
3.4. N-Diazeniumdiolates
Diazeniumdiolates, also known as NONOates, are zwitterionic structures as a result of storing two moles of NO (Figure 2D). Most diazeniumdiolates tether the NO to nitrogen or carbon atoms, although oxygen- and sulfur-bound diazeniumdiolates have been reported.[32] C-Diazeniumdiolates generally do not form NO upon decomposition, but rather nitrous oxide. As a result, N-diazeniumdiolates are by far the most intensively studied as NO donors due to their ability to spontaneously release NO. The properties and syntheses of diazeniumdiolates have been reviewed previously by Hrabie and Keefer.[32–33]
N-Diazeniumdiolates are formed through the direct reaction of secondary amines with gaseous NO in alkaline solutions. Nitric oxide release from N-diazeniumdiolates is initiated through a proton-mediated breakdown of the zwitterionic structure to produce two moles of NO per mole of diazeniumdiolate and regenerate the precursor amine molecule. The NO release of diazeniumdiolates in aqueous media is also temperature-dependent due to its pseudo first-order release kinetics.[34] Strong bases (e.g., sodium methoxide) are commonly employed as catalyst during synthesis to both improve NO loading and/or reduce NO loss due to decomposition. A number of functionalities, such as protecting groups, may be added directly to the NONOates, for example at the O2 site for N-diazeniumdiolates, to further tune their physical and chemical properties (e.g., charge, stability, reactivity, and hydrophobicity).
The NO-release kinetics from N-diazeniumdiolates are dependent on the structure of the precursor amine and the resulting diazeniumdiolate stability. For example, the NO-release half-lives for small molecule donors at pH 7.4 range from 2 s for N-diazeniumdiolate-modified proline (PROLI/NO) to 24 h for N-diazeniumdiolate-modified diethylenetriamine (DETA/NO).[33b] In the case of DETA/NO and similarly structured compounds, the terminal primary amines are believed to stabilize the NONOate via intramolecular ring formation, thus slowing down the rate of diazeniumdiolate decomposition and NO release.[35] The range of NO-release kinetics possible using structurally diverse amines makes N-diazeniumdiolate incredibly versatile NO donors.
3.5. Comparison of NO Donors for Antimicrobial Applications
While each NO donor class presents certain advantages for NO storage and release, it is important to appreciate their overall utility in the context of treating bacterial and biofilm infections. Organic nitrates are perhaps the most limited of the NO donors for antibacterial applications, as their NO release is primarily mediated by enzymes that are absent in bacteria. The NO release is also susceptible to deactivation by surrounding tissue where such enzymes are present.[36] The light-sensitive metal nitrosyls enable a NO burst profile, but only with the use of an external light trigger. Further, UV or visible light is generally required, presenting potential difficulties in treating internal infections where tissue penetration by these wavelengths is limited. The metal centers and ligands may also present toxicity concerns (e.g., cyanate ligands on sodium nitroprusside). While the multiple mechanisms associated with S-nitrosothiol decomposition presents certain challenges (e.g., storage stability), their ability to spontaneously release NO in biological milieu eliminates the need for external triggers. Due to homolytic generation of NO and thiyl radicals, S-nitrosothiols have a greater likelihood of exerting bactericidal action through trans-nitrosylation of thiolated proteins relative to other NO donors.[37] N-Diazeniumdiolates are also able to spontaneously release NO under biological conditions. The proton-mediated decomposition mechanism ensures storage stability in moisture-free environments. The ability to directly modify N-diazeniumdiolates with other chemical structures to further modulate NO-release kinetics or enhance bactericidal action offers another unique advantage. While toxicity associated with high concentrations of amines from N-diazeniumdiolate precursors may be of concern, the amines likely facilitate the disruption of bacterial membranes via electronic interactions. In summary, N-diazeniumdiolates are the most extensively utilized NO donor for studying NO-mediated biology due to their stability, versatility, and tunability.
4. Macromolecular Nitric Oxide Donors
While low molecular weight NO donors have proven useful in ascertaining the killing mechanisms of NO’s antibacterial and antibiofilm action in vitro,[15a] many of these donors have limitations (e.g., cytotoxicity and clearance), limiting their utility as antibacterial therapeutics.[5a] Low molecular weight donors are often toxic to mammalian cells, and thus have greater potential to induce off-target cytotoxicity when used as antibacterial agents.[5a] While excessive toxicity may be avoided by using endogenous donors (e.g., nitrosated glutathione or GSNO), the number of such donors is limited as is the ability to easily tune specific NO-release properties (e.g., flux, decay, duration, etc.). Additionally, therapeutic administration of low molecular weight NO donors may result in accelerated clearance (i.e., rapid diffusion away from the target) and concomitant reduced antibacterial activity.
Many of the problems associated with low molecular weight NO donors have been addressed through the design of macromolecular NO release scaffolds.[12a, 31, 38] To date, a variety of materials have been chemically modified to contain NO-releasing moieties or used to house or encapsulate low molecular weight NO donors. It is important to note that NO loading directly onto macromolecular scaffolds typically results in loading efficiencies below that of low molecular weight compounds, primarily attributed to loading conditions, steric hindrance, and functional group interactions preventing donor moiety formation across the entire material backbone.[31] However, macromolecular scaffolds are still capable of storing greater NO payloads per mole of material than low molecular weight donors owing to the larger number of NO loading sites. The conditions required to form NO donors (e.g., highly acidic conditions for forming RSNOs and basic conditions for forming N-diazeniumdiolates) are another important consideration for macromolecular scaffolds, as they may present stability concerns for some materials (e.g., degradable polymers), thus requiring tuning to maximize NO payloads while preserving the scaffold backbone. For low molecular weight NO donors loaded into an external matrix or scaffold, leaching must be evaluated to discern if toxicity or donor loss (i.e., reduced NO payloads and off-target NO delivery) may occur. Regardless, both macromolecular scaffolds directly modified to release NO and NO donors incorporated into macromolecular scaffolds have improved drug localization, reduced cytotoxicity, and enhanced the antibacterial action of NO due to their unique NO payloads and targeting mechanisms.[5a, 31]
Hetrick et al. first reported the utility of NO-releasing silica nanoparticles as antibacterial agents, and compared their antibacterial efficacy with low molecular weight donor PROLI/NO.[8] Relative to PROLI/NO, NO-releasing silica nanoparticles exhibited enhanced bactericidal action against P. aeruginosa, which the authors attributed to improved association of the scaffold to the bacteria. In cell toxicity studies, cell viabilities were maintained for NO-releasing silica nanoparticles but not for PROLI/NO at the concentrations relevant to bacterial killing. While this comparison is just a single example, macromolecules encompass a massive library of materials from which NO-release systems can be prepared. As a result of this diversity, NO-release scaffolds can be selected based on certain properties, including advantages for a specific application. The utility of NO-releasing macromolecular scaffolds for biomedical applications (e.g., cardiovascular diseases, wound healing, and anticancer applications) has been reviewed previously.[5a, 12a, 31, 38] Herein, our goal is to provide an overview of nanometer-scale NO-releasing materials that have been utilized or have potential as antibacterial and antibiofilm agents, with special attention given to recent progress. Of note, the studies of antimicrobial NO-releasing polymer coatings, including coatings doped with low molecular weight/macromolecular NO donors and coatings with covalently attached NO donors, are not a focus of this article. We direct interested readers to other recently published reviews.[12c, 31]
4.1. Inorganic Nanoparticles
Silica nanoparticles have been widely used for the storage and controlled release of NO due to their ease of synthesis, favorable NO-release profiles, and tolerability to cells.[39] Post grafting, co-condensation, and ion-exchange reactions have been explored to introduce NO donor precursors such as secondary amines or thiols, onto the silica backbone.[40] Recent studies pointed out the benefits of mesoporous silica nanoparticles with large surface area available for NO donor modification relative to non-porous silica nanoparticles.[40d, 41] As expected, the increased surface area led to greater NO donor loading and payloads. Munaweera et al. reported the incorporation of other bioactive compounds such as cisplatin, an anticancer drug, into the pores of NO-releasing mesoporous silica nanoparticles via adsorption, achieving multifunctional systems.[42]
Gold nanoparticles, iron oxide nanoparticles, and quantum dots also represent attractive materials for N-diazeniumdiolate- or S-nitrosothiol-modification to create NO-release systems.[38, 43] These materials have unique optical, magnetic, and electronic properties that enable potential multifunctional utility. For example, Liu et al. reported quantum dots-hyperbranched polyether hybrid nanospheres with an average size of 127 nm that were able to release ~0.3 μmol NO per mg of material.[44] This system was also shown capable of detecting the NO release in real-time based on fluorescence change (25 nM limit of detection).
Ford and co-workers reported on the systems of lanthanide-doped upconverting nanoparticles (UCnanoparticles) for photochemical delivery of NO.[45] Upon excitation with near infrared light (NIR), the emission of visible light triggered NO release from photoactivated metal-based NO donors (e.g., RBS). This photochemical system overcomes the limitations of traditional photoactivated NO donors that require visible light. Moving to the NIR that has improved tissue penetration ability increased the therapeutic action of the photoactivated NO donors. In addition, the light-triggered mechanism offered more precise spatiotemporal control over the NO release.
4.2. Biopolymeric Scaffolds
Biopolymers are classified as naturally-occurring, non-toxic, biodegradable, and biocompatible polymers. To date, sugar-based materials including chitosan, dextran, and hyaluronic acid have been successfully modified with S-nitrosothiols and N-diazeniumdiolates.[46] Lu et al. reported the synthesis of N-diazeniumdiolate-modified NO-releasing chitosan oligosaccharides (COS).[47] Compared to previously reported NO-releasing high molecular weight chitosan that was not water-soluble and had poor NO loading (~0.2 μmol mg−1), NO-releasing COS exhibited enhanced NO storage (~0.8 μmol mg−1) and water solubility.[47] The versatility of biopolymers (e.g., chitosan and alginate) enabled Seabra et al. to prepare nanoparticles capable of encapsulating small molecule NO donors.[48] Small NO donors such as S-nitrosoglutathione and S-nitrosomercaptosuccinic acid were incorporated into the nanoparticles to confer NO release.
4.3. Synthetic Polymers
A wide range of synthetic polymers and polymeric nanoparticles has been utilized for NO-release applications.[31, 38] Dendrimers are a particularly attractive class of synthetic polymers because of their multivalent surface and well-defined polymeric structure.[34] The high density of exterior functional groups has proven useful for modification with NO donors to achieve significant high NO payloads (up to 5 μmol mg−1).[34, 49] Control over NO-release kinetics is achieved by varying the generation and exterior structure (e.g., steric environment and hydrophobicity) of the dendrimers.[49–50] However, the synthesis of dendrimers is labor-intensive as a result of multistep purification requirements. Hyperbranched polymers, a subclass of dendritic polymer, are more readily prepared in bulk.[51] While hyperbranched polymers have greater structural defects than dendrimers, they retain a high density of functional groups. Recent research has demonstrated that hyperbranched polymers act as an effective alternative to dendrimers for NO-release applications, at a much lower synthetic cost.[52]
Hollow polymeric nanoparticles made of synthetic polymer (e.g., polymethacrylate and polydopamine) have unique properties such as low density, optical scattering, and good flow capacity.[53] In particular, the large surface area of hollow polymeric nanoparticles facilitates NO donor functionalization on both the inner and outer surfaces, leading to larger NO payloads. Li and co-workers developed NO-releasing silica/polymer core shell particles using distillation precipitation polymerization.[53a, 54] Hollow polymeric particles were obtained by removing the silica core with a hydrofluoric acid solution. Nitric oxide storage was increased for N-diazeniumdiolate-modified hollow polymeric particles (5.5 μmol NO mg−1) relative to their non-hollow silica/polymer counterparts (3.6 μmol NO mg−1). To achieve steady NO-release kinetics under various conditions (e.g., different pHs and temperatures), pH- and thermal-responsive segments (e.g., poly(2-(dimethylamino)ethyl methacrylate and poly-N-isopropylacrylamide) acting as environmentally protective sheaths were incorporated into the polymeric particles through a two-stage distillation precipitation polymerization.[55] The resulting N-diazeniumdiolate-modified double-layered polymeric particles exhibited nearly equivalent NO-release properties over wide pH (5–9) and temperature (20–55 °C) ranges.
Biodegradability is an equally important characteristic for NO-releasing macromolecular therapeutics, given the concern over potential accumulative toxicity for non-degradable scaffolds. Although several strategies describing the synthesis of biodegradable synthetic polymers either chemically modified with NO donors or prepared as polymeric nanoparticles for the encapsulation of NO-releasing small molecules have been reported, the majority of these materials are limited to polyesters such as poly(lactic-co-glycolic acid),[56] poly(sulfhydrylated polyester)[57], poly(glycerol-co-glutaric acid),[58] and poly(citric-co-maleic acid-co-1,8-octanediol).[59] Reynolds and co-workers have worked to expand this area by developing S-nitrosothiol-modified poly(organophosphazenes) (POP) systems that can degrade hydrolytically into non-toxic phosphate and ammonia.[60] The NO-release properties of POP is altered based on the incorporated thiol structure, with primary thiols providing faster NO release and tertiary thiols leading to sustained NO release.[60b]
4.4. Liposomes
Liposomes are colloidal nanocarriers composed of an inner aqueous core and a phospholipid bilayer outer shell, with an overall structure that mimics natural cell membranes. Both NO gas and NO donors (e.g., N-diazeniumdiolates, metal nitrosyls, and organic nitrites) have been encapsulated into liposomes to achieve controlled NO release.[61] Suchyta et al. recently reported on the ability to tune NO-release kinetics of N-diazeniumdiolate-encapsulated liposomes by altering the identity of NO donors and phospholipid composition.[62] The presence of hydrophobic phospholipid bilayers as a physical barrier was shown to slow proton-initiated decomposition of encapsulated N-diazeniumdiolates, thus enhancing the stability of the NO donors and extending concomitant NO release compared to the free NO donors.
5. Antibacterial Applications
The rapid development of NO-releasing macromolecular systems has provided researchers with ample opportunities to study the antibacterial efficacy of exogenous NO. To date, a wide range of NO-releasing macromolecular scaffolds have proven efficacious against bacteria. A careful comparison of antibacterial activity across these materials has not yet been established, most likely due to large discrepancies in the bacterial strains, NO measurement, and bacteria eradication assays employed. In addition, limited effort has been made by prior reviews to summarize the desired properties that would enhance the antibacterial action of NO-releasing macromolecular scaffolds. In this section, we review the impact of both the NO-release and physiochemical characteristics of a NO-releasing macromolecular scaffold on antibacterial efficacy, aiming to elucidate the favorable factors that should be taken into account in the design of such materials.
5.1. NO-Release Properties
The antimicrobial activity of exogenous NO is highly dependent on its flux levels. With respect to NO-releasing polymer coatings, Hetrick et al. reported that a minimum NO flux of ~20 pmol cm−2 s−1 was required to reduce P. aeruginosa adhesion by 65% over 2 h under dynamic flow conditions (0.2 mL min−1).[63] The bacteria adhered to the NO-releasing coatings were no longer viable at 7 h based on fluorescent viability staining. In a subsequent study, the NO flux was varied from 0.50 to 50 pmol cm−2 s−1, and the bacterial adhesion was investigated as a function of pathogen type.[64] Regardless of the bacterial species, a NO flux between 20 to 50 pmol cm−2 s−1 reduced bacterial adhesion by >80% reduction over a 1 h exposure period.
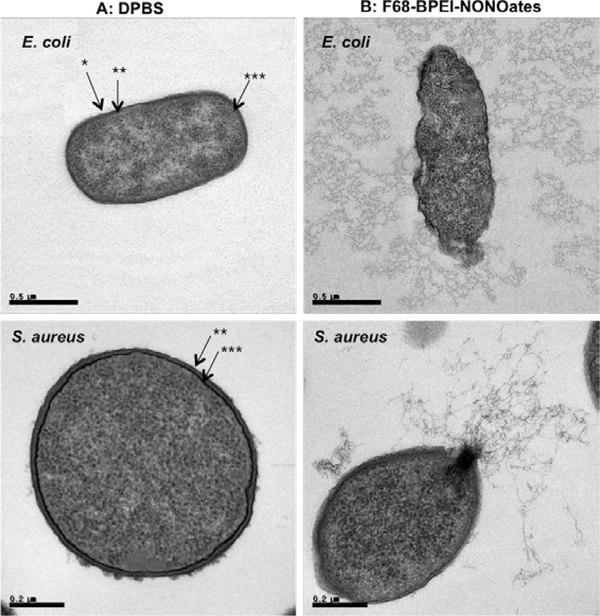
These previous studies suggest that NO-releasing macromolecular scaffolds with NO fluxes greater than 20 pmol cm−2 s−1 may also be suitable for antibacterial applications. Indeed, several reports support this notion.[28b, 41, 48a, 53b, 65–66] For example, Brandon et al. loaded photoactive manganese nitrosyl [Mn(PaPy3)(NO)](ClO4) NO donors into porous silicate materials. A rapid burst of NO (~10,000 pmole) was achieved by treating the material with low power visible light (10–100 mW) for 5 min, and this NO flux was maintained for a period of 60 min. Antibacterial efficacy was demonstrated against Acinetobacter baumannii in a soft tissue-infection model. Combining 50 mg of the NO-releasing hybrids with 1.5 h of illumination resulted in complete eradication (>5-log reduction in bacterial viability) of both drug-susceptible and drug-resistant A. baumannii. In another study, a pluronic thermo-responsive triblock polymer was conjugated with N-diazeniumdiolate-modified branched polyethylenimine (BPEI) to yield F68-BPEI-NONOates.[65] The ensuing large NO flux (730 pmol mg−1 s−1) resulted in eradication of a wide range of pathogens, including Gram-negative E. coli, Gram-positive S. aureus, and MRSA. Transmission electron microscopy (TEM) indicated the destruction of the bacterial membrane and discharge of intracellular compounds (e.g., DNA, RNA, and ribosomes), and an apoptosis-based killing mechanism (Figure 3).
Figure 3.
TEM image of E. coli and S. aureus. A: Treated with PBS as a control, B: Treated with F68-BPEI-NONOates. *: outer membrane,**: peptidoglycan, ***: cytoplasmic membrane bacteria. Reproduced with permission.[65] Copyright 2013, Elsevier.
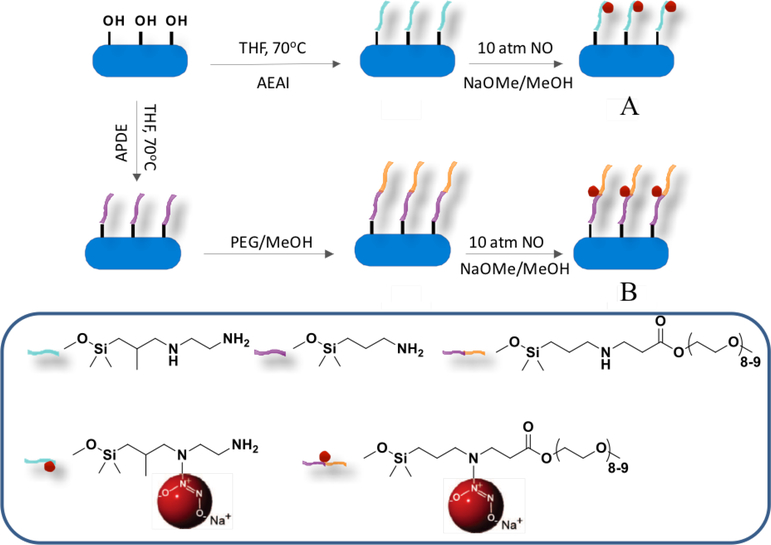
To determine the impact of NO release on antibacterial action, Lu et al. prepared N-diazeniumdiolate-modified silica nanorods of similar NO totals, but two distinct initial NO fluxes of 14000 ppb mg−1 s−1 and 2000 ppb mg−1 s−1 (i.e., 1834 pmol mg−1 s−1 and 262 pmol mg−1 s−1, respectively) with a half-life of 9 min and 45 min, respectively.[41] The greater NO flux and faster NO-release kinetics were achieved by modifying aminosilane (N-diazeniumdiolate NO donor precursor) with polyethylene glycol (PEG) to facilitate faster proton-initiated N-diazeniumdiolate decomposition to NO (Figure 4).[41] Antibacterial action was tested against Gram-negative P. aureginosa, with results indicating that greater NO flux with faster NO release enhanced bactericidal efficacy. Indeed, the minimum bactericidal concentrations (MBC), corresponding to a 3-log reduction in planktonic bacterial viability, of the faster and slower NO release silica nanorods were 0.25 mg mL−1 (NO dose ~0.07 μmol mL−1) and 4 mg mL−1 (NO dose ~1.1 μmol mL−1), respectively.
Figure 4.
Synthesis of N-diazeniumdiolate-functionalized silica nanorods with A: low NO flux with slower NO release, and B: high NO flux with faster NO release. Reproduced with permission.[41] Copyright 2013, Wiley-VCH.
In addition to scaffold design (e.g., NO donor structure), the NO-release properties are influenced by the environmental media for N-diazeniumdiolate-modified macromolecular systems. In this manner, acidic environments may elevate the bactericidal action, as lower pH results in greater initial NO flux and faster NO-release kinetics. Indeed, the antibacterial action of N-diazeniumdiolate-modified silica nanoparticles against Gram-positive Streptococcus mutans, dental caries-causing bacteria, was enhanced at pH 6.4 (MBC 32 mg mL−1 and NO dose 30.4 μmol mL−1) relative to pH 7.4 (MBC >48 mg mL−1 and NO dose > 51.8 μmol mL−1).[66] Confocal fluorescence microscopy using 4,5-diaminofluorescein diacetate (DAF-2 DA), an intracellular NO sensitive dye, confirmed more rapid NO delivery under more acidic conditions (Figure 5), as evidenced by greater intracellular NO levels detectable after 60 min at pH 6.4 (19.0 normalized fluorescence intensity) over pH 7.4 (5.6 normalized fluorescence intensity).
Figure 5.
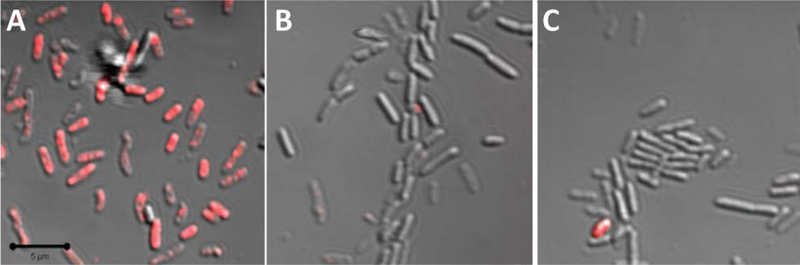
Overlay of fluorescence and bright-field scanning confocal microscopy images of P. aeruginosa treated with 10 μg mL−1 of A: 50, B: 100, and C: 200 nm rhodamine isothiocyanate (RITC)-modified silica nanoparticles for 10 min. Magnification is 63, and scale bar is 5 μm. Reproduced with permission.[74] Copyright 2011, American Chemical Society.
S-Nitrosothiol-modified macromolecular scaffolds (chitosan oligosaccharides and porous silicon nanoparticles) have also been developed as potential antibacterial therapeutics, respectively.[46d, 67] Of note, these materials only exhibited mild bacterial action, attributable to slow thermal decomposition of S-nitrosothiols that only provided a low NO flux under physiological conditions (pH 7.4, 37 °C). To improve their antibacterial activity, ascorbic acid, a strong reducing agent, was co-administered to accelerate S-nitrosothiol NO donor decomposition. Upon adding ascorbic acid, the materials exhibited one order magnitude of greater maximum NO flux and more rapid NO release with enhanced bacterial action.
Collectively, large bursts of NO and faster NO release are clearly beneficial for killing planktonic bacteria. A large instantaneous NO concentration is required to overload any protective mechanism inherent to bacteria for eliciting NO-mediated death. It is now known that Gram-negative P. aeruginosa uses nitric oxide reductase (NOR) as a detoxification enzyme against NO generated by the host immune system, thereby mitigating NO’s bactericidal activity at low levels.[68] Gram-positive bacteria employ thicker peptidoglycan bacterial membranes that resist antibacterial agent penetration more effectively than Gram-negative bacteria.[69] Without such protective mechanisms, sustained delivery of NO at low levels might be more beneficial to eradicate bacteria. Indeed, Gram-negative periodontal pathogens (i.e., Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans) that lack NORs have been reported as more susceptible to slower NO-release systems.[66] Additional bacterial strains, such as NOR-negative P. aeruginosa mutants, should be investigated to better elucidate the impact of NO-release kinetics on bactericidal efficacy.
5.2. Physical Properties
The physical properties of nanomaterials, such as size and shape, are known to influence their ensuing bactericidal activity, mainly by affecting particle-bacteria association. Silver, copper, and zinc oxide materials of smaller size generally exhibit enhanced killing.[70] For example, silver nanoparticles with a size range of ~1–10 nm were more effective against E. coli compared to larger silver particles (e.g., 100 nm) because of their capacity to directly interact with and penetrate the bacterial cell membranes, compromising membrane function and intracellular compounds (e.g., DNA).[70c] Agnihotri et al. synthesized monodisperse silver nanoparticles of precisely controlled sizes (i.e., 5, 7, 10, 15, 20, 50, 63, 85, 100 nm), and likewise found that silver nanoparticles smaller than 10 nm showed the greatest antibacterial efficacy, with silver nanoparticles of 5 nm having the fastest bactericidal action based on a time-kill assay.[71]
Particles with uneven surfaces and irregular shapes have more biologically and chemically reactive edges due to lower bonding coordination.[72] The increased reactivity at these sites facilitates association with bacteria and internalization of particles, with enhanced antibacterial action. A recent study showed that nanosheet cupric oxide with a larger reactive surface area elicited more potent bacterial killing relative to bulk and nanosphere cupric oxide.[73]
Clearly, the size and shape of NO-releasing materials should also impact their bactericidal action. Carpenter et al. was the first to evaluate the bactericidal efficacy of N-diazeniumdiolate-modified NO-releasing silica nanoparticles as a function of particle size (i.e., 50, 100, and 200 nm) against P. aeruginosa.[74] Analogous to the behavior observed for silver nanoparticles, the smallest NO-releasing silica nanoparticles (i.e., 50 nm) exhibited the greatest antibacterial activity, as evidenced by two-fold lower MBC and NO dose. Confocal fluorescence experiments using rhodamine isothiocyanate-modified silica nanoparticles confirmed that the smaller particles associated with the bacteria more rapidly (Figure 5), and thus delivered greater NO payloads into the bacteria.
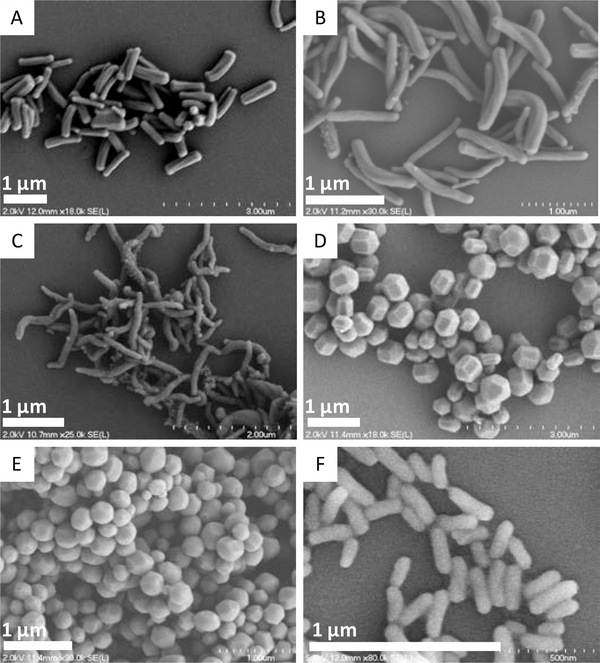
Lu et al. reported that in addition to size, the shape of the particle influenced the antibacterial activity of NO-releasing silica.[41] A series of silica nanorods with different aspect ratios (1–8) but similar NO-release properties and particle volumes (~0.02 μm3) were prepared by systematically adjusting silane concentration, pH, and temperature during the synthesis (Figure 6). The NO-releasing silica nanorods of greatest aspect ratio (AR: 8) were more effective against planktonic bacteria (P. aeruginosa and S. aureus) than the more spherical rods (AR: 1). For example, the MBC values for the AR 8 silica nanorods against P. aeruginosa were 150 μg mL−1 (NO dose ~0.10 μmol mL−1) vs 500 μg mL−1 for the AR 1 spherical rods (NO dose ~0.32 μmol mL−1), respectively. The authors attributed this improvement to greater AR 8 nanorod:bacteria association and intracellular NO delivery, that was confirmed by confocal fluorescence data.
Figure 6.
Scanning electron microscopy (SEM) images of silica nanorods with aspect ratios of A: 4.5 ± 0.9; B: 8.0 ± 1.0; C: 9.4 ± 0.7; D:1.2 ± 0.5; E:1.1 ± 0.1; and F: 3.1 ± 0.3. Reproduced with permission.[41] Copyright 2013, Wiley-VCH.
5.3. Surface Properties
In general, bacterial cell membranes possess a net negative charge. In this manner, the charge of a NO-releasing macromolecular scaffold is likely to influence bacterial-scaffold association via electrostatic interactions, with positively charged scaffolds capable of binding with the bacteria to deliver NO payloads more efficiently. For example, Nurhasni et al. reported NO-releasing nanoparticles by incorporating N-diazeniumdiolate-modified branched polyethyleneimine (BPEI) into poly(lactic-co-glycolic acid) (PLGA) nanoparticles with an average size of ~170 nm. The protonated amines of BPEI significantly increased the surface charge of PLGA nanoparticles from −24 mV to +35 mV.[75] As a result, a strong biocidal action against P. aeruginosa and MRSA was observed because of the beneficial interactions between the nanoparticles and bacteria. Likewise, Lu et al. compared primary and tertiary S-nitrosothiol-functionalized chitosan by modifying the primary amines of chitosan with 4-thiolbutylamidine (TBA) and 3-acetamido-4,4-dimethylthietan-2-one (NAP), respectively.[46d] The TBA modification (MBC 15 mg mL−1 and NO dose ~0.53 μmol mL−1) facilitated greater P. aeruginosa killing relative to NAP modification (MBC 30 mg mL−1 and NO dose ~7.20 μmol mL−1) as a result of the greater positive charge. Confocal microscopy indicated concomitantly that TBA-modified chitosan associated with bacteria more rapidly. In another study, NO-releasing silica nanoparticles (~150 nm) having similar NO-release properties but distinct surface charge were prepared by varying the identity of the incorporated aminosilane (N-(6-aminohexyl)aminopropyltrimethoxysilane (AHAP) and 3-methylaminopropyltrimethoxysilane (MAP), measuring as +31.4 and +20.9 mV, respectively).[66] As expected, the more positively charged AHAP nanoparticles elicited faster intracellular NO delivery and stronger bactericidal action over MAP nanoparticles against both P. gingivalis and A. actinomycetemcomitans.
The surface density of the NO donor will also affect the antibacterial action of NO-releasing macromolecular scaffolds. The earliest studies of functional group density were carried out using N-diazeniumdiolate-modified NO-releasing dendrimers (i.e., polypropylene imine (PPI) dendrimers and polyamidoamine (PAMAM) dendrimers).[76] Despite being larger in size (from generation 1 to generation 3 dendrimers), the greater N-diazeniumdiolate density afforded by higher generation dendrimers resulted in greater bactericidal action regardless of the type of dendrimer and bacterial strain (among P. aeruginosa, S. aureus, and MRSA). Confocal microscopy was used to confirm that the greater NO donor density enabled more efficient intracellular NO delivery to the bacteria.
5.4. Dual-Action Hybrid Materials
The combination of antibacterial agents has often proven beneficial in eradicating bacteria and reducing the incidence of resistance.[77] Likewise, the use of antibacterial functional groups with NO may improve the action of either the functional group or NO, or both.[37c, 76c, 76d, 78] Gehring et al. reported on mesoporous silica nanoparticles consisting of Rose Bengals (RB) and S-nitrosothiols that were capable of releasing both reactive oxygen species (ROS) and nitrogen species (i.e., NO) upon exposure to sunlight.[78a] The combination of ROS and NO is known to produce peroxynitrite anion (ONOO−), a strong oxidant implicated in lipid peroxidation. In this manner, the bacterial cellular constituents are effectively destroyed. The bactericidal assay against P. aeruginosa with sunlight revealed that the combination of NO and ROS release resulted in stronger biocidal action than either activity alone. Similarly, the Sortino group reported a polystyrene nanofiber comprised of NO photodonors and singlet oxygen O2(1Δg) photosensitizers.[78b] Upon irradiation by visible light, the nanofiber was capable of simultaneously releasing NO and O2(1Δg), with long (~100 μm) and short (10–100 nm) radii of antibacterial action, respectively. The combination of NO and O2(1Δg) significantly improved the antibacterial action of the nanofiber against E. coli compared to the nanofiber releasing O2(1Δg) alone.
Friedman and co-workers reported on the synthesis of NO-releasing nanoparticles via a nitrite containing hydrogel/glass composite.[37b] The bactericidal efficacy of these materials alone and in combination with glutathione (GSH) was evaluated against MRSA, E. coli, K. pneumoniae, and P. aeruginosa. In the presence of GSH, the authors suggested that the NO generated from the nanoparticles was rapidly converted to S-nitrosoglutathione (GSNO), a potent transnitrosylating agent, resulting in greater bactericidal action compared to the NO-releasing nanoparticles alone. The importance of using the nanoparticles to deliver NO was evidenced by negligible bactericidal efficacy of administering only GSNO. As GSH is not stable under ambient conditions, a freshly prepared suspension of GSH and nanoparticles is required, limiting the potential therapeutic utility. To circumvent such disadvantages, a more stable thiol-containing compound, captopril, was integrated into the nanoparticles.[37c] The new nanoparticles were capable of releasing NO and facilitating transnitrosylation through the formation of S-nitrosocaptopril. Of note, the transnitrosylation action of the new nanoparticles was greater than that of the nanoparticles/GSH suspension, producing a stronger antibacterial effect against the tested pathogenic bacteria (E. coli and MRSA).
Other work has focused on modifying NO-releasing silica nanoparticles and polyamidoamine (PAMAM) dendrimers with nondepleting antibacterial components that act via different mechanisms.[76c, 76d, 79] For example, NO-releasing silica nanoparticles were functionalized with quaternary ammonium (QA) groups of different alkyl chain lengths. Long chain QA (i.e., octyl or dodecyl) modifications led to better killing of S. aureus compared to solely NO-releasing silica nanoparticles, while negligible benefits were observed for modification with a short alkyl chain QA.[79a] Confocal microscopy revealed that the long alkyl chains disrupted the bacterial cell membranes prior to any intracellular accumulation of NO, corroborating previous reports of hydrophobic long alkyl chains intercalation into bacterial peptidoglycan layers, causing physical damage to bacterial membranes.[80] Such results indicate the advantages of combining long alkyl chains with NO release for antibacterial applications. Subsequent studies with dendrimers confirmed these observations and benefits.[76c, 76d, 79b] The antibacterial action of alkyl QA groups improved with increasing chain length, independent of bacterial strain. However, NO release did not influence the antibacterial action of long alkyl chain (e.g., hexyl, octyl, and dodecyl) QA-modified PAMAM dendrimers. Nevertheless, both NO-releasing silica and PAMAM dendrimer macromolecular systems exhibited less toxicity against mammalian cells compared to control (i.e., non-NO-releasing) materials at bactericidal concentrations. Bactericidal activity of the dual-action PAMAM dendrimers was also evaluated as a function of size (i.e., generation). Although the short alkyl chain modifications may have benefited from greater functional group density (i.e., higher dendrimers generation), no generation impacts were observed for long alkyl chains, indicating negligible influence of long alkyl chain concentration on bacteria eradication.
Future studies should focus on the incorporation of other ligands such as antibodies and/or sugars for modification of NO-releasing macromolecular scaffolds to enhance scaffold association with or promote uptake by bacterial cells. Indeed, other antibacterial materials have benefited from such strategies.[81] For example, Chen et al. reported on the conjugation of chloramphenicol (CAP), a traditional antibiotic, and an antibacterial peptide fragment, UBI29–41 with high affinity for bacteria.[81a] The CAP-UBI29–41 hybrid exhibited stronger antibacterial activity than CAP alone in an infection mouse model. These results support the potential benefits of incorporating a bacteria-targeting ligand (e.g., UBI29–41) when constructing antibacterial agents.
6. Antibiofilm Applications
Although studying the bactericidal action using planktonic bacteria is a viable means for screening new antibacterial agents, evaluating antibiofilm eradication is paramount for fully assessing their potential clinically utility. As mentioned earlier, the antibiofilm action of exogenous NO delivered by low molecular weight NO donors is dose-dependent, with necessary eradication concentration also being dependent on bacterial species. At low levels (pM to nM range), NO mediates biofilm formation and can disperse established biofilms via cell signaling pathways. Large concentrations of NO (μM to mM range) eradicate biofilms through direct killing of embedded bacteria. Below, the utility of NO-releasing macromolecular scaffolds for antibiofilm applications based on mode of action is detailed, with special focus on the correlation between antibacterial activity, NO flux, and physiochemical properties.
6.1. Biofilm Inhibition and Dispersion
Boyer and co-workers prepared a series of N-diazeniumdiolate-modified poly(oligo(ethylene glycol)methyl ether methacrylate) (POEGMA)-based block copolymers using reversible addition fragmentation transfer (RAFT) polymerization.[82] These materials released low levels of NO in a prolonged manner, which was exploited to inhibit new biofilm formation via cell signaling pathways. In their first study, NO-releasing spherical polymer/gold (Au) NP hybrids with an average particle size of 10 nm were synthesized by grafting secondary amine-containing block copolymers onto gold nanoparticles, followed by converting the secondary amines to N-diazeniumdiolate functionalities.[82a] The polymer/Au NP hybrid was capable of releasing NO over a 6 d period without burst of NO. The inhibitory effect of the NO-releasing polymer/Au NP hybrids on biofilm formation was tested against P. aeruginosa and quantified with a crystal violet assay that measured biofilm biomass. The NO-releasing hybrids (0.010 mg mL−1) elicited significant reduction (83%) in biofilm biomass without compromising the planktonic bacterial viability, indicating that the NO-releasing hybrids mainly acted via the cell signaling mechanism rather than as a biocidal agent. In a follow-on study, NO-releasing core cross-linked star polymer nanoparticles with an average size of 25 nm were prepared in a similar manner.[82b] A more sustained NO-release profile was observed for the star polymer relative to sodium nitroprusside (SNP), a low molecular weight NO donor, with comparable NO totals. The anti-biofilm assay against P. aeruginosa revealed that the NO-releasing star polymer induced greater inhibition of biofilm formation compared to SNP at similar NO doses. Mutant strains of P. aeruginosa impaired in key enzymes (i.e., dipA and rbdA phosphodiestrases) for the NO dispersal pathway were tested to better understand the antibiofilm action of NO. At the NO concentration capable of achieving almost complete dispersal of the normal P. aeruginosa biofilms, the NO-releasing star polymer had minimal effect on the mutant P. aeruginosa biofilms, suggesting that NO delivered in this manner stimulates phosphodiestrase activity to maintain lower intracellular c-di-GMP levels. The low expression of c-di-GMP confines bacterial growth to the planktonic mode, preventing biofilm formation.
Sadrearhami et al. evaluated the impact of NO-release kinetics on biofilm dispersal efficacy. In particular, NO-releasing polymeric nanoparticles with two morphologies, spheres (~40 nm) and worms (~80 nm), but similar NO storage (~0.6 μmol mg−1) were synthesized using the polymerization induced self-assembly method.[83] The NO-release kinetics of the polymeric nanoparticles proved highly dependent on their shape, with sphere nanoparticles (half-life ~4 h) showing faster NO-release kinetics than worm nanoparticles (half-life ~20 h). Although the worm-like nanoparticles exhibited less biofilm dispersal against P. aeruginosa under short treatment time (i.e., 30 min) relative to spherical nanoparticles, more significant biofilm dispersal was achieved for worm-like nanoparticles after extended periods (i.e., 60 min). In contrast, no improvement was observed for spherical nanoparticles. Despite differences in the morphologies, the authors concluded that NO-releasing materials with more sustained NO release would be beneficial for biofilm dispersal. As free-floating (i.e., planktonic) bacteria are more susceptible to antibacterial treatments relative to biofilms,[3a] these NO-releasing materials may enhance the antibiofilm efficacy of other antibacterial agents in/through combination therapies.
Boyer and co-workers developed a polymeric nanoparticle that simultaneously releases NO and gentamicin, a commercially available antibiotic, in an attempt to kill established biofilms with low levels of NO rather than only dispersing them to planktonic bacteria by one system.[82c] The NO released by this material dispersed established P. aeruginosa biofilms into an antibiotic-susceptible planktonic phase, allowing the co-delivered gentamicin to successfully exhibit bactericidal activity. By quantification of remaining biofilm metabolic activity via measuring ATP levels, the polymeric nanoparticle system showed strong biofilm eradication efficacy. Of note, no significant biofilm killing was achieved upon administering NO or gentamicin alone at the same dosage. The synergistic effect between low NO doses and gentamicin illustrates a promising strategy for eradicating established biofilms with only low levels of NO.[82c] Dong et al. reported on a similar synergistic concept by fabricating Roussin’s black salt (RBS)-loaded upconversion nanoparticles (UCnanoparticles) with quaternized ammonium chitosan (qC).[84] Under a near infrared (NIR) light trigger, the UCnanoparticles released low levels of NO and dispersed biofilm bacteria into the planktonic phase. The integrated qC then acted as a non-depleting bactericidal agent via disrupting the bacterial membrane (visualized by microscopy). The antibiofilm activity of this system was demonstrated using MRSA biofilms through plate counting method to determine the remaining viable bacteria embedded in the biofilms. A strong synergistic effect between qC and UCnanoparticles as an integrated nanoparticle platform was observed, as evidenced by a stronger antibiofilm potency relative to qC and UCnanoparticles treatment alone or simple addition of both agents. Although these previous studies demonstrate the utility of NO-releasing macromolecular scaffolds for inhibiting biofilm formation and dispersing established biofilms, the authors neither quantified or reported the therapeutic NO doses, making full appreciation of these materials difficult.
6.2. Biofilm Eradication
Nitric oxide is able to achieve complete eradication of established biofilms (>99.99% reduction in bacterial viability) at larger concentrations (μM-nM).[12a, 12b] The use of macromolecular NO-releasing scaffolds (i.e., silica nanoparticles, PAMAM dendrimers, and chitosan) for fully eradicating mature biofilms has been an active area of research.[47, 76d, 79b, 85] In many studies, the biocidal activity is quantified by determining the remaining viable bacterial colony forming units (CFU) using a plate counting method. The minimum biofilm eradication concentration (MBEC) is defined as the concentration of an NO-release material that results in complete biofilm eradication. Relative to minimal bactericidal concentrations for planktonic bacteria, MBECs were reported as 2–10 fold greater and attributed to the protective EPS structure innate to biofilms.[3] Traditional antibiotics require 1000–10000-fold greater doses to treat biofilm infections relative to planktonic bacteria,[3b] highlighting another benefit of exogenous NO.
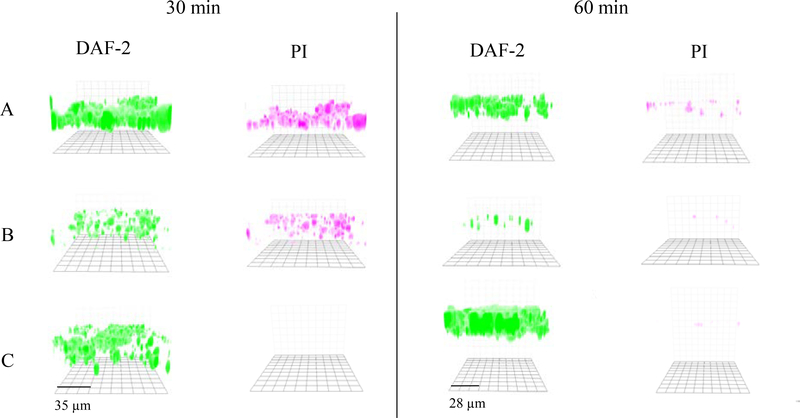
The biofilm killing activity of NO-releasing macromolecular scaffolds is also highly dependent on NO release and physiochemical characteristics. For example, Hetrick et al. evaluated the impact of NO-release kinetics on biofilm eradication using N-diazeniumdiolate-modified silica nanoparticles. Silica nanoparticles of greater initial NO burst (~24900 pmol mg−1 s−1) and faster release (4 min half-life) elicited >5-log (99.999%) killing against P. aeruginosa biofilms (cultured 24 h) at 8 mg mL−1, while silica nanoparticles of lower initial burst (~2800 pmol mg−1 s−1) and slower NO release (18 min half-life) administered at an equivalent concentration exhibited only 2-log (99%) killing. [85a] In a follow-on study, Slomberg et al. reported how the size and shape of NO-releasing silica nanoparticles affected biofilm eradication.[85b] Silica nanoparticles of three distinct sizes (14, 50, 150 nm) and aspect ratios (i.e., 1, 4, and 8) were prepared, respectively. Despite the differences in morphology, similar NO-release properties (i.e., NO totals and kinetics) were obtained for each system to enable direct study of particle size and shape. The antibiofilm assay revealed that NO-releasing silica nanoparticles of smaller sizes and larger aspect ratios were more effective against both P. aeruginosa and S. aureus. For example, the MBEC values for 14 nm and 150 nm silica nanoparticles against P. aeruginosa biofilms were 6 mg mL−1 (NO dose ~1.40 μmol mL−1) and 10 mg mL−1 (NO dose ~2.50 μmol mL−1), respectively. Confocal microscopy was used to visualize intracellular NO delivery and bacterial cell death using DAF-2 DA and PI fluorescent probes, respectively. As expected, the enhanced killing correlated directly with more significant NO accumulation throughout the biofilm EPS matrix that resulted in faster bacterial cell death (Figure 7).
Figure 7.
Fluorescent images of P. aeruginosa biofilm exposed to NO-releasing A: 14, B: 50, or C: 150 nm particles for 30 or 60 min at the same concentration (1 mg/mL) and NO dosage (∼250 μmol/L). DAF-2 (green fluorescence) indicates increased intracellular NO, and PI (red fluorescence) indicates compromised cell membranes (i.e., cell death). Reproduced with permission.[85b] Copyright 2013, American Chemical Society.
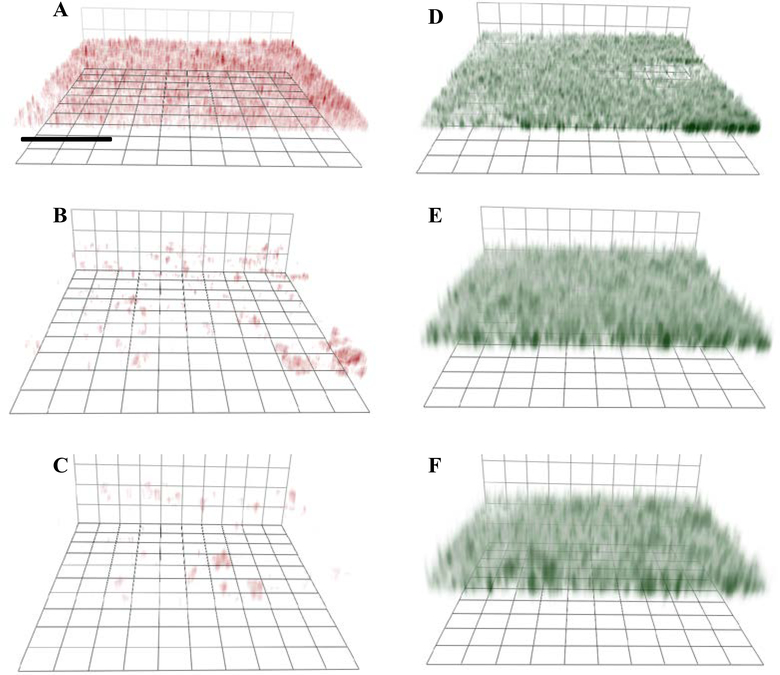
Lu et al. studied the antibiofilm action of NO-releasing chitosan oligosaccharides (COS/NO) as a function of polymer size.[47] Lower molecular weight (5 kDa) COS/NO elicited enhanced biofilm killing relative to the greater molecular weight (10 kDa) scaffold. Indeed, the MBEC values for 5 kDa and 10 kDa COS/NO were 400 μg mL−1 (NO dose ~0.35 μmol mL−1) and 600 μg mL−1 (NO dose ~0.49 μmol mL−1), respectively. The authors attributed this improvement to faster penetration of the smaller polymers into the biofilm matrix. Confocal microscopy allowed for visualization of this phenomenon (Figure 8). The surface charge of the COS/NO became neutral by modification of the primary amine of COS with a PEG moiety, shielding the positive charge with the now overall more neutral COS/NO exhibiting less antibiofilm activity versus cationic COS/NO. As most bacterial biofilms have a negatively charged EPS,[85a] the positively charged scaffolds would be expected to have better association with bacteria embedded in EPS (Figure 8).
Figure 8.
Confocal fluorescence images of RITC-labeled chitosan oligosaccharide association with P. aeruginosa in biofilms: A: COS/NO (cationic)-5k, B: PEG-modified COS (neutral)/NO-5k, C: COS/NO (cationic)- 10k and images of Syto 9 labeled biofilms incubated with D: COS/NO (cationic)-5k, E: PEG-modified COS (neutral)/NO-5k, and F: COS/NO (cationic)- 10k. Scale bar: 40 mm. Green fluorescence (Syto 9) indicates that the P. aeruginosa bacteria are embedded within the biofilms. Red fluorescence indicates the association of RITC-labeled COS with P. aeruginosa in biofilms. Reproduced with permission.[47] Copyright 2014, Elsevier.
The antibiofilm efficacy of NO-releasing dendrimers was evaluated as a function of dendrimer size (i.e., generation) against MRSA, S. aureus, and P. aeruginosa biofilms.[76d] Larger generations (e.g., G2, G3 and G4) of NO-releasing PAMAM dendrimers with their greater functional group densities generally lowered the doses required to achieve the same degree of biofilm killing as lower generation (e.g., G1) dendrimers. For example, the MBEC values for butyl-modified NO-releasing G1- and G3-PAMAM dendrimers against S. aureus biofilms (cultured 24 h) were 10 mg mL−1 (NO dose ~10.6 μmol mL−1) and 5 mg mL−1 (NO dose ~4.7 μmol mL−1), respectively. Among the higher generations, G3-PAMAM was identified as the most potent polymer. Confocal microscopy indicated that G4-PAMAM, due to its larger size, exhibited less penetration into the biofilm EPS matrix relative to smaller PAMAM dendrimers, consistent with previous findings for COS scaffolds.[47] The G3-PAMAM represents the optimal balance between size and functional group density to achieve maximum killing. Further studies involving even larger PAMAM generations (e.g,, G5, G6, and G7) are needed to confirm this observation, and were not a focus of this report.
To improve the antibiofilm activity further, dual-action NO-releasing PAMAM dendrimers were prepared via modification with long alkyl chain moieties.[76d, 79b] Similar to planktonic killing, biofilm eradication was significantly improved with the longer alkyl chain modifications. The authors attributed this enhancement to greater bacterial cell membrane damage and biofilm penetration by the longer alkyl chains. Of importance, neither the increased functional group density (i.e., higher generation) nor addition of NO-release capacity further improved the antibiofilm action, suggesting that the long alkyl chains, even at low densities (e.g., G1-PAMAM), were sufficiently capable of inducing lethal membrane damage. Unfortunately, the long alkyl chain modifications also resulted in undesirable cytotoxicity against mammalian cells due to its non-selective killing mechanisms. This cytotoxicity was mitigated with NO release, reflecting the benefits of combing static and actively releasing biocidal agents.
Collectively, these previous studies demonstrated the utility of NO-releasing macromolecular scaffolds (e.g., silica nanoparticles, dendrimers, and chitosan) as a broad-spectrum antibiofilm agents for eradicating mature biofilms (cultured 24 h). The favorable physiochemical properties (e.g., faster NO release, smaller size, and both greater positive charge and functional group densities) of the NO-releasing macromolecular scaffolds against planktonic bacteria remained beneficial for biofilm eradication.
7. Cytotoxicity
Assessing toxicity to mammalian cells at relevant bactericidal concentrations is paramount for understanding the therapeutic potential of new antibacterial agents. Certain characteristics of NO-releasing macromolecular scaffolds favorable to killing bacteria might also contribute to mammalian cell toxicity. For example, rapid and large bursts of NO, and overly hydrophobic or charged macromolecular scaffolds have been shown to disrupt membranes of mammalian cells. Likewise, NO-releasing silica nanoparticles with initial NO flux of ~24900 pmol mg−1 s−1 administered at their effective antibiofilm concentrations were shown to decrease mouse fibroblasts cell viability by 70% after 24 h.[85a] Dodecyl-modified NO-releasing G1-PAMAM dendrimers similarly reduced human gingival fibroblasts cell viability by ~80% after 2 h.[79b] Nevertheless, the cytotoxicity of these NO-releasing materials is minimal compared to common antiseptics. Pyo et al. reported that clinical doses of povidone iodine and chlorhexidine reduced human fibroblasts viability by ~90 and ~100%, respectively.[85a]
A number of strategies could be employed to further reduce cytotoxicity of NO-releasing materials. For example, of polyethylene glycol and poly(lactic-co-glycolic acid) modifications have been shown to reduce hydrophobicity and improve degradability of the scaffolds, respectively.[56, 65, 75−76, 82a] Recent work has begun to evaluate the use of naturally produced biopolymers as NO-release scaffolds, including dextran and chitosan with inherently favorable toxicity and biodegradability properties.[47, 86] For instance, Lu et al. reported on NO-releasing chitosan oligosaccharides that proved non-toxic to mouse fibroblasts at their MBEC, indicating an advantage of these materials compared to other antibacterial agents.[47] The use of dual-action materials is another desirable strategy for reducing cytotoxicity, due to lower concentrations required for bacterial killing. For example, dodecylQA-modified silica nanoparticles administered at 4 mg mL−1 (MBC against planktonic P. aeruginosa) resulted in a 94% decrease in mouse fibroblasts viability, whereas the MBC dose of dodecylQA-modified NO-releasing silica nanoparticles (1.5 mg mL−1) only reduced fibroblast viability by 67%.[97a]
8. Summary
In this article, we describe the utility of NO-releasing macromolecular scaffolds for antibacterial and antibiofilm applications as a function of both NO-release properties and physiochemical characteristics. Large NO fluxes and fast NO release are now regarded as favorable for eradicating planktonic bacteria and biofilms. Low NO flux and sustained NO release are more beneficial for inhibiting biofilm formation and/or biofilm dispersal. Macromolecular scaffolds of smaller size, greater positive surface charge, higher surface density of NO donors, and higher aspect ratio generally exhibit improved antimicrobial activity, attributable to faster association with the bacteria, greater penetration into the biofilms, and more efficient NO delivery. Designing macromolecular NO-release systems with chemical functionalities that can work synergistically with NO is promising for further improving antimicrobial action.
With respect to the comparison of macromolecular scaffolds, many are characterized with advantages and disadvantages. For example, NO-releasing G1-PAMAM dendrimers exhibit stronger bactericidal action relative to silica nanoparticles of similar NO-release properties (attributable to enhanced bacterial-scaffold association of the dendritic scaffold).[87] However, the synthetic cost (e.g., synthesis and purification) of dendrimers is much greater than that of silica, with significant scale up and manufacturing hurdles. As a potential solution, Yang et al. reported the synthesis of NO-releasing hyperbranched polymers via a one-pot reaction in bulk.[52b] Despite greater structural imperfections (i.e., defects), NO-releasing hyperbranched PAMAM exhibited nearly identical antibacterial efficacy against both Gram-positive and Gram-negative bacteria relative to that achieved with defect-free PAMAM dendrimers.
The use of naturally occurring (e.g., chitosan and dextran) biodegradable polymers for NO storage and release addresses concerns regarding potential accumulation toxicity of non-degradable scaffolds (e.g., silica and dendrimers). While promising, these biopolymers exhibit low NO storage (<1 μmol mg−1) than their non-degradable counterparts (e.g., silica and dendrimers). As such, larger concentrations of these materials may be required to achieve the similar therapeutic doses.
Nitric oxide-releasing synthetic polymeric nanoparticles is another class of emerging materials for antibacterial applications.[5a, 12a] To date, it remains challenging to simultaneously tune both physiochemical and NO-release properties. Given that a wide range of synthetic approaches are available for modification of polymeric nanoparticles, attaining a synthetic polymer with tunable properties represents a robust research area for next generation of NO-release systems.
In summary, the most favorable attributes of NO-releasing macromolecular scaffolds as antibacterial therapeutics include ease of synthesis, large NO payloads, tunable NO-release properties, biocompatibility, and degradability (for certain biopolymers).
9. Perspectives
9.1. Modification of NO Donors
The antimicrobial efficacy of low molecular weight NO donors may be improved via modification with another functionality.[88] For example, Kutty et al. designed a dual action antimicrobial agent consisting of NO release and fimbrolide by conjugating fimbrolide with a N-diazeniumdiolate NO donor.[88a] Fimbrolides are known for their ability to inhibit quorum sensing (QS), a cell-to-cell communication system responsible for biofilm formation. The fimbrolide-NO hybrids were able to act against biofilms through QS inhibition and NO signaling pathways. An in vitro antibiofilm assay against P. aeruginosa revealed this hybrid system exhibited greater efficacy relative to fimbrolide and NO treatments alone. Nitric oxide donors that release NO only in the presence of biofilms are equally attractive because they minimize exposure of exogenous NO to host tissues and maximize NO delivery to an area of interest. To this end, Kelso and co-workers reported on the synthesis of a cephalosporin-3’-diazeniumdiolate NO donor.[88b, 89] Upon reaction with a biofilm-specific enzyme (i.e., β-lactamase), the diazeniumdiolates become cleaved from the cephalosporin, thereby releasing NO. The cephalosporin-3’-diazeniumdiolate NO donor was capable of dispersing P. aeruginosa biofilms in a dose dependent manner while potentiating the biofilm killing efficacy of a co-administered traditional antibiotics (e.g., tobramycin). Given the advantages of using macromolecular scaffolds over low molecular weight scaffolds, future work should focus on modifying macromolecular scaffolds with these novel NO donors.
9.2. Reporting NO-Release Properties
Prior articles have stressed the importance of standardized measuring and reporting of NO release to allow for a clear comparison of results[21b, 90] With regard to NO-releasing materials for antibacterial and antibiofilm applications, several critical parameters should be but continue not to be clearly reported, including normalized NO storage, NO-release kinetics (i.e., NO flux and half-lives), and NO payloads (i.e., amount of NO released over time course of a biological milieu) and therapeutic NO dose (i.e., amount of NO required to achieve antibacterial or antibiofilm action).
9.3. In vitro Studies
The antimicrobial action of NO-releasing macromolecular scaffolds requires evaluation using in vitro models reflective of disease. However, most in vitro biofilm models used to date are single species, whereas biofilms associated with respiratory, gastrointestinal, and oral infections consist of multiple bacterial species. These collective communities are more resistant to antibiotic therapy compared to single species biofilms.[91] The antibiofilm efficacy of NO-releasing macromolecular scaffolds against multispecies biofilms has rarely been reported, with one example showing that 3 w/v% of N-diazeniumdiolate-modified polyacrylonitrile achieved 90% reduction in biomass of multispecies biofilms consisting of P. aeruginosa, S. aureus, and E. faecalis over 24 h.[92] Clearly more studies are required to fully evaluate the potential of exogenous NO for inhibiting or eradicating multispecies biofilms, particularly for those NO-releasing macromolecular scaffolds already proven effective against single-species biofilms. The analysis of changes in bacterial composition change in multi-species biofilms after NO treatment should also be carefully evaluated. Ideal NO-releasing materials should preferentially eliminate pathogenic bacterial populations without damaging commensal bacteria.[93]
9.4. In vivo Studies
Although in vitro bacterial and biofilm assays are effective for initial screening of new antibacterial scaffolds, in vivo studies are required to further ascertain the therapeutic utility. To date, reports on the in vivo antibacterial efficacy of macromolecular NO-release systems are somewhat scarce. The Meyerhoff group demonstrated the use of a polymeric film doped with low molecular weight NO donors to inhibit bacterial growth (A. baumannii) using a mouse burn wound model.[94] Polymeric films capable of releasing 1.28–1.45 nmol cm−2 min−1 resulted in 4-log reduction of bacterial viability compared to controls after 24 h in vivo. Similarly, Friedman and co-workers used a murine infection model to assess the ability of NO-releasing nanoparticles to decrease bacterial burden (e.g., S. aureus and A. baumannii) via topical treatment.[95] The work was followed up by demonstrating that the NO-releasing nanoparticles were capable of inhibiting MRSA biofilm formation in a rat central venous catheter model.[96] Of note, the authors did not evaluate the antibacterial efficacy in a dose-dependent manner, leaving the required (therapeutic) dose or NO payload in question. Furthermore, the NO release from the nanoparticles employed (nitrite-loaded hydrogel/glass composites) is likely difficult to control or tune.
Future preclinical (in vivo) studies should carefully evaluate the antibacterial efficacy as a function of NO payloads and physiochemical properties of the macromolecular systems, with an aim to validate the desired characteristics observed on the bench (in vitro). Additional characterization should include bio-distribution, clearance, and pharmacokinetics.[5a] More importantly, the toxicity of NO-releasing materials should be evaluated in vivo in a dose-dependent manner. Such information is critical for first-in-human testing.
9.5. Formulation
The majority of the literature on NO-releasing macromolecular scaffolds has evaluated the therapeutic potential of new material in powder form or dissolved in aqueous solution. For certain application, pharmaceutical formulations must be developed and evaluated. To date, most formulation work has focused on either the noncovalently doping of low molecular weight NO donors or covalently modifying NO donors within bandage or dressing-type materials for wound dressing applications.[97] As described in Section 4, low molecular weight NO donors are prone to leaching and/or have limited NO-release properties. The covalent attachment of NO donors requires a specific precursor (e.g., secondary amines and thiols), which restricts the selection of matrix materials. Given that NO-releasing macromolecular scaffolds store greater NO payloads per mole of materials with less toxicity, incorporation of NO-releasing macromolecular scaffolds into conventional/innovative pharmaceutical formulations represents an important future research opportunity. As a start, Kang et al. have incorporated F68-BPEI-NONOates (described in Section 5.1) into a PEG-based ointment that is a key ingredient for a commercial product.[98] The resulting NO-releasing ointment was capable of slowly releasing NO and accelerating wound healing. Worley et al. reported the fabrication of electrospun composite polyurethane fibers containing NO-releasing PAMAM dendrimers.[99] The fibers exhibited porosity and water adsorption capacities that should enable dynamic gas and fluid exchange, requirements for effective wound dressings. The release of NO-releasing PAMAM dendrimers facilitated antibacterial action. Such features illustrate the potential of rationally designed systems for biomedical applications.
9.6. Storage Stability
The storage stability of NO-releasing macromolecular scaffolds is another paramount factor for determining eventual therapeutic utility. To date, examination of the storage stability of NO-releasing materials has been somewhat limited.[40b, 100] Riccio et al. reported that the NO-release properties of S-nitrosothiol-modified silica nanoparticles after 2 months of storage at −20 °C in vacuo and dark were identical to freshly prepared particles.[40b] Given the greater stability of N-diazeniumdiolates versus S-nitrosothiols (described in Section 3.5), N-diazeniumdiolate-modified macromolecular scaffolds are expected to have greater storage stability under proper conditions (moisture-free and low temperature). Indeed, Neidrauer et al. reported that N-diazeniumdiolate-modified zeolite loaded ointment remained stable for 4 months in vacuum-sealed nylon/poly bags.[100] Future studies should continue to evaluate and report the storage stability of emerging NO-releasing materials in a systematic manner.
Acknowledgements
Financial support was provided by the National Institutes of Health (DE025207).
Biography
 Lei Yang is a graduate student in the Department of Chemistry at the University of North Carolina at Chapel Hill working under the direction of Prof. Mark Schoenfisch. His research is focused on the development of nitric oxide-releasing hyperbranched polymer systems for biomedical applications, with a particular interest in dental therapeutics. He holds a B.S. degree in Chemistry from the University of Science and Technology of China.
Lei Yang is a graduate student in the Department of Chemistry at the University of North Carolina at Chapel Hill working under the direction of Prof. Mark Schoenfisch. His research is focused on the development of nitric oxide-releasing hyperbranched polymer systems for biomedical applications, with a particular interest in dental therapeutics. He holds a B.S. degree in Chemistry from the University of Science and Technology of China.
 Evan S. Feura received his B.S. in Chemistry from the University of Pittsburgh. He is working toward his Ph.D. in Chemistry under the guidance of Prof. Mark Schoenfisch at the University of North Carolina at Chapel Hill. His research interests include the development of nitric oxide-releasing biopolymers and hydrogels for therapeutic applications.
Evan S. Feura received his B.S. in Chemistry from the University of Pittsburgh. He is working toward his Ph.D. in Chemistry under the guidance of Prof. Mark Schoenfisch at the University of North Carolina at Chapel Hill. His research interests include the development of nitric oxide-releasing biopolymers and hydrogels for therapeutic applications.
 Mona Jasmine R. Ahonen is a graduate student in the Department of Chemistry at the University of North Carolina at Chapel Hill working under the direction of Prof. Mark H. Schoenfisch. Her research is focused on the development of nitric oxide-releasing biopolymers for therapeutic applications, with a particular focus on new therapeutics for treating cystic fibrosis infections. She holds a B.S. degree in Chemistry from Iona College.
Mona Jasmine R. Ahonen is a graduate student in the Department of Chemistry at the University of North Carolina at Chapel Hill working under the direction of Prof. Mark H. Schoenfisch. Her research is focused on the development of nitric oxide-releasing biopolymers for therapeutic applications, with a particular focus on new therapeutics for treating cystic fibrosis infections. She holds a B.S. degree in Chemistry from Iona College.
Footnotes
Conflict of Interest
The authors declare the following competing financial interest(s): Mark H. Schoenfisch is a co-founder and maintains a financial interest in Novan, Inc. and Novoclem Therapeutics, Inc. Both companies commercialize macromolecular nitric oxide storage and release vehicles for clinical indications.
References
- [1].a) Rossolini GM, Arena F, Pecile P, Pollini S, Curr. Opin. Pharmacol 2014, 18, 56; [DOI] [PubMed] [Google Scholar]; b) Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, Cohen J, Findlay D, Gyssens I, Heure OE, Kahlmeter G, Kruse H, Laxminarayan R, Liébana E, López-Cerero L, MacGowan A, Martins M, Rodríguez-Baño J, Rolain JM, Segovia C, Sigauque B, Tacconelli E, Wellington E, Vila J, New Microbes New Infect 2015, 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Römling U, Balsalobre C, J. Intern. Med 2012, 272, 541. [DOI] [PubMed] [Google Scholar]
- [3].a) Manuel S, Curr. Med. Chem 2011, 18, 2129. [DOI] [PubMed] [Google Scholar]; b) Smith AW, Adv. Drug Delivery Rev 2005, 57, 1539. [DOI] [PubMed] [Google Scholar]
- [4].Flemming H-C, Wingender J, Nat. Rev. Microbiol 2010, 8, 623. [DOI] [PubMed] [Google Scholar]
- [5].a) Seabra AB, Justo GZ, Haddad PS, Biotechnol. Adv 2015, 33, 1370. [DOI] [PubMed] [Google Scholar]; b) Carpenter AW, Schoenfisch MH, Chem. Soc. Rev 2012, 41, 3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Hill BG, Dranka BP, Bailey SM, Lancaster JR, Darley-Usmar VM, J. Biol. Chem 2010, 285, 19699; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Förstermann U, Sessa WC, Eur. Heart J 2012, 33, 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Friedman A, Blecher K, Sanchez D, Tuckman-Vernon C, Gialanella P, Friedman JM, Martinez LR, Nosanchuk JD, Virulence 2011, 2, 217. [DOI] [PubMed] [Google Scholar]
- [8].Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, Schoenfisch MH, ACS Nano 2008, 2, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schairer DO, Chouake JS, Nosanchuk JD, Friedman AJ, Virulence 2012, 3, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wink D, Kasprzak K, Maragos C, Elespuru R, Misra M, Dunams T, Cebula T, Koch W, Andrews A, Allen J, a. et, Science 1991, 254, 1001. [DOI] [PubMed] [Google Scholar]
- [11].Privett BJ, Broadnax AD, Bauman SJ, Riccio DA, Schoenfisch MH, Nitric Oxide 2012, 26, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Quinn JF, Whittaker MR, Davis TP, J. Controlled Release 2015, 205, 190; [DOI] [PubMed] [Google Scholar]; b) Barraud N, J Kelso M, A Rice S, Kjelleberg S, Curr. Pharmaceutics Des 2015, 21, 31; [DOI] [PubMed] [Google Scholar]; c) Wo Y, Brisbois EJ, Bartlett RH, Meyerhoff ME, Biomater. Sci 2016, 4, 1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller C, McMullin B, Ghaffari A, Stenzler A, Pick N, Roscoe D, Ghahary A, Road J, Av-Gay Y, Nitric Oxide 2009, 20, 16. [DOI] [PubMed] [Google Scholar]
- [14].Miller CC, Hergott CA, Rohan M, Arsenault-Mehta K, Döring G, Mehta S, J. Cystic Fibrosis 2013, 12, 817. [DOI] [PubMed] [Google Scholar]
- [15].a) Arora DP, Hossain S, Xu Y, Boon EM, Biochemistry 2015, 54, 3717; [DOI] [PubMed] [Google Scholar]; b) Barraud N, Storey MV, Moore ZP, Webb JS, Rice SA, Kjelleberg S, Microb. Biotechnol 2009, 2, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaplan JB, J. Dent. Res 2010, 89, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hossain S, Nisbett L-M, Boon EM, Acc. Chem. Res 2017, 50, 1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barraud N, Hassett DJ, Hwang S-H, Rice SA, Kjelleberg S, Webb JS, J. Bacteriol 2006, 188, 7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Neufeld BH, Reynolds MM, Biointerphases 2016, 11, 031012. [DOI] [PubMed] [Google Scholar]
- [20].a) Duan J, Kasper DL, Glycobiology 2011, 21, 401; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR, Mutat. Res., Fundam. Mol. Mech. Mutagen 1999, 424, 37. [DOI] [PubMed] [Google Scholar]
- [21].a) Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, Wink DA, Biol. Chem 2004, 385, 1; [DOI] [PubMed] [Google Scholar]; b) Coneski PN, Schoenfisch MH, Chem. Soc. Rev 2012, 41, 3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nichols SP, Storm WL, Koh A, Schoenfisch MH, Adv. Drug Delivery Rev 2012, 64, 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Angus DC, Clermont G, Watson RS, Linde-Zwirble WT, Clark RH, Roberts MS, Pediatrics 2003, 112, 1351. [DOI] [PubMed] [Google Scholar]
- [24].Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ, Chem. Rev 2002, 102, 1091. [DOI] [PubMed] [Google Scholar]
- [25].Seth P, Fung H-L, Biochem. Pharmacol 1993, 46, 1481. [DOI] [PubMed] [Google Scholar]
- [26].McCleverty JA, Chem. Rev 2004, 104, 403. [DOI] [PubMed] [Google Scholar]
- [27].Matthews E, Seaton E, Forsyth M, Humphrey P, Br. J. Pharmacol 1994, 113, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].a) Halpenny GM, Gandhi KR, Mascharak PK, ACS Med. Chem. Lett 2010, 1, 180; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Heilman BJ, St. John J, Oliver SRJ, Mascharak PK, J. Am. Chem. Soc 2012, 134, 11573. [DOI] [PubMed] [Google Scholar]
- [29].Ghosh K, Eroy-Reveles AA, Avila B, Holman TR, Olmstead MM, Mascharak PK, Inorg. Chem 2004, 43, 2988. [DOI] [PubMed] [Google Scholar]
- [30].Williams DLH, Acc. Chem. Res 1999, 32, 869. [Google Scholar]
- [31].Riccio DA, Schoenfisch MH, Chem. Soc. Rev 2012, 41, 3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hrabie JA, Keefer LK, Chem. Rev 2002, 102, 1135. [DOI] [PubMed] [Google Scholar]
- [33].a) Keefer LK, ACS Chem. Biol 2011, 6, 1147; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Keefer LK, Annu. Rev. Pharmacol. Toxicol 2003, 43, 585. [DOI] [PubMed] [Google Scholar]
- [34].Stasko NA, Schoenfisch MH, J. Am. Chem. Soc 2006, 128, 8265. [DOI] [PubMed] [Google Scholar]
- [35].Shin JH, Schoenfisch MH, Chem. Mater 2007, 20, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Daiber A, Wenzel P, Oelze M, Münzel T, Clin. Res. Cardiol 2008, 97, 12. [DOI] [PubMed] [Google Scholar]
- [37].a) Hogg N, Free Radicals Biol. Med 2000, 28, 1478; [DOI] [PubMed] [Google Scholar]; b) Friedman AJ, Blecher K, Schairer D, Tuckman-Vernon C, Nacharaju P, Sanchez D, Gialanella P, Martinez LR, Friedman JM, Nosanchuk JD, Nitric Oxide 2011, 25, 381; [DOI] [PubMed] [Google Scholar]; c) Mordorski B, Pelgrift R, Adler B, Krausz A, da Costa Neto AB, Liang H, Gunther L, Clendaniel A, Harper S, Friedman JM, Nosanchuk JD, Nacharaju P, Friedman AJ, Nanomedicine 2015, 11, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim J, Saravanakumar G, Choi HW, Park D, Kim WJ, J. Mater. Chem. B 2014, 2, 341. [DOI] [PubMed] [Google Scholar]
- [39].Zhang H, Annich GM, Miskulin J, Stankiewicz K, Osterholzer K, Merz SI, Bartlett RH, Meyerhoff ME, J. Am. Chem. Soc 2003, 125, 5015. [DOI] [PubMed] [Google Scholar]
- [40].a) Shin JH, Metzger SK, Schoenfisch MH, J. Am. Chem. Soc 2007, 129, 4612; [DOI] [PubMed] [Google Scholar]; b) Riccio DA, Nugent JL, Schoenfisch MH, Chem. Mater 2011, 23, 1727; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Carpenter AW, Johnson JA, Schoenfisch MH, Colloids Surf., A 2014, 454, 144; [Google Scholar]; d) Soto RJ, Yang L, Schoenfisch MH, ACS Appl. Mater. Interfaces 2016, 8, 2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lu Y, Slomberg DL, Sun B, Schoenfisch MH, Small 2013, 9, 2189. [DOI] [PubMed] [Google Scholar]
- [42].Munaweera I, Shi Y, Koneru B, Patel A, Dang MH, Di Pasqua AJ, Balkus KJ, J. Inorg. Biochem 2015, 153, 23. [DOI] [PubMed] [Google Scholar]
- [43].a) Polizzi MA, Stasko NA, Schoenfisch MH, Langmuir 2007, 23, 4938; [DOI] [PubMed] [Google Scholar]; b) Zhang XF, Mansouri S, Mbeh DA, Yahia LH, Sacher E, Veres T, Langmuir 2012, 28, 12879; [DOI] [PubMed] [Google Scholar]; c) Tan L, Wan A, Li H, ACS Appl. Mater. Interfaces 2013, 5, 11163. [DOI] [PubMed] [Google Scholar]
- [44].Liu S, Gu T, Fu J, Li X, Chronakis IS, Ge M, Mater. Sci. Eng. C 2014, 45, 37. [DOI] [PubMed] [Google Scholar]
- [45].a) Garcia JV, Yang J, Shen D, Yao C, Li X, Wang R, Stucky GD, Zhao D, Ford PC, Zhang F, Small 2012, 8, 3800; [DOI] [PubMed] [Google Scholar]; b) Ford PC, Nitric Oxide 2013, 34, 56; [DOI] [PubMed] [Google Scholar]; c) Burks PT, Garcia JV, GonzalezIrias R, Tillman JT, Niu M, Mikhailovsky AA, Zhang J, Zhang F, Ford PC, J. Am. Chem. Soc 2013, 135, 18145. [DOI] [PubMed] [Google Scholar]
- [46].a) Damodaran VB, Place LW, Kipper MJ, Reynolds MM, J. Mater. Chem 2012, 22, 23038; [Google Scholar]; b) Di Meo C, Capitani D, Mannina L, Brancaleoni E, Galesso D, De Luca G, Crescenzi V, Biomacromolecules 2006, 7, 1253; [DOI] [PubMed] [Google Scholar]; c) Wan A, Gao Q, Li H, J. Appl. Polym. Sci 2010, 117, 2183; [Google Scholar]; d) Lu Y, Shah A, Hunter RA, Soto RJ, Schoenfisch MH, Acta Biomater 2015, 12, 62; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Lutzke A, Pegalajar-Jurado A, Neufeld BH, Reynolds MM, J. Mater. Chem. B 2014, 2, 7449. [DOI] [PubMed] [Google Scholar]
- [47].Lu Y, Slomberg DL, Schoenfisch MH, Biomaterials 2014, 35, 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].a) Cardozo VF, Lancheros CAC, Narciso AM, Valereto ECS, Kobayashi RKT, Seabra AB, Nakazato G, Int. J. Pharmaceutics 2014, 473, 20; [DOI] [PubMed] [Google Scholar]; b) D Marcato P, F Adami L, de Melo Barbosa R, S Melo P, R Ferreira I, de Paula L, Duran N, B Seabra A, Curr. Nanosci 2013, 9, 1. [Google Scholar]
- [49].Lu Y, Sun B, Li C, Schoenfisch MH, Chem. Mater 2011, 23, 4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stasko NA, Fischer TH, Schoenfisch MH, Biomacromolecules 2008, 9, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang D, Zhao T, Zhu X, Yan D, Wang W, Chem. Soc. Rev 2015, 44, 4023. [DOI] [PubMed] [Google Scholar]
- [52].a) Yang L, Lu Y, Soto RJ, Shah A, Ahonen MJR, Schoenfisch MH, Polym. Chem 2016, 7, 7161; [PMC free article] [PubMed] [Google Scholar]; b) Yang L, Wang X, Suchyta DJ, Schoenfisch MH, Bioconjugate Chem 2018, 29, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].a) Liu T, Zhang D, Yang X, Li C, Polym. Chem 2015, 6, 1512; [Google Scholar]; b) Park D, Kim J, Lee YM, Park J, Kim WJ, Adv. Healthcare Mater 2016, 5, 2019. [Google Scholar]
- [54].Liu T, Zhang W, Yang X, Li C, J. Colloid Interface Sci 2015, 459, 115. [DOI] [PubMed] [Google Scholar]
- [55].Liu T, Zhang W, Song T, Yang X, Li C, Polym. Chem 2015, 6, 3305. [Google Scholar]
- [56].a) Lautner G, Meyerhoff ME, Schwendeman SP, J. Controlled Release 2016, 225, 133; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Reger NA, Meng WS, Gawalt ES, Appl. Surf. Sci 2017, 401, 162; [Google Scholar]; c) Hasan S, Thomas N, Thierry B, Prestidge CA, J. Mater. Chem. B 2017, 5, 1005; [DOI] [PubMed] [Google Scholar]; d) Gomes AJ, Barbougli PA, Espreafico EM, Tfouni E, J. Inorg. Biochem 2008, 102, 757; [DOI] [PubMed] [Google Scholar]; e) Marino N, Perez-Lloret M, Blanco AR, Venuta A, Quaglia F, Sortino S, J. Mater. Chem. B 2016, 4, 5138. [DOI] [PubMed] [Google Scholar]
- [57].Seabra AB, Martins D, Simões MMSG, Da Silva R, Brocchi M, De Oliveira MG, Artif. Organs 2010, 34, E204. [DOI] [PubMed] [Google Scholar]
- [58].Coneski PN, Rao KS, Schoenfisch MH, Biomacromolecules 2010, 11, 3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yapor JP, Lutzke A, Pegalajar-Jurado A, Neufeld BH, Damodaran VB, Reynolds MM, J. Mater. Chem. B 2015, 3, 9233. [DOI] [PubMed] [Google Scholar]
- [60].a) Lutzke A, Neufeld BH, Neufeld MJ, Reynolds MM, J. Mater. Chem. B 2016, 4, 1987; [DOI] [PubMed] [Google Scholar]; b) Lutzke A, Tapia JB, Neufeld MJ, Reynolds MM, ACS Appl. Mater. Interfaces 2017, 9, 2104. [DOI] [PubMed] [Google Scholar]
- [61].Elnaggar MA, Subbiah R, Han DK, Joung YK, Expert Opin. Drug Delivery 2017, 14, 1341. [DOI] [PubMed] [Google Scholar]
- [62].a) Suchyta DJ, Schoenfisch MH, ACS Biomater. Sci. Eng 2017, 3, 2136; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Suchyta DJ, Schoenfisch MH, Mol. Pharmaceutics 2015, 12, 3569. [DOI] [PubMed] [Google Scholar]
- [63].Hetrick EM, Schoenfisch MH, Biomaterials 2007, 28, 1948. [DOI] [PubMed] [Google Scholar]
- [64].Nichols SP, Schoenfisch MH, Biomater. Sci 2013, 1, 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Park J, Kim J, Singha K, Han D-K, Park H, Kim WJ, Biomaterials 2013, 34, 8766. [DOI] [PubMed] [Google Scholar]
- [66].Backlund CJ, Worley BV, Sergesketter AR, Schoenfisch MH, J. Dent. Res 2015, 94, 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hasanzadeh Kafshgari M, Delalat B, Harding FJ, Cavallaro A, Makila E, Salonen J, Vasilev K, Voelcker NH, J. Mater. Chem. B 2016, 4, 2051. [DOI] [PubMed] [Google Scholar]
- [68].Kakishima K, Shiratsuchi A, Taoka A, Nakanishi Y, Fukumori Y, Biochem. Biophys. Res. Commun 2007, 355, 587. [DOI] [PubMed] [Google Scholar]
- [69].Beveridge TJ, J. Bacteriol 1999, 181, 4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].a) Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D, Nano-Micro Lett 2015, 7, 219; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kaweeteerawat C, Chang CH, Roy KR, Liu R, Li R, Toso D, Fischer H, Ivask A, Ji Z, Zink JI, Zhou ZH, Chanfreau GF, Telesca D, Cohen Y, Holden PA, Nel AE, Godwin HA, ACS Nano 2015, 9, 7215; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jose Ruben M, Jose Luis E, Alejandra C, Katherine H, Juan BK, Jose Tapia R, Miguel Jose Y, Nanotechnology 2005, 16, 2346.20818017 [Google Scholar]
- [71].Agnihotri S, Mukherji S, Mukherji S, RSC Adv 2014, 4, 3974. [Google Scholar]
- [72].Suresh AK, Pelletier DA, Doktycz MJ, Nanoscale 2013, 5, 463. [DOI] [PubMed] [Google Scholar]
- [73].Gilbertson LM, Albalghiti EM, Fishman ZS, Perreault F, Corredor C, Posner JD, Elimelech M, Pfefferle LD, Zimmerman JB, Environ. Sci. Technol 2016, 50, 3975. [DOI] [PubMed] [Google Scholar]
- [74].Carpenter AW, Slomberg DL, Rao KS, Schoenfisch MH, ACS Nano 2011, 5, 7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nurhasni H, Cao J, Choi M, Kim I, Lee BL, Jung Y, Yoo J-W, Int. J. Nanomed 2015, 10, 3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].a) Sun B, Slomberg DL, Chudasama SL, Lu Y, Schoenfisch MH, Biomacromolecules 2012, 13, 3343; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lu Y, Slomberg DL, Shah A, Schoenfisch MH, Biomacromolecules 2013, 14, 3589; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Worley BV, Slomberg DL, Schoenfisch MH, Bioconjugate Chem 2014, 25, 918; [DOI] [PubMed] [Google Scholar]; d) Worley BV, Schilly KM, Schoenfisch MH, Mol. Pharmaceutics 2015, 12, 1573. [DOI] [PubMed] [Google Scholar]
- [77].a) Allahverdiyev AM, Kon KV, Abamor ES, Bagirova M, Rafailovich M, Expert Rev. Anti-Infect. Ther 2011, 9, 1035; [DOI] [PubMed] [Google Scholar]; b) Fischbach MA, Curr. Opin. Microbiol 2011, 14, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].a) Gehring J, Trepka B, Klinkenberg N, Bronner H, Schleheck D, Polarz S, J. Am. Chem. Soc 2016, 138, 3076; [DOI] [PubMed] [Google Scholar]; b) Dolanský J. i., Henke P, Kubát P, Fraix A, Sortino S, Mosinger J, ACS ACS Appl. Mater. Interfaces 2015, 7, 22980; [DOI] [PubMed] [Google Scholar]; c) Di Bari I, Picciotto R, Granata G, Blanco AR, Consoli GM, Sortino S, Org. Biomol. Chem 2016, 14, 8047; [DOI] [PubMed] [Google Scholar]; d) Consoli GM, Di Bari I, Blanco AR, Nostro A, D’Arrigo M, Pistarà V, Sortino S, ACS Med. Chem. Lett 2017, 8, 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].a) Carpenter AW, Worley BV, Slomberg DL, Schoenfisch MH, Biomacromolecules 2012, 13, 3334; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Backlund CJ, Worley BV, Schoenfisch MH, Acta Biomater 2016, 29, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Simoncic B, Tomsic B, Text. Res. J 2010, 80, 1721. [Google Scholar]
- [81].a) Chen H, Liu C, Chen D, Madrid K, Peng S, Dong X, Zhang M, Gu Y, Mol. Pharmaceutics 2015, 12, 2505; [DOI] [PubMed] [Google Scholar]; b) Black KCL, Sileika TS, Yi J, Zhang R, Rivera JG, Messersmith PB, Small 2014, 10, 169; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhao Y, Tian Y, Cui Y, Liu W, Ma W, Jiang X, J. Am. Chem. Soc 2010, 132, 12349. [DOI] [PubMed] [Google Scholar]
- [82].a) Duong HTT, Adnan NNM, Barraud N, Basuki JS, Kutty SK, Jung K, Kumar N, Davis TP, Boyer C, J. Mater. Chem. B 2014, 2, 5003; [DOI] [PubMed] [Google Scholar]; b) Duong HTT, Jung K, Kutty SK, Agustina S, Adnan NNM, Basuki JS, Kumar N, Davis TP, Barraud N, Boyer C, Biomacromolecules 2014, 15, 2583; [DOI] [PubMed] [Google Scholar]; c) Nguyen T-K, Selvanayagam R, Ho KKK, Chen R, Kutty SK, Rice SA, Kumar N, Barraud N, Duong HTT, Boyer C, Chem. Sci 2016, 7, 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sadrearhami Z, Yeow J, Nguyen T-K, Ho KKK, Kumar N, Boyer C, Chem. Commun 2017, 53, 12894. [DOI] [PubMed] [Google Scholar]
- [84].Dong K, Ju E, Gao N, Wang Z, Ren J, Qu X, Chem. Commun 2016, 52, 5312. [DOI] [PubMed] [Google Scholar]
- [85].a) Hetrick EM, Shin JH, Paul HS, Schoenfisch MH, Biomaterials 2009, 30, 2782; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Slomberg DL, Lu Y, Broadnax AD, Hunter RA, Carpenter AW, Schoenfisch MH, ACS Appl. Mater. Interfaces 2013, 5, 9322; [DOI] [PubMed] [Google Scholar]; c) Reighard KP, Hill DB, Dixon GA, Worley BV, Schoenfisch MH, Biofouling 2015, 31, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pegalajar-Jurado A, Wold KA, Joslin JM, Neufeld BH, Arabea KA, Suazo LA, McDaniel SL, Bowen RA, Reynolds MM, J. Controlled Release 2015, 217, 228. [DOI] [PubMed] [Google Scholar]
- [87].Backlund CJ, Sergesketter AR, Offenbacher S, Schoenfisch MH, J. Dent. Res 2014, 93, 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].a) Kutty SK, Barraud N, Pham A, Iskander G, Rice SA, Black DS, Kumar N, J. Med. Chem 2013, 56, 9517; [DOI] [PubMed] [Google Scholar]; b) Yepuri NR, Barraud N, Mohammadi NS, Kardak BG, Kjelleberg S, Rice SA, Kelso MJ, Chem. Commun 2013, 49, 4791. [DOI] [PubMed] [Google Scholar]
- [89].Barraud N, Kardak BG, Yepuri NR, Howlin RP, Webb JS, Faust SN, Kjelleberg S, Rice SA, Kelso MJ, Angew. Chem., Int. Ed 2012, 51, 9057. [DOI] [PubMed] [Google Scholar]
- [90].a) Jen MC, Serrano MC, van Lith R, Ameer GA, Adv. Funct. Mater 2012, 22, 239; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hetrick EM, Schoenfisch MH, Annu. Rev. Anal. Chem 2009, 2, 409; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hunter RA, Storm WL, Coneski PN, Schoenfisch MH, Anal. Chem 2013, 85, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Elias S, Banin E, FEMS Microbiol. Rev 2012, 36, 990. [DOI] [PubMed] [Google Scholar]
- [92].Craven M, Kasper SH, Canfield MJ, Diaz-Morales RR, Hrabie JA, Cady NC, Strickland AD, J. Appl. Microbiol 2016, 120, 1085. [DOI] [PubMed] [Google Scholar]
- [93].Thein ZM, Seneviratne CJ, Samaranayake YH, Samaranayake LP, Mycoses 2009, 52, 467. [DOI] [PubMed] [Google Scholar]
- [94].Brisbois EJ, Bayliss J, Wu J, Major TC, Xi C, Wang SC, Bartlett RH, Handa H, Meyerhoff ME, Acta Biomater 2014, 10, 4136–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].a) Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR, Virulence 2010, 1, 62; [DOI] [PubMed] [Google Scholar]; b) Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, Friedman JM, J. Invest. Dermatol 2009, 129, 2463; [DOI] [PubMed] [Google Scholar]; c) Schairer DO, Martinez LR, Blecher K, Chouake JS, Nacharaju P, Gialanella P, Friedman JM, Nosanchuk JD, Friedman AJ, Virulence 2012, 3, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mihu MR, Cabral V, Pattabhi R, Tar MT, Davies KP, Friedman AJ, Martinez LR, Nosanchuk JD, Antimicrob. Agents Chemother 2017, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].a) Kim JO, Noh J-K, Thapa RK, Hasan N, Choi M, Kim JH, Lee J-H, Ku SK, Yoo J-W, Int. J. Biol. Macromol 2015, 79, 217; [DOI] [PubMed] [Google Scholar]; b) Lowe A, Bills J, Verma R, Lavery L, Davis K, Balkus KJ, Acta Biomater 2015, 13, 121; [DOI] [PubMed] [Google Scholar]; c) Schanuel FS, Raggio Santos KS, Monte-Alto-Costa A, de Oliveira MG, Colloids Surf., B 2015, 130, 182. [DOI] [PubMed] [Google Scholar]
- [98].Kang Y, Kim J, Lee YM, Im S, Park H, Kim WJ, J. Controlled Release 2015, 220, 624. [DOI] [PubMed] [Google Scholar]
- [99].Worley BV, Soto RJ, Kinsley PC, Schoenfisch MH, ACS Biomater. Sci. Eng 2016, 2, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Neidrauer M, Ercan UK, Bhattacharyya A, Samuels J, Sedlak J, Trikha R, Barbee KA, Weingarten MS, Joshi SG, J. Med. Microbiol 2014, 63, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]