Abstract
The authors describe a rapid and low-cost approach for multiplex microRNA(miRNA) assay on lateral flow nucleic acid biosensor (LFNAB). The principle of assay is based on sandwich-type nucleic acid hybridization reactions to produce gold nanoparticle (GNP)-attached complexes (ssDNA-microRNA-ssDNA/GNPs), which are captured and visualized on the test zone of LFNAB. By designing three different test zones on LFNAB, simultaneous detection of microRNA-21, microRNA-155 and microRNA-210 was achieved with an adding-measuring model by using GNP as visual tag. The method was challenged by testing the microRNAs in spiked serum samples with satisfied results. In our perception, the test is a particularly valuable tool for clinical application and biomedical diagnosis, particularly in limited resource settings.
Keywords: Lateral flow biosensor, microRNA, simultaneous detection, multiplex detection, serum sample
Introduction
MicroRNAs (miRNA) are class of short (usually ~18–25 nucleotides), single-stranded and endogenous noncoding RNA molecules, which are derived from long primary transcripts by the catalysis of Dicer enzyme [1, 2]. It is of crucial importance in regulating post-transcriptional gene expression in a broad range of animals, plant, and viruses [3–5]. More importantly, there is accumulating evidence that the expression levels of microRNAs are associated with a variety of pathological conditions including cancers, such as lung cancer, hepatocellular carcinoma, breast cancer and gastric carcinoma [2]. MicroRNAs have become a vital class of potential biomarker candidates for clinical and early diagnosis [6, 7]. MicroRNA detection is challenging due to its low abundances, small size, and high degree of sequence similarity presenting obstacles in the use of conventional analytical approaches [8, 9]. Meanwhile, some studies have also demonstrated that the variation of microRNA in cancer includes both downregulated and overexpressed microRNAs with putative tumor suppressive and oncogenic functions. For qualitative analysis, the amount of microRNA in suspicious samples is compared to that of normal cells or tissues. For quantitative measurement, the detection limit is the bottleneck for detection of microRNA downregulation. [9] Taking these issues into account, considerable effort has been devoted to the development of methods with both high sensitivity and specificity for microRNA detection [10, 11]. Sequencing-based methods have been used for microRNA detection, such as Northern blotting [12], reverse transcriptase-polymerase chain reaction (RT-PCR) [13], rolling circle amplification (RCA) [14] and microarrays [6, 15, 16]. Northern blotting protocol is highly quantitative and has been considered as the “gold standard” method in microRNA profiling, but it requires large amounts of sample (~10–30 μg) and is highly cumbersome and time-consuming, often taking days for completion [10, 17, 18]. RT-PCR has high dynamic range and is sensitive, but has low throughput and is less specific compared to standard PCR [19]. Microarray-based methods were limited in terms of sensitivity, selectivity, and specificity although they have the property of high-throughput [20]. In general, although these methods performed well on microRNA analysis with high sensitivity, high selectivity, or high throughput, they suffered from time-consumption, expensive equipment and complex operations [21, 22] which dramatically limited their further applications on point-of-care diagnostic testing at low-resource settings. Therefore, facile, rapid, straight-forward and economical experimental approaches are urgently needed for microRNA analysis [23]. Recently, with the extraordinary achievements of nanotechnology, nanomaterial-based biosensors have sprung out and been greatly developed due to the unique electronic, optical and catalytic properties of the nanomaterials, which offer the signal amplification to achieve high sensitivity [3, 23].
Lateral flow biosensors have been extensively used as an effective tool for detecting bacteria [24], parasite antigens [25, 26], and hormones [27, 28] owing to their obvious advantages of user-friendly format, short assay time, and low testing cost [29, 30]. Gold nanoparticles (GNPs) are the most widely used tag on lateral flow assays for colorimetric analysis [31]. Furthermore, lateral flow nucleic acid biosensors (LFNABs) which inspired by the traditional immunochromatography test were reported for rapid visual detection of DNA or RNA segments [32, 23]. The principle of LFNAB is based on sandwich-type DNA hybridization reactions, which were implemented on the traditional lateral flow device. Recently, Liu and co-workers applied LFNABs to detect microRNA 215 (miRNA-215) with a detection limit of 60 pM [23]. Disease diagnostics usually requires accurate measurement of a panel of biomarkers, while only single-microRNA detection is usually inadequate for accurate diagnosis of cancer [33–36]. To the best of our knowledge, simultaneous detection of multiple microRNAs on lateral flow device with satisfied sensitivity has not been reported. Herein, we present a lateral flow nucleic acid biosensor for multiplex microRNA assay in aqueous solution and spiked serum samples. MicroRNA-21 (miR-21), microRNA-155 (miR-155) and microRNA-210 (miR-210), which are related with cancers and considered as potential biomarkers of diseases, were chosen as model targets to evaluate the multiplex feasibility of LFNAB. The promising properties of the approach are reported in the following sections.
Materials and methods
2.1 Reagents
Streptavidin (SAV), bovine serum albumin (BSA), trisodium citrate, tween-20, deoxyadenosine triphosphate (dATP) and tris (2-carboxyethl)phosphine (TCEP) were purchased from Sangon Biotech (Shanghai) Co., Ltd. and used directly without further purification. HAuCl4 was purchased from J&K Chemical Scientific Ltd. (Shanghai, China). Cellulose fiber sample pads, conjugate pads, and laminated cards were purchased from Millipore (USA). Nitrocellulose membranes including CN 95 and Vivid 170 were purchased from Sartorisu Stedim Biotech GmbH (Goettingen, German) and PALL Corporation (New York, USA), respectively. All the solutions used in this research were prepared with double distilled water (> 18 MΩ). Target microRNAs used in this study were synthesized by Takara Biotechnoly (Dalian) Co., Ltd. All other deoxy-oligonucleotide probes used in this study were ordered from Sangon Biotech (Shanghai) Co., Ltd. The sequences of DNA probes and microRNAs are presented in Table 1.
Table 1.
Sequences of all the oligonucleotide probes used in this research
| Name of probes | Sequences Information |
|---|---|
| miRNA-21 (miR-21) | 5′-UAG CUU AUC AGA CUG AUG UUGA-3′ |
| miRNA-155 (miR-155) | 5′-UUA AUG CUA AUC GUG AUA GGGU-3′ |
| miRNA-210 (miR-210) | 5′-CUG UGC GUG UGA CAG CGG CUGA-3′ |
| C line probe (probe 1) | 5′-Biotin-ACA CGG TGT CTA GGG GG-3′ |
| T-21 probe (probe 2) | 5′-CTG ATA AG C TAC CCCC-Biotin-3′ |
| T-155 probe (probe 3) | 5′-GAT TAG CAT TAA CCC CC-Biotin-3′ |
| T-210 probe (probe 4) | 5′-ACA CGC ACA GCC CCC-Biotin-3′ |
| Detection probe to miRNA-21 (probe 5) | 5′-thiol-CCC CCT AGA CAC CGT GTT CAA CATC AGT-3′ |
| Detection probe to miRNA-155 (probe 6) | 5′-thiol -CCC CCT AGA CAC CGT GTA CCC CTA TCAC-3′ |
| Detection probe to miRNA-210 (probe 7) | 5′-thiol-CCC CCT AGA CAC CGT GTT CAG CCG CTGT-3′ |
2.2 Preparation of concentrated (10-fold) gold nanoparticles (GNPs)
Gold nanoparticles (GNPs) with average diameter of 25 nm were prepared by the classic trisodium citrate reduction method with slight modifications [30]. Briefly, 50 mL of 0.01% HAuCl4 were brought into a 400-mL conical flask and heated to boiling with vigorous stirring, and then 950 μL of 1% trisodium citrate was added rapidly. The color of the solution changed from dark red to purple, and finally to wine red. The solution was kept heating and stirring for another 10 min and then cooled to room temperature.
One milliliter above prepared GNP solution was centrifuged at a relative centrifugal force (RCF) of 9200 g for 7 min, and 900 μL of supernatant was decanted. The remaining pellet in solution was eddied to obtain the 10-fold GNP solution. The 10-fold GNPs were stored at 4 °C for subsequent use.
2.3 Preparation of GNP-X ssDNA conjugates (X means miRNA-21, miRNA-155 and miRNA-210)
Firstly, a mixture solution containing three thiol-modified ssDNA probes (Table 1) was prepared. The final concentration of each ssDNA probe in the mixture solution was 2 μM. Then 100 μL of the mixture solution was activated for 1 h in dark by adding 2 μL of 1 mM TCEP at room temperature (RT), which was prepared in 0.5 M acetate buffer. Following, 100 μL of 10-fold concentrated GNPs were added and the solution was kept stirring for 1 h. Afterward, 10 μL of 10 mM dATP was added to inhibit the nonspecific absorption on the GNP surface, and the solution was kept stirring for another 45 min. The GNP-X ssDNA conjugates were aged by adding 20 μL of 0.1 M NaCl and stayed at RT for 1 h. The solution could stand at 4 °C for additional 6 h. The conjugates were purified by centrifugation at 8600 g for 7 min and the pellet was re-suspended in 100 μL of re-suspension solution containing 1 mM Tris-HCl (pH 8.0), 5% BSA, 0.25% Tween-20, and 10% sucrose.
2.4 Preparation of lateral flow nucleic acid biosensor (LFNAB)
The diagram of LFNAB is shown in scheme 1. It is composed of four parts including an absorbent pad, a nitrocellulose membrane, a glass fiber membrane (conjugated pad) and a sample pad. Before assembling, the sample pad and the conjugated pad were treated with different buffer solutions. The buffer solution containing 50 mM Tris-HCl (pH 8.0), 0.15 mM NaCl and 0.25% Tritonx-100 was used to treat the sample pad. While the buffer solution to treat the conjugated pad was 10 mM PBS (pH 7.4) containing 5% sucrose, 1% Trehalose, 0.25% PEG, and 0.3% Tween 20. After the pretreatments, the sample pad and conjugated pad were dried at 37 °C for 6 h and stored in desiccator at RT. The biotin-modified ssDNA probe (T-21 probe, T-155 probe, T-210 probe and control line probe) was mixed with streptavidin in PBS (pH 7.4) solution. After purification with a filter, the resulting streptavidin-biotin-T probe and streptavidin-biotin-C probe solution were dispensed on the nitrocellulose membrane at the designed area named as the test line (T line) and control line (C line) by the Biojet BJQ 3000 dispenser. The four parts mentioned above were assembled on the sticky back plate orderly and overlapped with each part by 2 mm to ensure that the sample solution could migrate through the strip. The strip was cut into 4 mm in width and stored at 4 °C and kept dry for use.
Scheme 1.
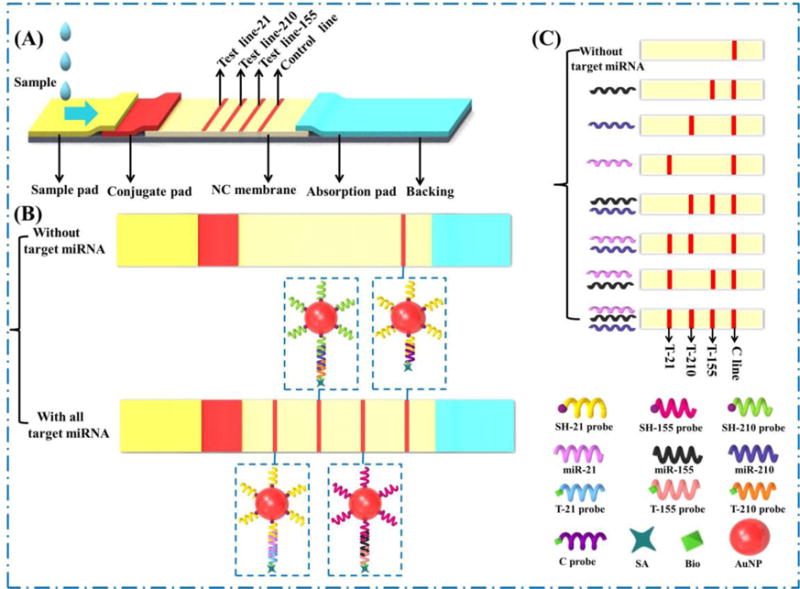
(A) Schematic diagram of lateral flow nucleic acid biosensor for multiplex microRNA assay; (B) In the absence and presence of three microRNA; (C) Principle of detecting multiple microRNAs on lateral flow nucleic acid biosensor.
2.5 Singleplex microRNA assay
Detection of microRNA-21 (miRNA-21) was implemented by applying 100 μL of sample solution containing a desired miRNA-21 concentration to the sample pad of LFNAB. After 10 min, the T line was observed by naked-eye and the intensity of the T line was obtained by taking the image of the T line and analyzing with ImageJ software. The detection of miR-155 and miR-210 was also conducted respectively with the same procedure.
2.6 Multiplex microRNA assay
Multiplex microRNA assay was carried out by applying 100 μL of sample solution containing different concentrations of miRNA-21, miRNA-155 and miRNA-210 to the sample pad of the biosensor. Ten min later, red bands on the LFNAB were observed by naked-eye indicating the accumulation of GNPs on the test lines and quantitative analysis was performed by ImageJ software.
2.7 Detection of microRNAs in serum samples
For evaluating the potential of the LFNAB for clinical application, the biosensor was applied to detect microRNAs in serum samples. Serum samples spiked different concentrations of miR-21, miR-155 and miR-210 were diluted 10-folds with running buffer. Then the spiked serum samples were tested on LFNAB as described in 2.6.
Results and discussions
3.1 Principle of multiplex microRNA assay on LFNAB
The principle of multiplex microRNA assay on LFNAB was based on sandwich-type hybridization reactions to produce a ssDNA-miRNA-ssDNA/GNPs complexes, which were captured and visualized on the test zones of LFNAB. (Scheme 1) Firstly, the four streptavidin-biotin -ssDNA probes (probe 1, 2, 3, 4 in scheme) were dispensed on the different zones of nitrocellulose membrane to form three test (T) lines and one control (C) line respectively. The thiol-modified ssDNA probes (probe 5, 6, 7 against miRNA 21, 155 and 210, respectively) were conjugated with the GNPs by self-assembling procedure. Then the resulting ssDNA/GNP conjugates were mixed and loaded on the conjugate pad of the LFNAB. In the presence of microRNAs in the sample solution, the sandwich-type complexes could be captured on the corresponding T line resulting in the accumulation of GNPs and visualized as red bands. The optical intensity of each T line was proportional to the concentration of each target miRNA, and the qualitative results could be achieved by naked-eye observation. The excess of thiol-ssDNA/GNP conjugates were captured by the ssDNA probe 1 on the C line and induced the red band, indicating the effectiveness of LFNAB. The intensities of T lines are analyzed with the ImageJ software.
3.2 Optimization of experimental parameters
In order to obtain the best performance of microRNA assay on LFNAB, the experimental parameters of the assay were optimized. Firstly, nitrocellulose membrane with different pore sizes would affect the migration rate of the GNP-ssDNA conjugates during the assay. Vivid 170 and CN 95 nitrocellulose membranes were used to prepare LFNABs and the performances of the biosensors were compared. Figure 1a presents the photo images of the LFNABs after testing different miRNA-21 concentrations. One can see the LFNAB prepared with CN 95 membrane could detect lower concentrations than that of the LFNABs prepared with Vivid 170 membrane. Therefore, CN 95 membrane was chosen to prepare the LFNAB in the following experiments. The amount of ssDNA used to prepare ssDNA-GNP conjugates was another important factor to affect the sensitivity of the LFNAB. The ssDNA with different volumes ranging from 0 to 20 μL was used to prepare the ssDNA-GNP conjugates, and the performances of the conjugates were evaluated by detecting 10 nM miRNA-21 (Figure 1b). One can see that the intensity of the test line increased with the increase of the ssDNA volume from 0 to 15 μL; further increment of the ssDNA volume led to a slight decreased intensity of the test line (Figure 1c), which could be attributed to the spatial hindrance effect from the excess of ssDNA on the GNP surface. Therefore, 15 μL of 2 μM ssDNA detection probe was adopted as the optimal volume to prepare the ssDNA-GNP conjugates. In addition, the amount of dATP used to block GNP surface was also optimized and the results were detailed in supporting information. Other parameters including the components of the running buffer and the diameter of GNPs were adopted from our previous study without future optimization.
Figure 1.
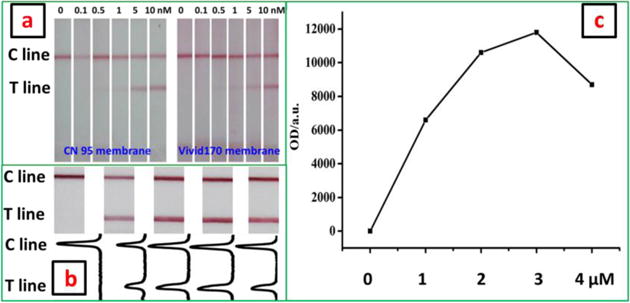
(a) Photo images of the CN 95-based LFNABs and Vivid 170-based LFNABs in the presence of different miRNA-21 concentrations; (b) Photo images (top) of LFNABs and corresponding responses (bottom) in the presence of 10 nM miRNA-21. The LFNABs were prepared with different ssDNA-GNP conjugates. The ssDNA-GNP conjugates were prepared with different ssDNA volume ranged from 0 to 20 μL. (c) Effect of ssDNA volume used in the preparation of ssDNA-GNP conjugates on the peak area of T line.
3.3 Analytical performance of microRNA assay on LFNAB
Under the optimal conditions, we examined the analytical performance of LFNAB for multiplex miRNA assay. The simultaneous determination results of three target microRNAs were shown in Figure 2. Figure 2a presents the typical photo images of LFNABs in the presence of different concentrations of three microRNAs and corresponding quantitative results. One can see that the optical intensity of the T line increased with the concentration increase of three analytes in the range from 0 to 10 nM. The threshold of visual detection of miRNA-155, miRNA-21 and miRNA-210 on LFNAB was 0.01, 0.01 and 0.05 nM, respectively. Based on the quantitative analysis results in Figure 2a, the calibration curves of three miRNAs were constructed and shown in Figure 2b. The R squares of three calibration curves for miRNA-155, miRNA-21 and miRNA-210 determination is 0.9973, 0,9987 and 0.9895, respectively. Besides, the calibration curve of each miRNA is also of litter difference in both the linear range and the detection limit (DL). Good linear relationship was achieved in the range from 0.01 to 5 nM, 0.1 to 10 nM, and 0.05 to 10 nM, for miRNA-155, miRNA-21and miRNA-210, respectively. The DL of three miRNAs were estimated to be 0.007 nM, 0.068 nM and 0.017 nM, respectively (signal to noise ratio, S/N=3), which are comparable to our previous report [23]. The DLs for three miRNAs are better than that of enzymatic based amplification methods [37, 38]. The concentrations of microRNAs in serum/plasma range from fmol/L to pmol/L, which depends on the examined microRNAs. [39] Further research will aim to improve the DLs of LFNAB by using signal amplification approaches developed in our laboratory. [32]
Figure 2.
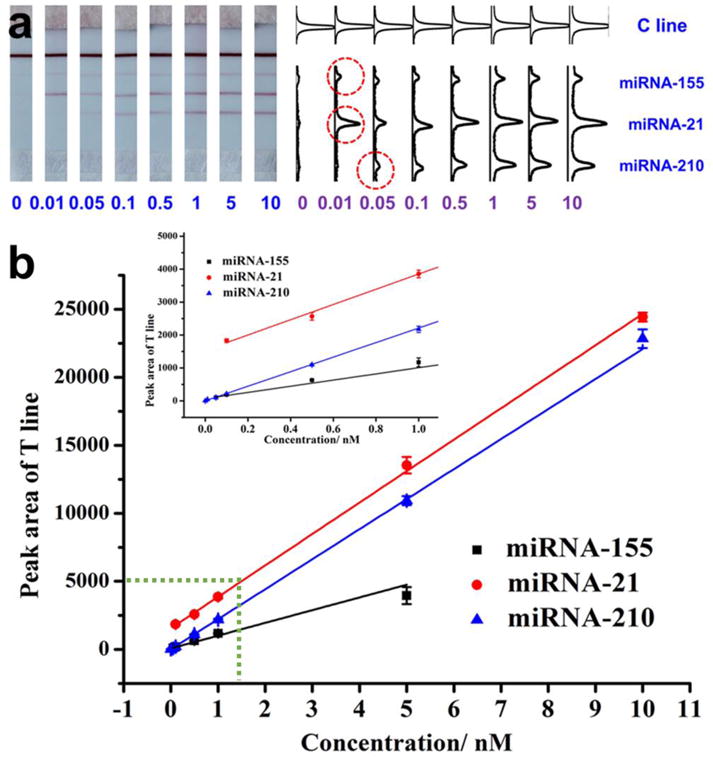
(a) Typical photo images of LFNABs in the presence of different concentrations of miRNA-21. (b) Corresponding optical responses; (c) Calibration curves of detecting individual miRNA on LFNAB.
The specificity of microRNA assay on LFNAB relies on the recognition ability of the designed ssDNA probes. Due to the sequence homology of the microRNA family members, it is important to check the specificity of microRNA assay on LFNAB. The specificity of microRNA assay on LFNAB was studied by detecting the target microRNA, single base-mismatched ssDNA (M1), two bases-mismatched ssDNA (M2), two other microRNAs with different sequences and a ssDNA with random sequence. Figure 3 presents the responses of T lines in the presence of 10 nM miRNA-21 and other mentioned analytes above. In this case, only a pair of ssDNA probes specific to miRNA-21 was used to prepare the LFNAB. The highest response was obtained with miRNA-21, and negligible responses were obtained with other miRNAs and ssDNA with random sequence, indicating there is no cross-reaction between the designed ssDNA and other miRNAs and single-stranded DNA. It is noted that the LFNAB could differentiate the target miRNA-21 from single base-mismatched and two bases-mismatched DNAs. We also tested the specificities of LFNABs prepared with other ssDNA probes, which are specific for miRNA-155 and miRNA-210, respectively. The LFNAB showed and excellent specificity. (see the details in Figure S4).
Figure 3.
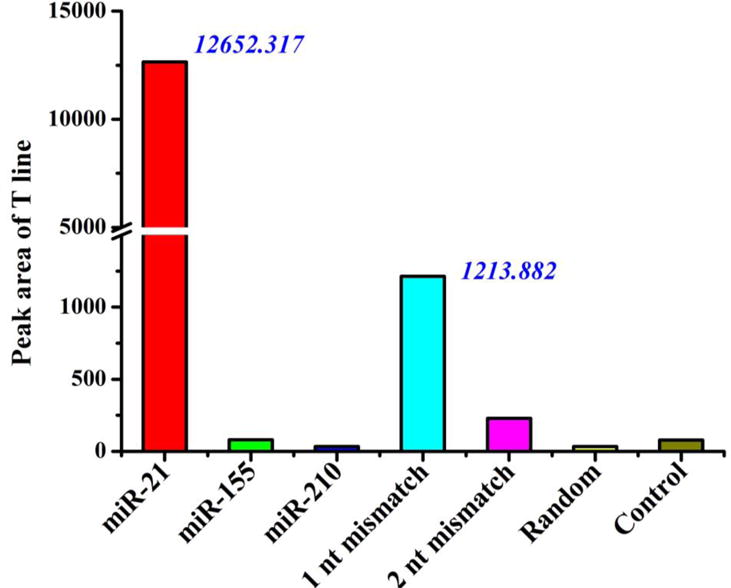
Histogram of the T line response of the LFNAB in the presence of 10-nM miR-21, 10-nM miR-155, 10-nM miR-210, 10-nM one-base mismatched DNA (1 nt mismatch), 10 nM two-base mismatched DNA (2 nt mismatch), 10-nM DNA with random sequence(Random) and 0-nM microRNA(control). The LFNABs were prepared with the ssDNA probes specific to miR-21.
3.4 Multiplex miRNA assay in serum samples
The capability of multiplex microRNA assay on LFNAB was studied by detecting microRNAs in spiked serum samples. Three test zones were prepared by dispensing three ssDNA probes on the nitrocellulose membrane of LFNAB. Firstly miRNA-21 was tested in spiked serum samples. As depicted in Figure 4A, two bright red bands (T line and C line) were observed on the LFNAB and the intensity of T line increases with the increase of miR-21 concentration in serum samples. In the case of testing the serum sample spiked either miR-21 or miR-155 or miR-210, T line appeared at different locations of LFNAB (Figure 4B). The serum samples spiked two microRNAs and three microRNAs were also tested on LFNABs. As expected, three red bands (two T lines and one C line) were observed on LFNAB when the serum samples were spiked with two miRNAs, and four red bands (three T lines and one C line) were observed when the serum samples were spiked with three microRNAs (Figure 4B). The results indicated that no cross-reaction occurred and the LFNAB can be used to detect miRNA without the influence of analogues or matrix in serum samples.
Figure 4.
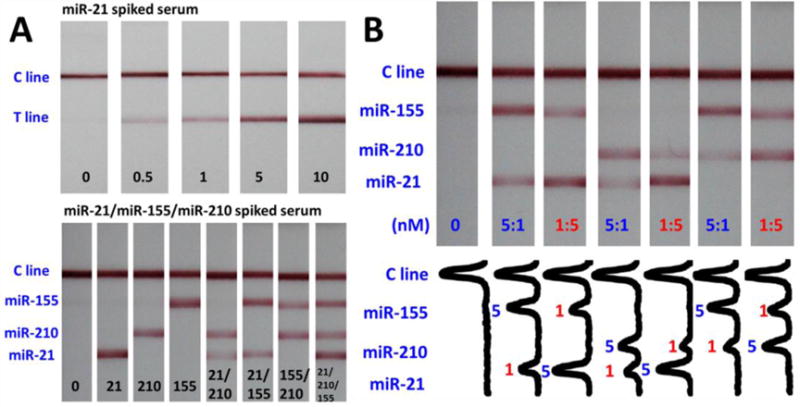
(A) Typical photo images of the LFNABs in the presence of different concentrations of miR-21 in spiked serum samples. (B) Typical photo images of the LFNABs in the absence and presence of individual microRNA and multiple microRNAs in spiked serum samples.
Conclusions
In this article, the lateral flow nucleic acid biosensor was used for multiplex microRNA assay. After systematic optimization, the biosensor was able to detect miRNA-21, miRNA-155 and miRNA-210 with detection limits of 0.073, 0.061 and 0.085 nM, respectively. The biosensor was successfully applied for simultaneous detection of multiple microRNAs in spiked serum samples without cross-reactivity and matrix-effect. The lateral flow nucleic acid biosensor could be a potential tool for rapid and on-site screening of microRNAs. Further work will aim to improve the sensitivity of the biosensor and test microRNAs in serum samples from patient and healthy controls.
Supplementary Material
Highlights.
Lateral flow nucleic acid biosensor was constructed for rapid detection of microRNAs.
Three target microRNAs were all successfully detected with good linearity and sensitivity.
MicroRNAs were detected in the spiked serum samples with this lateral flow nucleic acid biosensor.
Acknowledgments
This work is financially supported by the NSFC Grant of 21475030, the S&T Research Project of Anhui Province 15czz03109, the National 10000 Talents-Youth Top-notch Talent Program, and the Science and Technology Ministry of China (2015BAD17B02-3). G. Liu acknowledges financial support from the NIH, Centers of Biomedical Research Excellent (NIH, BOBRE, P20 GM109024).
Biographies
Wanli Zheng is a master student in school of food science and engineering, Hefei University of Technology. Her research is establishment of test paper sensors.
Li Yao is currently studying for Ph.D in school of food science and engineering, Hefei University of Technology. Her current research is lateral chromatographic strips and Raman based methods.
Jun Teng is currently studying for Ph.D in school of food science and engineering, Hefei University of Technology. He is dedicated to the detection of pathogenic bacterium.
Chao Yan is a Ph. D student in School of Food Science & Engineering, Hefei University of Technology. His current research focus on the development of rapid detection methods for food safety and early diagnosis.
Panzhu Qin is currently studying for his master degree in School of Food Science & Engineering, Hefei University of Technology. His current research is molecular biology based rapid detection methods.
Guodong Liu is an Associate Professor in Department of Chemistry and Biochemistry, North Dakota State University, USA. He received his Ph.D degree from Hunan University in 2001. His main research interests are in the development of nanomaterial-based biosensor platforms for the detection of protein and nucleic acid biomarkers associated with biomedically and environmentally relevant targets.
Wei Chen is a professor in Hefei University of Technology (HFUT), China. He received his Ph.D. degree from Jiangnan University in 2011 and started his group and research in HFUT. His current research interest includes the development of rapid detection methods in food safety, early diagnostic of disease and nanobiotechnology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dong HF, Lei JP, Ding L, Wen YQ, Ju HX, Zhang XJ. MicroRNA: function, detection, and bioanalysis. Chem Rev. 2013;113:6207–6233. doi: 10.1021/cr300362f. [DOI] [PubMed] [Google Scholar]
- 2.Zhu GC, Liang L, Zhang CY. Quencher-free fluorescent method for homogeneously sensitive detection of microRNAs in human lung tissues. Anal Chem. 2014;86:11410–11416. doi: 10.1021/ac503365z. [DOI] [PubMed] [Google Scholar]
- 3.Baigude H, Ahsanullah, Li ZH, Zhou Y, Rana TM. miR-TRAP: A Benchtop Chemical Biology Strategy to Identify microRNA Targets. Angew Chem Int Ed. 2012;51:5880–5883. doi: 10.1002/anie.201201512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2014;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.He L, Hannon GJ. Micro RNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 6.Yin BC, Liu YQ, Ye BC. One-step, multiplexed fluorescence detection of microRNAs based on duplex-specific nuclease signal amplification. J Am Chem Soc. 2012;134:5064–5067. doi: 10.1021/ja300721s. [DOI] [PubMed] [Google Scholar]
- 7.Degliangeli F, Kshirsagar P, Brunetti V, Pompa PP, Fiammengo R. Absolute and direct microRNA quantification using DNA-gold nanoparticle probes. J Am Chem Soc. 2014;136:2264–2267. doi: 10.1021/ja412152x. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Z, Zhou YY, Gao SX, Cheng YQ, Li ZP. Homogeneous and Sensitive Detection of microRNA with Ligase Chain Reaction and Lambda Exonuclease-Assisted Cationic Conjugated Polymer Biosensing. Appl Mater Inter. 2014;6:6181–6185. doi: 10.1021/am500883q. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Hui A, Pampalakis G, Soleymani L, Liu FF, Sargent EH, Kelley SO. Direct, Electronic MicroRNA Detection for the Rapid Determination of Differential Expression Profiles. Angew Chem Int Ed. 2009;48:8461–8464. doi: 10.1002/anie.200902577. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Schwarz G, Santiago JG. Rapid high-specificity microRNA detection using a two-stage isotachophoresis assay. Angew Chem Int Ed. 2013;52:11534–11537. doi: 10.1002/anie.201305875. [DOI] [PubMed] [Google Scholar]
- 11.Wark AW, Lee HJ, Corn RM. Multiplexed Detection Methods for Profiling MicroRNA Expression in Biological Samples. Angew Chem Int Ed. 2008;47:644–652. doi: 10.1002/anie.200702450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 13.Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 2005;39:519–525. doi: 10.2144/000112010. [DOI] [PubMed] [Google Scholar]
- 14.Chen CF, Ridzon DA, Broomer AJ, Zhou ZH, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ. Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng YQ, Zhang X, Li ZP, Jiao XX, Wang YC, Zhang YL. Highly Sensitive Determination of microRNA Using Target-Primed and Branched Rolling-Circle Amplification. Angew Chem Int Ed. 2009;48:3268–3272. doi: 10.1002/anie.200805665. [DOI] [PubMed] [Google Scholar]
- 16.Wu CC, Cansiz S, Zhang LQ, Teng I, Qiu LP, Li J, Liu Y, Zhou CS, Hu R, Zhang T, Cui C, Cui L, Tan WH. A Nonenzymatic Hairpin DNA Cascade Reaction Provides High Signal Gain of mRNA Imaging inside Live Cells. J Am Chem Soc. 2015;137:4900–4903. doi: 10.1021/jacs.5b00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao ZQ, Yang ZC. Detection of MicroRNAs Using Electrocatalytic Nanoparticle Tags. Anal Chem. 2006;78:1470–1477. doi: 10.1021/ac051726m. [DOI] [PubMed] [Google Scholar]
- 18.Wuchty S, Arjona D, Bozdag S, Bauer PO. Involvement of microRNA families in cancer. Nucleic Acids Res. 2012;40:8219–8226. doi: 10.1093/nar/gks627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persat A, Santiago JG. MicroRNA profiling by simultaneous selective isotachophoresis and hybridization with molecular beacons. Anal Chem. 2011;83:2310–2316. doi: 10.1021/ac103225c. [DOI] [PubMed] [Google Scholar]
- 20.Cissell KA, Rahimi Y, Shrestha S, Hunt EA, Deo SK. Bioluminescence-Based Detection of MicroRNA, miR21 in Breast Cancer Cells. Anal Chem. 2008;80:2319–2325. doi: 10.1021/ac702577a. [DOI] [PubMed] [Google Scholar]
- 21.Gao ZQ, Deng HM, Shen W, Ren YQ. A label-free biosensor for electrochemical detection of femtomolar microRNAs. Anal Chem. 2013;85:1624–1630. doi: 10.1021/ac302883c. [DOI] [PubMed] [Google Scholar]
- 22.Wu XY, Chai YQ, Zhang P, Yuan R. An Electrochemical Biosensor for Sensitive Detection of MicroRNA-155: Combining Target Recycling with Cascade Catalysis for Signal Amplification. Appl Mater Inter. 2015;7:713–720. doi: 10.1021/am507059n. [DOI] [PubMed] [Google Scholar]
- 23.Gao XF, Xu H, Baloda M, Gurung AS, Xu LP, Zhang T, Zhang XJ, Liu GD. Visual Detection of microRNA with Lateral Flow Nucleic Acid Biosensor. Biosens Bioelectron. 2014;54:578–584. doi: 10.1016/j.bios.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laderman EI, Whitworth E, Dumaual E, Jones M, Hudak A, Hogrefe W, Carney J, Groen J. Rapid, sensitive, and specific lateral-flow immunochromatographic point-of-care device for detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in serum and whole blood. Clin Vaccine Immunol. 2008;15:159–163. doi: 10.1128/CVI.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang GP, Li QM, Yang YY, Guo JQ, Li XW, Deng RG, Xiao ZJ, Xing GX, Yang JF, Zhao D, Cai SJ, Zang WM. Development of a One-Step Strip Test for the Diagnosis of Chicken Infectious Bursal Disease. Avian Dis. 2005;49:177–181. doi: 10.1637/7272-090704R. [DOI] [PubMed] [Google Scholar]
- 26.Zhang GP, Guo JQ, Wang XN, Yang JX, Yang YY, Li QM, Li XW, Deng RG, Xiao ZJ, Yang JF, Xing GX, Zhong D. Development and evaluation of an immunochromatographic strip for trichinellosis detection. Vet Parasitol. 2006;137:286–293. doi: 10.1016/j.vetpar.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Zhang GP, Wang XN, Yang JF, Yang YY, Xing GX, Li QM, Zhao D, Chai SJ, Guo JQ. Development of an immunochromatographic lateral flow test strip for detection of β-adrenergic agonist Clenbuterol residues. J Immunol Methods. 2006;312:27–33. doi: 10.1016/j.jim.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Song CM, Liu QT, Zhi AM, Yang JF, Zhi YB, Li QM, Hu XF, Deng RG, Casas J, Tang L, Zhang GP. Development of a Lateral Flow Colloidal Gold Immunoassay Strip for the Rapid Detection of Olaquindox Residues. J Agric Food Chem. 2011;59:9319–9326. doi: 10.1021/jf202213m. [DOI] [PubMed] [Google Scholar]
- 29.Zhu MY, Wang Y, Deng Y, Yao L, Adeloju SB, Pan DD, Xue F, Wu YC, Zheng L, Chen W. Ultrasensitive detection of mercury with a novel one-step signal amplified lateral flow strip based on gold nanoparticle-labeled ssDNA recognition and enhancement probes. Biosens Bioelectron. 2014;61:14–20. doi: 10.1016/j.bios.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 30.Mei ZL, Qu W, Deng Y, Chu HQ, Cao JX, Xue F, Zheng L, EI-Nezamic HS, Wu YC, Chen W. One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A (BPA) in aqueous samples. Biosens Bioelectron. 2013;49:457–461. doi: 10.1016/j.bios.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Song CM, Zhi AM, Liu QT, Yang JF, Jia GC, Shervin J, Tang L, Hu XF, Deng RG, Xu CL, Zhang GP. Rapid and sensitive detection of β-agonists using a portable fluorescence biosensor based on fluorescent nanosilica and a lateral flow test strip. Biosens Bioelectron. 2013;50:62–65. doi: 10.1016/j.bios.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 32.He YQ, Zhang SQ, Zhang XB, Baloda M, Gurung AS, Xu H, Zhang XJ, Liu GD. Ultrasensitive nucleic acid biosensor based on enzyme-gold nanoparticle dual label and lateral flow strip biosensor. Biosens Bioelectron. 2011;26:2018–2024. doi: 10.1016/j.bios.2010.08.079. [DOI] [PubMed] [Google Scholar]
- 33.Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, Calabro E, Croce CM, Pastorino U, Sozzi G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Pro Natl Acad Sci U S A. 2011;108:3713–3718. doi: 10.1073/pnas.1100048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng DL, Haddadin S, Wang Y, Gu LQ, Perry MC, Freter CE, Wang MX. Plasma microRNAs as novel biomarkers for early detection of lung cancer. Int J Clin Exp Pathol. 2011;4:575–586. [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang XY, Wang Y, Fricke BL, Gu LQ. Programming Nanopore Ion Flow for Encoded Multiplex MicroRNA Detection. Acs Nano. 2014;8:3444–3450. doi: 10.1021/nn406339n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CY, Hou F, Ma YC. Simultaneous quantitative detection of multiple tumor markers with a rapid and sensitive multicolor quantum dots based immunochromatographic test strip. Biosens Bioelectron. 2015;68:156–162. doi: 10.1016/j.bios.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 37.Cui L, Lin XY, Lin NH, Song YL, Zhu Z, Chen X, Yang CJ. Graphene oxide-protected DNA probes for multiplex microRNA analysis in complex biological samples based on a cyclic enzymatic amplification method. Chem Commun. 2012;48:194–196. doi: 10.1039/c1cc15412e. [DOI] [PubMed] [Google Scholar]
- 38.Harting JS, Grune I, Najafi-Shoushtari SH, Famulok M. Sequence-specific detection of MicroRNAs by signal-amplifying ribozymes. J Am Chem Soc. 2004;126:722–723. doi: 10.1021/ja038822u. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Zhu W, Wen W, Cheng W, Wang F, Wu Y, Qi L, Fan Y, Chen Y, Ding Y, Xu J, Qian J, Huang Z, Wang T, Zhu D, Shu Y, Liu P. Diagnostic value of a plasma microRNA signature in gastric cancer: a microRNA expression analysis. Scientific Reports. 2015;5:11251. doi: 10.1038/srep11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


