Abstract
Animals occupy territories in which resources such as food and shelter are often distributed unevenly. While studies of exploratory behavior have typically involved the laboratory rodent as an experimental subject, questions regarding what constitutes exploration have dominated. A recent line of research has utilized a descriptive approach to the study of rodent exploration, which has revealed that this behavior is organized into movement subsystems that can be readily quantified. The movements include home base behavior, which serves as a central point of attraction from which rats and mice organize exploratory trips into the remaining environment. In this review, we describe some of the features of this organized behavior pattern as well as its modulation by sensory cues and previous experience. We conclude the review by summarizing research investigating the neurobiological bases of exploration, which we hope will stimulate renewed interest and research on the neural systems mediating rodent exploratory behavior.
Keywords: locomotor, hyperactivity, open-field, spatial behavior, hippocampus, navigation, grid cells, place cell, head direction cells
1. Introduction
Animals occupy territories in which resources such as food and shelter are often distributed unevenly. Given the challenges involved in securing resources while at the same time minimizing the risk of predation, it is critical that animals optimize their movements to efficiently explore the space. While studies of exploratory behavior have been conducted in a wide number of animal species (Berlyne, 1960; Menzel, 1973; Renner, 1990), research involving the laboratory rodent as an experimental subject has largely dominated the field (Barnett, 1963; Drai et al., 2001; Eilam & Golani., 1989; Whishaw & Whishaw, 1996). The concentration on rodent research stems from the fact that their exploratory activity can be assessed in a variety of test situations including multi-choice mazes, cylinders, open-fields, and in response to brain manipulation (Clark et al., 2005; File, 1985; Gharbawie et al., 2004; Hall, 1934; O’Keefe & Nadel, 1978; Renner, 1990; Whishaw, 1974). An additional advantage of using rodents as subjects for experimental study is that, although behavior is variable from animal to animal, some movements are identifiable across tasks and rodent species. For instance, orientation responses towards environmental stimuli have been well characterized (Pavlov, 1927; Sokolov, 1963). Other movements that can be readily measured include the vertical movements made by animals when rearing up on their hind legs or against surfaces (Gharbawie et al., 2004; Lever et al., 2006), and general locomotor activity that takes an animal from one location to another (O’Keefe & Nadel, 1978).
Nevertheless, several challenges to the quantification of exploratory behavior remain. For example, rodent exploratory movements are often described as stochastic or random, and lacking moment-to-moment consistency (Morris, 1983; Tchernichovski, 1995). Others have argued that rodent behavior in open-fields and complex mazes are difficult to describe quantitatively, and much of the focus has remained on simple end-point measures such as the cumulative distance traveled, the number of photobeam crossings during a test, and locomotor speed. A second difficulty in quantification is whether exploratory behavior should be formulated in terms of the movements involved (Teitelbaum et al., 1980), or in relation to the underlying motivations or goals (Morris, 1983; Renner, 1990; Whishaw et al., 2006). Indeed, exploratory activity is often described in terms of concepts such as fear and anxiety (Blanchard et al., 1974; Gray, 1982; Montgomery, 1955; Russel, 1973), curiosity and information-gathering (Berlyne, 1960), and the acquisition of spatial “maps” of the environment (O’Keefe & Nadel, 1978).
A recent line of investigation has utilized a descriptive approach to the study of rodent exploratory behavior, which involves breaking the movements down into simpler behavioral subsystems which, when recombined, reconstitute the original full pattern of exploratory behavior (Golani, 2011; Teitelbaum et al., 1980; Wallace et al., 2003; Whishaw et al., 1994). Such descriptions have revealed that the structure of exploration is far from random, and is composed of movement subsystems that can be readily quantified (Eilam & Golani, 1989; Golani, 2011; Golani et al., 1993; Hines et al., 2005; Wallace et al., 2002; Whishaw et al., 2001). In this review, we describe some of the central features of this organized behavior pattern. Much of our discussion will center on the observation that rodent exploratory behavior is organized to encompass specific environmental locations termed “home bases” (Chance & Mead, 1955; Eilam & Golani, 1989). It has been argued that home bases serve as an organizational feature of rodent locomotor activity from which exploratory trips or excursions are made into the remaining environment. Although this rodent behavior pattern appears early in development and is likely a behavioral primitive (Loewen et al., 2005), the organization of these movements can be modulated by sensory cues as well as previous experience with environment stimuli (Clark et al., 2005; 2006; Hines et al., 2005; Lehmann et al., 2007). We conclude this review by summarizing work on the neurobiological bases of rodent exploration, which we hope will stimulate renewed interest and organize future thinking for the study of exploratory behavior.
2. Behavioral Subsystems of Rodent Exploration
2.1. Rat Home Base Behavior
Early studies investigating the exploratory movements of rodents have remarked on the natural tendency of animals to establish preferred “home” locations from which they make excursions into the remaining environment (Chance & Mead, 1955). For instance, feral rats maintain home burrows from which they organize their foraging and avoidance of predation (Barnett, 1963; Whishaw & Whishaw, 1996). Eilam and Golani (1989) provided one of the first experimental characterizations of home base behavior by placing wild rats in a large open environment devoid of a shelter or local cues. Over a 1-hour period, rats visited several locations, but restricted their visits to one or two of these locations. Rats tended to spend a disproportionate amount of time stopping at a single location (10 times more than the second location). A stop or pause was defined as the absence of active movement, forward or backwards, and lasting longer than one second. The duration of stops made at this preferred home base increased as a function of test duration. Eilam and Golani additionally observed a particular set of behaviors at the home base. Grooming, for instance, is almost exclusively expressed at the home base. Bouts of grooming are typically followed by excursions into the remaining environment, or prolonged crouching in place. Other behaviors at the home base included long duration rearing movements, and circling or pivoting behavior, that latter of which likely consists of sniffing the maze substrate. In sum, Eilam and Golani’s seminal study described a pattern of regionally restricted behavior characterized by grooming, rearing, and circling behaviors.
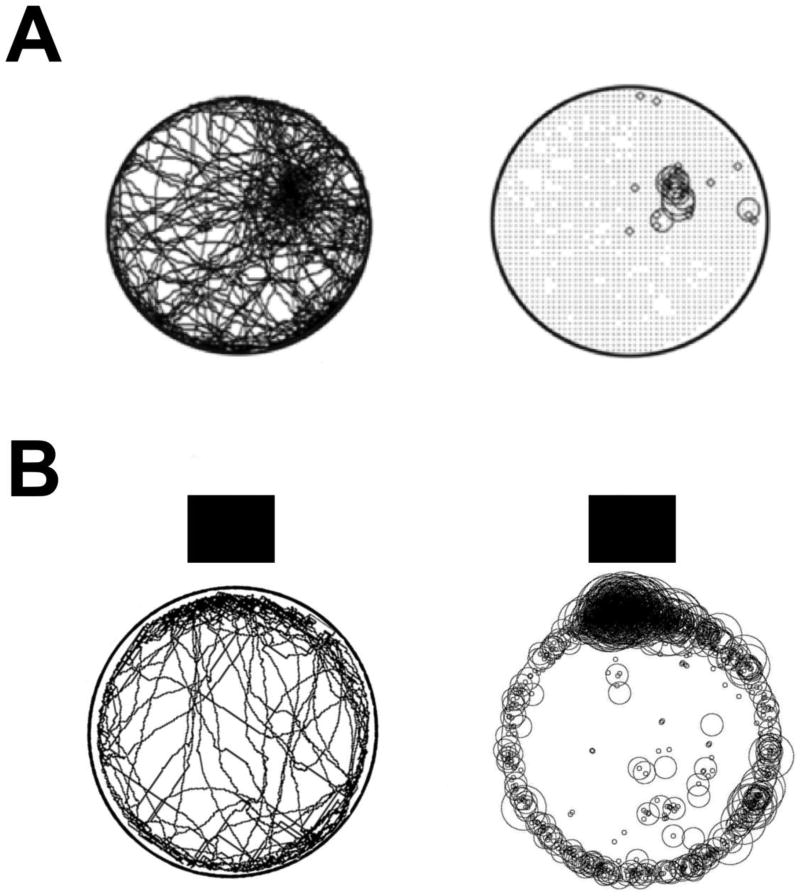
Home base behavior by rats has been reproduced in subsequent studies using featureless environments or in complete darkness (Fig. 1A) (Hines & Whishaw, 2005), but the behavior can also withstand changes in enclosure size and stimulus complexity (Eilam, 2004; 2014; Golani et al., 1993; Whishaw et al., 2006). Although these observations indicate that home base behavior is robust despite changing test situations and contextual features, Eilam and Golani (1989) noted a tendency for behavior to form at the edges of mazes, especially at the corners of square open-fields. Thus, home bases can be modulated to some degree by salient environmental stimuli and environment shape. Whishaw and colleagues investigated this relationship further by placing objects or small shelters in the proximity of the open-field (Clark et al., 2005; Hines et al., 2005; Lehmann et al., 2007; Wallace et al., 2002; Whishaw et al., 2001). For example, Hines & Whishaw (2005) placed a large dark cue next to an open field, but just out of reach of the rat. It has long been known that rats are attracted to dark locations in an environment (Whishaw, 1974), and it was therefore hypothesized that animals would establish their home bases near this cued location. As expected, rats rapidly visited the cued location and spent a significant amount of time in that segment of the open-field (Fig. 1B). Home base behavior at the cued location was similar to home base behavior in featureless environments, including the performance of circling, grooming, rearing, and slow lingering movements. In a follow-up test, the animal was removed from the field and the cue was moved to another location along the edge of the open-field. In response, rats changed their home bases locations so that it was maintained in relation to the moved cue. Hines and Whishaw (2005) reported that in some test sessions, rats would even establish home bases in relation to objects located along the testing room walls, such as a book shelf, suggesting that the behavior can be influenced by distant room cues.
Figure 1.
(A) Representative path (left) and stops (right) made by a rat (n = 1) during a 30 min exploration test in darkness. Stop duration is represented by the diameter of the circles (modified from Hines & Whishaw, 2005). (B) Composite of the paths (left) and stops (right) made by a group of rats (n = 6) tested in a 30min session in an open-field in lighted conditions (modified from Clark et al., 2005). The black square represents the location of a proximal visual cue placed next to the open-field. Note that rats display regional preferences in stopping behavior, but that the stops tend to cluster adjacent to the proximal cue when it is provided.
In some studies, a configuration of proximal cues has been provided within the confines of the open-field or just adjacent to the field (Lehmann et al., 2007; Yaski & Eilam, 2007). Whishaw and colleagues have reported a strong tendency for animals to evenly distribute their home bases across equivalent objects (Clark et al., 2006). However, when competing objects provide different sensory information, there is a clear preference for one cue over the other. For instance, Lehmann et al (2007) placed a large black box near one side of an open field, but far enough away from the maze such that the rat could not touch the object, but could still be seen. On the opposite side of the maze, a white wall occupied a small segment of the open-field, and was close enough to the field so that it could be used as a tactile cue. The two cues were available in a 30 min exploration session repeated over four days. Although rats formed two home bases, one adjacent to the black box and another next to the white wall, by the fourth day of testing there was a preference for engaging their home base next to the white wall (Fig. 2). Thus, rats spent a disproportionate amount of time and stopped more frequently at the wall segment. The preference for the wall over the large black box might be related to the perception that the wall offers greater security in the open field, i.e., rats could rest their bodies against the wall but were unable to do so against the black cue (Whishaw et al., 2006). Alternatively, the preference may reflect a hierarchy of sensory control over home base behavior, with tactile cues preferred over visual cues. Hierarchical control over behavior by sensory cues has been observed in other aspects of spatial navigation, with some reports concluding that cues located proximal to the animal, or related to the maze substrate, typically take precedence over other stimulus sources (Maaswinkel & Whishaw, 1999; Sanchez et al., 2016).
Figure 2.
Representative paths (left) and stops (right) from a rat tested in the presence of a cue and a segment of a white wall (pictured is the location of the black visual cue and the white wall segment; note that the black cue was located a short distance from the open-field but the while wall was continuous with the open-field). Stops made by rats tended to cluster near the visual cue on Day 1, but stops clustered near the wall on Day 4 (modified from Lehmann et al., 2007).
Several studies have reported that prior experience with environmental stimuli can modulate regional preferences in home base behavior (Eilam, 2014; Hines & Whishaw, 2005; Lehmann et al., 2007). Interestingly, Hines and Whishaw (2005) monitored the exploratory behavior of rats in an open field with a large proximal cue placed next to the arena. Animals were tested in five daily sessions in which the cue occupied the same position, but on the fifth day the proximal cue was removed from the testing room. Hines and Whishaw observed that despite the removal of the cue, rats continued to dwell in the location of the open-field. This observation indicated that animals can learn the fixed relationship between the cued home base location and the remaining room cues. The persistent behavior in these locations is reminiscent of the place behavior displayed by rats in the Morris water task and in other test procedures (Morris et al., 1982; Poucet, 1989). Similar observations have been reported using a distal cue instead of a proximal cue (Hines & Whishaw, 2005). Moreover, in studies in which rats established home bases next to two landmarks (e.g., a large black box and a segment of a white wall), animals continue to preferentially dwell in the two locations even in the absence of the cues (Lehmann et al., 2007). Nonetheless, it is important to point out that conditioned home base preferences tend to be short lived such that rats make several short trips back to the previously cued location, but do not perseverate in visiting these locations during long probe tests. Indeed, Travis et al (2010) observed that rats tended to orient and make direct trips back to the cued home base location, but the trips were largely concentrated in the first half of the 30min probe test. The authors concluded that after several visits to the previously cued location, rats likely established home bases in alternative locations.
The fact that rats express a conditioned preference for a previously cued location suggests the possibility that other forms of reinforcement can guide regional preferences for home base establishment. It has previously been speculated that the point of entry into an environment may serve as an organizing spatial feature to solve spatial problems and guide subsequent behavior (Clark et al., 2015; Eilam & Golani, 1988; Golani, 2011; Martin et al., 1997). In a seminal study by Golani et al (1981), it was shown that when rats are first placed in an unfamiliar location, animals subsequently display a sequence of “warm-up” behaviors composed of successive horizontal and vertical movements that escalate in size from the point of placement in the field. The expression of these warm-ups in relation to the point of entry point suggests that the first point of contact may form an organizational feature for subsequent exploration and home base behavior. Nemati and Whishaw (2007) tested this hypothesis by placing rats on a large circular open-field in the presence or absence of proximal cues, or in complete darkness. Regardless of test condition, rats showed a strong preference in establishing their home bases near the initial place of entry. Specifically, rats organized their excursions from and returns to this location, stopped at that location more frequently, and spent a significantly greater amount of time there. Home base behavior was exhibited at the entry point regardless of where the place was located in the open-field (edge vs. center of the arena). In addition, home base preference at the entry point was proportional to the salience of nearby proximal cues, suggesting that local stimuli can be rapidly associated with home base locations. Again, it is possible that home bases at entry may serve to optimize security, while at the same time organize subsequent exploratory movements. However, the behavior may also be organized in relation to path integration—a navigation strategy utilized in unfamiliar and featureless environments that enables accurate orientation in relation to a home location (Gallistel, 1990; Etienne & Jeffery, 2004; Whishaw & Tomie, 1997). Because path integration relies on self-movement cues (e.g., vestibular, motor, proprioceptive) in featureless environments, this form of navigation can be prone to errors and therefore requires frequent updating in relation to a stable reference. Thus, returns to the point of entry might be linked to a general need to correct inaccuracies in the path integration process (Hines & Whishaw, 2005; Nemati & Whishaw, 2007; Redish, 1999).
2.2. Mouse Home Base Behavior
It is well documented that wild mice set-up home sites or nests from which they secure resources and avoid predation (Blanchard et al., 2001). However, reports on the home base behavior of mice in laboratory settings have largely been inconsistent. Notably, several studies have reported that mice, when placed in a featureless open-field, generally differ from rats in that they fail to restrict their stops, grooming, and rearing to specific locations (Fig. 3) (Clark et al., 2006; Drai & Golani, 2001; Gorny et al., 2002). The absence of regionally restricted stopping behavior in featureless environments has been replicated in a number of mouse strains (Dvorkin et al., 2008; Fonio et al., 2006; Lipkind et al., 2004). It is important to note, however, that some studies have described home base-like behavior in featureless environments by mice. The behaviors reported includ tight circling movements restricted typically to a single location in an open-field (Dvorkin et al., 2010). Golani and colleagues have referred to this tight circling behavior as “knots” which are seemingly enhanced after the administration of fear or anxiety inducing stimuli. Thus, knot-behavior might be motivated to achieve security in a similar manner to the classic home base routines observed in the rat (Whishaw et al., 2006). Nonetheless, these knot-behaviors by mice are absent of other classic home base behaviors such as grooming, rearing, and long duration stops.
Figure 3.
Paths (top row), stops (middle row), and locomotor speed (bottom row) from representative mice tested in the presence of no cue, in darkness, with a single proximal cue, and with two proximal cues (black square = proximal cue) for 30 minutes (modified from Clark et al., 2006). Data from a different mouse is shown for each condition. Note that stops made by the mouse tended to cluster adjacent to the proximal cues, but do not display regional specificity in the absence of a proximal cue and in darkness. Locomotion speed is shown by color (green = 1– 20 cm/s, blue = 20–40 cm/s, red = 40+ cm/s). Note that higher locomotion speed (blue and red lines) occur in the dark and no cue conditions as well as in the center of the field in the cued conditions.
In contrast to the absence of clear-cut home base behavior in featureless test enclosures, several studies have reported that mice can establish home bases near physical objects or nesting material (Clark et al., 2006; Drai et al., 2001; Eilam, 2004; Fonio et al., 2009; Gorny et al., 2002). One notable exception is that the routine of movements near these physical bases do not include grooming and rearing, but involve long duration stops near the object. Importantly, mice appear to organize their exploratory excursions in relation to these cued sites (Drai et al., 2001; Gorny et al., 2002). This was demonstrated by Gorny et al (2002) who used bedding to encourage home base formation. The authors noted that mice performed long exploratory excursions from the nest that were indirect and interrupted by stops, especially along the edge of the open-field. The final stop in an excursion was followed by a direct and rapid return back to the nest. This pattern of indirect-outward and direct-inward trajectories has been well characterized in the rat (Wallace et al., 2002; Whishaw et al., 2001), and provides evidence that a cued home base can serve to organize the exploratory movements of mice in a similar manner.
Given that mice are seemingly more reliant on physical objects, Whishaw and colleagues (Clark et al., 2006) aimed to test the range of environmental and contextual cues that might influence mouse exploration. In this work, Clark et al (2006) were particularly interested in the relationship between visual stimuli and mouse home base behavior. This question stemmed from reports that mice have poor visual acuity relative to rats (Prusky et al., 2000), which could ultimately influence their reliance on environmental cues for home base establishment. In a series of experiments, Clark et al (2006) tested mice on a circular open table around which both visual cues and tactile cues were manipulated. As in the rat studies described in the previous section (Clark et al., 2005; Hines & Whishaw, 2005), visual cues were placed near the open-field, but were out of the reach of the mouse. First, mice were tested in conditions of impoverished cues, either in darkness or in normal light. Consistent with previous reports, mice displayed little regional preference in their movements, and no clear home base behaviors were exhibited (Fig. 3). However, when visual cues were placed adjacent to the table, mice spent a disproportionate amount of time, made a greater number of stops, and performed longer duration stops in the segment of the open field that was near the visual cue. When two identical visual cues were presented on opposite sides of the maze, mice distributed their home base behavior equally between the two cued locations.
In a second series of experiments, Clark et al (2006) placed the visual cue in competition with a tactile cue located on the opposite side of the maze. As in the studies with rats, the tactile cue was a white wall occupying a small segment of the open-field. Competition between the two cues was assessed across four days of testing. With both the white wall and visual cue present, home base behavior was initially equivalent in regions adjacent to the cues. However, after four days of repeated exposure to the open field and the cues, home base behavior in relation to the visual cue was significantly reduced. Thus, by the fourth day, mice generally preferred to form their bases next to the tactile cue. This pattern of behavior and preference for the tactile cue over a visual cue is consistent between both rat and mice species (Lehmann et al., 2007). Again, as with the experiments using rats, mice could rest their bodies against the wall but were unable to do so against the black cue. Thus, the preference for the wall over the visual cue could be explained by a simple unifying rule of finding the location that offers the greatest tactile security, and remaining at this location (Clark et al., 2006; Whishaw et al., 2006).
Lastly, as in the home base studies in rats, Clark et al (2006) examined whether experience with cued locations could influence the future home base behavior of mice. This was conducted similarly to previous studies with a cue removal probe test occurring 24-hours after four daily exposures to the cues (Hines & Whishaw, 2005; Lehmann et al., 2007). In contrast to rats, Clark et al found that mice showed relatively weak preferences for the previously cued home base location. A similar finding was observed even when the more salient tactile cue was used as a home base location, suggesting that mice fail to exhibit home bases in the absence of salient landmarks, even when locations are conditioned with repeated testing and experience. In contrast to this report, a recent study indicated that some home base-like behaviors (tight circling or “knot” behavior) are regionally specific across testing days even in the absence of environmental cues (Dvorkin et al., 2010). Nevertheless, our observations are consistent with studies using other forms of mouse place preference tests. For example, some studies have reported difficulties in encouraging mice to form place responses by escaping to a refuge from a Barnes maze, and in learning a hidden platform location in a Morris water task (Koopmans et al., 2003; Pompl et al., 1999; Whishaw, 1995; Whishaw & Tomie, 1996). Thus, although home base tests could possibly be used for the study of spatial memory in mice, it may not offer an obvious advantage over other procedures.
2.3. Excursions from the home base
The work above presents evidence that both rats and mice establish home bases which serve as a point of attraction from which animals appear to obtain their security. While the home base behavior of rats and mice tend to differ in the sense that mice do not set-up clear home bases in the absence of cues, the central features of exploratory behaviors away from these locations are persevered between species (Drai et al., 2001). When placed in a novel environment, animals initially make short exploratory excursions or trips away from the home base (Tchernichovski et al., 1998). These short warm-up excursions are followed by a gradual increase in excursion length, perhaps reflecting an animal’s familiarization with the environment. Typically, excursions away from the home site are longer, less direct, and interrupted by episodes of stopping and rearing, while returns to the home base are direct and occur at a greater speed with animals typically galloping or running (Eilam & Golani, 1989; Wallace et al., 2002; 2006; Whishaw et al., 2001). When stopping, animals perform lateral scanning head movements, and sniffing with their snout along the maze substrate (Drai et al., 2001; Golani et al., 1993; Whishaw et al., 1994). Golani and colleagues (1993) have noted that there is an upper limit to the number of stops made by animals during an excursion, with some estimates suggesting up to 8–10 stops. Nevertheless, the upper limit appears to be idiosyncratic to each animal, but once reached, animals conclude their excursions by making a rapid, and direct trip back to the home base. The pattern of indirect-excursion from, and direct-returns home, occurs similarly in darkness and in lighted conditions, suggesting that the organization of exploratory excursions constitute an unconditioned behavior (Gorny et al., 2002; Wallace et al., 2002; Whishaw et al., 2001).
While exploratory excursions can be characterized as a pattern of forward progressions that carry the animal from one place to another, stopping and scanning may allow animals to gather information regarding the test environment (Golani et al., 1993; Whishaw et al., 1994). Notably, head scanning movements during stops are reminiscent of the behaviors made by rodents at choice points in T-mazes and other similar environments, also known as vicarious trial and error behavior (see Redish, 2016 for review). Certainly, previous work showing that environmental context can influence the stopping behavior of rodents at home bases seems to support this view. Nevertheless, few studies have featured environmental stimuli as an experimental variable in the study of exploratory excursions. Further, some studies have reported poor replicability of mouse exploratory behavior between similar laboratory set-ups (Crabbe et al., 1999; Wahlsten, 2001). Again, this observation could be related to the fact that the environmental context has a modulating influence on exploratory excursions. Motivated by these issues, Clark et al (2006) sought to examine this relationship in mice by measuring the distribution of excursions, stops, and speed of movement in an open-field in varying contexts. First, mice were placed in a large circular open-field, and allowed to explore the environment during tests in which the room was lighted or was in complete darkness. In darkness, general locomotion was elevated, with a greater number of paths taken through the center of the maze (Fig. 3). In lighted conditions, and without the presence of proximal visual or tactile cues, locomotor activity was reduced compared to darkness. In addition, mice tended to shorten their paths and made a greater number of stops along the edges of the open-field, suggesting that simple changes in lighting condition produced significant changes in the excursion patterns of mice.
In other tests, Clark et al (2006) surrounded the open-field with visual and tactile wall cues. When two large visual cues were placed near the edge of the open-field, the distribution of mouse excursions and stops tended to be directed towards the cues (Fig. 3A). Interestingly, in some cases, mice would take repeated direct paths towards cues located on opposite sides of the maze, demonstrating that the presence of salient cues could significantly alter their trip organization. A similar observation was made when one of the visual cues was replaced with a tactile wall segment, however, movements toward the visual cue was reduced. To evaluate this further, Clark et al added walls to the open-field such that the walls now surrounded the open-field, but still included a dark cue along part of the wall. Again, under these conditions, excursions made toward the visual cue was limited in large part because stops and excursions were mostly restricted along the walls.
In addition to changes in the distribution of exploratory excursions and stops, Clark et al (2006) characterized changes in the locomotor speed of mice in response to contextual manipulations. Locomotion between stops were analyzed according to slow, medium, and fast movement speeds, which were conceptually similar to the first, second, and third “gears” of motion described by Drai et al (2000). In darkness or in a featureless lighted environment, animals typically made faster locomotion . When the cues were present, locomotion between stops were generally slower, especially near the cues and along the edge of the table (Fig. 3B). Faster modes of motion tended to occur in the center of the maze particularly along routes taken between the two cues. In an open-field that had surrounding walls, animals generally made faster movements in the presence of walls which surrounded an open-field. Again, faster locomotion were located in the center of the maze, while slower modes of motion occurred along the edge. The latter findings are consistent with the proclivity of rodents to stop near the edge of the maze and perform rapid and direct homeward trajectories across the center of the open field (Drai et al., 2000; Gorny et al., 2002; Wallace et al., 2002).
2.4. Summary, Conclusions, and Future Directions
To summarize, the work above shows that while mice and rats show similar preferences in forming home bases to environmental cues, mice fail to form home bases in the absence of salient environmental cues. Further, mice appear to express weaker home base memory in the absence of the cues. Nonetheless, the exploratory movements from home bases are similarly organized between species, with animals taking indirect excursions away from the home base, but short, direct, and fast returns to the home base.
The mechanism underlying species differences in home base formation in darkened or featureless environments is unclear, but may involve differences in the relative use of internal or external stimulus sources. For instance, tests in impoverished conditions may require path integration processes in which self-movement cues (e.g., proprioceptive, vestibular, and motor cues) are monitored to guide home base behavior and returns to the home location (Gallistel, 1990; Etienne & Jeffery, 2004; Whishaw & Tomie, 1997). Certainly, the fact that rats establish instantaneous home bases in cue deprived environments is supportive of this notion (Hines & Whishaw, 2005; Nemati & Whishaw, 2007). Previous studies have shown that when a physical home base is provided, mice can use path integration for accurate spatial orientation (Alyan, 1996; Gorny et al., 2002; Yoder et al., 2015). However, in the absence of external cues, path integration and self-motion cues might not be sufficient to guide home base establishment in the mouse. Recent evidence showing the loss of spatial specificity by entorhinal grid cells in the mouse while locomoting in a dark, featureless arena, seemingly supports this hypothesis (Chen et al., 2016). Weaker salience of self-movement cue processing could also explain the heightened sensitivity of mice to contextual features, the observed faster modes of motion in darkness and in the absence of a prominent cue, and the reports of poor replicability between lab environments (Crabbe et al., 1999; Wahlsten, 2001). Further work is needed to address this possibility, but to also discriminate this hypothesis from other alternatives including the possibility that mice might be responding to heightened anxiety in featureless environments. Finally, future work should be directed at determining the range of sensory cues (e.g., olfactory, thermal, etc.) that control exploratory behavior. A better understanding of the sensory hierarchy involved in exploration would facilitate future design and tests of locomotor behavior by mice.
3. Neural Subsystems in Rodent Exploratory Behavior
Very little is known regarding the neurobiological mechanisms of exploratory behavior. The organized patterns described in the previous sections however lends itself uniquely to measuring the effects of brain manipulations on exploration. If exploration is a mechanism for the acquisition of environmental information, then studies of spatial memory could benefit from a better understanding of the neural basis behind these movement subsystems. Indeed, the relationship between exploratory behavior and spatial memory was a central theme in O’Keefe & Nadel’s (1978) seminal work. Their summary of the literature led to a cognitive-mapping theory which suggested that as animals explore environments, the hippocampal formation generates spatial representations of that environment. This environmental map is then used to guide subsequent exploration and navigation to specific places in the same environment. Thus, a central hypothesis stemming from the spatial mapping theory is that damage to the hippocampus should abolish the expression of exploratory behaviors (O’Keefe & Nadel, 1978, pg. 242). Several studies have investigated this hypothesis in relation to novelty detection (Clark et al., 2000; Cohen et al., 2013; Mumby et al., 2002; Poucet, 2003; Save et al., 1992; Sutherland, 1985; Thinus-Blanc et al., 1991), but we focus our discussion below on the work measuring changes in home base behavior and exploratory excursion organization in animals with hippocampal lesions.
3.1. Home Bases in Hippocampal Lesioned Animals
A consistent observation in early lesion studies is that hippocampal damage results in heightened locomotion or “hyperactivity” in open-field tests (Jarrard, 1968; Kimble, 1963; O’Keefe & Nadel, 1978). Locomotor hyperactivity may have many sources, for example, the loss of normal home base behavior could lead to a wider distribution of movements in the environment and excessive locomotion over time. Hines and Whishaw (2005) investigated this possibility in a series of experiments where control and hippocampal lesioned rats explored an open-field either in complete darkness or with a prominent cue located near the table. In the first experiment, both control and hippocampal lesioned rats explored the table under lighted conditions and were found to perform long duration stops adjacent to the proximal cue. Behaviors typically associated with home bases were also selectively performed near the cue, suggesting that lesions of the hippocampus do not abolish this feature of exploratory behavior. Indeed, several subsequent studies have replicated this finding (Fig. 4A) (Clark et al., 2005; Lehmann et al., 2007; Travis et al., 2010), and has shown that home base behavior in lesioned animals persist in the cued locations, even when the cue is removed during a probe test.
Figure 4.
(A) Representative path (left) and stops (right) from a hippocampal lesioned rat tested in the presence of a cue and a segment of a white wall (modified from Lehmann et al., 2007). Note that the white wall was continuous with the open-field, but is separated in the illustration for display purposes. (B) Representative path (left) and stops (right) from a hippocampal lesioned rat tested in darkness (modified from Hines & Whishaw, 2005). Note that regional specificity in home base behavior is absent only in darkness.
The results above suggested that the hippocampus is not required for home base behavior when visual cues are available, but whether hippocampal lesioned animals can establish bases in the absence of visual cues was generally unknown. To evaluate this question, Hines and Whishaw (2005) allowed control and hippocampal lesioned animals to explore an open-field under darkened conditions, thereby removing access to the proximal and distal visual features of the environment. The authors observed that while control rats clearly established home bases in darkness, hippocampal rats did not (Fig. 4B). Specifically, hippocampal animals distributed their activity evenly throughout the maze and failed to restrict their stopping, grooming, and other home base behaviors to specific regions of the environment. In the same experiment, Hines and Whishaw tested rats with damage to the olfactory bulb in the darkened open-field, and observed that animals were shown to form normal home base behavior. In other reports, hippocampal animals are capable of establishing home base locations in darkness when a physical shelter is provided (Wallace & Whishaw, 2003). In sum, this work clearly illustrated that hippocampal lesions selectively disrupt home base behavior in darkness, but not when external cues are provided.
3.2. Exploratory Excursions by Hippocampal Lesioned Animals
Although impairments in home base behavior may provide an explanation for locomotor hyperactivity, alterations in other movement characteristics could also play a role. In particular, hyperactivity could be influenced by the distance travelled between stops, the speed of movement, or the duration of stops. With respect to stopping behaviors, fewer or shorter stops could encourage longer periods of travel. Whishaw and colleagues (1994) examined this possibility in rats with fimbria-fornix damage, which disconnects the hippocampus from its subcortical afferents. Whishaw et al confirmed that lesioned animals walked more (were hyperactive) in tests of open-field behavior, but reported that the source of heightened locomotion was related to the duration of their stops. Specifically, lesioned animals performed a greater number pauses during locomotion, but these stops were of a shorter duration compared to control animals, allowing for more overall movement. Nevertheless, locomotor speed of individual progressions did not differ between lesion and control groups. In sum, these results suggested that hippocampal lesioned animals made more stops, made more shorter stops, and therefore locomoted more than control animals. Thus, the impairment was not in their locomotor behavior, but in their stopping behavior. Because home bases are characterized by long duration stops, the proclivity for shorter stops in hippocampal lesioned animals could explain the absence of clear home bases in visually impoverished conditions.
A follow-up study by Clark et al (2005) replicated the observations above, but also determined that the exploratory movements by hippocampal lesioned animals fail to habituate with repeated testing, even after up to 4 days of exposure to same environment. Clark et al reported that on the first day of testing, both control and hippocampal rats formed a home base near a proximal cue, made equivalent numbers of exploratory excursions and stops away from the home base, and made similar numbers of head scans directed to different portions of the environment and to the cue. Although control rats and hippocampal rats were not different on the first test day, by the fourth day of testing, control animals were less active on all measures, except immobility near the cued home base. In contrast, the behavior of hippocampal rats was unchanged. Because the pattern of behavior exhibited by hippocampal rats on the fourth day resembles that of both groups on the first day, it could be argued that they were hyperexploratory rather than hyperactive. Whether the “hyperexploration” by lesioned animals on Day 4 reflects a general deficit in habituation and spatial mapping (O’Keefe & Nadel, 1978), or reflects heightened anxiety-like behavior (Whishaw et al., 2006), was not evaluated in the study. Nevertheless, provided that lesioned animals can retain a cued home base, even with the landmark removed from the environment (Clark et al., 2005; Lehmann et al., 2007; Travis et al., 2010), suggests that hippocampal lesioned animals can indeed acquire spatial information while exploring. Thus, the failure to modify their behavior with experience might not be related to impairments in spatial mapping.
Finally, it is important to note the studies reporting that lesions of the fimbria-fornix or hippocampus produce specific impairments in the homeward component of exploratory excursions (Gorny et al., 2002; Wallace et al., 2002; Wallace & Whishaw, 2003; Whishaw et al, 2001; Winter et al., 2013). As described in previous sections, normal rats typically make direct and rapid return trips to the home base. However, a similar pattern of homebound behavior is not observed in hippocampal lesioned rats. Animals with damage to the hippocampus tend to slowly follow the perimeter of the environment until they reach the base location. This tendency is much more pronounced under darkened conditions, suggesting that the impairment might be specific to the use of self-motion cues and dead reckoning. Additionally, measures of return velocity have indicated that control rats travel significantly faster than lesioned animals (Wallace et al., 2002; Wallace & Whishaw, 2003).
3.3. Neurophysiology and Exploratory Behavior
While much of the previous research has focused on examining the effects of hippocampal damage on exploratory movements, a large body of research has taken the approach of monitoring neural activity in the hippocampus and associated limbic regions while rodents locomote and explore environments (Moser et al., 2008; O’Keefe & Nadel, 1978; Taube, 2007). This work has led to the discovery of hippocampal place cells, which fire when an animal locomotes through specific locations within an environment (see Fig. 5) (O’Keefe & Dostrovsky, 1971). In the parahippocampal cortex, neurons also encode an animal’s environmental location, but discharge in multiple locations forming interlocking equilateral triangles called grid cells (Hafting et al., 2005; Boccara et al., 2010). A third cell type identified throughout the limbic system, called head direction (HD) cells, provide information regarding an animal’s directional orientation in space (Ranck, 1984; Taube et al., 1990). Finally, a fourth type of spatial cell located in parahippocampal cortex is specifically tuned to the borders of the environment, but most frequently a single border (Lever et al., 2009; Savelli et al., 2008; Solstad et al., 2008). Together, spatial signals in the hippocampus and limbic system provide information about position, distance, direction, and environmental boundaries, which are sufficient to construct an accurate representation of the animal’s changing position in an environment.
Figure 5.
Color-coded rate maps for a hippocampal place cell, a parahippocampal grid cell, and a border cell (Red = maximum firing rate; blue = minimum firing rate). The size of the testing arena is shown below each rate map. Polar plots of firing rate (spikes/sec) by direction are shown for an ensemble of head direction cells (P, peak firing rate in spikes/s). Place cell and head direction cells are from Berkowitz and Clark (unpublished), and grid and border cells are from Winter et al (2015a; 2015b).
Despite considerable interest in the relationship between the spatial signals above and navigation, few studies have provided a systematic assessment of their relationship with exploratory movements. Some exceptions comes from a study where HD cells have been recorded in a food hoarding task in which rats exit a home shelter located along the edge of a large open field, and return to this location after obtaining a food pellet in the open field. Interestingly, when the shelter is rotated to a new location along the edge of the open field, the preferred direction of HD cells have been observed to change their orientation by a similar angular distance and direction (Valerio & Taube, unpublished observations). Blair and Sharp (1996) made a similar observation, but in a circular open field without a home shelter. For each recording sessions, Blair and Sharp placed rats at a unique point of entry along the edge of the circular environment and found that HD cells changed their orientation by a corresponding magnitude. Thus, the orientation of HD cells were “set” by the animals initial entry position which, as noted in previous sections, has a significant influence on the location of home base establishment. Finally, Valerio & Taube (2012) have reported that HD cells can undergo changes, or “corrections”, in their orientation while the animal is at the home base, and these changes may influence subsequent navigation in the open field (see Winter et al., this issue for discussion). Thus, these collective observations point to the possibility that the home base can serve as a salient source of control over HD cell activity, but may additionally provide a locus for updating and processing spatial representations.
In addition to a general linkage between home bases and HD signal processing, recent work by Knierim and colleagues (Monaco et al., 2014) has indicated that hippocampal place cell activity is significantly influenced by the lateral head movements typically performed during stopping behavior. Specifically, the authors recorded hippocampal place cells while rats locomoted along a circular track. In this task, as in open field environments, rats punctuated their forward locomotion with stops, during which they would performed lateral head scanning movements. The authors showed that head scanning behavior at stops were correlated with the sudden onset of a place field at that location. In other cases, a place field appeared the very next time the rat visited that location. Thus, the study demonstrated that place cell activity can be potentiated at stop/scan locations in the environment, providing further support for the conclusion that exploration may serve to update and influence the expression of spatial representations (O’Keefe & Nadel, 1978).
3.4. Summary, Conclusions, and Future Directions
In summary, the work above shows that although hippocampal lesions do not completely abolish exploration, as predicted by spatial mapping theory, lesions can produce alterations to specific behavioral subsystems. Hyperlocomotor activity by hippocampal animals could be related to the reported absence of home base behavior in impoverished environments (Hines & Whishaw, 2005), shorter stop duration (Whishaw et al., 1994), and the failure to habituate exploration with repeated testing (Clark et al., 2005). Several hypotheses have been put forward to explain these behavioral changes, but the notion that hippocampal lesions disrupt the capacity to process self-movement cues for path integration has received considerable support (McNaughton et al., 1996; Wallace & Whishaw, 2003; Wallace et al., 2002; Whishaw & Tomie, 1997; Whishaw et al., 1994; 2001; 2006; Winter et al., 2013). The observations that hippocampal lesions impair home base behavior in darkness, and impair the ability to generate direct return paths to home locations in darkness is particularly supportive of this hypothesis (Wallace & Whishaw, 2003). In addition, disruptions of the vestibular system produce similar impairments in exploratory behavior (Avni et al., 2009; Blankenship et al., 2017; Wallace at al., 2002; Yoder et al., 2015; Zheng et al., 2006).
Finally, although the relationship between spatial signals in the hippocampus and limbic system (Fig. 5) and the subsystems of rodent exploratory behavior has received little attention, some notable observations have been made. First, previous work has shown that “corrections” or updates to HD cell orientation occur at the home base (Valerio & Taube, 2012). Secondly, stopping behavior, and the head scanning movements that typically occur at these locations, have a fundamental role in organizing the onset and later expression of place cell activity at those locations (Monaco et al., 2014). Collectively, these findings suggest that exploratory subsystems have a role in updating neural representations of space. Other features of spatial firing activity have yet to be investigated, notably the correlates of parahippocampal grid cell and border cell signals with exploratory movements. That grid/border cell activity and exploratory movements are both sensitive to environmental cue manipulations, such as borders and landmarks (Barry et al., 2007; Krupic et al., 2015; Lever et al., 2009), and responsive changes in self-motion stimuli (Winter et al., 2015b), lends itself well to this possibility.
4. General Conclusion
In the present review, we have summarized a large body of work directed toward understanding the movement subsystems involved in rodent exploratory behavior. We present extensive evidence suggesting that rodents readily establish home bases, defined as regionally restricted bouts of grooming, rearing, tight turning, and long duration stops (Eilam & Golani, 1989; Golani, 2014; Wallace & Whishaw, 2003). The home base therefore acts as a central feature of the organized pattern of exploratory movements made by rodents, and is possibly guided by the necessity to optimize security while securing resources in novel environments (Whishaw & Whishaw, 1996; Whishaw et al., 2006). Home bases are central to the organization of exploratory excursions to the rest of the environment, and are expressed such that animals initially make short trips followed by a gradual increase in excursion length over time (Golani et al., 1983; Tchernichovski et al., 1998; Wallace & Whishaw, 2003). Excursions away from the home site are longer, less direct, and interrupted by episodes of stopping, head scanning, and rearing, while returns to the home base are direct and occur at a greater speed with animals typically galloping or running (Wallace & Whishaw, 2003; Whishaw et al., 2001). Although these behavior subsystems have some variability from animal to animal and between test sessions, they have features of an action pattern that can be readily recognized and are therefore well-suited for the study of brain-behavior relationships (Krakauer et al., 2017).
This organization of exploratory behavior has been quantified and utilized to distinguish between rodent species and strains, and to examine the neurobiological basis of movement organization. Nonetheless, several major gaps in the literature exist. For example, research aimed at discriminating between theories of hippocampal involvement in exploration is needed. In addition, work focusing on the relationship between exploration and circuits beyond the hippocampus is greatly needed. A number of cortical and subcortical limbic circuits have been linked to the processing of spatial information, including circuits involved in the expression of place, grid, and head direction signals (Fig. 5) (Clark & Harvey, 2016; Clark & Taube, 2009; Clark et al., 2012; 2013; Taube, 2007; Wilber et al., 2015; Winter et al., 2015a). The relationship between these signals and exploratory behavior, and whether they play an important role in the organization of exploratory movements, will be of significant importance in developing a complete understanding of the neurobiology of spatial behavior. Our hope is that this review will assist future research examining the behavioral and neural subsystems underlying rodent exploration.
Acknowledgments
This manuscript was written while the authors were supported by grants from NIAAA (P50AA022534 and R21AA024983).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alyan SH. Evidence for resetting the directional component of path integration in the house mouse. Ethology. 1996;102:629–638. [Google Scholar]
- Avni R, Elkan T, Dror AA, Shefer S, Eilam D, Avraham KB, Mintz M. Mice with vestibular deficiency display hyperactivity, disorientation, and signs of anxiety. Behav Brain Res. 2009;202:210–7. doi: 10.1016/j.bbr.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Barnett SA. The Rat: A Study of Behavior. Aldine, Chicago: 1963. [Google Scholar]
- Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci. 2007;10:682–4. doi: 10.1038/nn1905. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Conflict, arousal, and curiosity. New York, NY, US: McGraw-Hill Book Company; 1960. [Google Scholar]
- Blair HT, Sharp PE. Visual and vestibular influences on head-directino cells in the anterior thalamus of the rat. Behavioral Neuroscience. 1996;110:643–60. doi: 10.1037//0735-7044.110.4.643. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Kelly MJ, Blanchard DC. Defensive reactions and exploratory behavior in rats. J Comp Physiol Psychol. 1974;87:1129–1133. [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–18. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Blankenship PA, Cherup LA, Donaldson TN, Brockman SN, Trainer AD, Yoder RM, Wallace DG. Otolith dysfunction alters exploratory movements in mice. Behav Brain Res. 2017 doi: 10.1016/j.bbr.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser MB. Grid cells in pre- and parasubiculum. Nat. Neurosci. 2010;13:987–994. doi: 10.1038/nn.2602. [DOI] [PubMed] [Google Scholar]
- Chance MRA, Mead AP. Competition between feeding and investigation in the rat. Behavior. 1955;8:174–181. [Google Scholar]
- Chen G, Manson D, Cacucci F, Wills TJ. Absence of visual input results in the disruption of grid cell firing in the mouse. Curr Biol. 2016;26:2335–42. doi: 10.1016/j.cub.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Harvey RH. Do the anterior and lateral thalamic nuclei make distinct contributions to spatial representation and memory. Neurobiology of Learning and Memory. 2016;133:69–78. doi: 10.1016/j.nlm.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Taube JS. Deficits in landmark navigation and path integration after lesions of the interpeduncular nucleus. Behavioral Neuroscience. 2009;123:490–503. doi: 10.1037/a0015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Brown JE, Taube JS. Head direction cell activity in the anterodorsal thalamus requires intact supragenual nuclei. Journal of Neurophysiology. 2012;108:2767–2784. doi: 10.1152/jn.00295.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Hong NS, Bettenson DJ, Woolford J, Horwood L, McDonald RJ. Maintained directional navigation across environments in the Morris water task is dependent on vestibular cues. Journal of Experimental Psychology: Animal Learning and Cognition. 2015;41:301–308. doi: 10.1037/xan0000066. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Hines DJ, Hamilton DA, Whishaw IQ. Movements of exploration intact in animals with hippocampal lesions. Behavioural Brain Research. 2005;163:91–9. doi: 10.1016/j.bbr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Rice JP, Akers KG, Candelaria-Cook FT, Taube JS, Hamilton DA. Lesions of the dorsal tegmental nuclei disrupt control of navigation by distal landmarks in cued, directional, and place variants of the Morris water task. Behavioral Neuroscience. 2013;127:566–581. doi: 10.1037/a0033087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Hamilton DA, Whishaw IQ. Motor activity (exploration) and formation of home bases in mice (C57BL/6) influenced by visual and tactile cues: Modification of movement distribution, distance, location, and speed. Physiology & Behavior. 2006;87:805–816. doi: 10.1016/j.physbeh.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW., Jr The rodent hippocampus is essential for nonspatial object memory. Current Biology. 2013;23:1685–90. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Drai D, Benjamini Y, Golani I. Statistical discrimination of natural modes of motion in rat exploratory behavior. Journal of Neuroscience Methods. 2000;96:119–31. doi: 10.1016/s0165-0270(99)00194-6. [DOI] [PubMed] [Google Scholar]
- Drai D, Kafkafi N, Benjamini Y, Elmer G, Golani I. Rats and mice share common ethologically relevant parameters of exploratory behavior. Behav Brain Res. 2001;125:133–140. doi: 10.1016/s0166-4328(01)00290-x. [DOI] [PubMed] [Google Scholar]
- Dvorkin A, Benjamini Y, Golani I. Mouse cognition-related behavior in the open-field: emergence of places of attraction. PLoS Comput Biol. 2008;4(2):e1000027. doi: 10.1371/journal.pcbi.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D. Locomotor activity in common spiny mice (Acomys cahirinuse): the effect of light and environmental complexity. BMC Ecol. 2004:16. doi: 10.1186/1472-6785-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D. Of mice and men: building blocks in cognitive mapping. Neurosci Biobehav Rev. 2014;47:393–409. doi: 10.1016/j.neubiorev.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Eilam D, Golani I. The ontogeny of exploratory behavior in the house rat (Rattus rattus): the mobility gradient. Dev Psychobiol. 1988;21:679–710. doi: 10.1002/dev.420210707. [DOI] [PubMed] [Google Scholar]
- Eilam D, Golani I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behavioural Brain Research. 1989;34:199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–92. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- File SE. What can be learned from the effects of benzodiazepines on exploratory behavior? Neurosci Biobehav. 1985;9:45–54. doi: 10.1016/0149-7634(85)90031-4. [DOI] [PubMed] [Google Scholar]
- Fonio E, Benjamini Y, Sakov A, Golani I. Wild mouse open field behavior is embedded within the multidimensional data space spanned by laboratory inbred strains. Genes Brain Behav. 2006;5:380–8. doi: 10.1111/j.1601-183X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Whishaw PA, Whishaw IQ. The topography of three-dimensional exploration: a new quantification of vertical and horizontal exploration, postural support, and exploratory bouts in the cylinder test. Behav Brain Res. 2004;151:125–135. doi: 10.1016/j.bbr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Gorny JH, Gorny B, Wallace DG, Whishaw IQ. Fimbria-fornix lesions disrupt the dead reckoning (homing) component of exploratory behavior in mice. Learn Mem. 2002;9:387–394. doi: 10.1101/lm.53002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. The MIT Press; Cambridge, Massachusetts: 1990. [Google Scholar]
- Golani I. The developmental dynamics of behavioral growth processes in rodent egocentric and allocentric space. Behav Brain Res. 2012;231:309–16. doi: 10.1016/j.bbr.2012.01.039. [DOI] [PubMed] [Google Scholar]
- Golani I, Bronchti G, Moualem D, Teitelbaum P. “Warm-up” along dimensions of movement in the ontogeny of exploration in rats and other infant mammals. Proc Natl Acad Sci U S A. 1981;78:7226–9. doi: 10.1073/pnas.78.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golani I, Benjamini Y, Eilam D. Stopping behavior: constraints on exploration in rats (Rattus norvegicus) Behavioural Brain Research. 1993;53:21–33. doi: 10.1016/s0166-4328(05)80263-3. [DOI] [PubMed] [Google Scholar]
- Gray JA. The Neuropsychology of anxiety. Oxford, UK: Clarendon Press; 1982. [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hall CS. Emotional behavior in the rat I Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18:385–403. [Google Scholar]
- Hines DJ, Whishaw IQ. Home bases formed to visual cues but not to self-movement (dead reckoning) cues in exploring hippocampectomized rats. Eur J Neurosci. 2005;22:2363–2375. doi: 10.1111/j.1460-9568.2005.04412.x. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Behavior of hippocampal lesioned rats in home cage and novel situations. Physiology of Behavior. 1968;3:65–79. [Google Scholar]
- Kimble DP. The effects of bilateral hippocampal lesions in rats. Journal of Comparative Physiological Psychology. 1963;56:273–83. doi: 10.1037/h0048903. [DOI] [PubMed] [Google Scholar]
- Koopmans G, Blokland A, van Nieuwenhuijzen P, Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol Behav. 2003;79:683–693. doi: 10.1016/s0031-9384(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghazanfer AA, Gomez-Marin A, MacIver MA, Poeppel D. Neuroscience needs behavior: correcting a reductionist bias. Neuron. 2017;93:480–490. doi: 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- Krupic J, Bauza M, Burton S, Barry C, O’Keefe J. Grid cell symmetry is shaped by environmental geometry. Nature. 2015;518:232–5. doi: 10.1038/nature14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Clark BJ, Whishaw IQ. Similar development of cued and learned home bases in control and hippocampal-damaged rats in an Open Field exploratory task. Hippocampus. 2007;17:370–380. doi: 10.1002/hipo.20274. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, O’Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17:111–33. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind D, Sakov A, Kafkafi N, Elmer GI, Benjamini Y, Golani I. New replicable anxiety-related measures of wall vs center behavior of mice in the open field. J Appl Physiol. 2004;97:347–359. doi: 10.1152/japplphysiol.00148.2004. [DOI] [PubMed] [Google Scholar]
- Loewen I, Wallace DG, Whishaw IQ. The development of spatial capacity in piloting and dead reckoning by infant rats: use of the huddle as a home base for spatial navigation. Dev Psychobiol. 2005;46:350–361. doi: 10.1002/dev.20063. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Whishaw IQ. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res. 1999;99:143–52. doi: 10.1016/s0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- Martin GM, Harley CW, Smith AR, Hoyles ES, Hynes CA. Spatial disorientation blocks reliable goal location on a plus-maze but does not prevent goal location in the Morris maze. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:183–193. doi: 10.1037//0097-7403.23.2.183. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J. Exp. Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- Menzel EW. Chimpanzee spatial memory organization. Science. 1973;182:943–5. doi: 10.1126/science.182.4115.943. [DOI] [PubMed] [Google Scholar]
- Monaco JD, Rao G, Roth ED, Knierim JJ. Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat Neurosci. 2014;17:725–31. doi: 10.1038/nn.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:132–136. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Neural subsystems of exploration in rats. In: Archer J, Birke LIA, editors. Exploration in animals and humans. Van Nostrand Reinhold (UK); Berkshire, England: 1983. [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Ann. Rev. Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Nadel L. The hippocampus and space revisited. Hippocampus. 1991;1:221–9. doi: 10.1002/hipo.450010302. [DOI] [PubMed] [Google Scholar]
- Nemati F, Whishaw IQ. The point of entry contributes to the organization of exploratory behaviour of rats on an open field: An example of spontaneous episodic memory. Behav Brain Res. 2007;182:119–128. doi: 10.1016/j.bbr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Pavlov IP. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford, England: Oxford Univ. Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucet B. Object exploration, habituation, and response to a spatial change in rats following septal or medial frontal cortical damage. Behavioural Neuroscience. 1989;103:1009–16. doi: 10.1037//0735-7044.103.5.1009. [DOI] [PubMed] [Google Scholar]
- Pompl PN, Mullan MJ, Bjugstad K, Arendash GW. Adaptation of the circular platform spatial memory task for mice: use in detecting cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer’s disease. J Neurosci Methods. 1999;87:87–95. doi: 10.1016/s0165-0270(98)00169-1. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity. Vision Res. 2000;40:2201–2209. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Head direction cells in the deep layer of dorsal presubiculum in freely moving rats. Soc. Neurosci. Abstr. 1984;10:599. [Google Scholar]
- Redish AD. Beyond the Cognitive Map: From Place Cells to Episodic Memory. MIT Press; 1999. [Google Scholar]
- Redish AD. Vicarious trial and error. Nat Rev Neurosci. 2016;17:147–59. doi: 10.1038/nrn.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner MJ. Neglected aspects of exploratory and investigatory behavior. Psychobiology. 1990;18:16–22. [Google Scholar]
- Russell PA. Relationships between exploratory behavior and fear: a review. Br J Psychol. 1973;64:417–433. doi: 10.1111/j.2044-8295.1973.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Sanchez LM, Thompson SM, Clark BJ. Influence of proximal, distal, and vestibular frames of reference in object-place paired associate learning in the rat. PloS ONE. 2016;11(9):e0163102. doi: 10.1371/journal.pone.0163102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli F, Yoganarasimha D, Knierim JJ. Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus. 2008;18:1270–1282. doi: 10.1002/hipo.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions; the orienting reflex. Annu Rev Physiol. 1963;25:545–80. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neuroscience Letters. 1982;31:271–6. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Ann. Rev. Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Golani I. A phase plane representation of rat exploratory behavior. Journal of Neuroscience Methods. 1995;62:21–7. doi: 10.1016/0165-0270(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Benjamini Y, Golani I. The dynamics of long-term exploration in the rat. Part I. The dynamics of long-term exploration in the rat. Biological Cybernetics. 1998;78:423–432. doi: 10.1007/s004220050446. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Schallert T, DeRyck M, Whishaw IQ, Golani I. Motor subsystems in motivated behavior. In: Thompson RF, Hicks LH, Shvyrokov VB, editors. Neural mechanisms of goal-directed behavior and learning. New York: Academic Press; 1980. pp. 127–143. [Google Scholar]
- Travis SG, Sparks FT, Arnold T, Lehmann H, Sutherland RJ, Whishaw IQ. Hippocampal damage produces retrograde but not anterograde amnesia for a cued location in a spontaneous exploratory task in rats. Hippocampus. 2010;20:1095–104. doi: 10.1002/hipo.20710. [DOI] [PubMed] [Google Scholar]
- Valerio S, Taube JS. Limbic system structures differentially contribute to exploratory trip organization of the rat. Nat Neurosci. 2012;15:1445–53. [Google Scholar]
- Wahlsten D. Standardizing tests of mouse behavior: reasons, recommendations, and reality. Physiol Behav. 2001;73:695–704. doi: 10.1016/s0031-9384(01)00527-3. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Hines DJ, Pellis SM, Whishaw IQ. Vestibular information is required for dead reckoning in the rat. J Neurosci. 2002;22:10009–17. doi: 10.1523/JNEUROSCI.22-22-10009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DG, Hines DJ, Whishaw IQ. Quantification of a single exploratory trip reveals hippocampal formation mediated dead reckoning. Journal of Neuroscience Methods. 2002;113:131–45. doi: 10.1016/s0165-0270(01)00489-7. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Whishaw IQ. NMDA lesions of Ammon’s horn and the dentate gyrus disrupt the direct and temporally paced homing displayed by rats exploring a novel environment: evidence for a role of the hippocampus in dead reckoning. European Journal of Neuroscience. 2003;18:513–23. doi: 10.1046/j.1460-9568.2003.02772.x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Light avoidance in normal rats and rats with primary visual system lesions. Physiological Psychology. 1974;2:143–147. [Google Scholar]
- Whishaw IQ. A comparison of rats and mice in a swimming pool place task and matching to place task: some surprising differences. Physiol Behav. 1995;58:687–693. doi: 10.1016/0031-9384(95)00110-5. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Of mice and mazes: similarities between mice and rats on dry land but not water mazes. Physiol Behav. 1996;60:1191–1197. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J. Piloting and dead reckoning dissociated by fimbria-fornix lesions in a rat food carrying task. Behav Brain Res. 1997;89:87–97. doi: 10.1016/s0166-4328(97)00068-5. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Whishaw GE. Conspecific aggression influences food carrying: Studies on a wild population of Rattus norvegicus. Aggressive Behav. 1996;22:47–66. [Google Scholar]
- Whishaw IQ, Cassal JC, Majchrzak M. “Short-stops” in rats with fimbria-fornix lesions: evidence for change in the mobility gradient. Hippocampus. 1994;4:577–582. doi: 10.1002/hipo.450040507. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behavioural Brain Research. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gharbawie OA, Clark BJ, Lehmann H. The exploratory behaviour of rats in an open environment optimizes security. Behav Brain Res. 2006;171:230–239. doi: 10.1016/j.bbr.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Demecha AJ, Mesina L, Vos JM, McNaughton BL. Cortical connectivity maps reveal anatomically distinct areas in the parietal cortex of the rat. Front Neural Circuits. 2015;8:146. doi: 10.3389/fncir.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SS, Koppen JR, Ebert TB, Wallace DG. Limbic system structures differentially contribute to exploratory trip organization of the rat. Hippocampus. 2013;23:139–52. doi: 10.1002/hipo.22075. [DOI] [PubMed] [Google Scholar]
- Winter SS, Clark BJ, Taube JS. Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science. 2015a;347:870–874. doi: 10.1126/science.1259591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SS, Mehlman MH, Clark BJ, Taube JS. Passive transport disrupts grid signals in the parahippocampal cortex. Curr Biol. 2015b;25:2493–502. doi: 10.1016/j.cub.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaski O, Eilam D. The impact of landmark properties in shaping exploration and navigation. Anim Cogn. 2007;10:415–28. doi: 10.1007/s10071-007-0073-8. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Goebel EA, Köppen JR, Blankenship PA, Blackwell AA, Wallace DG. Otolithic information is required for homing in the mouse. Hippocampus. 2015;25:890–9. doi: 10.1002/hipo.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Darlington CL, Smith PF. Impairment and recovery on a food foraging task following unilateral vestibular deafferentation in rats. Hippocampus. 2006;16:368–78. doi: 10.1002/hipo.20149. [DOI] [PubMed] [Google Scholar]