Abstract
Synapses are specialized cell-cell junctions that underlie the function of neural circuits by mediating communication between neurons. Both the formation and function of synapses require tight coordination between pre- and post-synaptic neurons. Trans-synaptic organizing molecules are important mediators of such signaling. Here we discuss how the EphB and ephrin-B families of trans-synaptic organizing proteins direct synapse formation during early development and regulate synaptic function and plasticity at mature synapses. Finally, we highlight recent evidence linking the synaptic organizing role of EphBs and ephrin-Bs to diseases of maladaptive synaptic function and plasticity.
Keywords: Ephs, ephrins, synaptogenesis, synaptic plasticity, maladaptive plasticity, neuropathic pain
1. Introduction
The trillions of synaptic connections in the brain are organized into precisely patterned neural circuits. The development of mature neural circuits involves the selection of the correct synaptic partners, a phase of rapid synaptogenesis, and then the pruning of synaptic connections (Biederer and Stagi, 2008; Dalva et al., 2007; Katz and Shatz, 1996). After this developmental period the adult brain remains capable of remarkable plasticity, particularly through activity-dependent modification of synaptic strength (Huganir and Nicoll, 2013). The importance of proper synapse formation and function are underscored by the prevalence of developmental disorders that involve dysregulation of synapse development, such as autism spectrum disorders, and by diseases of the mature nervous system such as Alzheimer’s disease and neuropathic pain that impact synaptic function (Betancur et al., 2009; Woolf and Salter, 2000).
Excitatory glutamatergic synapses are found primarily on actin-rich dendritic protrusions called dendritic spines. Spine synapses contain a post-synaptic density (PSD), the proteindense core of the synapse that contains many proteins including glutamate receptors and synaptic scaffolding proteins (Hering and Sheng, 2001; Sheng and Kim, 2011). Synaptogenesis appears to require thin, motile protuberances from the dendritic shaft called filopodia, which appear early in development, possibly allowing the neuron to sample the neuropil for appropriate axonal contacts (Hering and Sheng, 2001). Once a synapse is formed, activitydependent plasticity can modulate the structure and strength of mature synapses, which requires re-organization of the actin cytoskeleton and trafficking of glutamate receptors (Hlushchenko et al., 2016; Malinow and Malenka, 2002). Thus, key regulatory events in synapse development and plasticity may be the dynamic control of the actin cytoskeleton and recruitment of appropriate pre- and post-synaptic molecules such as glutamate receptors.
The development and maintenance of synaptic connections involves a host of different factors. Of particular importance to the formation and function of synapses are trans-synaptic synaptic organizing proteins (Biederer and Stagi, 2008; Dalva et al., 2007). Our review will focus on one key class of these molecules: the EphB family receptor tyrosine kinases and their ephrin-B ligands (Hruska and Dalva, 2012; Sheffler-Collins and Dalva, 2012). The EphB and ephrin-B families of proteins and their ability to signal to regulate the actin cytoskeleton, scaffolding molecules and glutamate receptors at synaptic sites have emerged as important mediators of synapse development and function (Hruska and Dalva, 2012; Sheffler-Collins and Dalva, 2012). There are excellent reviews on EphB-ephrin-B signaling in neuron-astrocyte communication so we will not cover this area (Goldshmit et al., 2006; Koeppen et al., 2018; Murai and Pasquale, 2011; Nikolakopoulou et al., 2016). In this review we highlight the roles of neuronal EphBs and ephrin-Bs at synapses and how dysregulated neuronal EphB-ephrin-B signaling is involved in human disease.
2. Structure and function of Ephs and ephrins
Eph (erythropoietin-producing hepatocellular) receptor tyrosine kinases and their ephrin ligands are membrane-associated proteins that typically function at sites where cells make contact with one another. Ephs and ephrins regulate a variety of cell-cell interactions during development including rhombomere formation, axon guidance, and hematopoiesis (Cayuso et al., 2015; Kania and Klein, 2016). In mammals there are 13 Ephs and 8 ephrins. The families of Ephs and ephrins are divided into A and B subclasses based on their structure and binding affinity. Ephrin-As (ephrin-A1–5 in mammals) are attached to the membrane by a glycosylphosphatidylinositol (GPI) moiety and interact primarily with EphAs (EphA1–8 in mammals). Unlike ephrin-As, ephrin-Bs (ephrin-B1–3) are single-pass transmembrane proteins which interact primarily with EphBs (EphB1–4 and 6 in mammals). In most cases, EphBs interact with ephrin-Bs and EphAs interact with ephrin-As. However, there is some promiscuity between A and B subclasses, as EphA4 can bind to ephrin-B2 and ephrin-B3, and EphB2 can bind to ephrin-A5 (Fig. 1A, B) (Hruska and Dalva, 2012; Sheffler-Collins and Dalva, 2012; Sloniowski and Ethell, 2012).
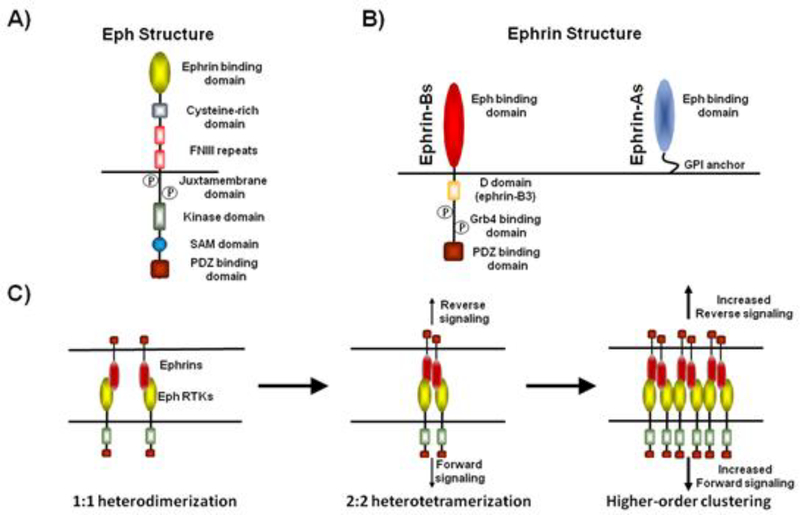
Figure 1.
Eph and ephrin domain structure. (A) EphA and EphB receptors are single-pass transmembrane proteins that contain several functional domains. The extracellular portion of Ephs consists of an ephrin-binding domain, cysteine-rich domain, and two fibronectin type III repeats. The intracellular side consists of a kinase domain which becomes autophosphorylated upon receptor clustering, a SAM domain, and a PDZ-binding domain. (B) Ephrin-Bs are single-pass transmembrane proteins that contain an Eph binding domain extracellularly, while the intracellular portion consists of a Grb4 binding domain and PDZ binding domain. Ephrin-B3 also contains a D domain which binds to Erk1/2. Ephrin-As contain an extracellular Eph-binding domain and are attached to the membrane by a glycosylphosphatidylinositol anchor. (C) Eph-ephrin activation proceeds by initial 1:1 Eph-ephrinheterodimerization followed by the formation of 2:2 Eph-ephrin heterotetramers. Heterotetramers canfurther oligomerize, likely increasing the signaling capacity of the complex.
Canonical binding of Eph and ephrin occurs in trans upon cell-cell contact and for the EphBs and ephrin-Bs results in bidirectional signaling mediated by both the Eph and ephrin (Fig. 1C). Upon cell-cell contact, Eph-ephrin interaction in trans results in “forward” signaling in the Eph-expressing cell and “reverse” signaling in the ephrin-expressing cell. Eph-ephrin binding induces the formation of high affinity Eph-ephrin heterodimers, which can then tetramerize to form 2:2 heterotetramers (Himanen and Nikolov, 2002; Himanen et al., 2001). These heterotetramers are thought to represent the minimal unit of signaling, but can further oligomerize, possibly increasing the signaling capacity of the complex (Himanen and Nikolov, 2003; Stein et al., 1998). Heterotetramerization of the Eph-ephrin complex brings the intracellular kinase domains of multiple Eph molecules together, permitting trans autophosphorylation that activates Eph kinase activity (Himanen and Nikolov, 2003; Himanen et al., 2010; Wybenga-Groot et al., 2001). Activation of Eph or ephrin signaling can be achieved experimentally by application of soluble Eph or ephrin ectodomains, but activation requires preclustering, suggesting that in a cellular environment Ephs or ephrins are pre-clustered prior to Eph binding. However, how pre-clustering might occur in cells remains unknown (Davis et al., 1994; Himanen and Nikolov, 2003). Eph-ephrin interactions can also occur in cis if both are expressed in the same cell. This mode of interaction is less understood but appears to attenuate rather than activate signaling (Carvalho et al., 2006; Egea and Klein, 2007).
Ephs contain multiple functional domains that mediate signaling. The Eph extracellular domain contains a globular N-terminal ephrin-binding domain, a cysteine-rich domain (CRD), and two fibronectin type III (FNIII) repeats (Fig. 1A). The CRD and FNIII regions are thought to participate in cis Eph-Eph interactions that mediate dimerization and higher-order clustering upon ephrin binding (Nikolov et al., 2013). The extracellular domain of EphBs 1–3 also mediates a direct interaction with the N-methyl-D-aspartate receptor (NMDAR) via a specific tyrosine residue (Y504) that is important for targeting and retention of NMDARs at synapses (Dalva et al., 2000; Hanamura et al., 2017; Nolt et al., 2011). The intracellular domain of Ephs contains a juxtamembrane domain, kinase domain, a sterile-α motif (SAM) domain, and a PDZ-binding domain (Fig. 1A) (Sloniowski and Ethell, 2012). The juxtamembrane domain contains two residues that are phosphorylated by Eph kinase activity and mediate interactions with SH2 domain containing proteins such as Src, Abl, Grb10 and Grb2 (Noren et al., 2006; Stein et al., 1996; Zisch et al., 1998). The SAM domain is thought to be important for Eph-Eph interactions. The PDZ-binding domain allows Ephs to interact with a host of PDZ-domain containing proteins including GRIP and PICK1 (Hoogenraad et al., 2005; Torres et al., 1998).Ephrin-Bs signal through a number of intracellular signaling pathways (Fig 1B). Ephrins bind Ephs via an N-terminal globular extracellular domain and contain a C-terminal PDZ-binding domain that mediates interactions with PDZ domain containing proteins such as GRIP1, GRIP2, and Syntenin (Bruckner et al., 1999; Lin et al., 1999; McClelland et al., 2009; Xu et al., 2011). Though they lack kinase activity, ephrin-Bs contain tyrosine residues that are phosphorylated by Src family kinases upon clustering and mediate interactions with SH2 domain containing proteins such as Grb4 (Cowan and Henkemeyer, 2001; Xu and Henkemeyer, 2009). Ephrin-B3 contains a D domain that is not present in ephrin-B1 or ephrin-B2 which mediates an interaction with the mitogen-activated protein kinase (MAPK) Erk1/2 (Fig. 1B) (Hruska et al., 2015).
When EphBs bind to ephrin-Bs both forward (Eph) and reverse (ephrin) signaling cascades are activated. This bidirectional signaling capacity of Ephs and ephrins makes them well-suited to coordinate signaling between pre- and post-synaptic neurons (Fig. 2A) (Hruska and Dalva, 2012; Klein, 2009; Sloniowski and Ethell, 2012). For example, reverse signaling by post-synaptic ephrin-B3 regulates synapse density in cortical neurons and can initiate presynaptic differentiation when expressed in a heterologous cell synapse formation assay. The synaptogenic activity of ephrin-B3 in this assay requires EphB2 in axons (McClelland et al., 2010). Forward signaling by post-synaptic EphBs promotes synaptogenesis and spinogenesis, while reverse signaling by their pre-synaptic binding partners, ephrin-B1 and ephrin-B2 are required for pre-synaptic development in cortical neurons (Kayser et al., 2008; McClelland et al., 2009). The reduction in synapse number following EphB2 knockdown can be rescued by combined expression of kinase-inactive EphB2 and a constitutively active form of the downstream effector PAK, but not if the ephrin-binding domain of EphB2 is deleted (Kayser et al., 2008). This finding underscores the importance of coordinated forward and reverse signaling between pre- and post-synaptic neurons.
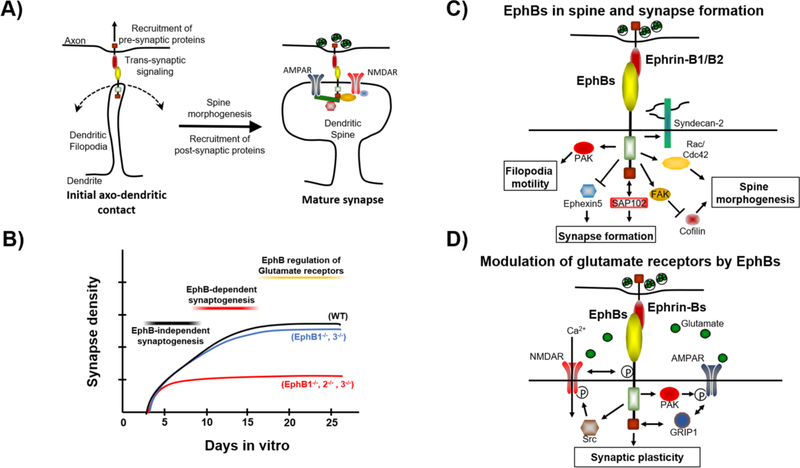
Figure 2.
Post-synaptic EphBs regulate synapse formation and glutamate receptor signaling. (A) Post-synaptic EphBs are required for dendritic filopodia motility, which is necessary for synapse formation. Synapse formation also requires the ability of EphBs to interact with pre-synaptic ephrinBs. (B) EphBs are required for synaptogenesis specifically from approximately DIV7–14 in cortical neurons, after which they remain at synapses and regulate glutamate receptor localization and function. In synaptogenesis EphBs appear to act in a compensatory fashion, with presence of any EphB protein able to rescue the loss of other proteins. This is in contrast to NMDAR localization were loss of EphB2 alone is sufficent to result in decreased synaptic NMDARs both in cortex and hippocampus (2D). (C) EphBs signal through multiple pathways to regulate synapse development. EphB kinase activity results in downstream activation of PAK, which is required for filopodia motility. EphBs also promote synapse formation by promoting degradation of the Rho GEF Ephexin5, and by interacting with the MAGUK scaffolding protein SAP102. EphBs promote spine morphogenesis by interacting with the proteoglycan syndecan-2, by activation of Rac1 GEFs Kalirin and Tiam and the Cdc42 GEF intersectin, and by activation FAK which in turn inhibits the actin severing protein cofilin. Post-synaptic EphBs interact with pre-synaptic ephrin-B1 and ephrin-B2 which mediate pre-synaptic differentiation through interaction with Syntenin-1. (D) At mature synapses, post-synaptic EphBs regulate glutamate receptor signaling. EphBs interact directly with NMDARs and induce Src-mediated phosphorylation of the NMDAR subunit GluN2B at Y1472 which enhances calcium flux through the channel. EphBs also interact indirectly with AMPARs, likely via GRIP1 and induce PAK-mediated phosphorylation of the GluA1 subunit of AMPARs which stabilizes them at the cell surface. EphBs promote NMDAR-dependent LTP, while pre-synaptic ephrin-Bs are required for mossy fiber LTP, which is NMDAR-independent and expressed pre-synaptically.
2.1. Postnatal expression of EphBs and ephrin-Bs in the forebrain
Ephs and ephrins have diverse roles in the nervous system beginning in embryonic development (Palmer and Klein, 2003). Here we focus on postnatal expression in the developing and mature forebrain. The expression patterns of EphBs and ephrin-Bs have been extensively characterized in the hippocampus. EphB2 is highly expressed throughout all regions of the hippocampus, while EphB1 is expressed specifically in the CA3 and dentate gyrus regions and EphB3 is expressed in CA1 (Grunwald et al., 2004; Henderson et al., 2001). Unlike EphBs 1–3, EphB4 is not normally expressed in nervous tissue (Shin et al., 2001) but may be up-regulated in the CNS of EphB1−/−, 2−/−, 3−/− (TKO) mice (M.B.D. and W.Z., unpublished observations). All three ephrin-Bs are also expressed in hippocampus. Ephrin-B1 is expressed at relatively low levels in the dentate gyrus (Grunwald et al., 2001). In contrast, ephrin-B3 is highly expressed in the dentate gyrus and in the CA1 region of the hippocampus, while ephrinB2 is enriched in the CA1 region.
The expression of EphBs and ephrin-Bs in cortex has not been as thoroughly characterized. EphB2 is expressed in super- and sub-granular layers of cortex where it localizes to both pre- and post-synaptic compartments (Bouvier et al., 2008; Kayser et al., 2011), while EphB3 was found to be enriched in layer V pyramidal neurons of somatosensory cortex (Willson et al., 2006). Ephrin-B1 was found localized diffusely throughout the neuropil in cortex and is localized to axons, while ephrin-B2 has been shown to be localized to layers II/III of cortex in neurons and astrocytes (McClelland et al., 2009; Migani et al., 2009). Ephrin-B3 has been shown to function post-synaptically in cortical neurons, where it is localized to dendrites of neurons in all pyramidal cell layers (Hruska et al., 2015; McClelland et al., 2010).
Knockouts of these molecules indicate that while the synaptic functions of the EphBs 1–3 may be redundant, the ephrin-B have non-redundant functions (Hruska and Dalva, 2012; Sheffler-Collins and Dalva, 2012; Sloniowski and Ethell, 2012). Both ephrin-B1 knockout mice and ephrin-B2 knockout mice die during embryonic development (Davy et al., 2004; Wang et al., 1998), while ephrin-B3 knockout mice survive but display distinct synaptic deficits and motor coordination defects (Hruska et al., 2015; Kullander et al., 2001; McClelland et al., 2010; Yokoyama et al., 2001), suggesting that ephrin-Bs are not functionally redundant (Wang et al., 1998). Many of the specific functions of EphBs and ephrin-Bs can be understood from the subcellular localization of these proteins. In the following sections of this review we go into further detail with regards to the specific localization and function of EphBs focusing on role of EphB2 and on each of the three ephrin-Bs in synaptic development and function.
3. Regulation of synapse development and function by EphBs
3.1. EphBs in synapse development
A number of lines of evidence indicate that EphBs are essential for the formation of many excitatory synapses. EphB1−/−, 2−/−, 3−/− triple knockout mice (TKO) have severe deficits in spine and synapse number and fail to thrive. Evidence both in vitro and in vivo indicates a loss of ~25% of excitatory synapses in hippocampus and ~40% in cortex (Henkemeyer et al., 2003; Kayser et al., 2006). Single knockouts of EphB1, EphB2, or EphB3 do not display a defect in spine or synapse number, indicating overlapping functions. Importantly however, defects in synapse formation can be rescued by post-synaptic expression of EphB2 suggesting that EphB2 functions cell-autonomously to regulate spine synapse formation in cortical and hippocampal neurons.
Several lines of evidence suggest that EphBs function to regulate synapse formation during a certain developmental time window (Fig. 2B). Overexpression of EphB2 can rescue synapse density in cortical neurons or brain slices from EphB TKO mice prior to ten days in vitro (DIV10) but fails to so when expressed later. Likewise, knockdown of EphB2 reduces synapse density prior to, but not after DIV14 (Kayser et al., 2008). Consistent with an early function of EphBs in synaptogenesis, EphB2-mediated synapse development requires EphB2 to interact selectively with the membrane-associated guanylate kinase (MAGUK) SAP102, the expression of which is developmentally regulated (Fig. 2C) (Murata and Constantine-Paton, 2013; Sans et al., 2000). SAP102 expression begins early in development, coinciding with EphB-dependent synaptogenesis between DIV7–14. In contrast, the expression of the related MAGUKs PSD-95 and PSD-93 begin later than SAP102 and increase more over time (Kayser et al., 2008; Murata and Constantine-Paton, 2013; Sans et al., 2000). These data suggest that EphBs regulate the formation of synaptic connections during specific periods of development. EphBs appear to regulate synapse formation that is mediated by motile dendritic filopodia. Knockdown or knockout of EphBs reduces filopodia motility and synapse formation, and these effects can be rescued only by approaches that induce both increased filopodia movement and EphBephrin-B binding (Kayser et al., 2008). Moreover, dendritic EphB2 and axonal ephrin-B1 colocalize at nascent synaptic release sites at the tips of filopodia (Mao et al., 2018). These data suggeste that EphBs might function to regulate the ability of filopodia to select the correct synaptic partners or reject incorrect synaptic partners. How might EphB signaling regulate this selection process? A study using a newly developed ratiometric EphB kinase indicator, called GPhosEphB, shed light on this question. GPhos indicators are genetically encoded tyrosine kinase indicators comprised of a circularly permutated EGFP core flanked by two kinasespecific phosphorylation consensus sequences at the N terminus and an SH2 domain on the C terminus that binds phosphorylated tyrosine residues. Thus, phosphorylation at the kinasespecific consensus sequence allows the N and C terminals to bind, increasing the fluorescence of the indicator (Mao et al., 2018). Using this tool in combination with live-cell imaging of motile dendritic filopodia in cultured cortical neurons revealed that the kinetics of EphB signaling in the tips of dendritic filopodia likely underlie the decision to accept or reject a potential synaptic contact. No difference in the magnitude of EphB kinase activity was observed between filopodia that contact an axon and retract and filopodia that contact an axon and remain stable. Instead, the kinetics of kinase activation was sufficient to explain filopodia behavior. Filopodia that immediately retracted upon contacting an axon exhibited a rapid increase in kinase activation, while filopodia that became stabilized exhibited a more gradual increase in EphB kinase activity, and that activity remained elevated as long as the filipodia remained in contact with the axon. Importantly, sites of contact between filopodia and axons with synaptic marker proteins also showed higher levels of EphB kinase activity. Thus, the rate of EphB activation in filopodia appears to be related to whether or not the filopodia accepts or rejects a potential synaptic contact. It will be interesting to determine what accounts for these differences in EphB signaling kinetics (Mao et al., 2018).
Molecular replacement studies in EphB knockouts and after EphB2 knockdown indicate that EphB2 kinase activity is required for normal synapse development (Henkemeyer et al., 2003; Kayser et al., 2008). However, using a genetically modified mouse model where the three neuronally expressed EphBs (EphB1–3) are mutated so as to be sensitive to inhibition with a selective drug, inhibition of EphB kinase activity did not alter synapse density (Soskis et al., 2012). These findings are consistent with a model where EphB-dependent synapse formation is principally mediated by the EphB-ephrin-B interactions and suggest a model where EphB signaling may act to select appropriate potential synaptic contacts or rejected incorrect connections (Mao et al., 2018). One prediction of this model is that EphB kinase blockade should result in defective target selection, generating defects in the specificity of synaptic connections, but not necessarily fewer synaptic contacts. It will be important to test whether this is the case. Regardless, these studies support the necessity of EphBs in the early steps of synaptogenesis.
By what downstream signaling mechanisms do EphB kinases regulate synapse formation? A key element of EphB-dependent regulation of synaptic development is the regulation of the actin cytoskeleton (Fig. 2C). EphBs can modulate the activity of Rho, Rac, and Cdc42 GTPases by regulating guanine nucleotide exchange factors (GEFs), which activate GTPases by binding to them and facilitating the exchange of GDP for GTP (Spiering and Hodgson, 2011). EphBs appear to be positioned as a central regulator of the actin cytoskeleton, acting to both release the brakes on synapse formation and potentiate mechanisms that promote the formation of new contacts. EphBs phosphorylate and activate the Rac1 GEFs Kalirin and Tiam1, and the Cdc42 GEF intersectin which promote spine and synapse maturation (Fig. 2C) (Irie and Yamaguchi, 2002; Penzes et al., 2003; Tolias et al., 2007). In contrast to the positive regulation of these GEFs, EphBs negatively regulate the RhoA GEF Ephexin5, which normally restricts synapse number by activating RhoA (Margolis et al., 2010). Upon activation by ephrin-Bs, EphB2 catalyzes the phosphorylation of Ephexin5, which targets Ephexin5 for ubiquitination by Ub3a and proteasomal degradation. Thus, EphB2 functions in this case to relieve the inhibitory effect of Ephexin5 on synapse formation.
Early in development, EphB-mediated activation of Rac1 and p21-activated kinase (PAK) drives the motility of dendritic filopodia, which is required for synapse formation (Fig. 2A-C) (Kayser et al., 2008). Interestingly, SAP102 was found in a complex with the Rac1 GEF Kalirin7 and PAK, both of which have been shown to function downstream of EphB kinase activity to mediate synapse formation via regulation of the actin cytoskeleton (Kayser et al., 2008; Murata and Constantine-Paton, 2013; Penzes et al., 2003). Other pathways by which EphBs drive spine development are through the phosphorylation and clustering of the cell-surface proteoglycan syndecan-2 (Ethell et al., 2001), and by phosphorylating and activating focal adhesion kinase (FAK), which inhibits the actin-severing protein cofilin (Moeller et al., 2006; Shi et al., 2009). Thus, EphBs localize to sites of nascent synaptic contact, signal to select appropriate targets, and activate signaling cascades that coordinate the early steps in synaptogenesis.
3.2. Regulating of glutamate receptors by EphBs.
EphBs continue to be expressed and localized to pre- and post-synaptic terminals into adulthood (Bouvier et al., 2008; Kayser et al., 2011; Kayser et al., 2008; Liebl et al., 2003). Indeed, a recent study using cryoelectron tomography of mature dendritic spines and superresolution imaging indicated that EphB2 is enriched at the core of the post-synaptic density in mature synapses that form on dendritic spines (Perez de Arce et al., 2015). However, in mature neurons, EphBs are not required to maintain existing synaptic contacts (Kayser et al., 2008).
At the mature synapse, EphBs regulate the localization of glutamate receptors via both direct binding and through indirect mechanisms mediated by adaptor proteins (Fig. 2D). EphBs 1–3 bind directly with NMDARs through the extracellular domains of each protein. Not only do these proteins interact, but the EphB-NMDAR interaction is important for the recruitment and retention of NMDARs at synaptic sites and modulates the function of the NMDAR (Dalva et al., 2000; Hanamura et al., 2017; Henderson et al., 2001; Kayser et al., 2006; Nolt et al., 2011). Consistent with these interactions with the NMDAR, EphB2−/− mice exhibit decreased NMDAR currents, deficits in NMDAR-dependent long-term potentiation (LTP) and long-term depression (LTD), and impaired memory in the Morris water maze task (Grunwald et al., 2001; Henderson et al., 2001). EphB TKO mice have significant reductions in levels of synaptic NMDARs, but no decrease in the total amount of NMDAR expressed. Activation of EphBs by ephrin-Bs not only clusters NMDARs at synapses, it also leads to Src kinase-dependent phosphorylation of the GluN2B NMDAR subunit, which increases calcium flux through the receptor (Takasu et al., 2002) and increased channel open time (Nolt et al., 2011). Thus, the EphB-NMDAR interaction is crucial for proper synaptic localization and function of NMDARs.
The EphB-NMDAR interaction is mediated by a specific tyrosine, Y504, which is located in the extracellular C-terminal fibronectin type III repeat (FNIII) region of EphB2 (Hanamura et al., 2017). Phosphorylation of Y504 on EphB2 is induced by activation of EphB2 by ephrin-Bs, occurs on the cell surface, and is necessary for the EphB2-NMDAR interaction. A phosphomimetic version of EphB2 (Y504E) interacts with the NMDAR independent of ephrin-B activation of EphB2, while a non-phosphorylatable version of EphB2 (Y504F) fails to interact with NMDAR, even after ephrin-B activation. Consistent with the importance of the EphBNMDAR interaction for the synaptic localization of NMDARs, expression of constitutively interacting EphB2, but not mutants unable to interact, increased the NMDAR component of evoked and miniature excitatory post-synaptic currents (mEPSCs) and increased levels of NMDARs at spine synapses. Together, these results reveal a novel role for extracellular phosphorylation in regulating the EphB2-NMDAR interaction and further illustrate how EphB2, a key factor in synapse development, acts at mature synapses to regulate NMDAR localization and function. Although extracellular phosphorylation is a relatively unexplored phenomenon, Y504 of EphB2 and the surrounding residues are highly conserved among FNIII-containing proteins suggesting that extracellular phosphorylation may be an underappreciated mechanism mediating protein-protein interactions. Consistent with this model, EphA8, which contains a domain identical to EphB2, was found to interact with the NMDAR when expressed in HEK293T cells (Hanamura et al., 2017).
EphBs regulate the localization of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) by interacting with adaptor proteins and regulating intracellular signaling cascades. EphBs colocalize with AMPA receptor clusters in cultured neurons, and treatment with ephrin-B2 increases the surface retention of GluA2-containing AMPARs (Kayser et al., 2006). Unlike the interaction with the NMDAR, clustering of AMPARs requires the PDZbinding domain of EphBs. The interaction with AMPARs likely occurs indirectly through interactions with PDZ domain proteins such as PICK1 and GRIP1, which also interact with AMPARs (Hoogenraad et al., 2005; Torres et al., 1998). Interestingly, the AMPAR component of mEPSCs was reduced by EphB2 knockdown and increased by EphB2 overexpression in DIV21–23 neurons but EphB2 knockdown had no effect at DIV9–11, suggesting that EphBs selectively regulate AMPAR subtype or levels later in development after most synapse formation has occurred (Kayser et al., 2006; Nolt et al., 2011).
In addition to the regulation of the surface/synaptic localization of AMPARs, EphB2 activates intracellular signaling cascades that increase phosphorylation of GluA1 at serine 863 (S863), a site which was found to regulate AMPAR surface localization (Hussain et al., 2015). EphBs can induce PAK3-mediated phosphorylation of GluA1 via the Cdc42-GEF Zizimin1, which interacts with both EphB2 and GluA1. PAK3 phosphorylates GluA1 at S863 in vitro, and PAK3-mediated phosphorylation of GluA1 is increased by Zizimin1. Activation of EphB2 by ephrin-B2 treatment increased the surface expression of GluA1 as well as its interaction with EphB2, and mutation of S863 blocked this effect. Interestingly, phosphorylation of GluA1 at S863 was also increased by activity blockade by tetrodotoxin (TTX) in a manner that depended on PAK activation, providing a potential link between EphB2 and activity-dependent regulation of AMPARs (Hussain et al., 2015). Together, these findings suggest that, like neuronal activity, EphB2 can regulate AMPAR content at synaptic sites (Hussain et al., 2015; Kayser et al., 2006).
Together, these studies suggest a model where EphBs are crucial early in development for synapse and spine formation, then at mature synapses for localizing glutamate receptors to synapses and for modulating glutamate receptor function. EphBs promote synaptogenesis and spinogenesis early in development (approximately DIV 7–14) (Kayser et al., 2008). At mature synapses, EphBs regulate glutamate receptor signaling. EphBs interact directly with NMDARs through the extracellular FNIII repeat region of EphBs and also modulate NMDAR function by inducing Src-dependent phosphorylation of the GluN2B NMDAR subunit (Dalva et al., 2000; Nolt et al., 2011; Takasu et al., 2002). Interestingly, increases in phosphorylation levels of the extracellular Y504 site on EphB2 parallel these changes in NMDAR function (Hanamura et al., 2017). In addition, EphBs interact indirectly via PDZ-binding domain interactions with AMPARs and can modulate AMPAR surface localization (Hussain et al., 2015; Kayser et al., 2006). These data support a model where like the receptor tyrosine kinase MuSK at the neuromuscular junction (Bowen et al., 1998; Burden et al., 2013; Wu et al., 2010), EphBs function early to regulate synaptogenesis then remain at synaptic sites as they mature to regulate glutamate receptor localization and function. Interestingly, de novo nonsense mutations in the EPHB2 gene were recently linked to autism (Sanders et al., 2012). As autism is thought to result from synaptic dysfunction (Zoghbi and Bear, 2012), it is possible that loss of EphB2 and resulting defects in synaptogenesis or synaptic function may be related to autism.
4. Role of ephrin-Bs at synaptic sites
Ephrin-Bs have diverse and important functions at synaptic sites. The function of ephrin-Bs is determined in part by whether they are localized pre- or post-synaptically. When localized pre-synaptically, ephrin-Bs act as ligands for post-synaptic EphBs and mediate the development and plasticity of pre-synaptic terminals. When localized post-synaptically they have more diverse functions that we will discuss below.
4.1. Ephrin-Bs in pre-synaptic development and function
Ephrin-B1 and ephrin-B2 are found to co-localize and in the case of ephrin-B1, to co-localize with pre-synaptic markers (Mao et al., 2018; McClelland et al., 2009). In a widely used coculture assay for synaptogenic activity (Kayser et al., 2006; Scheiffele et al., 2000), expression of EphB2 in non-neuronal cells induces pre-synaptic differentiation in the axons of cortical neurons (McClelland et al., 2009). EphB-dependent synapse induction requires ephrin-B1 and ephrin-B2, but not ephrin-B3, suggesting ephrin-B1 and ephrin-B2 function as pre-synaptic ligands for post-synaptic EphBs in cortical neurons (Fig. 2C). The tandem PDZ domaincontaining protein Syntenin-1, which interacts with the active zone organizing protein ERC2/CAST1 and with ephrin-Bs through their PDZ-binding domains, was also found to be required for EphB-dependent synapse induction (Ko et al., 2006; Torres et al., 1998), suggesting that it acts in the same pathway as ephrin-B1/2 to mediate synapse development. Consistent with this model, ephrin-B1 and ephrin-B2 were required for the localization of Syntenin-1 to synapses (McClelland et al., 2009). Consistent with these findings, cortical pyramidal neurons in ephrin-B1 deficient mice were found to have reduced numbers of dendritic spines (Arvanitis et al., 2014).
Additional evidence for the functional significance of pre-synaptic ephrin-B1 comes from the Xenopus retinotectal system. Ephrin-B1 is localized to retinal ganglion cell (RGC) axons, and expression of dominant-negative ephrin-B1 reduced the density of retinotectal synapses (Lim et al., 2008). Likewise, activation of ephrin-Bs by infusion of EphB2 ectodomain into the tectum increased the number of pre-synaptic release sites (Lim et al., 2008). In addition to the effects on synapse number, activation of ephrin-Bs by infusion of EphB2 ectodomain increased the magnitude of LTP at retinotectal synapses. Consistent with this effect on synaptic plasticity, a separate study found that overexpression of ephrin-B1 in Xenopus RGCs increased activityinduced receptive field shifts in the ventral tectum (Lim et al., 2010). Thus, pre-synaptic ephrinB1 likely regulates both the development and plasticity of RGC axons in this system.
Ephrin-Bs also act pre-synaptically in axonal projections made by the hippocampus. Transsynaptic ephrin-B3-EphB2 interactions are important for the development and function of projections from the CA1 region of the hippocampus to the basolateral amygdala (BLA) (Zhu et al., 2016a; Zhu et al., 2016b). In this system, ephrin-B3 is found in the axons of neurons in CA1 neurons that project to the BLA. In ephrin-B3−/− mice, axons from CA1 to BLA are mistargeted, and BLA neurons exhibit a reduction in dendritic spine density. More work will be needed to determine whether the defects in synapse development are due principally to the reduction in the number of axons from the CA1 that are properly targeted to BLA or if the loss is due to a direct effect on synaptogenesis. Regardless, mice lacking ephrin-B3 in the BLA have decreased in anxiety-like and fear behavior in the elevated plus maze and in response to predator odor respectively, and a decrease in the number c-Fos+ BLA neurons following these stimuli. Consistent with the model that ephrin-B3 may be important for synapse development, the behavioral phenotypes were rescued by re-expression of ephrin-B3 at P16 in within CA1 alone, suggesting that ephrin-B3 acts pre-synaptically in CA1 axons. Interestingly, EphB2−/− mice display the same behavioral deficits and reduction in c-Fos+ cells following fear and anxiety inducing stimuli, as well as a decrease in spine and synapse density in BLA neurons. In contrast to ephrin-B3−/− mice, these phenotypes are rescued by re-expression of EphB2 in BLA neurons, showing that EphB2 acts post-synaptically (Zhu et al., 2016b).
Similar to their role in promoting plasticity at retinotectal synapses (Lim et al., 2010), presynaptic ephrin-Bs also appear to regulate synaptic plasticity in hippocampus. These data come from studies of mossy fiber LTP, a form of NMDA-receptor independent LTP that is expressed pre-synaptically at synaptic terminals of mossy fibers originating in the granule neurons of the Dentate Gyrus (Armstrong et al., 2006; Harris and Cotman, 1986). Using an ephrin-B3lacZ mouse model in which the cytoplasmic domain of endogenous ephrin-B3 is replaced with β-Galactosidase (ephrin-B3-βGal), investigators found that ephrin-B3-βGal was localized to axons and pre-synaptic terminals of mossy fibers originating in CA3. Induction of mossy fiber LTP was impaired in these mice, suggesting that the cytoplasmic domain of ephrin-B3 is required for this form of plasticity. However, mossy fiber LTP was found to be normal in ephrin-B3−/− mice, suggesting that other ephrin-Bs may compensate for the total absence of ephrin-B3. Alternatively, ephrin-B3-βGal could act in a dominant negative fashion, inhibiting the function of other pre-synaptic ephrin-Bs. Another study found that infusion of a peptide corresponding to the C-terminus of EphB2 which interferes with EphB signaling into post-synaptic CA3 neurons blocked mossy fiber LTP (Contractor et al., 2002). Together, these results suggest that transsynaptic EphB-ephrin-B signaling is required for this form of plasticity.
4.2. Ephrin-Bs in post-synaptic development
Ephrin-Bs also have post-synaptic functions in hippocampus and cortex (Fig. 3). Postsynaptic ephrin-B signaling has been reported to promote spine morphogenesis in hippocampal neurons, which depends on the recruitment of GIT1 to synapses by ephrin-Bs through an interaction between ephrin-Bs and the adaptor protein Grb4 (Segura et al., 2007). GIT1 promotes spine morphogenesis by forming a complex with the GTPase Rac and PAK, suggesting that ephrin-B signaling in spine morphogenesis is linked to this pathway (Fig. 3A) (Zhang et al., 2005).
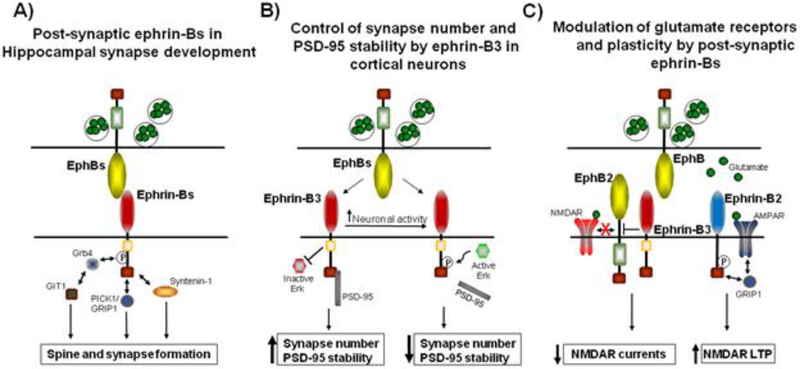
Figure 3.
Post-synaptic ephrin-Bs regulate synapse development and function. (A) Ephrin-Bs regulate post-synaptic development by interacting the Syntenin-1, interacting with the AMPAR-binding proteins PICK1 and GRIP1, and by interacting with Grb4, which recruits GIT1 to synapses. (B) In cortical neurons, ephrin-B3 controls synapse number by inhibiting Erk1/2 and by interacting with the MAGUK scaffolding protein PSD-95. Upon increased neuronal activity, Erk1/2 becomes activated and phosphorylates ephrin-B3 at S332. Phosphorylation of ephrin-B3 disrupts the interaction with PSD-95 and disperses PSD-95 from synapses. (C) Ephrin-Bs regulate glutamate receptor signaling at synapses. Ephrin-B2 interacts with AMPARs through GRIP1, which promotes the surface retention of AMPARs and promotes NMDAR-dependent LTP. Ephrin-B3 interacts in cis with EphB2, which attenuates EphB2 activation. Decreased EphB2 activity results in a decrease in phosphorylation of GluN2B at Y1472 which in turn decreases NMDAR currents.
Among the ephrin-Bs, the post-synaptic functions of ephrin-B3 have been the most wellstudied. In cortical neurons, ephrin-B3 is enriched at post-synaptic densities, and knockdown of ephrin-B3 decreases synapse density, suggesting that ephrin-B3 is involved in specifying synapse number (Fig. 3B) (Hruska et al., 2015; McClelland et al., 2010). Ephrin-B3 can induce pre-synaptic differentiation in a heterologous cell culture assay, and this effect depends on the presence of pre-synaptic EphB2 (Hruska et al., 2015; McClelland et al., 2010). However, Ephrin-B3 knockout neurons exhibit normal synapse density in vitro and in vivo, suggesting that ephrin-B3 is not required for synapse formation. Mixed cultures of ephrin-B3 knockout and wildtype neurons have instead shown that relative differences in ephrin-B3 expression between neurons control synapse density. Ephrin-B3−/− neurons, which form normal numbers of synapses in homotypic ephrin-B3−/− cultures, receive fewer synapses when co-cultured with wild-type neurons at a 1:10 ratio. Likewise, wild-type neurons receive more synapses when co-cultured with ephrin-B3−/− at a 1:10 ratio than wild-type neurons in homotypic cultures (McClelland et al., 2010). It is tempting to speculate from this data that ephrin-B3 might mediate a cell-cell competition for synapses, possibly by competing for a limiting amount of their EphB2 ligand in axons (McClelland et al., 2010). However, this hypothesis has yet to be validated in a system where competition can be measured directly. Suitable systems where winners and losers of such a competition could be identified might be a simplified culture system such as a microisland (Tarsa and Goda, 2002) or an in vivo model system such as the mosaic analysis with double markers (MADM) mouse model where genetically distinct neurons can be fluorescently labeled (Espinosa et al., 2014; Zong et al., 2005). Control of synapse number by ephrin-B3 requires an interaction with Erk1/2 through a D-domain found only in the intracellular region of ephrin-B3, and not in ephrin-B1 or ephrin-B2. Loss of ephrin-B3 resulted in increased nuclear Erk1/2, and synapse number was rescued by expression of dominant-negative MEK, the upstream activator of Erk1/2 (McClelland et al., 2010. These results suggest that ephrin-B3 normally inhibits Erk1/2 function.
Ephrin-B3 is enriched at mature synaptic sites, where it appears to be a key regulator of the activity-dependent localization of PSD-95 to spines (Fig. 3B). Ephrin-B3 interacts directly with PSD-95 selectively at synaptic sites, and ephrin-B3−/− mice have significantly reduced synaptic levels of PSD-95 in cortex, suggesting that ephrin-B3 functions specifically at synapses to anchor PSD-95. Consistent with this, fluorescence recovery after photobleaching (FRAP) experiments showed that synaptic PSD-95-EGFP mobility was higher in cultured neurons expressing ephrin-B3 shRNA compared to wild-type, and PSD-95 was localized diffusely throughout the dendritic shaft in ephrin-B3−/− neurons in cortical slices and in vitro (Hruska et al., 2015). Ephrin-B3-dependent regulation of PSD-95 relies on regulation by Erk1/2 (Fig. 3B). Erkdependent phosphorylation of a MAPK consensus phosphorylation site near the PDZ-binding domain of ephrin-B3, centered around S332, negatively regulates the direct interaction between ephrin-B3 and PSD-95 and destabilizes PSD-95 in spines. Wild-type ephrin-B3 and a nonphosphorylatable ephrin-B3 mutant (S332A), but not a phosphomimetic S332D ephrin-B3 mutant interacted directly with PSD-95 in an in vitro binding assay. S332A, but not S332D ephrin-B3 was able to rescue synaptic localization and stability of PSD-95 in brain slices from ephrin-B3−/− mice and following ephrin-B3 knockdown (Hruska et al., 2015). Together, these results indicate that a direct interaction between PSD-95 and ephrin-B3, which is regulated by MAPK signaling, likely anchors PSD-95 at synapses. Importantly, ephrin-B3 remained at synapses following knockdown of PSD-95, indicating that ephrin-B3 localizes to synapses independently of PSD-95 (Hruska et al., 2015). Thus, trans-synaptic interactions mediated by ephrin-B3 may act as in instructive cue that localizes PSD-95, an intracellular protein, to sites of axo-dendritic contact.
Ephrin-B3 regulates the synaptic localization of PSD-95 in an activity-dependent fashion. When neuronal activity was reduced in vivo by trimming whiskers on only one side of a mouse’s snout to create unilateral sensory deprivation, the deprived sensory cortex had significantly reduced ephrin-B3 S332 phosphorylation and increased levels of ephrin-B3-PSD-95 interaction. These data indicate that sensory-driven neuronal activity negatively regulates ephrin-B3 phosphorylation and that this increases synaptic PSD-95, providing a potential bridge between neuronal activity and the dynamic reorganization of PSD-95. The molecular mechanism for these events appears to be driven by MAPK-dependent phosphorylation of S332. Phosphorylation of S332 was induced by increasing neuronal activity with KCl treatment in vitro and was blocked by inhibition of the upstream Erk1/2 activator, MEK, consistent with a role for MAPKs in regulating phosphorylation of ephrin-B3. A number of lines of evidence indicate that MAPK signaling negatively regulates the ephrin-B3-PSD-95 interaction, including experiments that indicate that the acute inhibition of MEK rescued stability to control levels in FRAP experiments following reduction of ephrin-B3 levels by ephrin-B3 knockdown (Hruska et al., 2015). These data support a model where neuronal activity induces activation of Erk kinasedependent phosphorylation of ephrin-B3, reducing the ephrin-B3-PSD-95 interaction and synaptic levels of PSD-95. In the absence of activity, Erk-dependent phosphorylation of S332 is reduced, increasing the ephrin-B3-PSD-95 interaction and levels of synaptic PSD-95.
Ephrin-B3 appears to function post-synaptically in the hippocampus to regulate excitatory synapse number (Fig. 3A). However, there are conflicting reports as to whether spine synapses or dendritic shaft synapses are affected (Aoto et al., 2007; Rodenas-Ruano et al., 2006; Sugiura et al., 2015; Xu et al., 2011). One potential explanation for these different findings comes from differences in PDZ domain interactions: studies on ephrin-B3 in the regulation of shaft synapse density link ephrin-B3 to GRIP1 and Syntentin-1 (Aoto et al., 2007; Sugiura et al., 2015), while ephrin-B3’s links to spine synapse formation are via Syntenin-1 and PICK1 (Fig. 3A) (Xu et al., 2011). Additional efforts will be needed to clarify these findings and to determine whether ephrin-B3 interacts with PSD-95 in hippocampal neurons as it does in cortical neurons (Hruska et al., 2015).
Taken together with the role of ephrin-B3 in establishing synapse number, the importance of ephrin-B3 in stabilizing a key synaptic organizing MAGUK protein, PSD-95, at synapses suggests a model in which ephrin-B3 acts post-synaptically as a link between neuronal activity and synapse formation (Hruska et al., 2015; McClelland et al., 2010). Interestingly, ephrin-B3 exerts its effects in part through inhibiting Erk1/2 signaling, which itself can inhibit ephrin-B3 function by phosphorylation of ephrin-B3 on S332. These findings suggest a feedback or feedforward relationship where increased activity activates Erk1/2, which downregulates the ephrin-B3-PSD-95 interaction. Likewise, decreased activity levels would reduce Erk1/2 activity and promote the ephrin-B3-PSD-95 interaction. Since ephrin-B3 can also bind to and inhibit Erk1/2, the level of ephrin-B3 expression in a cell may create a homeostatic set point for excitatory drive. Because PSD-95 has been shown to play important roles in plasticity, learning and memory (Migaud et al., 1998; Sun and Turrigiano, 2011), it will be interesting to examine whether ephrin-B3-dependent stabilization of PSD-95 at synapses is required for processes such as LTP, LTD or homeostatic scaling. Indeed, post-synaptic ephrin-Bs have been found to be involved in NMDAR-dependent LTP in hippocampus (see below).
4.3. Ephrin-Bs in post-synaptic plasticity
Post-synaptic ephrin-Bs also regulate glutamate receptor signaling and plasticity (Fig. 3C).Ephrin-B2 conditional knockout mice have deficits in NMDAR-dependent LTP and LTD at CA3-CA1 synapses (Grunwald et al., 2004). The role of ephrin-Bs in CA3-CA1 LTP may be at least in part due to an interaction with AMPARs. Ephrin-B2 stabilizes AMPARs at the cell surface by interacting with GRIP1, which in turn interacts with AMPARS (Essmann et al., 2008). Ephrin-B2/- neurons thus exhibit increased constitutive internalization of AMPARs and a decrease in AMPAR-mediated synaptic currents. The association between ephrin-B2, GRIP1 and AMPARs depends on phosphorylation of a serine residue (S328) near the C-terminal PDZ-binding domain of ephrin-B2. Phosphorylation of this residue is induced by depolarization with KCl, suggesting that this interaction may be regulated by activity (Essmann et al., 2008). Interestingly, the homologous serine residue in ephrin-B3 is a perfect Erk phosphorylation consensus site, and negatively regulates the interaction between ephrin-B3 and PSD-95, but both ephrin-B1 nor ephrin-B2 lack the prefect Erk consensus and fail to co-immunoprecipate with PSD-95 at synaptic sites (Hruska et al., 2015). Neurons from hippocampus and cortex of ephrin-B3−/− mice also exhibit a reduction in mEPSC amplitude, suggesting ephrin-B3 may serve a similar role in stabilizing AMPARs at synapses (Antion et al., 2010; McClelland et al., 2010). Conflicting results on the involvement of ephrin-B3 in hippocampal have been published using different ephrin-B3 knockout mouse lines, one showing defective LTP and the other not (Antion et al., 2010; Rodenas-Ruano et al., 2006). The reason for this discrepancy is unclear but may be due to the different strains of ephrin-B3−/− mice used in the studies. Ephrin-B3 also appears to regulate synaptic function in CA1 neurons through a cis interaction with EphB2 that inhibits EphB2 kinase activity (Antion et al., 2010). As discussed above, EphB2 promotes increases in NMDAR currents by retaining NMDARs at synapses and by inducing Src-dependent phosphorylation of the GluN2B subunit of the NMDAR, which increases calcium flux (Dalva et al., 2000; Nolt et al., 2011; Takasu et al., 2002). Thus, in ephrin-B3−/− mice there is an increase in phosphorylation of both EphB2 and GluN2B, and an increase in the ratio of NMDAR/AMPAR synaptic currents (Antion et al., 2010). These data support the importance of ephrin-Bs and EphBs in the regulation of synaptic function and plasticity.
In summary, the data indicate that the function of ephrin-Bs is largely determined by where they are localized. In cortex, pre-synaptic ephrin-B1 and ephrin-B2 regulate pre-synaptic development through interactions with Syntenin-1, while ephrin-B3 acts post-synaptically to control excitatory synapse number and to anchor PSD-95 at synapses (Hruska et al., 2015; McClelland et al., 2010; McClelland et al., 2009). In CA1 neurons, post-synaptic ephrin-B3 acts to control synapse and spine development, while post-synaptic ephrin-B2 is important for interacting with and stabilizing AMPARs (Antion et al., 2010; Essmann et al., 2008; RodenasRuano et al., 2006; Xu et al., 2011). Pre-synaptic ephrin-B3 in CA1 axons that project to the amygdala mediate the development of CA1-BLA synapses, and at CA3 mossy fibers to promote mossy fiber LTP (Armstrong et al., 2006; Zhu et al., 2016a; Zhu et al., 2016b). In the retinotectal system of Xenopus, ephrin-B1 is localized pre-synaptically in RGC axons and promotes the development and plasticity of retinotectal synapses (Lim et al., 2010; Lim et al., 2008). Thus, ephrin-Bs play diverse roles at synapses that depend on brain region, subcellular localization, and their interacting partners.
5. EphB-ephrin-B signaling in disease
Given the importance of EphB-ephrin-B signaling in synapse development and plasticity, it comes as little surprise that EphBs and ephrin-Bs have been implicated in many diseases of the nervous system. Here, we focus on the most studied roles of EphBs/ephrin-Bs in this context, which involve dysregulation of post-synaptic EphB signaling (Sheffler-Collins and Dalva, 2012). Importantly, both up-regulation and down-regulation of EphB signaling have been linked to disease, suggesting that either too much or too little EphB activation can be maladaptive.
5.1. Neuropathic pain involves increased EphB signaling
Pain is crucial for survival, allowing for the identification and avoidance of physically harmful stimuli. However, damage to the neural circuits involved in transducing painful stimuli can result in chronic, maladaptive neuropathic pain (Cohen and Mao, 2014). Under non-pathological conditions, pain-inducing stimuli activate the peripheral processes of primary nociceptive neurons that reside in the dorsal root ganglia (DRG). DRG neurons synapse onto second order neurons in the dorsal horn (DH) of the spinal cord, the axons of which transmit pain signals to the brainstem, thalamus, and finally the cerebral cortex (Fig. 4A) (Costigan et al., 2009; Woolf and Salter, 2000). Pathophysiological phenomena such as mechanical damage, toxic chemicals, and chronic inflammation among others can disrupt the pain transduction pathway and result in neuropathic pain (Scholz and Woolf, 2007; Woolf and Salter, 2000). One important mechanism in neuropathic pain is known as central sensitization, which involves an increase in the responsiveness of DH neurons to excitatory input from peripheral nociceptors (Woolf and Salter, 2000). Potentiation of DRG-DH synapses occurs in a NMDAR-dependent manner which, similar to the well-studied form of LTP in hippocampal CA1 neurons, requires calcium influx and activation of downstream effectors such as CamKII and PKC (Woolf and Salter, 2000). EphBephrin-B signaling has been linked to these processes in a number of different models of neuropathic pain (Fig. 4B) (Sheffler-Collins and Dalva, 2012).
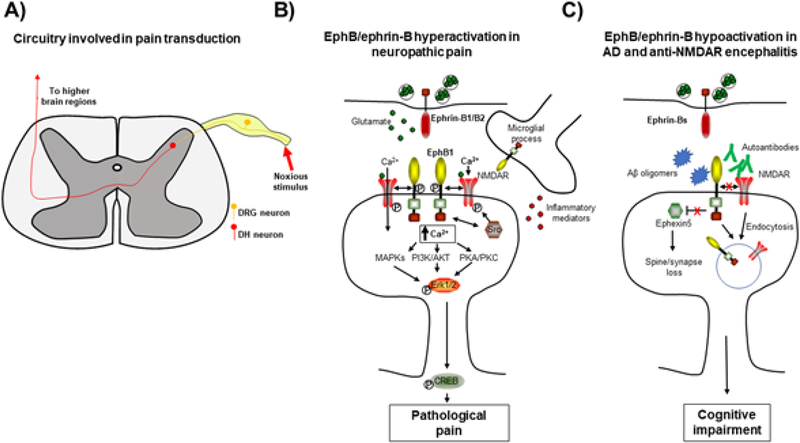
Figure 4.
Eph-ephrin signaling in disease. (A) Model of spinal circuitry involved in pain transduction. (B) Pathological increases in EphB1 signaling in dorsal horn neurons result in neuropathic pain. Activation of EphB1 by pre-synaptic ephrin-Bs results in enhanced NMDAR currents, activating MAPK, PI3K/AKT, PKA and PKC signaling pathways and inducing pathological pain. (C) Disruption of post-synaptic EphB signaling in Alzheimer’s disease (AD) and anti-NMDAR encephalitis results in cognitive impairment. In AD, Aβ oligomers bind to EphB2, which results in depletion of synaptic NMDARs either through disruption of the EphB2-NMDAR interaction or by an as yet undetermined mechanism involving the PDZ binding domain of EphB2. Knockout of Ephexin5 rescues behavioral deficits in APP mice, suggesting that reduced levels of EphB2, which normally inhibits Ephexin5, may play a role in synapse loss in this mouse model of AD. In anti-NMDAR encephalitis, autoantibodies bind to NMDARs and disrupt the EphB2-NMDAR interaction, resulting in endocytosis of NMDARs.
EphB1 is expressed in the dorsal horn region of the spinal cord which receives primary nociceptive afferents (Battaglia et al., 2003) and EphB1 expression is up-regulated in the dorsal horn in multiple pain models (Dong et al., 2011; Liu et al., 2011; Song et al., 2008a). The first evidence for the functional involvement of EphB-ephrin-B signaling in chronic pain states came from a study in which intrathecal injection of EphB1 ectodomain blocked mechanical allodynia and thermal hyperalgesia induced by intraplantar injection of carrageenan, a potent inflammatory agent (Battaglia et al., 2003). EphB1 has subsequently been demonstrated to be required for multiple forms of neuropathic pain resulting from injury, inflammation, and cancer (Cibert-Goton et al., 2013; Han et al., 2008; Liu et al., 2011). Consistent with importance of EphB1 in DH neurons, ephrin-B2 was found to be up-regulated in DRG neurons in multiple pain models (Kobayashi et al., 2007; Liu et al., 2011), and is required specifically in DRG nociceptive neurons for neuropathic pain (Zhao et al., 2010), suggesting that ephrin-B2 may be the presynaptic ligand for EphB1. Other studies have found up-regulation of ephrin-B1 in the spinal cord and DRG in injury pain models and in one cancer-induced pain model (Orikawa et al., 2010; Song et al., 2008a; Song et al., 2008b), while ephrin-B1 was down-regulated in the DRG in a bone cancer pain model (Dong et al., 2011). Together, these studies provide evidence for a model where post-synaptic EphB1 and pre-synaptic ephrin-Bs at DRG-DH synapses are crucial for neuropathic pain.
5.2. EphB-dependent potentiation of NMDAR function underlies neuropathic pain
Many pathways downstream of EphB-ephrin-B signaling have been implicated in neuropathic pain, but the strongest link is the EphB-dependent potentiation of NMDAR function (Fig. 4B) (Battaglia et al., 2003; Ruan et al., 2010; Slack et al., 2008; Xia et al., 2014; Zhou et al., 2015b). A number of lines of evidence support this conclusion. Intrathecal injection of ephrin-B1 or ephrin-B2 ectodomains induced thermal hyperalgesia that was blocked by the NMDAR antagonist, MK-801 (Battaglia et al., 2003; Ruan et al., 2010; Xia et al., 2014; Zhou et al., 2015b). Consistent with the role of EphBs in the regulation of NMDAR function (Takasu et al., 2002), intrathecal ephrin-B2 ectodomain injection caused an increase in the amount of activated Src kinase that co-immunoprecipitated with EphBs in spinal cord lysates (Battaglia et al., 2003), activation of EphBs induced phosphorylation of GluN2B (Slack et al., 2008), and GluN2B phosphorylation is decreased after knocking out ephrin-B2 in nociceptors (Slack et al., 2008; Zhao et al., 2010). Interestingly, phosphorylation of GluN2B at Y1472, which potentiates NMDAR function, has been shown to occur in injury-induced pain and in cancer pain, suggesting that it is a common mechanism in neuropathic pain states (Abe et al., 2005; Liu et al., 2011; Orikawa et al., 2010).
Consistent with the link between EphBs and the NMDAR in the context of pain, the direct EphB-NMDAR interaction appears to be crucial for the maintenance of neuropathic pain (Hanamura et al., 2017). A model of surgical pain and expression of constitutively interacting forms of EphB2 in the spinal cord increase NMDAR levels in the dorsal horn, induce the EphBNMDAR interaction and drive NMDARs to synaptic sites. Consistent with these results, induction of the extracellular EphB-NMDAR interaction by expression of constitutively interacting (Y504E) but not interaction deficient (Y504F) EphB2 induced long-lasting allodynia (2 months). Remarkably, injection of a membrane-impermeable kinase inhibitor that blocks Y504 phosphorylation blocked pain behavior following injury or after expression of WT, but not Y504E EphB2 in the dorsal horn of the spinal cord (Hanamura et al., 2017). These data suggest that sustained EphB-NMDAR interaction may play a key role in some forms of long-lasting allodynia. Consistent with the idea that excess EphB-dependent plasticity occurs at DRG-DH synapse in chronic pain states, activation of EphBs promote NMDAR-dependent LTP at DRG-DH synapses (Liu et al., 2009; Song et al., 2008a). Taken together, these findings suggest that EphBmediated recruitment of NMDARs to synaptic sites and potentiation of receptor function is an important mechanism for chronic pain.
Consistent with the importance of EphBs in pain, EphB signaling has been linked to several intracellular signaling cascades in the context of pain, most of them downstream of NMDAR activity (Fig. 4B). Injection of ephrin-B1 ectodomain to activate EphB signaling has been shown in multiple models to activate the MAPKs Erk1/2, p38, and JNK. Inhibition of MAPK signaling prevented pain behavior induced by ephrin-B1 ectodomain injection, demonstrating that MAPKs are necessary for EphB-ephrin-B mediated pain (Cao et al., 2008; Ruan et al., 2010). Another signaling cascade associated with activation of EphBs in the pain transduction pathway is the PI3 kinase (PI3K) cascade. PI3K was activated in response to both peripheral and intrathecal ephrin-B1 ectodomain injection. In both paradigms, blocking PI3K activation was sufficient to prevent pain behavior (Guan et al., 2010; Yu et al., 2012). Protein kinase C (PKC) and protein kinase A (PKA), and the downstream effector of PKA, CREB, are activated in chronic constriction injury (CCI), tibia bone cavity tumor cell implantation (TCI) and formalin injection models and by intrathecal injection of ephrin-B2 ectodomain. Knocking down PKC or blocking PKA signaling prevented pain induction by ephrin-B2 ectodomain injection (Zhou et al., 2015a; Zhou et al., 2015b).
There is also evidence that EphB signaling is linked to inflammatory responses in glial cells that are involved in neuropathic pain (Fig. 4B) (Scholz and Woolf, 2007). TCI was found to increase the expression of EphB1 and Toll-like receptor 4 (TLR4) in astrocytes and microglial cells. EphB1 and TLR4 appeared to be colocalized in glial cells, and knockdown of TLR4 or blockade of EphB activation each blocked the increases in IL-1β and TNFα expression, and also reduced thermal hypersensitivity (Liu et al., 2013). Consistent with a potential role for EphB1 in glial cells, microglial activation in multiple pain models was decreased in EphB1−/− mice (Cibert-Goton et al., 2013). These studies provide intriguing evidence that in addition to the role of EphB signaling in neurons, EphB1 may regulate inflammatory responses in glial cells. Upregulation of EphBs and ephrin-Bs has also been observed in reactive astrocytes in other non pain-inducing injury models, suggesting that EphBs and ephrin-Bs might be involved in the glial response to multiple types of insult to the nervous system (Du et al., 2007; Goldshmit et al., 2006; Koeppen et al., 2018; Wang et al., 2005).
In summary, an abundance of evidence from pain models to suggest that upregulation of EphBs and ephrin-Bs are of central importance in generating chronic pain. The mechanism by which EphBs and ephrin-Bs regulate pain may be in the modulation of the extracellular interaction between EphBs and the NMDAR, which is sufficient to induce pain in the absence of injury. Importantly, evidence suggests that blocking or reducing this interaction can reverse even months long allodynic states. These data suggest that the EphB-NMDAR interaction is a target worth further exploration. However, much remains unknown. For instance, what controls the up-regulation of EphB1 and ephrin-Bs in the context of pain? What kinase phosphorylates the EphB FNIII repeat region, promoting the EphB-NMDAR interaction? As therapeutic strategies for treating neuropathic pain are limited, further insights into these mechanisms will be of significant potential impact.
5.3. Decreased EphB signaling in Alzheimer’s disease and Anti-NMDAR encephalitis
While neuropathic pain involves pathological increases in EphB-ephrin-B signaling, too little Eph signaling can also have deleterious effects. Notably, decreased EphB function linked to the EphB-NMDAR interaction has been implicated in both Alzheimer’s disease and antiNMDAR encephalitis. In this section we discuss recent findings regarding the involvement of EphBs in these diseases.
Alzheimer’s disease (AD) is the most common neurodegenerative disease, with an estimated 40 million patients worldwide and a projected three-fold increase in prevalence by 2050 (Huang and Mucke, 2012; Selkoe and Hardy, 2016). The core clinical feature of AD is gradual, ultimately profound memory impairment which is followed in late stages with more extensive language and cognitive impairments (Selkoe, 2002). At the histological level, AD is characterized by amyloid plaques, and neurofibrillary tangles which contain a hyperphosphorylated form of the microtubule binding protein Tau (Selkoe and Hardy, 2016). Amyloid plaques are composed of the amyloid-β (Aβ) peptide, which is generated by cleavage of amyloid precursor protein (APP). Although our understanding of the mechanisms by which Aβ causes toxicity has undergone several revisions, it is generally accepted that Aβ is an important feature of AD pathogenesis (Selkoe and Hardy, 2016).
One of the early features of AD is synaptic and NMDAR dysfunction, yet for years little was known about the underlying mechanisms (Selkoe, 2002). In early stages of AD, EphB2 expression is downregulated in both human and mouse models (Simon et al., 2009), suggesting that EphBs, with their links to both synaptic and NMDAR function, may be linked to the disease. Indeed, Aβ can bind to the FNIII domain in the extracellular region of EphB2 in vitro. Aβ binding causes EphB2 to be removed from the cell membrane and degraded in the proteasome (Fig. 4C) (Cisse et al., 2011). Removal of EphB2 from the cell surface also reduced levels of NMDARs. Importantly, overexpressing EphB2 in the dentate gyrus of human APP overexpressing mice (hAPP), rescued the deficits in memory and NMDAR-dependent plasticity normally observed in the mice (Cisse et al., 2011). EphB2 overexpression was also able to rescue memory deficits a second mouse model, the APP/PS1 AD mouse model. In this model, EphB2 overexpression also rescued decreases in the synaptic expression of the GluN2B and GluN1 subunits of the NMDAR in hippocampus. A peptide designed to block the interaction between soluble Aβ oligomers and EphB2 rescued GluN2B and GluN1 expression and reversed impaired learning and memory in APP/PS1 mice (Shi et al., 2016). EphB2 overexpression also blocked toxicity induced by treatment with Aβ oligomers and increased synaptic NMDAR content (Geng et al., 2013). Interestingly, there may also be a link between EphA4 and AD. In contrast to EphB2, which is reduced following Aβ treatment, Aβ treatment results in activation of EphA4 signaling. Blockade of EphA4 signaling was found to rescue synaptic transmission and synaptic plasticity defects following Aβ treatment and in the APP/PS1 mouse model (Fu et al., 2014).
Rescue of memory, plasticity deficits, and GluN2B surface expression by overexpression of EphB2 in hAPP mice (Cisse et al., 2011; Hu et al., 2017), suggests that a primary pathological effect of decreased EphB2 expression in AD is a decrease in NMDAR function. This is consistent with the role of EphB2 in regulating NMDAR localization and function via direct extracellular interaction (Dalva et al., 2000; Hanamura et al., 2017; Takasu et al., 2002). However, whether this mechanism is responsible for the synaptic and memory deficits in AD is unclear. Indeed, a recent study suggested that although EphB2 does rescue Aβ-induced depletion of NMDARs, this effect requires neuronal activity, the PDZ-binding motif of EphB2, and is independent of EphB2 kinase activity (Miyamoto et al., 2016). Since the interaction with the NMDAR and modulation of NMDAR function by EphB2 require the extracellular domain and kinase activity of EphB2 respectively, it was concluded that in the context of AD EphB2 may promote NMDAR surface retention indirectly through PDZ-domain interactions. Now that the region of the FNIII repeat domain of EphB2 responsible for interacting with NMDARs has been identified (Hanamura et al., 2017), it would be interesting to test constitutively interacting and interaction deficient mutants of EphB2 in order to directly assess the involvement of the extracellular EphB2-NMDAR interaction in AD.
EphB2 may also play other roles in AD pathogenesis. Ephexin5, which negatively regulates synapse number and is itself negatively regulated by EphBs (Margolis et al., 2010), was found to be up-regulated in the hippocampus of hAPP mice (Sell et al., 2017). The upregulation of Ephexin5 is likely to be functionally significant, as knockdown of Ephexin5 in hAPP mice rescued dendritic spine loss in hippocampal neurons of hAPP mice as well as the associated behavioral deficits. Remarkably, knockdown of Ephexin5 in pre-symptomatic hAPP mice was able to prevent the mice from developing cognitive impairments (Sell et al., 2017). Since EphBs normally promote excitatory synapse formation by triggering the degradation of Ephexin5 (Margolis et al., 2010), an intriguing possibility is that decreased EphB2 expression in the context of AD results in a pathological increase in Ephexin5, which results in dendritic spine loss and functional impairments.
Disrupted EphB2 signaling has also been implicated in anti-NMDAR encephalitis, the most frequent synaptic autoimmune encephalitis (Fig. 4C) (Mikasova et al., 2012; Planaguma et al., 2016). Anti-NMDAR encephalitis occurs when antibodies are generated against NMDARs, resulting in psychiatric symptoms such as paranoia and delusional thinking followed by more severe symptoms such as seizures, dyskinesia and breathing instability (Dalmau et al., 2011). Recently it was discovered that autoantibodies against the NMDAR from patient cerebrospinal fluid (CSF) are targeted against the N-terminal domain of the GluN1 subunit and disrupt the interaction between the extracellular domains of EphB2 and the NMDAR (Gleichman et al., 2012; Mikasova et al., 2012). Single-particle tracking and FRAP experiments revealed that antiNMDAR autoantibodies increase the diffusive mobility of synaptic GluN2A-containing NMDARs, which are normally anchored at synapses. This effect on the mobility of NMDARs was accompanied by an increase in the mobility of EphB2, and a decrease in the EphB2-NMDAR interaction as measured by co-immunoprecipitation. Treatment with ephrin-B2 ectodomain was able to rescue NMDAR diffusion to control levels (Mikasova et al., 2012). The therapeutic potential of ephrin-B2 treatment in anti-NMDAR encephalitis was recently assessed using a mouse model of the disease in which patient CSF is delivered through ventricular catheters (Planaguma et al., 2016). In this model, infusion of patient CSF results in deficits in synaptic plasticity and memory, depressive symptoms, and decreased surface and synaptic EphB2 and NMDAR. Remarkably, infusion of ephrin-B2 prevented the development of these symptoms, emphasizing the functional importance of the EphB-NMDAR interaction and suggesting that this may be an effect therapeutic strategy (Planaguma et al., 2016). While more work will be needed to determine the precise mechanism at work, these data provide a promising avenue for further investigation. Antibodies against the NMDAR have also been detected in cases of schizophrenia and narcolepsy (Tsutsui et al., 2012), while antibodies against EphB2 were recently detected in a patient with acute necrotizing encephalopathy (Shirai et al., 2013), suggesting that EphB-NMDAR interaction might be an important therapeutic target for several complex neurological diseases.
6. Conclusions and future directions
Trans-synaptic EphB-ephrin-B signaling performs a remarkable array of functions throughout the lifetime of the synapse. During synaptogenesis, EphBs are required for dendritic filopodia motility, which appears to allow filopodia to ‘interview’ potential synaptic partners in a contact-dependent manner (Kayser et al., 2008; Mao et al., 2018). Indeed, our recently published paper indicates that differences in the rate of EphB activation enable filopodia to make this choice (Mao et al., 2018) As filopodia transition to mature spines, post-synaptic EphBs and ephrin-Bs facilitate structural maturation by regulating the actin cytoskeleton, and functional maturation by recruiting glutamate receptors and scaffolding proteins primarily through PDZ domain interactions (Hruska and Dalva, 2012; Hruska et al., 2015; Murata and Constantine-Paton, 2013; Sloniowski and Ethell, 2012). At mature synapses, EphBs and ephrinBs regulate NMDAR-receptor dependent (Grunwald et al., 2004; Grunwald et al., 2001) and NMDAR-independent (Armstrong et al., 2006) LTP and have been implicated in homeostatic responses to activity deprivation (Frank et al., 2009; Hruska et al., 2015; Hussain et al., 2015). Diseases in which either too much or too little EphB activation has pathological effects highlight the importance of EphB-ephrin-B signaling. Excessive EphB activation in the spinal cord is necessary and sufficient for neuropathic pain (Battaglia et al., 2003; Cibert-Goton et al., 2013; Hanamura et al., 2017), while diminished EphB surface expression is associated with Alzheimer’s disease and anti-NMDAR encephalitis (Cisse et al., 2011; Mikasova et al., 2012; Miyamoto et al., 2016; Planaguma et al., 2016). Further study of EphBs and ephrin-Bs in the context of disease should not only illuminate our understanding of the function of these molecules, they may also lead to new therapeutic interventions.
Highlights.
Trans-synaptic Eph-ephrin interactions coordinate pre- and post-synaptic signaling.
Post-synaptic EphBs regulate synapse and spine formation early in development.
Ephrin-Bs play diverse pre- and post-synaptic roles depending on their localization.
EphBs and ephrin-Bs regulate glutamate receptors and plasticity at mature synapses.
Both too little and too much EphB signaling are involved in CNS diseases.
Acknowledgments
We thank Rachel Hodge and Martin Hruska for their helpful comments and suggestions throughout the writing of this paper.
Funding
Dr. Matthew Dalva’s work was supported by grants RO1MH100093, R01NS106906 and RO1DA022727 from the National Institutes of Health.
Abbreviations:
- βGal
β-Galactosidase
- Aβ
amyloid-β
- Abl
Abelson murine leukemia viral oncogene homolog
- AD
Alzheimer’s disease
- AMPAR
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- APP
amyloid precursor protein
- BLA
basolateral amygdala
- CamKII
calcium/calmodulin-dependent kinase II
- CAST1
CAZ-associated structural protein 1
- CCI
chronic constriction injury
- Cdc42
cell division cycle 42
- CSF
cerebrospinal fluid
- c-Fos
cellular FBJ-osteosarcmoa
- CRD
cysteine-rich domain
- CREB
cyclic AMP response element binding protein
- DIV
day-in-vitro
- DH
dorsal horn
- DRG
dorsal root ganglion
- EGFP
enhanced green fluorescent protein
- Eph
erythropoietin-producing hepatocellular
- ephrin
Eph interacting protein
- ERC2
ELKS/RAB6-interacting/CAST family member 2
- Erk
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- FNIII
fibronectin type III
- FRAP
fluorescence recovery after photobleaching
- GIT1
G protein receptor-coupled kinase interactor 1
- GPI
glycosylphosphatidylinositol
- GRIP
glutamate receptor-interacting protein
- Grb
growth factor receptor bound-protein
- GDP
guanosine diphosphate
- GEF
guanine nucleotide exchange factor
- GTP
guanosine triphosphate
- HEK-293T
human embryonic kidney cells 293
- IL-1β
interleukin 1β
- JNK
Jun kinase
- KCl
potassium chloride
- LTD
long-term depression
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- MAGUK
Membrane-associated guanylate kinase
- MEK
MAPK/Erk kinase
- mEPSC
miniature excitatory post-synaptic current
- MuSk
muscle-specific kinase
- NMDAR
N-methyl D-aspartate receptor
- PAK
p21-activated kinase
- PDZ
PSD-95, DlgA, zo-1
- PI3K
phosphoinositide-3 kinase
- PICK
protein interacting with PRKCA
- PKA
protein kinase A
- PKC
protein kinase C
- PSD
postsynaptic density
- PS1
presenilin-1
- PSD-95
post-synaptic density protein 95
- PSD-93
postsynaptic density protein 93
- Rac1
Ras-related C3 botulinum substrate
- RGC
retinal ganglion cell
- SAM
sterile-α motif
- SH2
Src homology 2
- shRNA
short-hairpin RNA
- Src
sarcoma
- SAP102
synapse-associated protein 102
- Tiam1
T-cell lymphoma invasion and metastasis 1
- TCI
tibia bone cavity implantation
- TNFα
tumor necrosis factor α
- TKO
triple knockout
- TLR4
Toll-like receptor 4
- TTX
tetrodotoxin
Footnotes
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, et al. (2005). Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci 22, 1445–1454. [DOI] [PubMed] [Google Scholar]
- Antion MD, Christie LA, Bond AM, Dalva MB, and Contractor A (2010). Ephrin-B3 regulates glutamate receptor signaling at hippocampal synapses. Mol Cell Neurosci 45, 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Ting P, Maghsoodi B, Xu N, Henkemeyer M, and Chen L (2007). Postsynaptic ephrinB3 promotes shaft glutamatergic synapse formation. J Neurosci 27, 7508–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JN, Saganich MJ, Xu NJ, Henkemeyer M, Heinemann SF, and Contractor A (2006). B-ephrin reverse signaling is required for NMDA-independent long-term potentiation of mossy fibers in the hippocampus. J Neurosci 26, 3474–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis DN, Behar A, Drougard A, Roullet P, and Davy A (2014). Cortical abnormalities and non-spatial learning deficits in a mouse model of CranioFrontoNasal syndrome. PLoS One 9, e88325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia AA, Sehayek K, Grist J, McMahon SB, and Gavazzi I (2003). EphB receptors and ephrin-B ligands regulate spinal sensory connectivity and modulate pain processing. Nat Neurosci 6, 339–340. [DOI] [PubMed] [Google Scholar]
- Betancur C, Sakurai T, and Buxbaum JD (2009). The emerging role of synaptic celladhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci 32, 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, and Stagi M (2008). Signaling by synaptogenic molecules. Curr Opin Neurobiol 18, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier D, Corera AT, Tremblay ME, Riad M, Chagnon M, Murai KK, Pasquale EB, Fon EA, and Doucet G (2008). Pre-synaptic and post-synaptic localization of EphA4 and EphB2 in adult mouse forebrain. J Neurochem 106, 682–695. [DOI] [PubMed] [Google Scholar]
- Bowen DC, Park JS, Bodine S, Stark JL, Valenzuela DM, Stitt TN, Yancopoulos GD, Lindsay RM, Glass DJ, and DiStefano PS (1998). Localization and regulation of MuSK at the neuromuscular junction. Dev Biol 199, 309–319. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, and Klein R (1999). EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22, 511–524. [DOI] [PubMed] [Google Scholar]
- Burden SJ, Yumoto N, and Zhang W (2013). The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb Perspect Biol 5, a009167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Ruan JP, Ling DY, Guan XH, Bao Q, Yuan Y, Zhang LC, Song XJ, and Zeng YM (2008). Activation of peripheral ephrinBs/EphBs signaling induces hyperalgesia through a MAPKs-mediated mechanism in mice. Pain 139, 617–631. [DOI] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, and Drescher U (2006). Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci 9, 322–330. [DOI] [PubMed] [Google Scholar]
- Cayuso J, Xu Q, and Wilkinson DG (2015). Mechanisms of boundary formation by Eph receptor and ephrin signaling. Dev Biol 401, 122–131. [DOI] [PubMed] [Google Scholar]
- Cibert-Goton V, Yuan G, Battaglia A, Fredriksson S, Henkemeyer M, Sears T, and Gavazzi I (2013). Involvement of EphB1 receptors signalling in models of inflammatory and neuropathic pain. PLoS One 8, e53673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, et al. (2011). Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature 469, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SP, and Mao J (2014). Neuropathic pain: mechanisms and their clinical implications. BMJ 348, f7656. [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, and Heinemann SF (2002). Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science 296, 1864–1869. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, and Woolf CJ (2009). Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, and Henkemeyer M (2001). The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 413, 174–179. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, and Balice-Gordon R (2011). Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 10, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, and Kayser MS (2007). Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci 8, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, and Greenberg ME (2000). EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103, 945–956. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, and Yancopoulos GD (1994). Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science 266, 816–819. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, and Soriano P (2004). Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev 18, 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Mao-Ying QL, Chen JW, Yang CJ, Wang YQ, and Tan ZM (2011). Involvement of EphB1 receptor/ephrinB1 ligand in bone cancer pain. Neurosci Lett 496, 163167. [DOI] [PubMed] [Google Scholar]
- Du J, Tran T, Fu C, and Sretavan DW (2007). Upregulation of EphB2 and ephrin-B2 at the optic nerve head of DBA/2J glaucomatous mice coincides with axon loss. Invest Ophthalmol Vis Sci 48, 5567–5581. [DOI] [PubMed] [Google Scholar]
- Egea J, and Klein R (2007). Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol 17, 230–238. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Tea JS, and Luo L (2014). Mosaic analysis with double markers (MADM) in mice. Cold Spring Harb Protoc 2014, 182–189. [DOI] [PubMed] [Google Scholar]
- Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, and Acker-Palmer A (2008). Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci 11, 1035–1043. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Irie F, Kalo MS, Couchman JR, Pasquale EB, and Yamaguchi Y (2001). EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron 31, 1001–1013. [DOI] [PubMed] [Google Scholar]
- Frank CA, Pielage J, and Davis GW (2009). A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron 61, 556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu AK, Hung KW, Huang H, Gu S, Shen Y, Cheng EY, Ip FC, Huang X, Fu WY, and Ip NY (2014). Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer’s disease. Proc Natl Acad Sci U S A 111, 9959–9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng D, Kang L, Su Y, Jia J, Ma J, Li S, Du J, and Cui H (2013). Protective effects of EphB2 on Abeta1–42 oligomer-induced neurotoxicity and synaptic NMDA receptor signaling in hippocampal neurons. Neurochem Int 63, 283–290. [DOI] [PubMed] [Google Scholar]
- Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, and Lynch DR (2012). Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci 32, 11082–11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, McLenachan S, and Turnley A (2006). Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev 52, 327–345. [DOI] [PubMed] [Google Scholar]
- Grunwald IC, Korte M, Adelmann G, Plueck A, Kullander K, Adams RH, Frotscher M, Bonhoeffer T, and Klein R (2004). Hippocampal plasticity requires postsynaptic ephrinBs. Nat Neurosci 7, 33–40. [DOI] [PubMed] [Google Scholar]
- Grunwald IC, Korte M, Wolfer D, Wilkinson GA, Unsicker K, Lipp HP, Bonhoeffer T, and Klein R (2001). Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron 32, 1027–1040. [DOI] [PubMed] [Google Scholar]
- Guan XH, Lu XF, Zhang HX, Wu JR, Yuan Y, Bao Q, Ling DY, and Cao JL (2010). Phosphatidylinositol 3-kinase mediates pain behaviors induced by activation of peripheral ephrinBs/EphBs signaling in mice. Pharmacol Biochem Behav 95, 315–324. [DOI] [PubMed] [Google Scholar]
- Han Y, Song XS, Liu WT, Henkemeyer M, and Song XJ (2008). Targeted mutation of EphB1 receptor prevents development of neuropathic hyperalgesia and physical dependence on morphine in mice. Mol Pain 4, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamura K, Washburn HR, Sheffler-Collins SI, Xia NL, Henderson N, Tillu DV, Hassler S, Spellman DS, Zhang G, Neubert TA, et al. (2017). Extracellular phosphorylation of a receptor tyrosine kinase controls synaptic localization of NMDA receptors and regulates pathological pain. PLoS Biol 15, e2002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EW, and Cotman CW (1986). Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett 70, 132–137. [DOI] [PubMed] [Google Scholar]
- Henderson JT, Georgiou J, Jia Z, Robertson J, Elowe S, Roder JC, and Pawson T (2001). The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron 32, 1041–1056. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, and Ethell IM (2003). Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol 163, 1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, and Sheng M (2001). Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci 2, 880–888. [DOI] [PubMed] [Google Scholar]
- Himanen JP, and Nikolov DB (2002). Purification, crystallization and preliminary characterization of an Eph-B2/ephrin-B2 complex. Acta Crystallogr D Biol Crystallogr 58, 533–535. [DOI] [PubMed] [Google Scholar]
- Himanen JP, and Nikolov DB (2003). Eph signaling: a structural view. Trends Neurosci 26, 46–51. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Rajashankar KR, Lackmann M, Cowan CA, Henkemeyer M, and Nikolov DB (2001). Crystal structure of an Eph receptor-ephrin complex. Nature 414, 933–938. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, et al. (2010). Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A 107, 10860–10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlushchenko I, Koskinen M, and Hotulainen P (2016). Dendritic spine actin dynamics in neuronal maturation and synaptic plasticity. Cytoskeleton (Hoboken) 73, 435–441. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, and Sheng M (2005). GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci 8, 906–915. [DOI] [PubMed] [Google Scholar]
- Hruska M, and Dalva MB (2012). Ephrin regulation of synapse formation, function and plasticity. Mol Cell Neurosci 50, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska M, Henderson NT, Xia NL, Le Marchand SJ, and Dalva MB (2015). Anchoring and synaptic stability of PSD-95 is driven by ephrin-B3. Nat Neurosci 18, 1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Wei P, Jin L, Zheng T, Chen WY, Liu XY, Shi XD, Hao JR, Sun N, and Gao C (2017). Overexpression of EphB2 in hippocampus rescues impaired NMDA receptors trafficking and cognitive dysfunction in Alzheimer model. Cell Death Dis 8, e2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, and Mucke L (2012). Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL, and Nicoll RA (2013). AMPARs and synaptic plasticity: the last 25 years. Neuron 80, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NK, Thomas GM, Luo J, and Huganir RL (2015). Regulation of AMPA receptor subunit GluA1 surface expression by PAK3 phosphorylation. Proc Natl Acad Sci U S A 112, E5883–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie F, and Yamaguchi Y (2002). EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci 5, 1117–1118. [DOI] [PubMed] [Google Scholar]
- Kania A, and Klein R (2016). Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol 17, 240–256. [DOI] [PubMed] [Google Scholar]
- Katz LC, and Shatz CJ (1996). Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138. [DOI] [PubMed] [Google Scholar]
- Kayser MS, Lee AC, Hruska M, and Dalva MB (2011). Preferential control of basal dendritic protrusions by EphB2. PLoS One 6, e17417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, and Dalva MB (2006). Intracellular and transsynaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci 26, 12152–12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, Nolt MJ, and Dalva MB (2008). EphB receptors couple dendritic filopodia motility to synapse formation. Neuron 59, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R (2009). Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci 12, 15–20. [DOI] [PubMed] [Google Scholar]
- Ko J, Yoon C, Piccoli G, Chung HS, Kim K, Lee JR, Lee HW, Kim H, Sala C, and Kim E (2006). Organization of the presynaptic active zone by ERC2/CAST1-dependent clustering of the tandem PDZ protein syntenin-1. J Neurosci 26, 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Kitamura T, Sekiguchi M, Homma MK, Kabuyama Y, Konno S, Kikuchi S, and Homma Y (2007). Involvement of EphB1 receptor/EphrinB2 ligand in neuropathic pain. Spine (Phila Pa 1976) 32, 1592–1598. [DOI] [PubMed] [Google Scholar]
- Koeppen J, Nguyen AQ, Nikolakopoulou AM, Garcia M, Hanna S, Woodruff S, Figueroa Z, Obenaus A, and Ethell IM (2018). Functional Consequences of Synapse Remodeling Following Astrocyte-Specific Regulation of Ephrin-B1 in the Adult Hippocampus. J Neurosci 38, 5710–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, et al. (2001). Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev 15, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl DJ, Morris CJ, Henkemeyer M, and Parada LF (2003). mRNA expression of ephrins and Eph receptor tyrosine kinases in the neonatal and adult mouse central nervous system. J Neurosci Res 71, 7–22. [DOI] [PubMed] [Google Scholar]
- Lim BK, Cho SJ, Sumbre G, and Poo MM (2010). Region-specific contribution of ephrinB and Wnt signaling to receptive field plasticity in developing optic tectum. Neuron 65, 899–911. [DOI] [PubMed] [Google Scholar]
- Lim BK, Matsuda N, and Poo MM (2008). Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci 11, 160–169. [DOI] [PubMed] [Google Scholar]
- Lin D, Gish GD, Songyang Z, and Pawson T (1999). The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem 274, 3726–3733. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu WT, Liu YP, Dong HL, Henkemeyer M, Xiong LZ, and Song XJ (2011). Blocking EphB1 receptor forward signaling in spinal cord relieves bone cancer pain and rescues analgesic effect of morphine treatment in rodents. Cancer Res 71, 4392–4402. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu YP, Song WB, and Song XJ (2013). EphrinB-EphB receptor signaling contributes to bone cancer pain via Toll-like receptor and proinflammatory cytokines in rat spinal cord. Pain 154, 2823–2835. [DOI] [PubMed] [Google Scholar]
- Liu WT, Han Y, Li HC, Adams B, Zheng JH, Wu YP, Henkemeyer M, and Song XJ (2009). An in vivo mouse model of long-term potentiation at synapses between primary afferent C-fibers and spinal dorsal horn neurons: essential role of EphB1 receptor. Mol Pain 5, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, and Malenka RC (2002). AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25, 103–126. [DOI] [PubMed] [Google Scholar]
- Mao YT, Zhu JX, Hanamura K, Iurilli G, Datta SR, and Dalva MB (2018). Filopodia Conduct Target Selection in Cortical Neurons Using Differences in Signal Kinetics of a Single Kinase. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, Lipton DM, Mandel-Brehm C, Wills ZP, Mardinly AR, Hu L, Greer PL, Bikoff JB, Ho HY, et al. (2010). EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell 143, 442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland AC, Hruska M, Coenen AJ, Henkemeyer M, and Dalva MB (2010). Transsynaptic EphB2-ephrin-B3 interaction regulates excitatory synapse density by inhibition of postsynaptic MAPK signaling. Proc Natl Acad Sci U S A 107, 8830–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland AC, Sheffler-Collins SI, Kayser MS, and Dalva MB (2009). Ephrin-B1 and ephrin-B2 mediate EphB-dependent presynaptic development via syntenin-1. Proc Natl Acad Sci U S A 106, 20487–20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migani P, Bartlett C, Dunlop S, Beazley L, and Rodger J (2009). Regional and cellular distribution of ephrin-B1 in adult mouse brain. Brain Res 1247, 50–61. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, et al. (1998). Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439. [DOI] [PubMed] [Google Scholar]
- Mikasova L, De Rossi P, Bouchet D, Georges F, Rogemond V, Didelot A, Meissirel C, Honnorat J, and Groc L (2012). Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 135, 1606–1621. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Kim D, Knox JA, Johnson E, and Mucke L (2016). Increasing the Receptor Tyrosine Kinase EphB2 Prevents Amyloid-beta-induced Depletion of Cell Surface Glutamate Receptors by a Mechanism That Requires the PDZ-binding Motif of EphB2 and Neuronal Activity. J Biol Chem 291, 1719–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller ML, Shi Y, Reichardt LF, and Ethell IM (2006). EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J Biol Chem 281, 1587–1598. [DOI] [PubMed] [Google Scholar]
- Murai KK, and Pasquale EB (2011). Eph receptors and ephrins in neuron-astrocyte communication at synapses. Glia 59, 1567–1578. [DOI] [PubMed] [Google Scholar]
- Murata Y, and Constantine-Paton M (2013). Postsynaptic density scaffold SAP102 regulates cortical synapse development through EphB and PAK signaling pathway. J Neurosci 33, 5040–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolakopoulou AM, Koeppen J, Garcia M, Leish J, Obenaus A, and Ethell IM (2016). Astrocytic Ephrin-B1 Regulates Synapse Remodeling Following Traumatic Brain Injury. ASN Neuro 8, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov DB, Xu K, and Himanen JP (2013). Eph/ephrin recognition and the role of Eph/ephrin clusters in signaling initiation. Biochim Biophys Acta 1834, 2160–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolt MJ, Lin Y, Hruska M, Murphy J, Sheffler-Colins SI, Kayser MS, Passer J, Bennett MV, Zukin RS, and Dalva MB (2011). EphB controls NMDA receptor function and synaptic targeting in a subunit-specific manner. J Neurosci 31, 5353–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, Foos G, Hauser CA, and Pasquale EB (2006). The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol 8, 815–825. [DOI] [PubMed] [Google Scholar]
- Orikawa Y, Kato H, Seto K, Kobayashi N, Yoshinaga K, Hamano H, Hori Y, Meyer T, and Takei M (2010). Z-360, a novel therapeutic agent for pancreatic cancer, prevents upregulation of ephrin B1 gene expression and phosphorylation of NR2B via suppression of interleukin-1 beta production in a cancer-induced pain model in mice. Mol Pain 6, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A, and Klein R (2003). Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev 17, 1429–1450. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, and Huganir RL (2003). Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron 37, 263–274. [DOI] [PubMed] [Google Scholar]
- Perez de Arce K, Schrod N, Metzbower SW, Allgeyer E, Kong GK, Tang AH, Krupp AJ, Stein V, Liu X, Bewersdorf J, et al. (2015). Topographic Mapping of the Synaptic Cleft into Adhesive Nanodomains. Neuron 88, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planaguma J, Haselmann H, Mannara F, Petit-Pedrol M, Grunewald B, Aguilar E, Ropke L, Martin-Garcia E, Titulaer MJ, Jercog P, et al. (2016). Ephrin-B2 prevents Nmethyl-D-aspartate receptor antibody effects on memory and neuroplasticity. Ann Neurol 80, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas-Ruano A, Perez-Pinzon MA, Green EJ, Henkemeyer M, and Liebl DJ (2006). Distinct roles for ephrinB3 in the formation and function of hippocampal synapses. Dev Biol 292, 34–45. [DOI] [PubMed] [Google Scholar]
- Ruan JP, Zhang HX, Lu XF, Liu YP, and Cao JL (2010). EphrinBs/EphBs signaling is involved in modulation of spinal nociceptive processing through a mitogen-activated protein kinases-dependent mechanism. Anesthesiology 112, 1234–1249. [DOI] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, ErcanSencicek AG, DiLullo NM, Parikshak NN, Stein JL, et al. (2012). De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell, J.W., and Wenthold, R.J. (2000). A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci 20, 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, and Serafini T (2000). Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669. [DOI] [PubMed] [Google Scholar]
- Scholz J, and Woolf CJ (2007). The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10, 1361–1368. [DOI] [PubMed] [Google Scholar]
- Segura I, Essmann CL, Weinges S, and Acker-Palmer A (2007). Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci 10, 301–310. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2002). Alzheimer’s disease is a synaptic failure. Science 298, 789–791. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, and Hardy J (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell GL, Schaffer TB, and Margolis SS (2017). Reducing expression of synapse-restricting protein Ephexin5 ameliorates Alzheimer’s-like impairment in mice. J Clin Invest 127, 1646–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler-Collins SI, and Dalva MB (2012). EphBs: an integral link between synaptic function and synaptopathies. Trends Neurosci 35, 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, and Kim E (2011). The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XD, Sun K, Hu R, Liu XY, Hu QM, Sun XY, Yao B, Sun N, Hao JR, Wei P, et al. (2016). Blocking the Interaction between EphB2 and ADDLs by a Small Peptide Rescues Impaired Synaptic Plasticity and Memory Deficits in a Mouse Model of Alzheimer’s Disease. J Neurosci 36, 11959–11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Pontrello CG, DeFea KA, Reichardt LF, and Ethell IM (2009). Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci 29, 8129–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MA Jr., and Anderson DJ (2001). Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol 230, 139–150. [DOI] [PubMed] [Google Scholar]
- Shirai T, Fujii H, Ono M, Watanabe R, Shirota Y, Saito S, Ishii T, Nose M, and Harigae H (2013). A novel autoantibody against ephrin type B receptor 2 in acute necrotizing encephalopathy. J Neuroinflammation 10, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, de Maturana RL, Ricobaraza A, Escribano L, Schiapparelli L, CuadradoTejedor M, Perez-Mediavilla A, Avila J, Del Rio J, and Frechilla D (2009). Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer’s disease. J Alzheimers Dis 17, 773–786. [DOI] [PubMed] [Google Scholar]
- Slack S, Battaglia A, Cibert-Goton V, and Gavazzi I (2008). EphrinB2 induces tyrosine phosphorylation of NR2B via Src-family kinases during inflammatory hyperalgesia. Neuroscience 156, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloniowski S, and Ethell IM (2012). Looking forward to EphB signaling in synapses. Semin Cell Dev Biol 23, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XJ, Cao JL, Li HC, Zheng JH, Song XS, and Xiong LZ (2008a). Upregulation and redistribution of ephrinB and EphB receptor in dorsal root ganglion and spinal dorsal horn neurons after peripheral nerve injury and dorsal rhizotomy. Eur J Pain 12, 1031–1039. [DOI] [PubMed] [Google Scholar]
- Song XJ, Zheng JH, Cao JL, Liu WT, Song XS, and Huang ZJ (2008b). EphrinBEphB receptor signaling contributes to neuropathic pain by regulating neural excitability and spinal synaptic plasticity in rats. Pain 139, 168–180. [DOI] [PubMed] [Google Scholar]
- Soskis MJ, Ho HY, Bloodgood BL, Robichaux MA, Malik AN, Ataman B, Rubin AA, Zieg J, Zhang C, Shokat KM, et al. (2012). A chemical genetic approach reveals distinct EphB signaling mechanisms during brain development. Nat Neurosci 15, 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiering D, and Hodgson L (2011). Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr 5, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Cerretti DP, and Daniel TO (1996). Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem 271, 23588–23593. [DOI] [PubMed] [Google Scholar]
- Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, and Daniel TO (1998). Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev 12, 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Yasuda S, Katsurabayashi S, Kawano H, Endo K, Takasaki K, Iwasaki K, Ichikawa M, Kobayashi T, Hino O, et al. (2015). Rheb activation disrupts spine synapse formation through accumulation of syntenin in tuberous sclerosis complex. Nat Commun 6, 6842. [DOI] [PubMed] [Google Scholar]
- Sun Q, and Turrigiano GG (2011). PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J Neurosci 31, 6800–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, and Greenberg ME (2002). Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science 295, 491–495. [DOI] [PubMed] [Google Scholar]
- Tarsa L, and Goda Y (2002). Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A 99, 1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, and Greenberg ME (2007). The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A 104, 7265–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, and Yancopoulos GD (1998). PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron 21, 1453–1463. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Kanbayashi T, Tanaka K, Boku S, Ito W, Tokunaga J, Mori A, Hishikawa Y, Shimizu T, and Nishino S (2012). Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, and Anderson DJ (1998). Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor EphB4. Cell 93, 741–753. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ying GX, Liu X, Wang WY, Dong JH, Ni ZM, and Zhou CF (2005). Induction of ephrin-B1 and EphB receptors during denervation-induced plasticity in the adult mouse hippocampus. Eur J Neurosci 21, 2336–2346. [DOI] [PubMed] [Google Scholar]
- Willson CA, Foster RD, Onifer SM, Whittemore SR, and Miranda JD (2006). EphB3 receptor and ligand expression in the adult rat brain. J Mol Histol 37, 369–380. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, and Salter MW (2000). Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769. [DOI] [PubMed] [Google Scholar]
- Wu H, Xiong WC, and Mei L (2010). To build a synapse: signaling pathways in neuromuscular junction assembly. Development 137, 1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybenga-Groot LE, Baskin B, Ong SH, Tong J, Pawson T, and Sicheri F (2001). Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell 106, 745–757. [DOI] [PubMed] [Google Scholar]
- Xia WS, Peng YN, Tang LH, Jiang LS, Yu LN, Zhou XL, Zhang FJ, and Yan M (2014). Spinal ephrinB/EphB signalling contributed to remifentanil-induced hyperalgesia via NMDA receptor. Eur J Pain 18, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NJ, and Henkemeyer M (2009). Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci 12, 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NJ, Sun S, Gibson JR, and Henkemeyer M (2011). A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat Neurosci 14, 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, and Henkemeyer M (2001). Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron 29, 85–97. [DOI] [PubMed] [Google Scholar]
- Yu LN, Zhou XL, Yu J, Huang H, Jiang LS, Zhang FJ, Cao JL, and Yan M (2012). PI3K contributed to modulation of spinal nociceptive information related to ephrinBs/EphBs. PLoS One 7, e40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Niu S, and Horwitz AF (2005). A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci 25, 3379–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yuan G, Cendan CM, Nassar MA, Lagerstrom MC, Kullander K, Gavazzi I, and Wood JN (2010). Nociceptor-expressed ephrin-B2 regulates inflammatory and neuropathic pain. Mol Pain 6, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XL, Wang Y, Zhang CJ, Yu LN, Cao JL, and Yan M (2015a). PKA is required for the modulation of spinal nociceptive information related to ephrinB-EphB signaling in mice. Neuroscience 284, 546–554. [DOI] [PubMed] [Google Scholar]
- Zhou XL, Zhang CJ, Wang Y, Wang M, Sun LH, Yu LN, Cao JL, and Yan M (2015b). EphrinB-EphB signaling regulates spinal pain processing via PKCgamma. Neuroscience 307, 64–72. [DOI] [PubMed] [Google Scholar]
- Zhu XN, Liu XD, Sun S, Zhuang H, Yang JY, Henkemeyer M, and Xu NJ (2016a). Ephrin-B3 coordinates timed axon targeting and amygdala spinogenesis for innate fear behaviour. Nat Commun 7, 11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XN, Liu XD, Zhuang H, Henkemeyer M, Yang JY, and Xu NJ (2016b). Amygdala EphB2 Signaling Regulates Glutamatergic Neuron Maturation and Innate Fear. J Neurosci 36, 10151–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisch AH, Kalo MS, Chong LD, and Pasquale EB (1998). Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene 16, 2657–2670. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, and Bear MF (2012). Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Espinosa JS, Su HH, Muzumdar MD, and Luo L (2005). Mosaic analysis with double markers in mice. Cell 121, 479–492. [DOI] [PubMed] [Google Scholar]