Abstract
Campylobacteriosis is a widespread infectious disease, leading to a major health and economic burden. Chickens are considered as the most common infection source for humans. Campylobacter mainly multiplies in the mucus layer of their caeca. No effective control measures are currently available, but passive immunisation of chickens with pathogen-specific maternal IgY antibodies, present in egg yolk of immunised chickens, reduces Campylobacter colonisation. To explore this strategy further, anti-Campylobacter nanobodies, directed against the flagella and major outer membrane proteins, were fused to the constant domains of chicken IgA and IgY, combining the benefits of nanobodies and the effector functions of the Fc-domains. The designer chimeric antibodies were effectively produced in leaves of Nicotiana benthamiana and seeds of Arabidopsis thaliana. Stable expression of the chimeric antibodies in seeds resulted in production levels between 1% and 8% of the total soluble protein. These in planta produced antibodies do not only bind to their purified antigens but also to Campylobacter bacterial cells. In addition, the anti-flagellin chimeric antibodies are reducing the motility of Campylobacter bacteria. These antibody-containing Arabidopsis seeds can be tested for oral passive immunisation of chickens and, if effective, the chimeric antibodies can be produced in crop seeds.
Introduction
The incidence of campylobacteriosis has been increasing in the last years in both the developed and the developing world [1,2]. The majority of the human infections are caused by Campylobacter jejuni and Campylobacter coli, and broilers, where Campylobacter mainly colonises the intestinal tract [3], are the most common source of infection in industrialised countries. Symptoms of Campylobacter infection are diarrhoea, headache and fever and are mostly self-limiting [4,5]. In some cases, the infection has more severe consequences, like other gastrointestinal illnesses such as inflammatory bowel disease, colorectal cancer and the autoimmune diseases Guillain-Barré and Miller Fisher [1]. Colonisation of broilers by Campylobacter is typically asymptomatic [6]. During the first two to three weeks after hatching, broilers are protected against Campylobacter colonisation by the presence of Campylobacter-specific maternal antibodies. Sahin et al. [7] demonstrated the contribution of maternal antibodies to the absence of Campylobacter bacteria in chicks. Protection ultimately ceased, which led to a rapid spread of Campylobacter within the broiler flock by horizontal transmission, by the faecal-oral route, through feed and water [8]. Effective transmission causes a high prevalence of Campylobacter in broilers at slaughter age, typically at an age of six to seven weeks, leading to a high risk of carcass contamination [9]. Successful protection of broilers against Campylobacter is needed and passive immunisation-based strategies are promising for colonisation control [7,10]. Reduction of the Campylobacter load in the chicken caecum should result in a decrease of the number of human infections [11]. Because no efficient control strategies are available, the potential of novel methods needs more thorough exploration [9,12]. The use of antibiotics in animal feed to control colonisation leads to the rise of resistant strains [13]. The young age at which broilers are slaughtered and the time needed to induce antibody production in case of vaccination complicate the development of an effective vaccine [7,14]. However, previous studies have shown the potential of passive immunisation. Reduction of the C. jejuni count in the caeca of infected chickens was observed after feeding egg yolks, rich in IgY, from C. jejuni-immunised hens [10,15].
Nanobodies (Nb), the antigen-binding domains of camelid heavy-chain antibodies, possess several advantageous characteristics, which make their use as diagnostics and therapeutics interesting. Nanobodies show high affinity, specificity and stability and they can remain functional under harsh chemical and thermal conditions [16]. Because of their extended complementarity determining region 3 (CDR3), they have the capability of binding to buried epitopes and recognising a broader range of epitopes on the antigen [17]. However, single nanobodies are monovalent and are rapidly cleared from the host. These disadvantages can be circumvented by the fusion of nanobodies to the Fc-domain of an immunoglobulin, combining the benefits of a nanobody with an effector function. This leads to multiple valences, which makes agglutination of the bacteria possible [18]. Fusion of the nanobodies to the Fc-domain will lead to an extended half-life in vivo, by an increase in size and interaction with Fc-receptors [19,20] and could result in lowering the doses required for therapeutic treatment [21]. Another advantage of the Nb-Fc constructs is that the fusions are encoded by only one gene. In case of classical antibodies, the genes encoding the light and the heavy chain must be co-expressed [22].
Virdi et al. [23] successfully used nanobodies, generated against the F4-fimbriae of enterotoxigenic E. coli (ETEC), fused to the Fc-domain of pig IgA, for the passive vaccination of piglets against ETEC infections. The chimeric antibodies were expressed in Arabidopsis thaliana seeds and administered to the piglets via their feed. Bacterial colonisation was significantly reduced after challenge with an F4-positive ETEC strain. Expression in plants of recombinant proteins is cost-effective and can easily be scaled-up. Correct folding and the desired post-translational modifications are commonly achieved [24]. Production of recombinant proteins in seeds allows stable storage for long periods [25]. Other advantages are the ease of oral administration of the seeds via the animal feed and the potential protection of the chimeric antibodies against digestion by proteases in the stomach [26].
In this study, a strategy similar to that used by Virdi et al. [23] was followed to produce antibodies that can be applied for passive immunisation of broilers against Campylobacter, by means of chimeric antibodies expressed in seeds of A. thaliana. Campylobacter-specific nanobodies directed against the major outer membrane protein (MOMP) [27] and flagella, both important virulence factors, were selected. The MOMP is crucial for the viability of the bacterial cells and is involved in adhesion to intestinal cells, whereas flagella are essential for motility, colonisation and pathogenesis [28–30]. These nanobodies were fused to the constant domains of chicken immunoglobulins (Ig). The major serum immunoglobulin of chickens is IgY, whereas the antibody most abundantly found in the intestinal tract is IgA [31,32]. In this study, the construction and expression of chimeric IgY and IgA antibodies is described. These were transiently expressed in Nicotiana benthamiana leaves and stably in A. thaliana seeds, under the control of the seed-specific β-phaseolin promoter. We show that these chimeric antibodies recognise not only their respective target antigens but also the Campylobacter bacteria.
Materials and methods
Growth conditions of Campylobacter
C. jejuni KC40 [33] was grown on Nutrient Broth Nr.2 solidified with 1.5% agar (NB2, CM0067; Thermo Fisher Scientific) under microaerobic conditions (Oxoid CampyGen, Thermo Fisher Scientific) for 48 hours at 42°C.
Purification of MOMP and flagella
MOMP was purified from a total membrane extract, essentially as described by Hobb et al. and Bolla et al. [34,35]. Further purification was performed by anion-exchange chromatography, using a Resource Q column (GE Healthcare Life Sciences). The latter was equilibrated with 20 mM sodium phosphate buffer pH 6.0 supplemented with 0.6% poly(ethylene glycol) octyl ether (octyl-POE) and the extracted proteins were loaded on the equilibrated column. A linear gradient to 1 M NaCl was used for elution. The eluted fractions were analysed by SDS-PAGE and Coomassie blue staining. Pure fractions were dialysed against 20 mM sodium phosphate buffer pH 7.6 with 0.6% octyl-POE. Flagellins were isolated from C. jejuni KC40 (S1 File), as described by Logan and Trust [36].
Isolation of nanobodies targeting Campylobacter
Nanobodies against the MOMP (Nb5 and Nb23) [27] and flagella (Nb2Flag8, Nb2Flag24 and Nb2Flag67) of Campylobacter were obtained from a nanobody library by phage display [27,37] (S2 File). A C-terminal histidine-tag was added to the nanobodies, by subcloning the nanobody-encoding genes in the expression vector pHEN6c, a derivative of the pHEN6 vector [38]. The In-Fusion HD Cloning Kit (Takara Bio USA, Inc) was used for the introduction of the nanobodies in the pHEN6c vector, digested with PstI and BstEII. The nanobody-encoding sequences were amplified with the primers IF-NB1 (5’-TGGCCCAGGTGCAGCTGCAGGAGTCTGGAG-3’) and IF-NB2 (5’-TGAGGAGACGGTGACCTGGGTCC-3’). The reaction mix was transformed into CaCl2-competent E. coli DH5α [39] and transformants were selected on LB-agar plates with 100 μg/ml carbenicillin. The expression vector from positive transformants was introduced in E. coli WK6 for expressing the nanobodies. The bacterial cells were grown in the presence of carbenicillin (100 μg/ml) at 37°C. When an OD660 nm 0.6–0.8 was reached, the addition of 1 mM isopropyl β-D-1 thiogalactopyranoside (Thermo Fisher Scientific) led to the expression of the nanobodies. After overnight incubation, a periplasmic extract was prepared and the His-tagged nanobodies were further purified by nickel-affinity chromatography. Therefore, the sample in 20 mM Tris-HCl, 1M NaCl, pH 8.0 was loaded on a HisTrap HP column (GE Healthcare Life Sciences) and nanobodies were eluted using a linear gradient to 1 M imidazole. Finally, pure fractions were stored at -20°C in phosphate-buffered saline (PBS).
Thermal and pH stability of nanobodies
The stability of the nanobodies under different pH conditions was confirmed using SYPRO orange dye with the Thermofluor assay [40]. The details of the experimental approach are extensively described in S3 File.
Fusion of Campylobacter-specific nanobodies to the constant domain of chicken antibodies
Synthetic genes were designed for the fusion of Nb5 to the Fc-domain of chicken IgA [41] and the constant domains of chicken IgY [42], in which the codon usage is optimized for expression in plants (S4 File). The nanobody-encoding sequence was preceded by the signal sequence of the seed storage protein 2S2 of A. thaliana for targeting to the endoplasmic reticulum. At the C-terminus of the synthetic gene, a histidine-tag (His) and a KDEL signal were added (Fig 1). The latter is necessary for retention in the lumen of the endoplasmic reticulum. The attachment sites (attB1 and attB2), at both ends of the synthetic gene, make Gateway recombination possible. A BP reaction was used for cloning of the synthetic gene in the pDONR221 Gateway donor vector (Gateway Technology). The other nanobodies were introduced in the entry clone, encoding the Nb5-Fc fusion, by exchanging Nb5. The latter was removed by restriction of the entry clone with PstI and BstEII. For the amplification of the nanobody-encoding sequences, the primers T-NbS1 and T-NbS2 were used (S4 File). In-Fusion cloning was subsequently applied for the insertion of the sequences encoding Nb23, also directed against the MOMP, and V1, against the F4-fimbriae of enterotoxigenic E. coli [23], that was used as a control. The anti-flagellin nanobodies (Nb2Flag8, Nb2Flag24 and Nb2Flag67) were similarly fused to the Fc-domain of IgA.
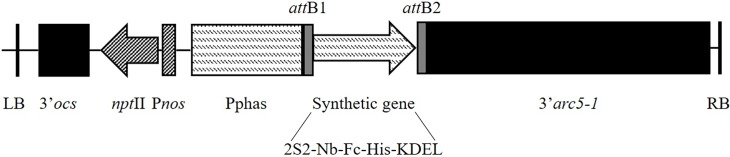
Fig 1. T-DNA construct used for the expression of the chimeric antibodies.
(LB) left border, (3’OCS) octopine synthase terminator, (nptII) neomycin phosphotransferase II gene, (Pnos) nopaline synthase promoter, (Pphas) β-phaseolin promoter, (attB1 & attB2) attachment sites for Gateway recombination, (2S2) signal peptide of the 2S2 seed storage protein, (Nb-Fc) Fusion of anti-Campylobacter nanobody to chicken IgA or IgY, (His) histidine-tag, (KDEL) endoplasmic retention peptide, (3’arc5-1) arcelin terminator and (RB) right border.
Transient expression in N. benthamiana leaves
For transient expression, the plasmid pEAQ-HT-DEST1 (43) was used. The entry clones were introduced in this vector using an LR reaction (Gateway Technology). The resulting clones (Table 1) were transformed via electroporation in A. tumefaciens LBA4404 [44,45]. The A. tumefaciens strains were subsequently used for the infiltration of N. benthamiana leaves [46]. One week later, the infiltrated areas of the leaves were harvested and protein extracts were prepared as described [46].
Table 1. The pEAQ-HT-DEST1 and pPhasGW expression plasmids encoding nanobodies fused to the constant domains of IgA or IgY.
| Plasmid | Characteristics |
|---|---|
| pEAQ-HT | pEAQspecialK with CPMV-HT cassette [43] |
| pEAQ-HT-DEST1 | Gateway-compatible pEAQ-HT destination vector [43] |
| pGV5689 | pEAQ-HT-DEST1 + Nb5-IgY |
| pGV5679 | pEAQ-HT-DEST1 + Nb5-IgA |
| pGV5923 | pEAQ-HT-DEST1 + Nb2Flag8-IgA |
| pGV5925 | pEAQ-HT-DEST1 + Nb2Flag24-IgA |
| pGV5927 | pEAQ-HT-DEST1 + Nb2Flag67-IgA |
| pPhasGW | Gateway-compatible vector [47] |
| pGV5774 | pPhasGW + V1-IgY |
| pGV5768 | pPhasGW + V1-IgA |
| pGV5772 | pPhasGW + Nb5-IgY |
| pGV5778 | pPhasGW + Nb5-IgA |
| pGV5776 | pPhasGW + Nb23-IgY |
| pGV5770 | pPhasGW + Nb23-IgA |
Stable expression in A. thaliana seeds
For stable expression in seeds, the entry clones were inserted in the Gateway-compatible pPhasGW vector (Table 1), via an LR reaction. In the T-DNA, the chimeric genes are under the control of the strong seed-specific β-phaseolin promoter. The T-DNA also encodes the kanamycin resistance gene nptII, allowing selection of transformed plants (Fig 1). The resulting clones were subsequently transformed via electroporation [45] in A. tumefaciens C58C1 RifR (pMP90) [48]. A. thaliana Columbia (Col-0) plants were transformed with A. tumefaciens C58C1 RifR (pMP90) harbouring the corresponding constructs in the pPhasGW vector (pGV5768, pGV5774, pGV5772, pGV5778, pGV5770 and pGV5776) (Table 1) via the floral dip method [49].
Samples (about 10 mg) of the obtained T1 seeds were surface-sterilized by washing with 70% ethanol for 2 minutes, followed by incubation in commercial bleach solution (10°Chl) supplemented with 0.1% Tween-20 for 15 minutes and washed three times with sterile water. Four ml 0.3% agar was added to the sterile seeds and these were plated on 20 ml K1 medium [46] supplemented with Timentin (160 μg/ml), nystatin (50 μg/ml) and kanamycin (50 μg/ml) in 9.4-cm Petri dishes sealed with gas-permeable tape. After storage at 4°C for 48 hours, the dishes were incubated at 24°C under a 16-hours light / 8-hours dark cycle. After 3 weeks, the resistant seedlings were transferred to commercial potting mix in the greenhouse for seed production by spontaneous self-pollination.
From the kanamycin-resistant T1 plants, T2 seeds were harvested and seed extracts were prepared. For each chimeric antibody construct, seeds of twenty plants were tested. As a negative control, protein extractions were performed on seeds of untransformed A. thaliana Columbia plants.
Protein extraction from A. thaliana seeds
For protein extraction, 10 mg seeds were weighed in a 2 ml microcentrifuge tube and two 4 mm stainless steel balls were added. The tubes were frozen in liquid nitrogen and the seeds were pulverised using the Retsch Mixer Mill MM 300 during 2 minutes at 25 Hz. The crushed seeds were resuspended in extraction buffer (50 mM NaH2PO4 pH 7.8, 300 mM NaCl, 10 mM EDTA, 0.1% Tween-20) supplemented with cOmplete Protease Inhibitor Cocktail (Roche Diagnostics) in a 1:100 ratio (mg seeds/μl extraction buffer). The suspension was centrifuged at 20000 g for 5 minutes at 4°C and the supernatant was mixed with glycerol (final concentration 20% v/v) and stored at -20°C.
ELISA
Seed extracts of independent A. thaliana transformants, 100 μl of a 1/20 dilution, were coated in 96-well plates in coating buffer (150 mM Na2CO3, 46 mM NaHCO3). Overnight incubation at 4°C was followed by five wash steps with PBS + 0.05% Tween-20. Then 200 μl 5% bovine serum albumin (BSA) was added to each well and incubated for 2 hours at room temperature. Subsequently, the wells were washed five times and 100 μl goat anti-Chicken IgA (1/5000) or rabbit anti-Chicken IgY (1/1250), both conjugated to HRP (horseradish peroxidase) (Thermo Fischer Scientific), was added to each well and incubated for 1 hour at room temperature. The ELISA was developed by addition of TMB Substrate Solution (Thermo Fisher Scientific). The reaction was stopped using 0.16 M H2SO4 (100 μl/well) and the signal was read at 450 nm.
For the interaction assay of the nanobodies or the chimeric antibodies with their antigen, purified MOMP or flagellins were coated in the 96-well plates at a concentration of 1 μg/ml. Whole-cell ELISA was used to assess their interaction with C. jejuni KC40 bacteria. Bacteria were grown on NB2 medium for 48 hours and harvested with PBS. The cells were pelleted at 3600 g for 15 minutes, resuspended in PBS and fixed by the addition of 2.5% (v/v, final concentration) of methanol-stabilized 37% formaldehyde solution (Merck) and incubation at 42°C for 100 minutes. Then the bacterial cells were pelleted, resuspended in coating buffer and the OD660 was adjusted to 0.3 before coating. After blocking with 200 μl 5% BSA and washing with PBS + 0.05% Tween-20, His-tagged nanobodies (50 μg/ml) or chimeric antibodies were added. Bound His-tagged nanobodies were detected using mouse anti-histidine tag monoclonal antibody (1/1000) (AbD Serotec) and goat anti-mouse IgG conjugated to alkaline phosphatase (AP) (1/5000) (Sigma-Aldrich). The ELISA was developed by adding 100 μl of developer solution, 2 mg/ml para-nitrophenyl phosphate (p-NPP) in ELISA buffer (100 mM Tris-HCl, pH 9.5, 5 mM MgCl2, 100 mM NaCl), and the OD was read at 405 nm. For the detection of bound IgA or IgY chimeric antibodies, goat anti-Chicken IgA (1/5000) or goat anti-Chicken IgY (1/1250), both conjugated to HRP (Abcam), were added.
Western blotting
Protein extracts (200 μl) were TCA-precipitated and resuspended in 20 μl deionised water and 20 μl 2X loading buffer. The TCA-precipitated extracts were boiled and loaded on a 12.5% acrylamide gel. After running, the gel was stained with Coomassie blue dye or the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, which was previously activated with methanol. The PVDF membrane was then washed five times with PBS + 0.2% Triton X-100 and incubated with blocking buffer (PBS + 10% milk powder) for 1 hour at 4°C. The presence of the chimeric antibodies was subsequently detected with mouse anti-histidine tag monoclonal antibody (1/1000) (AbD Serotec) and anti-mouse IgG conjugated to HRP (1/5000). The western blot was developed using the Pierce ECL Western Blotting Substrate. Images were made with the Molecular Imager ChemiDoc XRS+ (Biorad).
Protein-protein interactions were detected by western blotting on native purified antigens (5 μM). The samples were loaded on a 12.5% acrylamide gel in loading buffer without DTT and running buffer composed of 14.4 g glycine, 3.03 g Tris and 0.250 g SDS per litre. Electrophoresis was followed by staining with Coomassie blue dye or transfer to a PVDF membrane. A 1/20 dilution of the extracts was used to examine the interaction with their antigen. Subsequently, the western blot was developed using goat anti-IgA (1/5000) or goat anti-IgY (1/1250) conjugated to HRP (Abcam).
Immunofluorescence microscopy
C. jejuni bacteria were grown microaerobically on NB2 agar plates. After two days, the cells were harvested with NB2 medium and fixed by the addition of 2.5% (v/v, final concentration) of methanol-stabilized 37% formaldehyde solution (Merck). Afterwards, the cells were pelleted and resuspended in PBS. Of the fixed bacterial cells, 10 μl was spotted on 0.1% poly-L-lysine-treated glass slides and incubated for 15 minutes. The slides were treated with 5% BSA for 15 minutes and then 30 μl of anti-Campylobacter nanobodies (50 μg/ml) or undiluted seed extract were added. After 1 hour incubation at room temperature, the bound His-tagged nanobodies were detected using a mouse anti-histidine tag monoclonal antibody (1/200) (AbD Serotec) and anti-mouse IgG conjugated to Alexa Fluor 488 (1/250) (Thermo Fisher Scientific).
The chimeric antibodies in the seed extracts were detected using goat anti-IgA (1/200) or goat anti-IgY (1/200) (Abcam) and anti-goat IgG conjugated to Alexa Fluor 488 (1/250) (Abcam). The antibodies were each time incubated for 30 minutes at room temperature. After each step, the glass slides were washed with PBS. The nanobody V1 was used as a negative control (23). Images were acquired using an inverted epifluorescence microscope (Nikon Eclipse TE2000-U) (Objective = 100x) and a FITC filter block.
Motility assay
A motility assay was performed with the chimeric antibodies. Seed extract containing anti-flagellin chimeric antibodies (± 50 μg/ml) (S1 Table) was pre-incubated with equal volumes of C. jejuni KC40 (OD660 0.3) for 1 hour at room temperature. Of the suspension, 10 μl was spotted on an NB2 plate with 0.4% agar and incubated at 42°C under microaerobic conditions. After 24h, 48h and 72h, the diameter of the bacterial migration zone was measured.
Results
Design of chimeric genes encoding MOMP-recognising antibodies
Nb5 and Nb23 are directed against the MOMP and in a previous study it was shown that these nanobodies have a broad host specificity, interacting with multiple Campylobacter strains [27]. The interaction of the nanobodies with different isolates was confirmed by immunofluorescence microscopy (S1 Fig). The latter makes them an interesting choice for the development of chimeric antibodies. Bivalent nanobody constructs were obtained by the fusion of these nanobodies with the codon-optimised Fc-domain of IgA or the constant domains of IgY. Similar constructs, with the anti-E. coli nanobody V1, were made as controls. This resulted in six constructs: Nb5-IgA, Nb5-IgY, Nb23-IgA, Nb23-IgY, V1-IgA and V1-IgY.
MOMP-specific chimeric antibodies are transiently expressed in leaves
Transient expression in leaves is an excellent method for rapidly assessing whether a gene is expressed and to obtain a small quantity of recombinant protein. For this aim, the Nb5 fusion constructs were transiently expressed in leaves of N. benthamiana by infiltration with A. tumefaciens LBA4404 harbouring the plasmids pGV5679 and pGV5689 (Table 1). As a negative control, leaves were infiltrated with A. tumefaciens LBA4404 containing the pEAQ-HT plasmid. Extracts of the infiltrated leaves were screened for the presence of Nb5-Fc fusion proteins. After SDS-PAGE and Coomassie Blue staining, no clear difference in protein bands was observed between these extracts and the negative control (Fig 2A). Based on the amino acid sequence, molecular weights of approximately 50 and 63 kDa were expected for the IgA and IgY fusion constructs, respectively. Western blot, using an anti-histidine tag monoclonal antibody, showed bands of approximately 60 kDa and 70 kDa, for the IgA and IgY fusion constructs, respectively (Fig 2B). Glycosylation is probably responsible for the higher molecular weight.
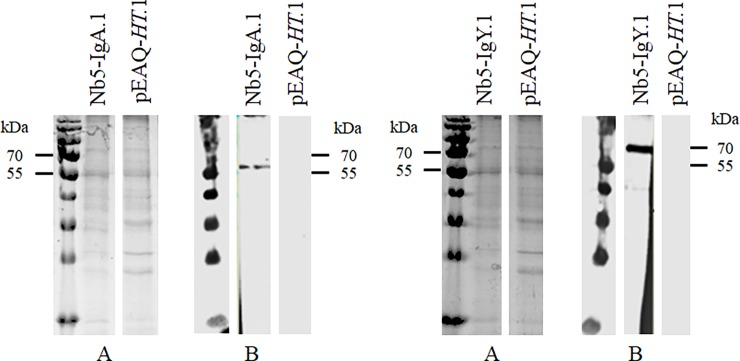
Fig 2. Transient expression of chimeric antibodies carrying Nb5 in leaves of N. benthamiana.
Protein extracts were analysed using (A) SDS-PAGE stained with Coomassie blue dye and (B) western blot developed with mouse anti-histidine tag monoclonal antibody. Extracts of N. benthamiana leaves transformed with Nb5-IgA and Nb5-IgY fusion constructs were tested. Leaves infiltrated with A. tumefaciens harbouring the vector pEAQ-HT were used as negative control.
Stable expression of chimeric antibodies in seeds
The previous results show the feasibility of the expression of the chicken antibody constructs in plants. However, for further work, larger quantities are required. Therefore, the chimeric genes, encoding V1-IgA, V1-IgY, Nb5-IgA, Nb5-IgY, Nb23-IgA and Nb23-IgY, were stably expressed in A. thaliana seeds under the control of the β-phaseolin promoter.
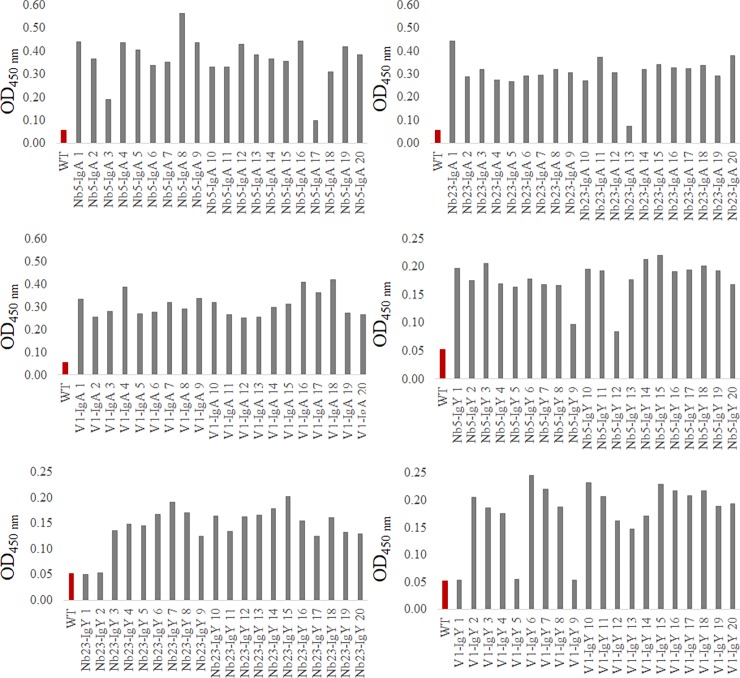
The expression of Nb-IgA and Nb-IgY constructs in T2 seeds was tested via ELISA, using polyclonal anti-IgA or anti-IgY antibodies, respectively (Fig 3). Except for a few transformants, all extracts were positive in ELISA. The variations in expression levels between most of the positive seed extracts were small. Positive transformants were chosen for further experiments.
Fig 3. Expression of chimeric antibodies in transgenic A. thaliana seeds.
Extracts of T2 seeds were coated and analysed by ELISA. The presence of chimeric antibodies in the extracts was tested, using anti-chicken IgA or IgY conjugated to HRP. As a negative control, extract of wild-type A. thaliana seeds was used. The results of seed extracts from A. thaliana plants transformed with Nb5-IgA, Nb23-IgA, V1-IgA, Nb5-IgY, Nb23-IgY and V1-IgY are shown.
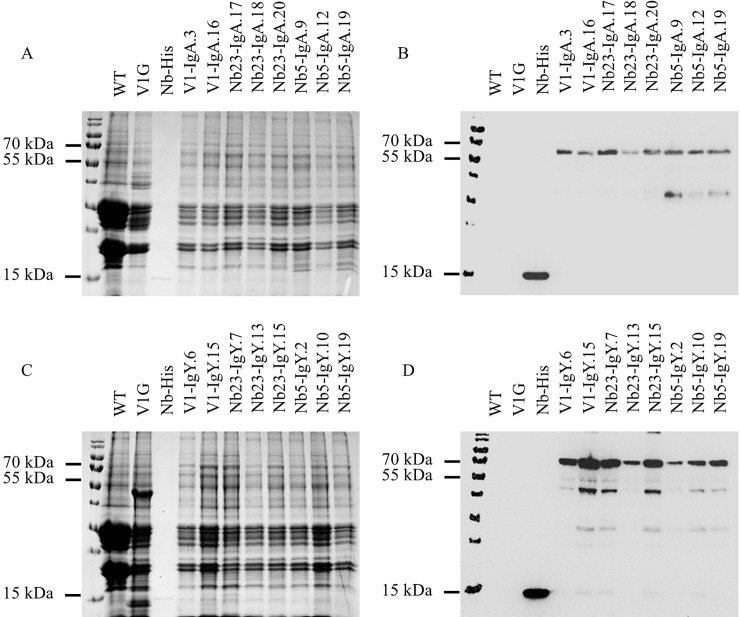
Seed extracts were analysed by SDS-PAGE and western blot, to confirm expression (Fig 4). Like in the results of the transient expression, no clear differences in band pattern were observed after Coomassie Blue staining, between the extract of wild-type A. thaliana seeds and the ones transformed with any of the six chimeric antibody constructs. However, the western blot with mouse anti-histidine tag monoclonal antibody clearly confirmed the presence of the chimeric antibodies in the seeds of transformed plants. Besides the intact protein, lower molecular weight bands were also observed, presumably as a consequence of proteolytic activity. An additional negative control, used in this western blot, was the V1G chimeric antibody, lacking the His-tag, fused with the Fc of porcine IgG, directed against F4-positive enterotoxigenic E. coli [23]. As a positive control, a His-tagged nanobody was used, that produced a protein band with a molecular weight of approximately 15 kDa.
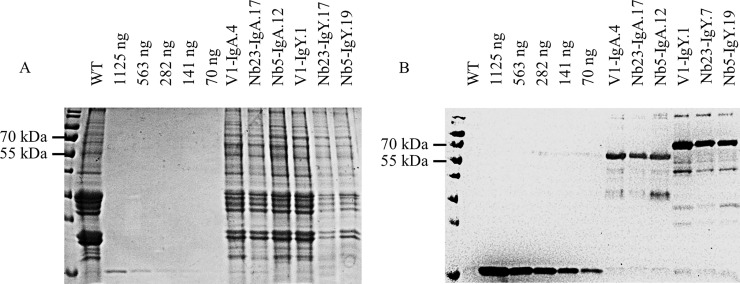
Fig 4.
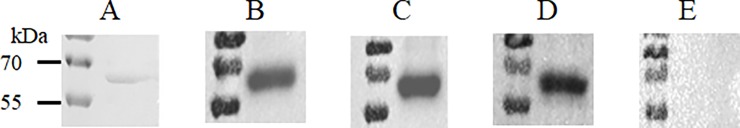
Screening for the expression of chimeric antibodies in A. thaliana seeds using SDS-PAGE (A, C) and western blot (B, D). The expression of chimeric antibodies in seeds was confirmed for extracts positive in ELISA. Western blots were developed with a mouse anti-histidine tag monoclonal antibody and a goat anti-mouse antibody conjugated to HRP. As negative controls, extract of wild-type seeds and the V1G chimeric antibody lacking the His-tag [23] were used. A His-tagged nanobody was used as a positive control, resulting in a protein band with a molecular weight of approximately 15 kDa. (A) IgA chimeric antibodies with V1, Nb23 and Nb5, (C) IgY chimeric antibodies with V1, Nb23 and Nb5.
The use of an anti-His antibody, for the screening of IgA as well as IgY constructs makes it possible to compare the quantity of chimeric antibodies present in the extracts. For this aim, an SDS-PAGE and a western blot, developed with a mouse anti-histidine tag monoclonal antibody were used (Fig 5). Concentrations were determined by comparison of the intensity of the band of a His-tagged nanobody, having a known concentration (1.2 mg/ml), with that of the band corresponding to chimeric antibodies, using the ImageJ program (https://imagej.nih.gov/ij/). On this basis, expression levels between 1 and 8% of the total soluble proteins (TSP) were estimated for the four chimeric antibodies (Table 2).
Fig 5. Determination of the concentration of chimeric antibodies present in seed extracts.
Intensities on (A) Coomassie blue stained SDS-PAGE and (B) western blot of protein bands corresponding to a His-tagged nanobody and the ones corresponding to chimeric antibodies were compared. A serial dilution of a His-tagged nanobody with a concentration of 1.2 mg/ml was made. As a negative control, seed extract of wild-type A. thaliana plants was used. The western blot was developed with a mouse anti-histidine tag monoclonal antibody and goat anti-mouse IgG conjugated to HRP.
Table 2. Chimeric antibody concentration in extracts of transformed A. thaliana seeds.
| Chimeric antibody | Concentration (μg/ml) | μg/mg seed | %TSP* |
|---|---|---|---|
| Nb5-IgA | 24.3 | 2.4 | 1.2 |
| Nb23-IgA | 21.6 | 2.2 | 1.1 |
| Nb5-IgY | 92.7 | 9.3 | 4.6 |
| Nb23-IgY | 156.4 | 15.6 | 7.8 |
*TSP: total soluble protein.
In seed produced chimeric antibodies bind native MOMP and C. jejuni bacteria
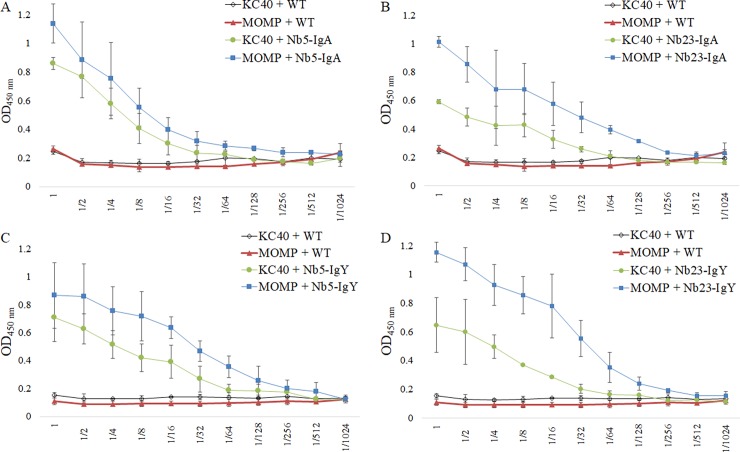
To verify whether the chimeric antibodies produced in seeds bind their corresponding recombinant antigens and C. jejuni bacteria, ELISA was performed (Fig 6). The binding between coated purified MOMP or KC40 bacterial cells and twofold serial dilutions of the seed extracts, ranging from undiluted to 1/1024, was measured. Extract of non-transformed A. thaliana seeds was used as a negative control. The obtained binding curves show interaction at low concentration of the chimeric antibodies with the bacteria as well as with the purified MOMP. The same downward trend was observed in both cases. The binding of the chimeric antibodies with C. jejuni KC40 was confirmed by immunofluorescence microscopy (Fig 7). Results also indicated the binding of the chimeric antibodies with two additional Campylobacter isolates, the C. coli isolate K43/5 and the human clinical C. jejuni isolate Cam12/0156. The negative control, the V1 nanobody fused to chicken IgA or IgY, showed no interaction with the selected isolates.
Fig 6. Binding of chimeric antibodies to C. jejuni KC40 bacteria and purified MOMP.
KC40 bacteria and MOMP (1 μg/ml) were coated in an ELISA plate. Subsequently, the interaction of twofold serial dilutions of the seed extracts was assessed. Therefore, anti-IgA and anti-IgY antibodies, conjugated to HRP, were used for the detection of chimeric antibodies, bound to the bacteria or to the purified native MOMP antigen. As a negative control, the binding of extracts of wild-type A. thaliana seeds with the bacteria and MOMP was measured. (A) Nb5-IgA, (B) Nb23-IgA, (C) Nb5-IgY and (D) Nb23-IgY. The error bars correspond to the standard deviations.
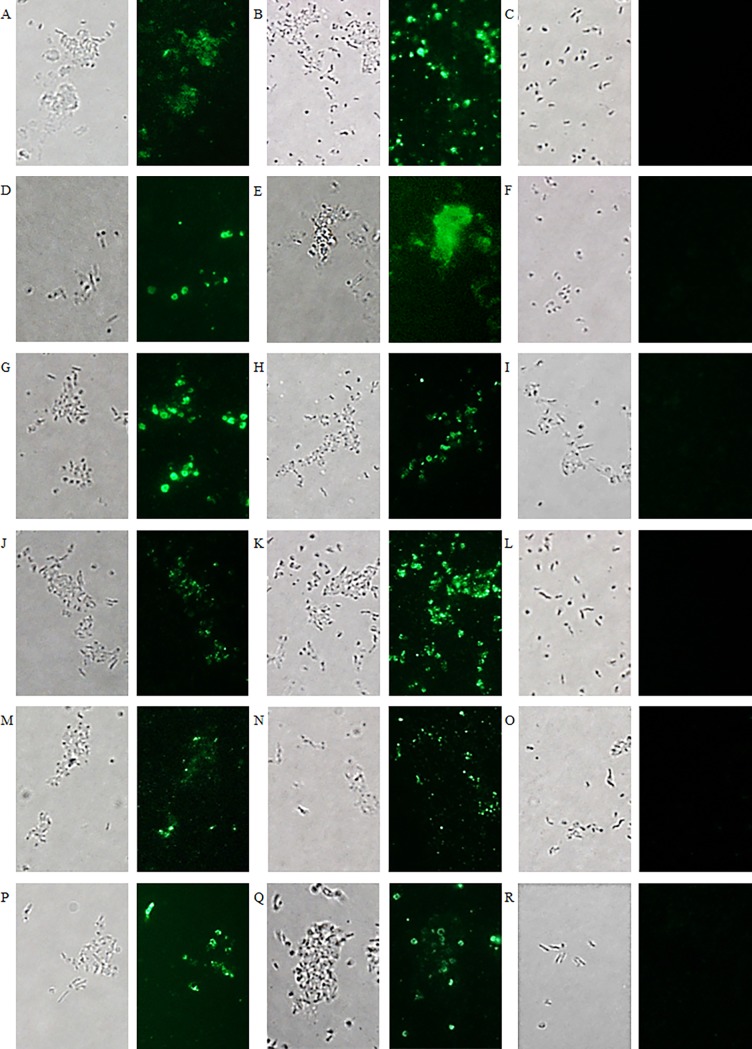
Fig 7. Visualisation of the binding of chimeric anti-MOMP antibodies in seed extract with Campylobacter isolates.
(A, B, C, J, K, L) C. jejuni strain KC40, (D, E, F, M, N, O) C. jejuni strain Cam12/0156 and (G, H, I, P, Q, R) C. coli strain K43/5. Binding with the different Campylobacter isolates is shown with seed extract containing (A, D, G) Nb5-IgA, (B, E, H) Nb23-IgA, (C, F, I) V1-IgA, (J, M, P) Nb5-IgY, (K, N, Q) Nb23-IgY and (L, O, R) V1-IgY. As a negative control, the nanobody V1 against F4-fimbriated E. coli was used. Bright field microscopy was used for the visualisation of the corresponding bacterial cells.
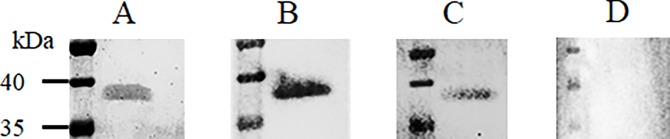
Comparable expression levels were obtained for the Nb23 chimeric antibodies in the T4 seeds of the obtained homozygous and heterozygous plants in ELISA (S2 Fig). The interaction of the chimeric antibodies in the seed extracts of the homozygous plants with the MOMP protein was confirmed in a western blot (Fig 8). Because nanobodies typically interact with conformational epitopes [37,50–52], a non-denaturing SDS-PAGE was used. Native MOMP corresponds in SDS-PAGE with a protein band with an apparent molecular weight of 38 kDa. A clear band was observed when seed extract was used of plants transformed with the Nb23-IgA and Nb23-IgY constructs, confirming the interaction.
Fig 8. Interaction of chimeric antibodies in seed extract from homozygous plants with their antigen.
SDS-PAGE and a western blot were performed on purified MOMP under non-denaturing conditions. (A) SDS-PAGE with purified native MOMP stained with Coomassie blue dye. The results of the western blot confirm the interactions of (B) Nb23-IgA14D and (C) Nb23-IgY12C, with MOMP. (D) Wild-type extract was used as a negative control. The western blot was developed with anti-IgA or anti-IgY antibodies conjugated to HRP.
The anti-flagellin nanobodies bind to C. jejuni bacterial cells
Purified flagella (S1 File) were used for the isolation of clones encoding anti-flagellin nanobodies from the nanobody library (S2 File). After panning, three anti-flagellin nanobodies (Nb2Flag8, Nb2Flag24 and Nb2Flag67) (Figure C in S2 File) were selected. ELISA confirmed the interaction of the three nanobodies with purified flagella and motile C. jejuni KC40 bacteria (S3 Fig). Immunofluorescence microscopy validated this result (Fig 9) and also revealed their binding with the human clinical C. jejuni isolate Cam12/0156, and the C. coli isolate K43/5. This indicates that these nanobodies may interact with conserved regions of the flagellins. The V1 nanobody, directed against the FaeG subunit of E. coli F4 fimbriae, showed no interaction.
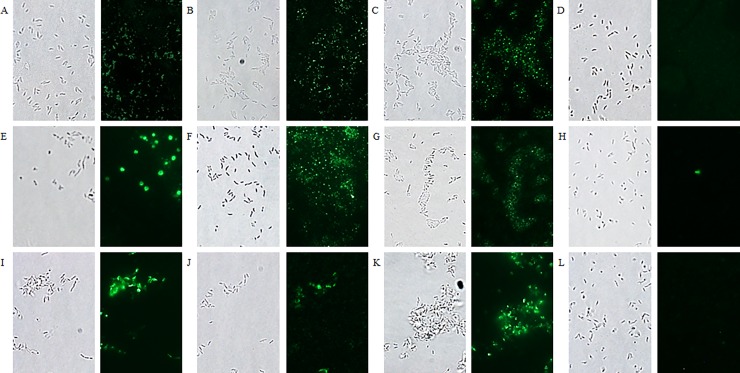
Fig 9. Fluorescence microscopy visualising the binding of labelled anti-flagellin nanobodies with different Campylobacter isolates.
(A, B, C, D) C. jejuni strain KC40, (E, F, G, H) C. jejuni strain Cam12/0156 and (I, J, K, L) C. coli strain K43/5. Interaction with the different isolates is shown with (A, E, I) Nb2Flag8, (B, F, J) Nb2Flag24 and (C, G, K) Nb2Flag67. (D, H, L) The negative control, fluorescently labelled V1 directed against F4-fimbriated E. coli, did not bind with the Campylobacter bacteria. The corresponding bacterial cells were visualised by bright field microscopy.
Transient expression of anti-flagellin nanobodies anchored to the Fc region of chicken IgA in leaves
Nanobodies (Nb2Flag8, Nb2Flag24 and Nb2Flag67) directed against the flagellins of C. jejuni were fused to the Fc-domain of chicken IgA and cloned in the pEAQ-HT-DEST1 vector. The obtained expression vectors (pGV5923, pGV5925 and pGV5927) (Table 1) were transformed into A. tumefaciens LBA4404, which was subsequently used for infiltration of N. benthamiana leaves. Extracts of these leaves were analysed for the presence of chimeric antibodies via ELISA and western blotting. ELISA plates were coated with a serial dilution of the leaf extracts (Fig 10A). Development, using anti-IgA conjugated to HRP, clearly confirmed the transient expression of the chimeric antibodies in leaves. The ELISA results indicated no significant difference in expression between the three constructs. The interaction of the chimeric antibodies with purified flagellins and motile C. jejuni KC40 was confirmed by ELISA (Fig 10C and 10D). To test the interaction with flagellins and bacterial cells, serial dilutions of the extracts were used. In both cases, clear dosage-dependent binding was observed. The results show a lower binding for the extract containing Nb2Flag67-IgA chimeric antibodies, while previous ELISA results showed that the Nb2Flag67 nanobody is not a poorer binder than Nb2Flag8 or Nb2Flag24 (S3 Fig). This can possibly be explained by the more extensive degradation of the Nb2Flag67-IgA construct in the leaf extract, which was observed in the western blot (Fig 10B). The bands of lower molecular weight show extensive proteolytic degradation. The interaction of the chimeric antibodies with native purified flagellins was confirmed in a non-denaturing western blot (Fig 11). The flagellins correspond with the protein band of an apparent molecular weight of 63 kDa.
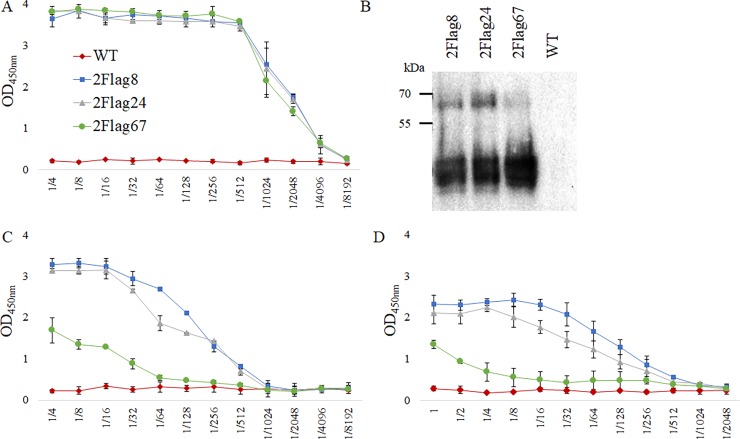
Fig 10. Transient expression of anti-flagellin chimeric antibodies in N. benthamiana leaves and the interaction with purified flagellins and motile C. jejuni KC40.
(A) ELISA for the detection of chimeric antibodies in serial dilutions (1/4–1/8192) of extracts of infiltrated leaves. (B) Western blot results of leaf extracts with Nb2Flag8, Nb2Flag24 and Nb2Flag67 nanobodies fused to the Fc-domain of chicken IgA. The western blot was developed with anti-IgA conjugated to HRP. As a negative control, leaf extract of wild-type N. benthamiana was used. (C) Binding curve of twofold serial dilutions (1/4–1/8192) of leaf extract with coated purified flagellins. (D) Binding curve of twofold serial dilutions (undiluted– 1/2048) of leaf extract with coated C. jejuni KC40 bacteria. Anti-IgA conjugated to HRP was used for the development of the ELISA.
Fig 11. Visualisation of the interaction of chimeric antibodies in extracts of N. benthamiana leaves with purified flagellins.
Non-denatured purified flagellins were used in SDS-PAGE and a western blot. (A) Purified flagellins on SDS-PAGE stained with Coomassie blue dye. Western blotting confirms the interactions of (B) Nb2Flag8-IgA, (C) Nb2Flag24-IgA and (D) Nb2Flag67-IgA. (E) Wild-type extract was used as a negative control. Anti-IgA antibodies conjugated to HRP were used for the development of the western blot.
Motility of Campylobacter is reduced by anti-flagellin chimeric antibodies produced in seeds
Similarly as for the expression of the anti-MOMP antibodies, the constructs encoding anti-flagellin Nb-IgA antibodies were cloned in the pPhasGW vector. The resulting plasmids (pGV5923, pGV5926 and pGV5928) (Table 1) were transformed into A. tumefaciens C58C1 RifR (pMP90). The resulting strains were used for transformation of A. thaliana Col-0 using the floral dip method [49]. Three plants were transformed with each construct. The T2 seeds of ten kanamycin-resistant plants were analysed for each anti-flagellin chimeric antibody (Fig 12).
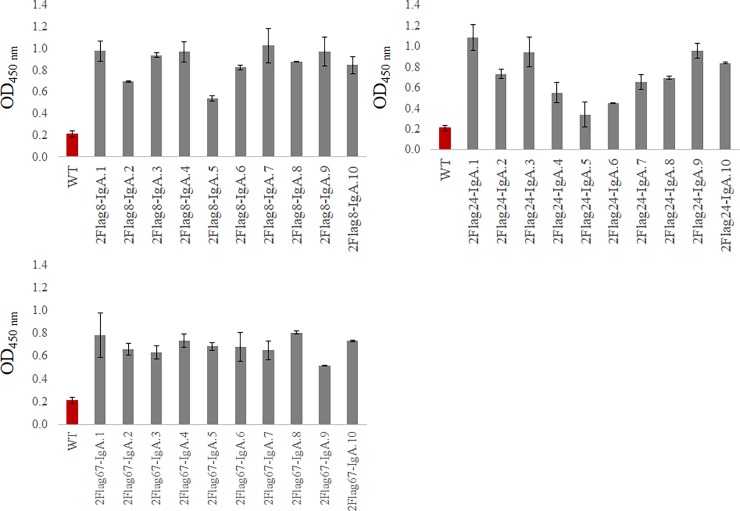
Fig 12. Expression of chimeric antibodies in transgenic A. thaliana seeds.
Extracts of T2 seeds were analysed by ELISA. The presence of chimeric antibodies in the extracts was tested, using anti-chicken IgA conjugated to HRP. An extract of wild-type A. thaliana seeds was used as a negative control. The error bars represent the standard deviation.
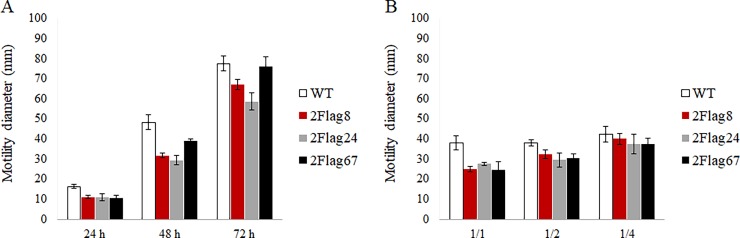
Seed extracts containing the anti-flagellin antibodies were used in a soft-agar assay to assess the influence of the chimeric antibodies on the motility of Campylobacter bacteria (Fig 13). C. jejuni KC40 bacteria were incubated with extract containing Nb2Flag8-IgA.1, Nb2Flag24-IgA.1 and Nb2Flag67-IgA.1. An extract of wild-type Arabidopsis thaliana Col-0 seeds was used as a control. After 48 hours, a reduction was observed in the bacterial motility for the three chimeric antibodies (Fig 13A). The influence of different dilutions of the chimeric antibodies on the Campylobacter motility after 48 hours of incubation was analysed. Fig 13B shows that the inhibitory effect of the chimeric antibodies diminished when the seed extracts were diluted.
Fig 13. Motility of C. jejuni KC40 after incubation with chimeric antibodies produced in seeds.
The bacterial spread through the soft-agar, represented by the diameter of the circle, was measured. (A) The mean diameter (mm ± SD) was plotted versus the incubation time. (B) The influence of different dilutions (1/1–1/4) of the seed extract containing the chimeric antibodies on the motility of Campylobacter after 48 h, was analysed. The mean diameter (mm ± SD) was plotted.
Discussion
Passive immunisation, using pathogen-specific antibodies, can be successfully applied in human and veterinary medicine. Oral delivery of IgY antibodies, derived from chicken eggs, effectively inhibited colonisation of broilers by different pathogens, such as Salmonella and Campylobacter [10,53–55]. Specific egg-derived IgY antibodies protected piglets against enterotoxigenic E. coli [56]. Passive immunisation using IgA derivatives, also protected against enterotoxigenic E. coli [23,57].
Nanobody-based chimeric antibodies have been developed in this study for a similar passive immunisation strategy to prevent colonisation of chickens by Campylobacter or reducing pathogen loads in the intestinal tract. Recombinant bivalent chimeric heavy-chain only antibodies were constructed, via fusion of anti-Campylobacter nanobodies with the effector domains of IgA or IgY. Nanobodies against the MOMP and flagella of Campylobacter were isolated and shown to interact with different Campylobacter strains. The latter can be important, because broilers can be colonised by a large variety of Campylobacter strains [58,59]. MOMP is a virulence factor and has an essential transport and structural function [30,60]. The ability of multimers of these anti-MOMP nanobodies to agglutinate Campylobacter bacteria, has been demonstrated [27]. Another important virulence factor is the flagellum, required for motility, chemotaxis and transport of non-flagellar proteins [61–63]. Nanobodies directed against Campylobacter flagellin, fused to a pentameric protein, were shown to inhibit the motility of Campylobacter and the colonisation of chicks [64].
Chimeric antibodies, directed against MOMP or flagella, were produced in plants as additional tools to develop novel strategies for the protection of chicks against Campylobacter colonisation. Easy genetic manipulation is possible in plants and the absence of endotoxins and mammalian pathogens are an advantage for biosecurity and for therapeutic use [65,66]. The cost of expression of recombinant proteins in plants, is estimated to be lower compared with other production platforms [67]. Transient expression of the constructs in leaves of N. benthamiana showed that antibodies recognising their respective antigens were produced at low expression levels. In addition, smaller proteins, possibly generated by proteolytic degradation, were also observed in the western blots with monoclonal anti-His antibody, notwithstanding the use of a cocktail of proteinase inhibitors in the extraction buffer. Proteolytic degradation is also observed in other studies [23,68]. Whether these degraded proteins are present in the plant tissues or are generated by residual proteolytic activity during extraction is unclear.
For passive immunisation of broilers, sufficient amounts of antibodies are required. Therefore, the genes encoding the chimeric antibodies were expressed in seeds of transgenic Arabidopsis plants. Seeds are ideal for the expression and storage of recombinant proteins because they are natural storage organs of the plant, containing large quantities of proteins, and show low protease activity during storage [69]. Oral administration of pea seeds expressing a single chain Fv antibody mitigated Eimeria infections in chickens [26]. Also, addition of A. thaliana seeds expressing a chimeric porcine IgA directed against a fimbrial adhesin to the feed was already used successfully to protect piglets against enterotoxigenic E. coli infection [23]. No adverse effects were reported in these studies. The safety and the performance of genetically modified crops in diets of broiler chickens and laying hens were evaluated in several poultry nutrition studies. These studies showed that transgenic crops provided comparable performance, carcass and egg yields, and meat and egg composition, when compared with conventional grains (reviewed in Tufarelli et al., 2015) [70]. Other studies also evaluated whether foreign DNA and proteins could be detected in meat, egg, and tissue samples from broiler chickens and laying hens fed diets containing transgenic crops. None of these studies could detect transgenic DNA or proteins in food products derived from these animals, using the most sensitive detection methods available (reviewed in Tufarelli et al., 2015) [70].
The results of this study show that the chimeric antibodies were produced in comparable quantities in seeds of heterozygous as well as homozygous plants. With a few exceptions, the variation of the expression levels was relatively low and little degradation products were observed in western blots. The accumulation level of the IgA chimeric antibodies in Arabidopsis seeds is around 1% of total soluble protein (TSP), and for the IgY chimeric antibodies yields of up to 8% TSP were obtained. These results are comparable with those observed previously [71,72]. ELISA and western blot confirmed that the chimeric antibodies in seed extracts recognise the corresponding purified antigen and also bind intact Campylobacter bacteria. The motility assay showed a significant reduction of the motility of C. jejuni KC40 in the presence of anti-flagellin chimeric antibodies produced in planta. Higher concentrations of the chimeric antibodies or the addition of the antibodies to the soft agar medium may lead to an higher reduction.
In conclusion, functional chimeric antibodies, recognising flagella and MOMP, were successfully produced in N. benthamiana leaves and A. thaliana seeds. The homozygous transgenic lines were upscaled to obtain sufficient quantities of transgenic seeds to test the in vivo effect of passive immunisation on colonisation of chickens by Campylobacter. If the results are positive, the chimeric antibodies can be expressed in seeds of crop plants, to produce amounts of antibodies needed in field conditions.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
Immunofluorescence microscopy confirms the interaction of Nb5 and Nb23 with Campylobacter isolates. (A, B, C) C. jejuni strain KC40, (D, E, F) C. jejuni strain Cam12/0156 and (G, H, I) C. coli strain K43/5. The binding of Nb5 is shown in A, D and G and the binding of Nb23 in B, E and H. As a negative control, the fluorescently labelled (C, F, I) V1 nanobody was used.
(TIF)
ELISA was used for the analysis of seed extracts from A. thaliana plants transformed with (A) Nb23-IgA constructs and (B) Nb23-IgY constructs. The results of the extracts of the homozygous plants are visualised by the histogram with hatched shading. Extract of wild-type A. thaliana seeds was used as a negative control. The ELISA was developed using anti-IgA or anti-IgY conjugated to HRP. The error bars correspond to the standard deviation.
(TIF)
ELISA for the confirmation of the interaction of anti-flagellin nanobodies with (A) purified flagellins and (B) C. jejuni KC40. Bound His-tagged nanobodies were detected with mouse anti-histidine monoclonal antibodies and goat anti-mouse IgG. The error bars correspond to the standard deviation.
(TIF)
(PDF)
Acknowledgments
We thank F. De Meyer, F. Verlé and G. Raeymaekers for their help and J. Nolf and F. Deboeck for excellent technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research that yielded these results, was funded by the Belgian Federal Public Service of Health, Food Chain Safety and Environment (www.health.belgium.be) through the contract RF 13/6272 CampyNanoCure. The funding was received by HDG, JPH, FP, AM and FH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev. 2015;28(3):687–720. 10.1128/CMR.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014 European Food Safety Authority European Centre for Disease Prevention and Control. EFSA J. 2014;13(12):4329. [Google Scholar]
- 3.Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl Enviromental Microbiol. 2003;69(8):4343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis. 1997;176(Suppl 2):S103–5. [DOI] [PubMed] [Google Scholar]
- 5.Moore JE, Corcoran D, Dooley JSG, Fanning S, Lucey B, Matsuda M, et al. Campylobacter. Vet Res. 2005;36(3):351–82. 10.1051/vetres:2005012 [DOI] [PubMed] [Google Scholar]
- 6.Näther G, Alter T, Martin A, Ellerbroek L. Analysis of risk factors for Campylobacter species infection in broiler flocks. Poult Sci. 2009;88(6):1299–305. 10.3382/ps.2008-00389 [DOI] [PubMed] [Google Scholar]
- 7.Sahin O, Luo N, Huang S, Zhang Q. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl Enviromental Microbiol. 2003;69(9):5372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MD, Newell DG. Campylobacter in poultry: filling an ecological niche. Avian Dis. 2006. March;50(1):1–9. 10.1637/7474-111605R.1 [DOI] [PubMed] [Google Scholar]
- 9.Lin J. Novel approaches for Campylobacter control in poultry. Foodborne Pathog Dis. 2009. September;6(7):755–65. 10.1089/fpd.2008.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans D, Van Steendam K, Verbrugghe E, Verlinden M, Martel A, Seliwiorstow T, et al. Passive immunization to reduce Campylobacter jejuni colonization and transmission in broiler chickens. Vet Res. 2014;45:27 10.1186/1297-9716-45-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messens W, Hartnett E, Gellynck X, Viaene J, Halet D, Herman L, et al. Quantitative risk assessment of human campylobacteriosis through the consumption of chicken meat in Belgium. 18th Eur Symp Qual Poult Meat; 12th Eur Symp Qual Eggs Egg Prod. 2007;167–8. [Google Scholar]
- 12.Hermans D, Van Deun K, Messens W, Martel A, Van Immerseel F, Haesebrouck F, et al. Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Vet Microbiol. 2011. September;152(3–4):219–28. 10.1016/j.vetmic.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 13.Moore JE, Barton MD, Blair IS, Corcoran D, Dooley JSG, Fanning S, et al. The epidemiology of antibiotic resistance in Campylobacter. Microbes Infect. 2006;8(7):1955–66. 10.1016/j.micinf.2005.12.030 [DOI] [PubMed] [Google Scholar]
- 14.de Zoete MR, van Putten JPM, Wagenaar JA. Vaccination of chickens against Campylobacter. Vaccine. 2007;25(30):5548–57. 10.1016/j.vaccine.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 15.Al-Adwani SR, Crespo R, Shah DH. Production and Evaluation of Chicken Egg-Yolk-Derived Antibodies Against Campylobacter jejuni Colonization-Associated Proteins. Foodborne Pathog Dis. 2013;10(7):624–31. 10.1089/fpd.2012.1313 [DOI] [PubMed] [Google Scholar]
- 16.Muyldermans S, Baral TN, Retamozzo VC, De Baetselier P, De Genst E, Kinne J, et al. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol. 2009;128(1–3):178–83. 10.1016/j.vetimm.2008.10.299 [DOI] [PubMed] [Google Scholar]
- 17.Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 2007;77(1):13–22. 10.1007/s00253-007-1142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche AM, Richard AL, Rahkola JT, Janoff EN, Weiser JN. Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 2015;8(1):176–85. 10.1038/mi.2014.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontermann RE. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs. 2009;23(2):93–109. 10.2165/00063030-200923020-00003 [DOI] [PubMed] [Google Scholar]
- 20.Hutt M, Färber-Schwarz A, Unverdorben F, Richter F, Kontermann RE. Plasma half-life extension of small recombinant antibodies by fusion to immunoglobulin-binding domains. J Biol Chem. 2012;287(7):4462–9. 10.1074/jbc.M111.311522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Current Opinion in Biotechnology. 2011. p. 22:868–876. 10.1016/j.copbio.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 22.Schirrmann T, Al-halabi L, Dübel S, Hust M. Production systems for recombinant antibodies. Front Biosci. 2008;13(13):4576–94. [DOI] [PubMed] [Google Scholar]
- 23.Virdi V, Coddens A, De Buck S, Millet S, Goddeeris BM, Cox E, et al. Orally fed seeds producing designer IgAs protect weaned piglets against enterotoxigenic Escherichia coli infection. Proc Natl Acad Sci U S A. 2013;110(29):11809–14. 10.1073/pnas.1301975110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yusibov V, Kushnir N, Streatfield SJ. Antibody production in plants and green algae. Annu Rev Plant Biol. 2016;67(1):669–701. [DOI] [PubMed] [Google Scholar]
- 25.Rademacher T, Arcalis E, Stoger E. Production and localization of recombinant pharmaceuticals in transgenic seeds. Faye L, Gomord V, editors. Methods Mol Biol. 2009;483:69–87. 10.1007/978-1-59745-407-0_5 [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann J, Saalbach I, Jahn D, Giersberg M, Haehnel S, Wedel J, et al. Antibody expressing pea seeds as fodder for prevention of gastrointestinal parasitic infections in chickens. BMC Biotechnol. 2009;9:79 10.1186/1472-6750-9-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanmarsenille C, Díaz del Olmo I, Elseviers J, Hassanzadeh Ghassabeh G, Moonens K, Vertommen D, et al. Nanobodies targeting conserved epitopes on the major outer membrane protein of Campylobacter as potential tools for control of Campylobacter colonization. Vet Res. 2017;48(1):86 10.1186/s13567-017-0491-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007;15(10):456–61. 10.1016/j.tim.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 29.Moser I, Schroeder W, Salnikow J. Campylobacter jejuni major outer membrane protein and a 59-kDa protein are involved in binding to fibronectin and INT 407 cell membranes. FEMS Microbiol Lett. 1997;157(2):233–8. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan RC, Neal-McKinney JM, Dhillon AS, Miller WG, Konkel ME. Examination of Campylobacter jejuni putative adhesins leads to the identification of a new protein, designated FlpA, required for chicken colonization. Infect Immun. 2009;77(6):2399–407. 10.1128/IAI.01266-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebacq-Verheyden A-M, Vaerman J-P, Heremans JF. Quantification and distribution of chicken immunoglobulins IgA, IgM and IgG in serum and secretions. Immunology. 1974;27(4):683–92. [PMC free article] [PubMed] [Google Scholar]
- 32.Carlander D, Stålberg J, Larsson A. Chicken antibodies. Ups J Med Sci. 1999;104(3):179–90. [DOI] [PubMed] [Google Scholar]
- 33.Van Deun K, Pasmans F, Ducatelle R, Flahou B, Vissenberg K, Martel A, et al. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet Microbiol. 2008;130(3–4):285–97. 10.1016/j.vetmic.2007.11.027 [DOI] [PubMed] [Google Scholar]
- 34.Hobb RI, Fields JA, Burns CM, Thompson SA. Evaluation of procedures for outer membrane isolation from Campylobacter jejuni. Microbiology. 2009. March;155(Pt 3):979–88. 10.1099/mic.0.024539-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolla JM, Loret E, Zalewski M, Pages JM. Conformational analysis of the Campylobacter jejuni porin. J Bacteriol. 1995;177(15):4266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logan SM, Trust TJ. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983;42(2):675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardon E, Laeremans T, Triest S, Rasmussen SGF, Wohlkönig A, Ruf A, et al. A general protocol for the generation of nanobodies for structural biology. Nat Protoc. 2014. March;9(3):674–93. 10.1038/nprot.2014.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conrath KE, Lauwereys M, Galleni M, Matagne A, Frère J-M, Kinne J, et al. β-Lactamase inhibitors derived from single-domain antibody fragments elicited in the Camelidae. Antimicrob Agents Chemother. 2001. October;45(10):2807–12. 10.1128/AAC.45.10.2807-2812.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagert M, Ehrlich SD. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979;6(1):23–38. [DOI] [PubMed] [Google Scholar]
- 40.Huynh K, Partch CL. Analysis of protein stability and ligand interactions by thermal shift assay. Curr Protoc protein Sci. 2015;79:28.9.1–28.9.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansikka A. Chicken IgA H chains. Implications concerning the evolution of H chain genes. J Immunol. 1992;149(3):855–61. [PubMed] [Google Scholar]
- 42.Parvari R, Avivi A, Lentner F, Ziv E, Tel-Or S, Burstein Y, et al. Chicken immunoglobulin gamma-heavy chains: limited VH gene repertoire, combinatorial diversification by D gene segments and evolution of the heavy chain locus. EMBO J. 1988;7(3):739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sainsbury F, Thuenemann EC, Lomonossoff GP. pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J. 2009;7(7):682–93. 10.1111/j.1467-7652.2009.00434.x [DOI] [PubMed] [Google Scholar]
- 44.Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA. A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature. 1983;303:179–80. [Google Scholar]
- 45.McCormac AC, Elliott MC, Chen DF. A simple method for the production of highly competent cells of Agrobacterium for transformation via electroporation. Mol Biotechnol. 1998;9(2):155–9. 10.1007/BF02760816 [DOI] [PubMed] [Google Scholar]
- 46.De Buck S, Virdi V, De Meyer T, De Wilde K, Piron R, Nolf J, et al. Production of camel-like antibodies in plants. Methods Mol Biol. 2012;911:305–24. 10.1007/978-1-61779-968-6_19 [DOI] [PubMed] [Google Scholar]
- 47.Morandini F, Avesani L, Bortesi L, Van Droogenbroeck B, De Wilde K, Arcalis E, et al. Non-food/feed seeds as biofactories for the high-yield production of recombinant pharmaceuticals. Plant Biotechnol J. 2011;9(8):911–21. 10.1111/j.1467-7652.2011.00605.x [DOI] [PubMed] [Google Scholar]
- 48.Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204(3):383–96. [Google Scholar]
- 49.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–43. [DOI] [PubMed] [Google Scholar]
- 50.Rudolph MJ, Vance DJ, Cheung J, Franklin MC, Burshteyn F, Cassidy MS, et al. Crystal structures of ricin toxin’s enzymatic subunit (RTA) in complex with neutralizing and non-neutralizing single-chain antibodies. J Mol Biol. 2014;426(17):3057–68. 10.1016/j.jmb.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassanzadeh-Ghassabeh G, Devoogdt N, De Pauw P, Vincke C, Muyldermans S. Nanobodies and their potential applications. Nanomedicine. 2013;8(6):1013–26. 10.2217/nnm.13.86 [DOI] [PubMed] [Google Scholar]
- 52.Braun MB, Traenkle B, Koch PA, Emele F, Weiss F, Poetz O, et al. Peptides in headlock–a novel high-affinity and versatile peptide-binding nanobody for proteomics and microscopy. Sci Rep. 2016;6:19211 10.1038/srep19211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahimi S, Shiraz ZM, Salehi TZ, Torshizi MAK, Grimes JL. Prevention of Salmonella infection in poultry by specific egg-derived antibody. Int J Poult Sci. 2007;6(4):230–5. [Google Scholar]
- 54.Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annu Rev Food Sci Technol. 2012;3:163–82. 10.1146/annurev-food-022811-101137 [DOI] [PubMed] [Google Scholar]
- 55.Baxter D. Active and passive immunity, vaccine types, excipients and licensing. Occup Med (Chic Ill). 2007;57(8):552–6. [DOI] [PubMed] [Google Scholar]
- 56.Yokoyama H, Peralta RC, Diaz R, Sendo S, Ikemori Y, Kodama Y. Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets. Infect Immun. 1992;60(3):998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corthésy B. Recombinant immunoglobulin A: powerful tools for fundamental and applied research. Trends Biotechnol. 2002;20(2):65–71. [DOI] [PubMed] [Google Scholar]
- 58.Höök H, Fattah MA, Ericsson H, Vågsholm I, Danielsson-Tham ML. Genotype dynamics of Campylobacter jejuni in a broiler flock. Vet Microbiol. 2005;106(1–2):109–17. 10.1016/j.vetmic.2004.12.017 [DOI] [PubMed] [Google Scholar]
- 59.EFSA. Scientific report of EFSA: analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008. EFSA J. 2010;8(03):1503. [Google Scholar]
- 60.Wu Z, Periaswamy B, Sahin O, Yaeger M, Plummer P, Zhai W, et al. Point mutations in the major outer membrane protein drive hypervirulence of a rapidly expanding clone of Campylobacter jejuni. Proc Natl Acad Sci U S A. 2016;113(38):10690–5. 10.1073/pnas.1605869113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilbreath JJ, Cody WL, Merrell DS, Hendrixson DR. Change is good: variations in common biological mechanisms in the epsilonproteobacterial genera Campylobacter and Helicobacter. Microbiol Mol Biol Rev. 2011;75(1):84–132. 10.1128/MMBR.00035-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nachamkin I, Yang XH, Stern NJ. Role of Campylobacter jejuni flagella as colonization factors for three-day-old chicks: analysis with flagellar mutants. Appl Environ Microbiol. 1993;59(5):1269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Konkel ME, Klena JD, Rivera-Amill V, Monteville MR, Biswas D, Raphael B, et al. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J Bacteriol. 2004;186(11):3296–303. 10.1128/JB.186.11.3296-3303.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riazi A, Strong PCR, Coleman R, Chen W, Hirama T, van Faassen H, et al. Pentavalent single-domain antibodies reduce Campylobacter jejuni motility and colonization in chickens. Mantis NJ, editor. PLoS One. 2013;8(12):e83928 10.1371/journal.pone.0083928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pogue GP, Vojdani F, Palmer KE, Hiatt E, Hume S, Phelps J, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol J. 2010;8(5):638–54. [DOI] [PubMed] [Google Scholar]
- 66.Porro D, Sauer M, Branduardi P, Mattanovich D. Recombinant protein production in yeasts. Mol Biotechnol. 2005;31(3):245–60. 10.1385/MB:31:3:245 [DOI] [PubMed] [Google Scholar]
- 67.Chen M, Liu X, Wang Z, Song J, Qi Q, Wang PG. Modification of plant N-glycans processing: the future of producing therapeutic protein by transgenic plants. Med Res Rev. 2005;25(3):343–60. 10.1002/med.20022 [DOI] [PubMed] [Google Scholar]
- 68.De Wilde K, De Buck S, Vanneste K, Depicker A. Recombinant antibody production in Arabidopsis seeds triggers an unfolded protein response. Plant Physiol. 2013;161(2):1021–33. 10.1104/pp.112.209718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boothe J, Nykiforuk C, Shen Y, Zaplachinski S, Szarka S, Kuhlman P, et al. Seed-based expression systems for plant molecular farming. Plant Biotechnol J. 2010;8(5):588–606. 10.1111/j.1467-7652.2010.00511.x [DOI] [PubMed] [Google Scholar]
- 70.Tufarelli V, Selvaggi M, Dario C, Laudadio V. Genetically Modified Feeds in Poultry Diet: Safety, Performance, and Product Quality. Crit Rev Food Sci Nutr. 2015; [DOI] [PubMed] [Google Scholar]
- 71.De Jaeger G, Scheffer S, Jacobs A, Zambre M, Zobell O, Goossens A, et al. Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulgaris regulatory sequences. Nat Biotechnol. 2002;20(12):1265–8. 10.1038/nbt755 [DOI] [PubMed] [Google Scholar]
- 72.De Buck S, Nolf J, De Meyer T, Virdi V, De Wilde K, Van Lerberge E, et al. Fusion of an Fc chain to a VHH boosts the accumulation levels in Arabidopsis seeds. Plant Biotechnol J. 2013;11(8):1006–16. 10.1111/pbi.12094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
Immunofluorescence microscopy confirms the interaction of Nb5 and Nb23 with Campylobacter isolates. (A, B, C) C. jejuni strain KC40, (D, E, F) C. jejuni strain Cam12/0156 and (G, H, I) C. coli strain K43/5. The binding of Nb5 is shown in A, D and G and the binding of Nb23 in B, E and H. As a negative control, the fluorescently labelled (C, F, I) V1 nanobody was used.
(TIF)
ELISA was used for the analysis of seed extracts from A. thaliana plants transformed with (A) Nb23-IgA constructs and (B) Nb23-IgY constructs. The results of the extracts of the homozygous plants are visualised by the histogram with hatched shading. Extract of wild-type A. thaliana seeds was used as a negative control. The ELISA was developed using anti-IgA or anti-IgY conjugated to HRP. The error bars correspond to the standard deviation.
(TIF)
ELISA for the confirmation of the interaction of anti-flagellin nanobodies with (A) purified flagellins and (B) C. jejuni KC40. Bound His-tagged nanobodies were detected with mouse anti-histidine monoclonal antibodies and goat anti-mouse IgG. The error bars correspond to the standard deviation.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.