Abstract
To identify the risk factors for developing renal involvement and severe kidney disease in Chinese childhood Henoch–Schönlein purpura (HSP) patients.
This was a retrospective study of 2731 children with HSP diagnosed between 2012 and 2015. We analyzed their demographic data, clinical manifestations, and laboratory tests retrospectively. Multivariate logistic regression analysis was used to assess the risk factors.
Renal involvement occurred in 844 HSP patients (35.60%), and severe kidney disease occurred in 104 HSP patients (4.39%). Age over 6 years old at onset, colder season, more than 8 days interval between symptom onset and diagnosis, residence in rural, recurrence, angioedema, and the central nervous system (CNS) involvement were the significant risk factors for renal involvement. At the same time, age over 6 years at onset, more than 8 days interval between symptom onset and diagnosis, recurrence, angioedema, and CNS involvement were highly associated with severe kidney disease. Angioedema, longer interval between symptom onset and diagnosis, older age at HSP onset, and recurrence are prognostic indicators for renal involvement and severe kidney disease in children with HSP. The onset in colder season and rural residence associated with an increased risk for renal involvement, and the CNS involvement had an increased risk for severe kidney disease.
HSP tends not to be self-limiting, and could progress into renal involvement or severe kidney disease for some of the HSP patients. Pediatricians should pay more attention to the children diagnosed with HSP, who also have these risk factors, for potential to develop renal involvement, and severe kidney disease, especially.
Keywords: children, Chinese, Henoch–Schönlein purpura, renal involvement, risk factors, severe kidney disease
1. Introduction
Henoch–Schönlein purpura (HSP), also known as an aphylactic purpura or allergic purpura, is the most common small vessel vasculitis in childhood. The dominant clinical features are nonthrombocytopenic purpura, abdominal pain, gastrointestinal bleeding, arthritis, and renal involvement.
Renal involvement, also called Henoch–Schönlein purpura nephritis (HSPN), has been reported to occur in 20% to 80% of the patients with HSP,[1–3] and is clinically manifested by microscopic or macroscopic hematuria, proteinuria, nephrotic syndrome, and reduced kidney function.[4] HSP is considered to be self-limiting and most of HSP patients with renal involvement have a good prognosis in general. However, 1% to 7% patients develop end-stage renal disease (ESRD).[5–7] Renal involvement in HSP is one of the major causes of chronic renal failure in children, and the long-term principal prognostic determinant depends on the severity of renal damage.[8] The children with hematuria alone or accompanied with mild proteinuria have an almost favorable prognosis, while increasing severity of proteinuria is associated with progressively worse outcome. Therefore, the progression of renal involvement is considered to contribute to the outcome of HSP. More severe form of HSPN is called severe kidney disease.[9] Thus, it is important to assess the risk factors for developing renal involvement and severe kidney disease in childhood HSP.
Many studies had focused on the prognostic factors of developing renal involvement in HSP, but the results were not exactly consistent. The first multivariate analysis in 1998 showed that severe abdominal symptoms, persistent purpura, and decreased coagulation factor XIII activity were significant risk factors for renal involvement in HSP, and some analyses identified other risk factors of renal involvement.[10–13] Besides, several studies in Chinese population were limited by a small sample size and did not consider socioeconomic factor and other initial alterations of this disease, such as HSP triggers and central nervous system (CNS) involvement.[2,14–16] Furthermore, there was a scarcity of studies with pediatric HSP patients to evaluate the risk factors associated with severe kidney disease.
We therefore evaluated multiple clinical and laboratory parameters of 2371 Chinese patients with pediatric HSP and examined the risk factors for not only development of renal involvement in HSP but also development of severe kidney disease.
2. Methods
We performed a retrospective study on 2731 children hospitalized for HSP between January 2012 and December 2015 at the West China Second University Hospital of Sichuan University for Children, in Chengdu, China. The HSP was diagnosed if a typical purpura without thrombocytopenia was recognized.[17] Subjects were chosen for this study if they were no more than 19 years old, were at their first diagnosis, and had no clinical and/or laboratory evidences of microscopic polyangiitis, Wegener granulomatosis, systemic lupus erythematosus, antiphospholipid syndrome, recent serious systemic infection, clotting disorders, and liver disease. This study was approved by the ethics committee of West China Second University Hospital, Sichuan University.
For each hospitalized patient, we abstracted data from electronic and paper medical records. Patients’ data consisted of demographic characteristics (age at onset, sex, and weight), interval between symptom onset and diagnosis, onset season, and clinical and laboratory data, including serum creatinine, serum IgA, urinalysis, urinary protein excretion level, and urinary erythrocytes level. The symptoms onset refers to any initial clinical symptoms associated with HSP, such as purpura, abdominal symptom, skin manifestations, angioedema, arthritis, etc. Other features included known HSP triggers, skin manifestations, abdominal pain, arthritis, recurrences of HSP, angioedema, CNS involvement, renal involvement, and severe kidney disease. The known triggers of HSP included exposure to infection, allergens, food, vaccines, and drugs prior onset of HSP symptom. Arthritis was defined as swelling joints or painful periarticular soft tissue edema. The skin manifestations were classified into none, mild skin rash, severe skin rash, and digestive tract purpura. Mild skin rashes were only manifested in limbs. Severe skin rashes presented at both limbs and trunk. And digestive tract purpura was confirmed during the surgery for acute abdominal pain or gastrointestinal endoscopy. The patients manifesting abdominal pain with gastrointestinal bleeding, such as guaiac-positive stools or grossly bloody stools, were classified into severe abdominal symptoms group. And the patients with only abdominal pain, but not other symptoms, were classified into mild abdominal symptoms group. The symptoms of headaches, convulsions, and coma were grouped into CNS involvement. Renal involvement was clinically diagnosed by macroscopic or microscopic hematuria (>3 red blood cells per high-power microscopic field in a centrifuged specimen) with or without proteinuria.[10,18] Severe kidney disease was defined by the presence of macroscopic hematuria, proteinuria, nephritic syndrome, and nephritic syndrome with or without acute kidney injure.[9] In our analysis, proteinuria was defined as proteinuria >0.15 g/d.[12] Nephrotic syndrome was defined as massive proteinuria 40 mg/m2 per day or 50 mg/kg body weight per day or a random sample of urinary protein-to-creatinine ratio exceeding 2.0, which effectively resulted in severe hypoalbuminemia (<2.5 g/dL).[19]
The statistical analyses were performed with SAS (version 9.4 for Windows), with the chi-squared test for categorical variables and Student 2-tailed t test for continuous variables. A multivariate logistic regression model was used to assess the risk factors for renal involvement and severe kidney disease. Statistical significance was set at 0.05.
3. Results
3.1. Demographic characteristics and principal clinical features
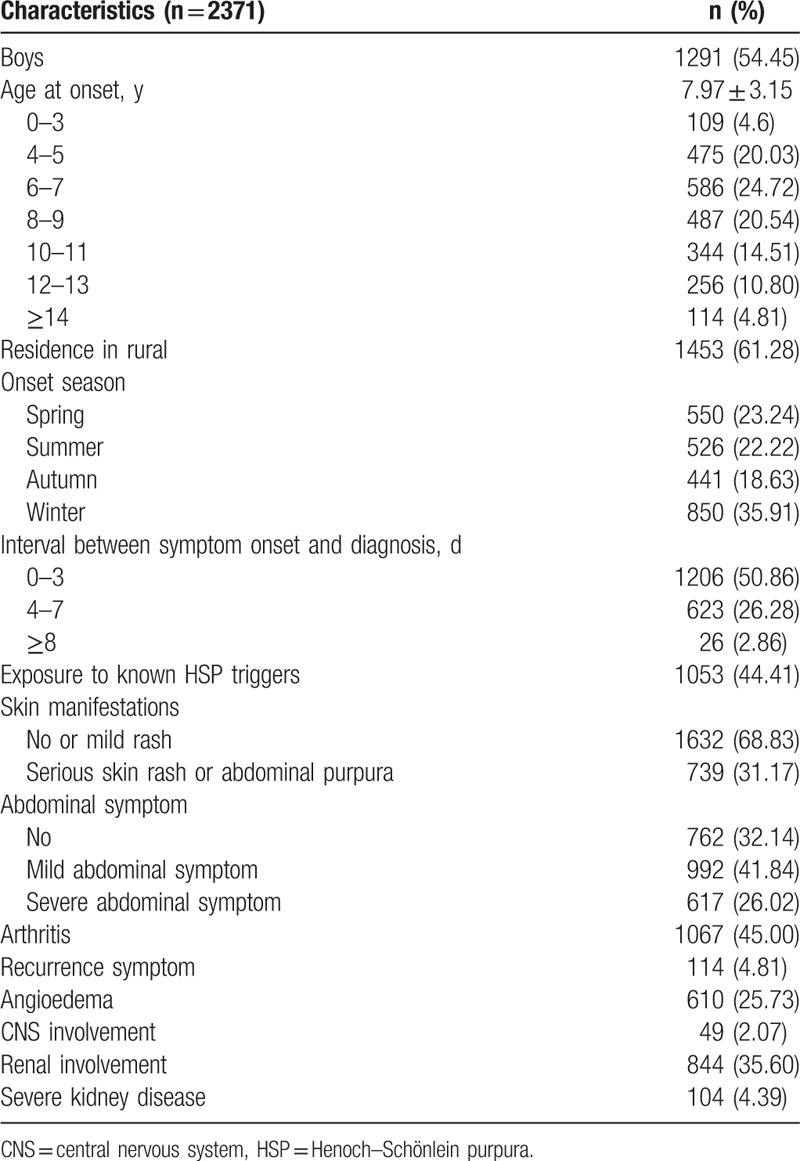
A total of 2731 subjects with HSP were identified (Table 1), including 1291 boys (54.45%) and 1440 girls (45.55%). The mean onset age at HSP diagnosis was 7.97 (3.15) years. Most cases occurred between 4 and 11 years (79.80% of total cases). More than half of the subjects resided in rural areas (approximately 61%) and were older than 6 (75.37%). Thirty-six percent of these subjects had the disease onset in winter, 23.24% in spring, 22.22% in summer, and 18.63% in autumn. Half of the subjects (50.86%) had interval between symptom onset and diagnosis <4 days; 1053 (44.41%) subjects had obvious exposure to the HSP triggers, 31.17% had severe rash or abdominal purpura symptom, 26.02% had severe abdominal symptoms, 45.00% had arthritis, 4.81% had a recurrence symptom, 25.73% had angioedema, 2.07% had CNS involvement, 35.60% had renal involvement, and 4.39% had severe kidney disease.
Table 1.
Demographic characteristics and clinical features in 2371 cases of HSP.
3.2. Risk factors for renal involvement and severe kidney disease
We used multivariate logistic regression analysis with renal involvement and severe kidney disease as dependent variable, individually. Independent variables included gender, onset age, residence, the onset season, interval between symptom onset and diagnosis, exposure to known HSP triggers, skin manifestations, abdominal symptom, arthritis, recurrence symptom, angioedema, and CNS involvement.
Onset at older age, colder season, longer interval between symptom onset and diagnosis, rural residency, recurrence, and angioedema were significantly associated with an increased risk of renal involvement (Fig. 1).
Figure 1.
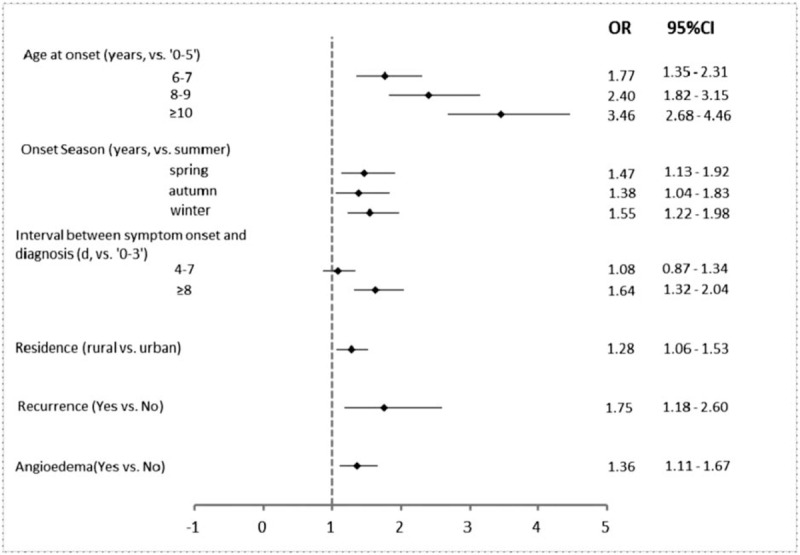
Multivariate analysis by logistic regression of related factors in 2371 patients associated with renal involvement in Henoch–Schönlein purpura.
Comparing to children with onset age at 5 or younger, children with onset age at 6 or older had higher risk for renal involvement (odds ratio [OR]: 1.77, 95% confidence interval [CI]: 1.35–2.31), and the risk increased as the onset age increased. The subject with HSP onset in winter had a higher risk for renal involvement (OR: 1.55, 95% CI: 1.22–1.98) comparing to summer. Children who lived in rural area had a higher risk for renal involvement than those from urban area (OR: 1.28, 95% CI: 1.06–1.53). Comparing to subjects with interval between onset of symptoms and diagnosis <4 days, subjects with interval more than 8 days had higher risk for renal involvement (OR: 1.64, 95% CI: 1.32–2.04). Children with recurrence of HSP (OR 1.75, 95% CI: 1.18–2.60) or angioedema (OR: 1.36, 95% CI: 1.11–1.67) had a higher risk for renal involvement than those did not have recurrence or angioedema.
Onset at older age, longer interval between symptom onset and diagnosis, recurrence, angioedema, and CNS involvement were significantly associated with an increased risk of severe kidney disease (Fig. 2). Comparing to children with onset age at 5 or younger, children with onset age at 6 or older had higher risk for severe kidney disease (OR: 3.46, 95% CI: 1.45–8.24), and the risk increased as the onset age increased. Comparing to subjects with interval between onset of symptoms and diagnosis <4 days, subjects with interval more than 8 days had higher risk for renal involvement (OR: 1.95, 95% CI: 1.20–3.16). Children with recurrence of HSP (OR 3.42, 95% CI: 1.83–6.42) or angioedema (OR: 3.09, 95% CI: 2.00–4.78) had a higher risk for severe kidney disease than those had no recurrence or angioedema. Children with CNS involvement had higher risk for severe kidney disease (OR: 2.81, 95% CI: 1.06–7.45).
Figure 2.
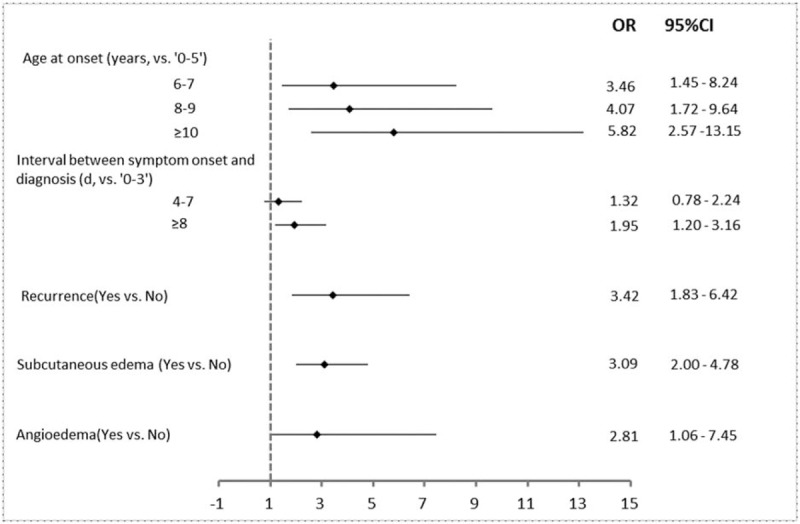
Multivariate analysis by logistic regression of related factors in 2371 patients associated with severe kidney disease in Henoch–Schönlein purpura.
4. Discussion
In this study, we retrospectively reviewed the demographic, clinical, and laboratory findings of 2371 children with HSP at West China Second University Hospital in a 4 years period. To our knowledge, this is the largest recent survey of this disease in China. The possible risk factors of renal involvement in children with HSP have been reported in the past 20 years. This study attempted to explore comprehensively the effect of a multitude of possible risk factors for renal involvement and severe kidney disease in childhood HSP. Even though most children with HSP accompanied with renal involvement have a benign prognosis, some children with severe kidney disease would suffer from ESRD. We therefore examined the risk factors of severe kidney disease as well as renal involvement.
In our series, 35.6% of admitted children had renal involvement. Our result is similar with a previous report conducted in Chinese children, in which the incidence of renal involvement was 31.9%,[14] and a retrospective report conducted in Japanese children, in which the incidence of renal involvement was 33.5%.[10] The incidence of renal involvement in different reports may have different results, which could be explained by reasons such as race, regions, study types (retrospective or prospective), the definition of the renal involvement, and the observation period of patients with HSP. In this study, we found that 104 of the 844 (12.33%) patients had severe kidney disease. Similarly, Narchi found that one-fifth of proteinuria and/or hematuria patients were in association with nephritic or nephrotic syndrome.[1] In our study, the majority of patients were diagnosed at age between 4 and 13. The peak age of HSP onset was between the ages 4 to 7, which was similar to the studies from the United Kingdom and Taiwan.[20,21]
Our study showed that onset age over 6 was a significant risk factor of renal involvement, which is similar to Mao's[2] and Zhao et al's[14] finding in Chinese children. While, according to Sano et al's finding in Japan,[12] onset age over 4 was a risk factor for developing nephritis, and Jauhola et al[22] concluded that onset age older than 8 was an independent risk factor for renal involvement. But age 4 or 8 also were around 6. In conclusion, these studies, like one meta-analysis said,[13] showed that children with HSP onset at older age were at higher risk of renal involvement than onset at younger age. At the same time, our study found that the risk of renal involvement and severe kidney disease increased with the increasing of onset age. Therefore, more attention should be paid to the children with HSP onset at age 6 or older.
In contrast to the previous studies,[11,23,24] our data did not find that the gender was a risk factor for renal involvement and severe kidney disease. The epidemiological survey from Gardner-Medwin et al, and other studies showed that boys were at higher risk for renal involvement than girls.[23] However, there were also studies reported that girls were more likely to have a HSPN.[11,24] The differences in gender among these studies may be due to different ethnic origin and/or having different environmental factors and ascertainment of cases (hospitalized medical records, questionnaire data, insurance claim data).
In this study, patients with onset in winter had a higher risk of renal involvement compared to those with onset in summer, while there were no association between onset seasons and developing severe kidney disease. The previous studies had never explored the correlation between the weather and the risk of renal involvement. The reason that children with HSP in winter at onset were prone to have renal involvement deserves a further study.
Our results also suggested that more than 8 days’ interval between symptoms onset and diagnosis was at higher risk for both renal involvement and severe kidney disease than those patients with interval <4 days. This risk factor had rarely been reported in previous studies. This indicated that HSP is a self-limiting disease in most cases, but for a small portion of the patients, HSP may not be self-limiting and it will progress into renal involvement or severe kidney diseases. The finding is similar to the opinion of Davin et al.[25,26] These results suggested that earlier diagnosis and attention could benefit children with HSP.
Our data showed that children from rural area had a higher risk for renal involvement than those from urban area. We speculated that it might be related to less medical resources, poor medical knowledge, and backward economy in rural area. Previous studies had less contention to the region and risk of renal involvement. Therefore, pediatrician should pay some attention to the patients with HSP from rural area.
Our finding that abdominal symptom and renal involvement were not correlated was similar to a retrospective study of 141 HSP patients from Zhao et al and Rigante's study.[14,27] However, many other studies indicated that digestive tract symptoms (abdominal pain, gastrointestinal bleeding, and severe bowel angina) were strongly correlated with renal involvement.[11,12,28] Abdominal pain, bleeding, and severe bowel angina are unpleasant when children suffer gastrointestinal involvement in HSP, so parents may pay more attention to the symptoms and thus children can get earlier diagnosis and intervention, which will be conducive to the development of HSP. Of course, further research should be carried out to explore this difference.
Most published studies reported that there was a close association between persistent purpura or relapse and HSPN. According to our data, both renal involvement and severe kidney disease of children with HSP were related to the recurrence of purpura. Therefore, careful attention should be paid especially to children with HSP recurrence.
In addition, we found that children with angioedema had a higher risk for renal involvement and severe kidney disease than those without. But there is no published study about the association between angioedema and renal involvement or severe kidney disease.
Our study had the largest number of subjects among the similar studies and could provide useful information regarding the risk factors for the development of renal involvement and severe kidney disease in childhood HSP patient in China. However, this study has the limitations of being a retrospective study. The HSP patients enrolled in our study had their renal involvement and severe kidney conditions identified at the same time of HSP diagnosing, and there was no follow-up. Thus, there is no causal relationship inferred between accessed risk factors in our study and developing renal involvement and severe kidney disease in HSP patients. Detailed long-term cohort study is needed to clarify this point.
5. Conclusions
In conclusion, our results show that angioedema and longer interval between symptom onset and diagnosis have higher risk for renal involvement and severe kidney disease in children with HSP, and reconfirm that older age at onset of HSP and recurrence are the independent risk factors of renal involvement and severe kidney disease as previously reported. The colder onset season and residence in rural had an increased risk for renal involvement, and the CNS involvement had an increased risk for severe kidney disease. Moreover, our study found that HSP may not be a self-limiting disease for a part of the patient with HSP, which could progress into renal involvement or severe kidney disease. Therefore, HSP children who have the risk factors for renal involvement, especially severe kidney disease, should be closely observed. The present study was retrospective and further prospective studies should be conducted to get results that are more reliable.
Acknowledgments
The authors are grateful to individuals who participated in the study. The authors would also like to thank the clinicians and staff who contributed to the data collection for this study.
Author contributions
Data curation: Xiaomei Sun, Yang Cao, Liang Dai, Feiyang Sun.
Conceptualization: Ping Yu, Liqun Dong.
Data curation: Ke Wang, Liqun Dong.
Formal analysis: Ke Wang, Ping Yu, Liqun Dong.
Investigation: Xiaomei Sun, Yang Cao, Liang Dai, Feiyang Sun.
Methodology: Ping Yu, Liqun Dong.
Writing – original draft: Ke Wang, Liqun Dong.
Writing – review & editing: Ke Wang, Ping Yu, Liqun Dong.
Footnotes
Abbreviations: CI = confidence interval, CNS = central nervous system, ESRD = end-stage renal disease, HSP = Henoch–Schönlein purpura, HSPN = Henoch–Schönlein purpura nephritis, OR = odds ratio.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Narchi H. Risk of long term renal impairment and duration of follow up recommended for Henoch–Schönlein purpura with normal or minimal urinary findings: a systematic review. Arch Dis Child 2005;90:916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mao Y, Yin L, Huang H, et al. Henoch–Schönlein purpura in 535 Chinese children: clinical features and risk factors for renal involvement. J Int Med Res 2014;42:1043–9. [DOI] [PubMed] [Google Scholar]
- [3].Ghrahani R, Ledika MA, Sapartini G, et al. Age of onset as a risk factor of renal involvement in Henoch–Schönlein purpura. Asia Pac Allergy 2014;4:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saulsbury FT. Henoch–Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine 1999;78:395–409. [DOI] [PubMed] [Google Scholar]
- [5].Coppo R, Mazzucco G, Cagnoli L, et al. Long-term prognosis of Henoch–Schönlein nephritis in adults and children. Italian Group of Renal Immunopathology Collaborative Study on Henoch–Schönlein purpura. Nephrol Dial Transplant 1997;12:2277–83. [DOI] [PubMed] [Google Scholar]
- [6].Stewart M, Savage JM, Bell B, et al. Long term renal prognosis of Henoch–Schönlein purpura in an unselected childhood population. Eur J Pediatr 1988;147:113–5. [DOI] [PubMed] [Google Scholar]
- [7].Spasojevic-Dimitrijeva B, Kostic M, Peco-Antic A, et al. Henoch–Schönlein purpura outcome in children: a ten-year clinical study. Srp Arh Celok Lek 2011;139:174–8. [DOI] [PubMed] [Google Scholar]
- [8].Albaramki J. Henoch–Schönlein purpura in childhood a fifteen-year experience at a tertiary hospital. J Med Liban 2016;64:13–7. [DOI] [PubMed] [Google Scholar]
- [9].Hahn D, Hodson EM, Willis NS, et al. Interventions for preventing and treating kidney disease in Henoch–Schönlein purpura (HSP). Cochrane Database Syst Rev 2015;8:CD005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kaku Y, Nohara K, Honda S. Renal involvement in Henoch–Schönlein purpura: a multivariate analysis of prognostic factors. Kidney Int 1998;53:1755–9. [DOI] [PubMed] [Google Scholar]
- [11].de Almeida JL, Campos LM, Paim LB, et al. Renal involvement in Henoch–Schönlein purpura: a multivariate analysis of initial prognostic factors. J Pediatr 2007;83:259–66. [DOI] [PubMed] [Google Scholar]
- [12].Sano H, Izumida M, Shimizu H, et al. Risk factors of renal involvement and significant proteinuria in Henoch–Schönlein purpura. Eur J Pediatr 2002;161:196–201. [DOI] [PubMed] [Google Scholar]
- [13].Chan H, Tang YL, Lv XH, et al. Risk factors associated with renal involvement in childhood Henoch–Schönlein purpura: a meta-analysis. PLoS ONE 2016;11:e0167346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao YL, Liu ZJ, Bai XM, et al. Obesity increases the risk of renal involvement in children with Henoch–Schönlein purpura. Eur J Pediatr 2015;174:1357–63. [DOI] [PubMed] [Google Scholar]
- [15].Zhu JJ, Zhu-Wen YI, Huang JH, et al. Clinical analysis of 118 cases of Henoch–Schönlein purpura in children. J Clin Dermatol 2014;43:336–9. [Google Scholar]
- [16].Wang X, Zhu Y, Gao L, et al. Henoch–Schönlein purpura with joint involvement: analysis of 71 cases. Pediatr Rheumatol 2016;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kraft DM, McKee D, Scott C. Henoch–Schönlein purpura: a review. Am Fam Physician 1998;58:405–8. [PubMed] [Google Scholar]
- [18].Ronkainen J, Nuutinen M, Koskimies O. Henoch–Schönlein purpura in children. Duodecim Laaketieteellinen Aikakauskirja 2000;116:2339–46. [PubMed] [Google Scholar]
- [19].Kodner C. Nephrotic syndrome in adults: diagnosis and management. Am Fam Physician 2009;80:1129–34. [PubMed] [Google Scholar]
- [20].Gardner-Medwin JM, Dolezalova P, Cummins C, et al. Incidence of Henoch–Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002;360:1197–202. [DOI] [PubMed] [Google Scholar]
- [21].Yang YH, Hung CF, Hsu CR, et al. A nationwide survey on epidemiological characteristics of childhood Henoch–Schönlein purpura in Taiwan. Rheumatology 2005;44:618–22. [DOI] [PubMed] [Google Scholar]
- [22].Jauhola O, Ronkainen J, Koskimies O, et al. Renal manifestations of Henoch–Schönlein purpura in a 6-month prospective study of 223 children. Arch Dis Child 2010;95:877–82. [DOI] [PubMed] [Google Scholar]
- [23].Davin JC, Weening JJ. Henoch–Schönlein purpura nephritis: an update. Eur J Pediatr 2001;160:689–95. [DOI] [PubMed] [Google Scholar]
- [24].Calvino MC, Llorca J, Garcia-Porrua C, et al. Henoch–Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine 2001;80:279–90. [DOI] [PubMed] [Google Scholar]
- [25].Davin JC, Coppo R. Henoch–Schönlein purpura nephritis in children. Nat Rev Nephrol 2014;10:563–73. [DOI] [PubMed] [Google Scholar]
- [26].Alfredo CS, Nunes NA, Len CA, et al. Henoch–Schönlein purpura: recurrence and chronicity. J Pediatr 2007;83:177–80. [DOI] [PubMed] [Google Scholar]
- [27].Rigante D, Candelli M, Federico G, et al. Predictive factors of renal involvement or relapsing disease in children with Henoch–Schönlein purpura. Rheumatol Int 2005;25:45–8. [DOI] [PubMed] [Google Scholar]
- [28].Hung SP, Yang YH, Lin YT, et al. Clinical manifestations and outcomes of Henoch–Schönlein purpura: comparison between adults and children. Pediatr Neonatol 2009;50:162–8. [DOI] [PubMed] [Google Scholar]


