Supplemental Digital Content is available in the text
Keywords: diagnosis, idiopathic sudden sensorineural hearing loss, meta-analysis, neutrophil-to-lymphocyte ratio, prognosis
Abstract
Background:
Chronic inflammation has been regarded as one of the causes of idiopathic sudden sensorineural hearing loss (ISSHL). Several individual studies have reported the association between neutrophil-to-lymphocyte ratio (NLR) and ISSHL. However, the findings have been inconsistent, and these data have not been systematically evaluated. Thus, we conducted this meta-analysis to further explore the predictive value of NLR on formation and prognosis of ISSHL.
Methods:
A comprehensive literature search was performed to identify eligible studies based on PubMed, Embase, Web of Science, and China National Knowledge Infrastructure. The Standardized mean deviation (SMD) with its 95% confidence interval (CI) was applied to be the effect size estimate.
Results:
A total 10 papers with 15 retrospective case-control studies, which included 1029 ISSHL patients (the case group) and 1020 healthy people (the control group), were selected for the meta-analysis of the relationship between NLR and onset of ISSHL. The NLR levels in the case group were observed to be higher than the control group (SMD = 1.65, 95% CI = 1.20–2.09, P < .001). The pooled results did not significantly change by the subgroup analyses based on study region, baseline matching, and laterality. Moreover, 9 publications with 12 retrospective cohort studies, which included 590 recovered ISSHL patients and 438 unrecovered ISSHL patients, explored the association between NLR and ISSHL prognosis, and the combined data showed that the NLR value was much higher in unrecovered patients rather than recovered patients (SMD = 1.27, 95% CI: 0.62–1.92, P < .001). The subgroup analyses based on study region, laterality, type of steroid, medication administration, maintenance treatment, follow-up period, and definition of “recovered” further supported these results.
Conclusion:
The results of this meta-analysis suggest that NLR might be a useful biomarker to determine the onset and prognosis of ISSHL.
1. Introduction
Idiopathic sudden sensorineural hearing loss (ISSHL) is defined as a hearing loss of more than 30 dB that occurs in at least 3 consecutive frequencies occurring within 3 days, which is a common otologic emergency presenting mostly as an acute hearing loss with an abrupt occurrence.[1] The incidence of ISSHL is estimated to be approximately 10 cases per 100,000 person-years, but it was only the statistics for patients who sought treatment.[2] A number of causes of ISSHL have been raised, including viral, bacterial or protozoan infections, vascular incident, labyrinthine membrane rupture, blood disorders, ototoxicity drugs, neoplastic, metabolic conditions, and autoimmune disorders. These theories are proposed based on certain laboratory and postmortem findings, but each has its own flaws, and none of them could fully explain the pathogenesis of ISSHL.[3–5] Therefore, the real origins for ISSHL remain a matter of debate.
In recent years, hypotheses about the mechanism of ISSNHL have focused on chronic inflammation. Chronic inflammations can cause microvascular injury and atherogenesis, which increase the risk of ischemia in a direct manner.[6] Because the blood supply of cochlea is mainly reliant on a single, terminal artery (the labyrinthine artery), while cochlear hair cells presents high oxygen consumption and poor tolerance of hypoxia, the inner ear is extremely prone to circulatory alterations. Thus, the pathophysiology of ISSHL may be closely related to the vascular background of the inner ear. Animal studies have shown that, after a transient injury of internal auditory artery, cochlea is more susceptive to suffer functional impairment rather than vestibule.[7] Inflammation can lead to an endothelial dysfunction which accelerates the development of a pro-thrombotic state due to the thickening of the vascular wall, even at the level of the ear.[8] When the endothelium cannot explicitly perform its own duties, the sudden and transient thrombotic event caused by adhesion molecules, endothelial progenitor cells, or proinflammatory vascular conditions could alter the blood supply to the inner ear.[9] On the other hand, under the inflammatory conditions, endothelial dysfunction facilitates the onset of atherosclerosis, and then promotes the occurrence of microvascular disease involving the stria vascularis. Stria vascularis is critical for the amplification function of the cochlea which functions as an electrochemical pump to generate the endolymphatic potential.[10] Blood flow reduction in the stria vascularis leads to a loss of the endolymphatic potential, and finally results in diminishment of cochlear amplification.[11] All the aforementioned mechanisms could explain the nature of ISSHL.
Moreover, it has been proved that circulating inflammatory molecules have a negative impact on tissues of the cochlear vasculature, both proximally in the spiral modiolar artery and distally among the capillaries of the stria vascularis.[12] Some evidences have proved that inflammation may involve in the development of ISSHL, and has affected the prognosis. Berrettini et al[13] reported that high-intensity signals in the affected inner ear on 3-dimensional fluid-attenuated inversion-recovery magnetic resonance imaging were closely correlated with the severity of hearing loss, and identified the occurrence of mild hemorrhage, acute inflammation, and presence or absence of blood-labyrinth or nerve barrier breakdown during the process of ISSHL. Also, Masuda et al[14] found that a neutrophil count above the reference range was related to severe illness and poor prognosis of ISSHL. Therefore, the inflammatory biomarkers may have potential values to some extent in the prediction of ISSHL diagnosis and prognosis.
To date, several measures of inflammatory status have been proved to be significantly associated with ISSHL in clinical practice, including white blood cell (WBC) count, WBC subtypes counts, interleukin 6 (IL-6), C-reactive protein, tumor necrosis factor-α (TNF-α), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR).[15] Among these inflammatory biomarkers, NLR, represented as a combination of circulating neutrophils and lymphocytes counts, has been achieved great interest especially. Since hematological test is a routine examination in all hospitals, calculation of NLR is a very simple and cheap method when compared with the other inflammatory cytokines, such as IL-6, C-reactive protein, and TNF-a. NLR has been reported to be a useful prognostic indicator in various diseases, including community acquired pneumonia, ischemic heart disease, ulcerative colitis, appendicitis, and cancers.[16–18] Recently, the predictive value of NLR on diagnosis and prognosis of ISSHL had been individually investigated, and some findings had been achieved in this research field. However, due to the small sample size and the interstudy heterogeneity, the results were inconsistent and there were still some controversies among these studies. Considering that meta-analyses were useful to synthesize data from individual studies for a specified outcome, we conducted this meta-analysis to comprehensively estimate the predictive value of NLR on diagnosis and prognosis in patients with ISSHL.
2. Methods
2.1. Search strategy
A systematic search was conducted in multiple bibliographic databases such as the PubMed, Embase, Web of Science, and China National Knowledge Infrastructure by using the following terms: “neutrophil-to-lymphocyte ratio OR neutrophil-lymphocyte ratio OR neutrophil lymphocyte ratio OR NLR (all fields),” AND “sudden hearing loss OR hearing loss OR hearing disorders OR sudden deafness OR deafness (all fields),” AND “onset OR diagnosis OR predictive or prediction (all fields),” OR “prognosis OR prognostic OR recovery (all fields).” No language limitation was indicated, and the articles’ inclusion period was until August 2018. We also screened the references of the relevant studies for a further comprehensive search.
2.2. Inclusion and exclusion criteria
Publications were considered eligible for this meta-analysis when they fit all of the following criteria: the study was designed as randomized controlled trial, cohort study, or case-control study; the study compared the NLR value between ISSHL patients and healthy people, and (or) compared the NLR value between “recovered” and “unrecovered” ISSHL patients according to the hearing outcome at the end of follow-up period; measured NLR value before the patients was administered any treatments; provided sufficient data, including the sample size, the mean, and standard deviation of NLR level, to infer the results. When multiple studies addressed the same population, the most representative study was included, whose study hypothesis was best fitted to ours. We excluded the following studies: reviews, conference abstracts, comments, editorials, letters, case reports, basic research, or animal experiments.
2.3. Data extraction and quality assessment
Two authors (JW and GZ) independently extracted relevant information from each eligible study, and disagreement was resolved by consensus. Data extracted from the studies included the name of the first authors, publication year, study region, duration period, sample size, baseline matching, laterality, pretreatment NLR measurement, therapeutic regimen, medication administration, follow-up time, definition of “recovered,” and quality scores.
The Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of the case-control or cohort study.[19] The NOS is a semiquantitative scale, and the total score ranges from 0 to 9 with higher scores indicating higher quality. We considered studies as high quality if they met at least 6 scores.
2.4. Statistical analysis
STATA 11.0 software (STATA Corporation, College Station, TX) was used for all statistical analyses. Standardized mean deviation (SMD) and 95% confidence intervals (CI) were used as effect measure to assess the difference of pretreatment NLR level between the groups. The statistical significance of the pooled result was determined through Z-test. The results were considered statistically significant if P < .05. Heterogeneity assumption was qualitatively examined through the χ2 test, and was considered statistically significant when P < .05. Heterogeneity was also quantitatively estimated using the I2 metric (0% to 25% indicates no heterogeneity, 25% to 50% indicates moderate heterogeneity, and 50% to 100% indicates extreme heterogeneity).[20] When significant heterogeneity had been observed among the studies (P < .05 or I2 > 50%), a random-effects model (DerSimonian and Laird method) was used to calculate the pooled outcomes.[21] Otherwise, a fixed-effects model was applied (Mantel–Haenszel method).[22]
If extreme heterogeneity existed between studies, subgroup analyses were conducted to explore the potential confounding factors. The subgroup analysis was performed according to study region, baseline matching, laterality, type of steroid, medication administration, maintenance treatment, follow-up period, and definition of “recovered.”
Sensitivity analysis was conducted by sequentially omitting each individual study to validate the stability of the pooled outcomes. Publication bias was statistically assessed by Begg and Egger asymmetry tests (P < .05 was defined as statistically significance),[23] and was visually evaluated using funnel plots. If significant publication bias existed, trim, and fill method was performed to validate the robust of the meta-analysis results.[24]
3. Results
3.1. Study characteristics
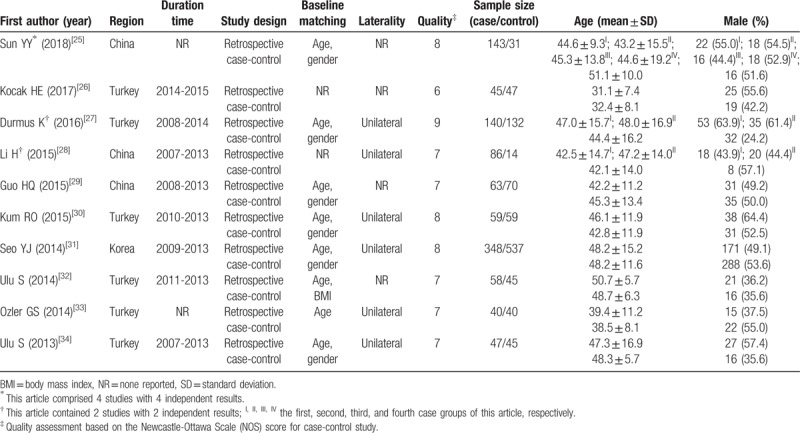
The process of literature search was shown in Fig. 1. Initially, 365 papers were generated in the primary electronic search in the major databases. Ten full-text publications designed as retrospective case-control study met the inclusion criteria of our meta-analysis for the outcome of ISSHL diagnosis,[25–34] of which the main characteristics were listed in Table 1. The article written by Sun et al[25] comprised 4 studies, while both the papers written by Durmus et al[27] and Li et al[28] included 2 studies. Therefore, a total of 15 studies published from 2013 to 2018 were selected for our meta-analysis. A total of 1029 ISSHL patients (the case group) and 1020 healthy people (the control group) were included, of which the number ranged from 33 to 348 in the case groups, and the number ranged from 14 to 537 in the control groups. These studies originated from Turkey,[26,27,30,32–34] China,[25,28,29] and Korea,[31] respectively. Ten studies used age and gender as the matching factors,[25,27,29–31,34] one study presented age and BMI as matching factors,[32] 1 study only used age as the matching factor,[33] and even 3 study did not report the matching factor.[26,28] Eight studies included ISSHL patients with unilateral hearing loss, but 7 studies did not report the laterality.[25,26,29,32] According to the quality criteria, all case-control studies had scores of 6 or more and were of high quality (Supplement Table 1).
Figure 1.
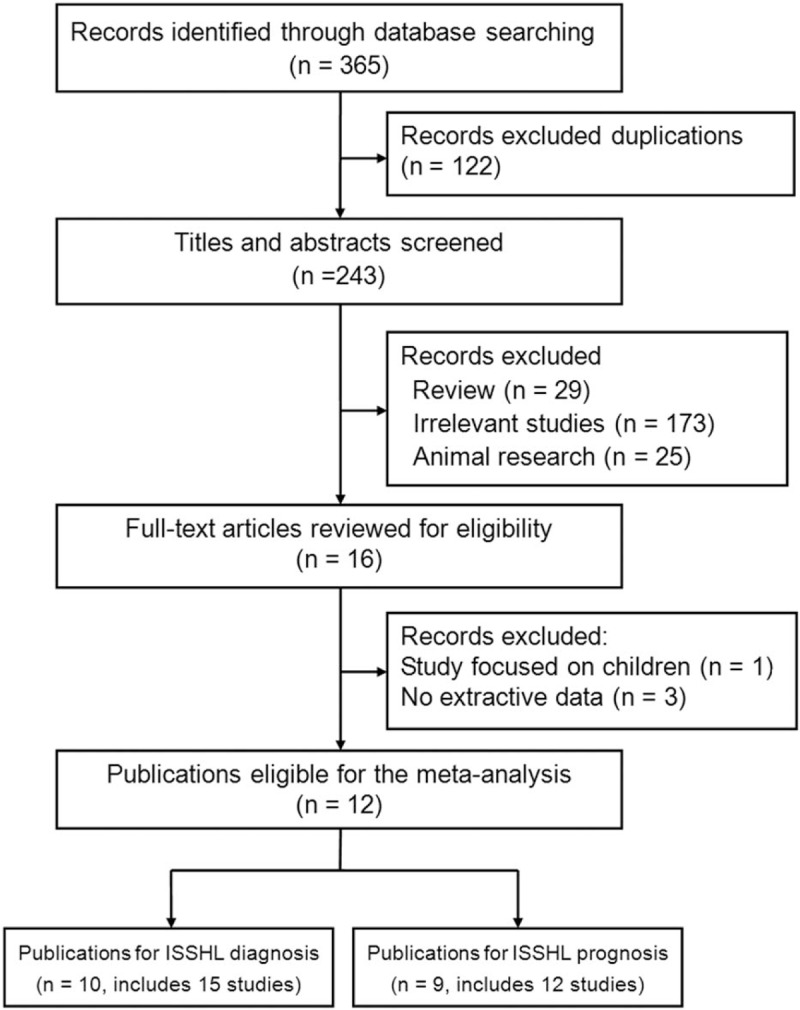
Flow diagram of the study selection process and specific reasons for exclusion in the meta-analysis.
Table 1.
Main characteristics of the included studies in the meta-analysis of the association of neutrophil-to-lymphocyte ratio in idiopathic sudden sensorineural hearing loss diagnosis.
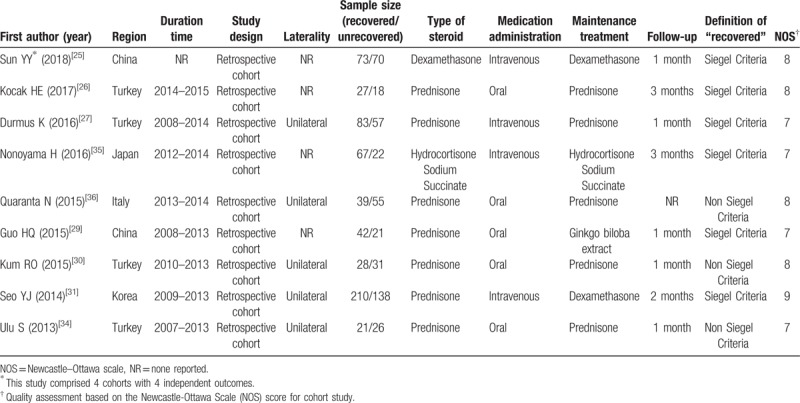
Moreover, 9 retrospective cohort studies reported the comparative results of the pretreatment NLR between “recovered” patients and “unrecovered” patients, and then were included in the meta-analysis for the outcome of ISSHL prognosis,[25–27,29–31,34–36] of which the main features were summarized in Table 2. The article written by Sun et al[25] contained 4 studies, thus, there were 12 studies published from 2013 to 2018 that were included for this meta-analysis. The therapeutic regimen, follow-up period, and recovery condition were reported in all these 12 studies. Nine studies applied the Siegel criteria as the hearing improvement criteria, which defined ISSHL patients with more than 15 dB hearing gain after treatment as “recovered” patients,[25–27,29,31,35] whereas 3 studies used a non-Siegel criteria to classify the curative response, which regarded ISSHL cases with more than 10 dB hearing gain after therapy as “recovered” patients.[30,34,36] According to the quality assessment, all cohort studies had scores of 7 or more and were of high quality (Supplement Table 2).
Table 2.
Main characteristics of the included studies in the meta-analysis of the association of neutrophil-to-lymphocyte ratio in idiopathic sudden sensorineural hearing loss prognosis.
3.2. Meta-analysis of NLR in ISSHL diagnosis
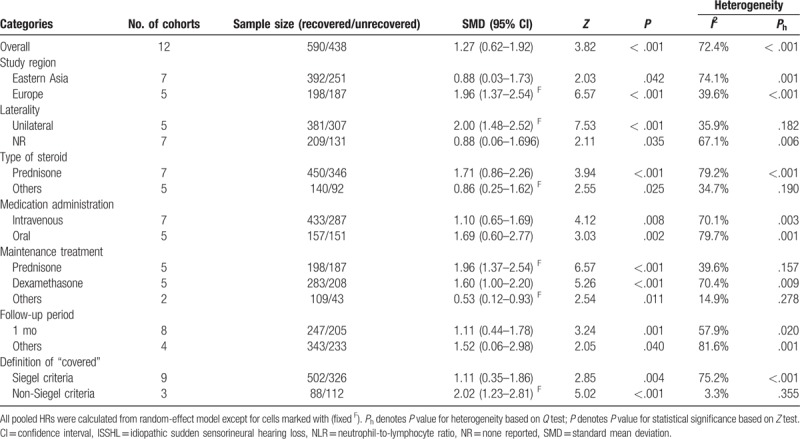
Ten publications with 15 studies reported the effects of pretreatment NLR on the ISSHL diagnosis, which included 1029 ISSHL patients and 1020 healthy controls. The heterogeneity test revealed that there was extreme heterogeneity across the included studies (I2 = 88.0%, P < .001), so a random-effect model was conducted to calculate the pooled result. The result declared that the pretreatment NLR level was higher in the ISSHL patients rather than the healthy controls (SMD = 1.65, 95% CI: 1.20–2.09, P < .001; Fig. 2). When stratified by study regions, significant differences were observed between ISSHL patients and healthy populations in the subgroups regarding Turkey (SMD = 1.62, 95% CI: 0.97–2.27, P < .001, random-effect model), China (SMD = 1.46, 95% CI: 0.93–1.98, P < .001, fixed-effect model), and Korea (SMD = 2.65, 95% CI: 2.23–3.07, P < .001, random-effect model). Similarly, the differences were also observable between ISSHL patients and healthy controls regardless of the subgroup analyses of baseline matching, and laterality (Table 3).
Figure 2.
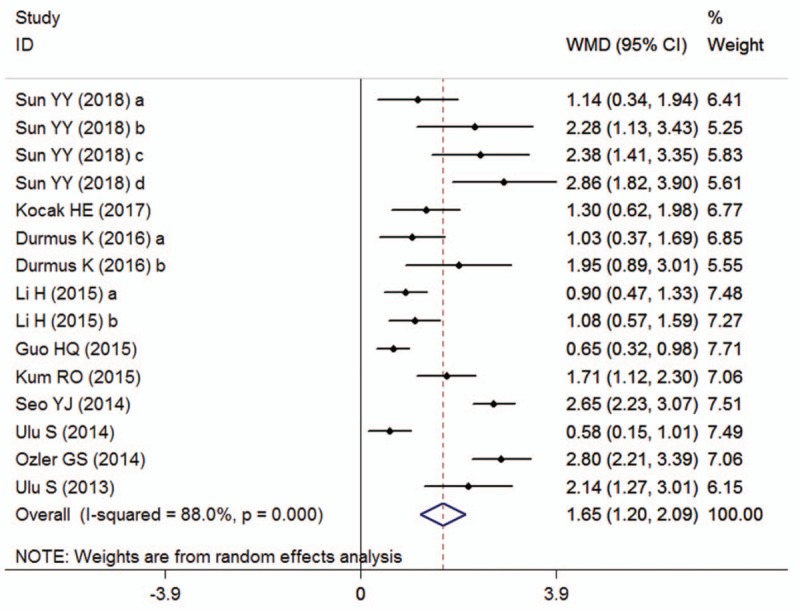
Forest plots showing the overall outcome of the meta-analysis of neutrophil-to-lymphocyte ratio for the case group (patients with ISSHL) versus the control group (healthy people). CI = confidence interval, ISSHL = idiopathic sudden sensorineural hearing loss, SMD = standardized mean deviation.
Table 3.
Summary of the meta-analysis results for the predictive value of NLR in ISSHL diagnosis.
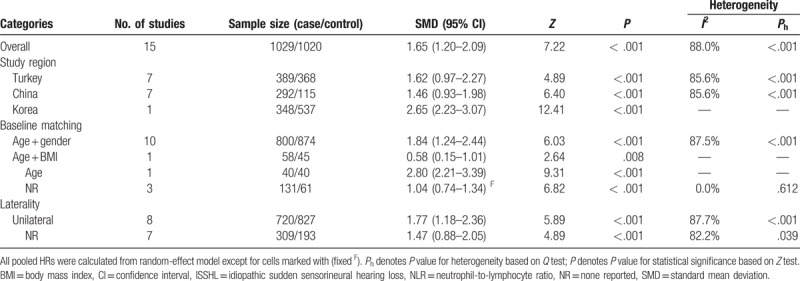
3.3. Meta-analysis of NLR in ISSHL prognosis
Nine papers with 12 studies comprising 590 “recovered” and 438 “unrecovered” ISSHL patients reported the outcomes for ISSHL prognosis, and the heterogeneity test indicated that extreme heterogeneity was found among the included studies (I2 = 72.4%, P < .001); therefore, a random-effect model was used to estimate the overall outcomes. The conjoined result indicated that the pretreatment NLR level in “unrecovered” patients was markedly higher than that in the “recovered” patients (SMD = 1.27, 95% CI: 0.62–1.92, P < .001; Fig. 3). When the data were stratified by medication administration, significant differences were found in the subgroups concerning intravenous administration (SMD = 1.10, 95% CI: 0.65–1.69, P = .008 random-effect model), and oral administration (SMD = 1.69, 95% CI: 0.60–2.77, P = .002, random-effect model). When grouped based on definition of “recovered,” significant differences were also observed in all subgroups, including “Siegel criteria” (SMD = 1.11, 95% CI: 0.35–1.86, P = .004, random-effect model), and “non-Siegel criteria” (SMD = 2.02, 95% CI: 1.23–2.81, P < .007, fixed-effect model). Moreover, the heavy distinction of pretreatment NLR levels between these 2 groups did not change regardless of the subgroup analyses of study region, laterality, type of steroid, maintenance treatment, and follow-up period (Table 4).
Figure 3.
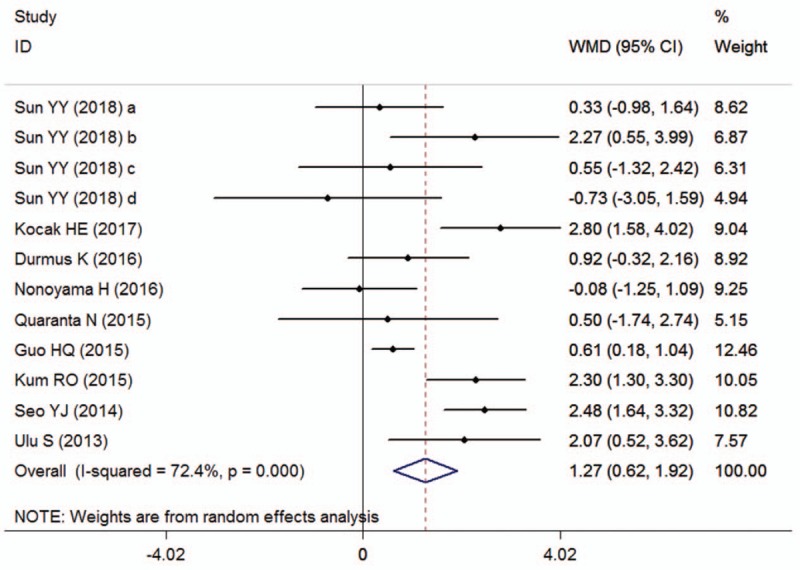
Forest plots showing the overall outcome of the meta-analysis of neutrophil-to-lymphocyte ratio for the case group (unrecovered ISSHL patients) versus the control group (recovered ISSHL patients). CI = confidence interval, ISSHL = idiopathic sudden sensorineural hearing loss, SMD = standardized mean deviation.
Table 4.
Summary of the meta-analysis results for the predictive value of NLR in ISSHL prognosis.
3.4. Sensitivity analysis and publication bias
Sensitivity analysis was conducted to evaluate the robustness of the meta-analysis by leave-one-out approach, and the results suggested that no individual study significantly changed the overall SMDs and their 95% CIs of our meta-analysis for ISSHL diagnosis (Fig. 4A) and ISSHL prognosis (Fig. 4B).
Figure 4.
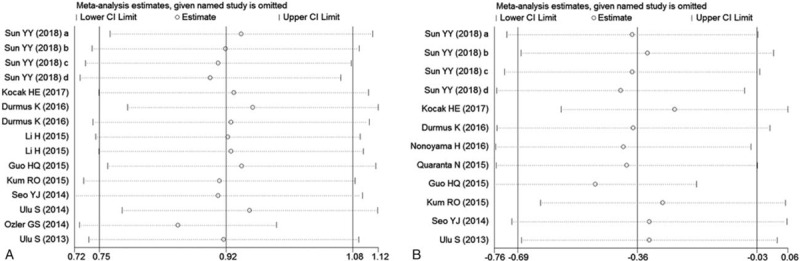
Effect of individual studies on pooled hazard ratios (HR) for the relationship between neutrophil-to-lymphocyte ratio and ISSHL. CI = confidence interval, ISSHL = idiopathic sudden sensorineural hearing loss. A, Sensitivity analysis for ISSHL formation. B, Sensitivity analysis for ISSHL prognosis.
No evidence of publication bias was found for the studies used for ISSHL diagnosis (Begg test: P = .101; Egger test: P = .083), and ISSHL prognosis (Begg test: P = .537; Egger test: P = .596). Moreover, the shape of the funnel plot did not show any obvious evidence of asymmetry for the outcomes of ISSHL diagnosis (Fig. 5A), and ISSHL prognosis (Fig. 5B). Hence, the results of the meta-analysis were robust and reliable.
Figure 5.
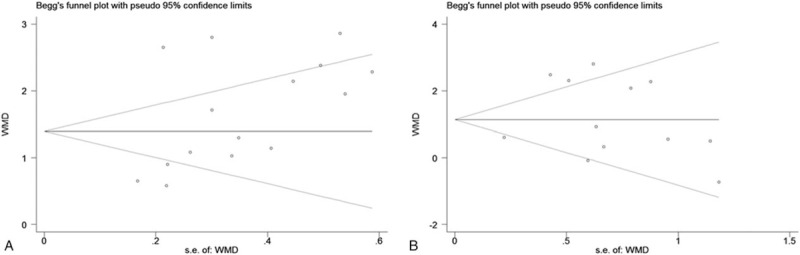
Funnel plots for assessment of potential publication bias in studies of neutrophil-to-lymphocyte ratio in patients with ISSHL. A, Funnel plot of publication bias for studies reporting ISSHL formation. B, Funnel plot of publication bias for studies reporting ISSHL prognosis.
4. Discussion
The key to successful treatment of diseases lies in the understanding of their etiopathogenesis. Although several conflicting hypotheses have been proposed regarding the origin of ISSHL, its exact cause is still unclear, because of the complete lack of unequivocal diagnostic and pathogenetic elements in the patients’ history.[37] Perhaps, ISSHL could be more accurately described as a clinical syndrome, for which there is no single identifiable cause for all cases. As steroids performs an anti-inflammatory property by reducing inflammation and edema in the inner ear, and has been labeled as the gold standard for treatment of ISSHL, the hypothesis that inflammation plays an important role in the pathogenesis of ISSHL has been recently highlighted.[38] Chronic inflammation can be caused by viral or bacterial infections directly, which results in secondary vascular insufficiency through the precipitation of hemagglutination, capillary edema, or induction of a hypercoagulable state.[39] It is also probable that chronic inflammation leads to alterations within the cochlea via a similar mechanism by which it has been shown to affect the central nervous system, despite the “blood–brain barrier.”[40] Under inflammatory conditions, the cochlea is susceptible to injury through a variety of vascular insults due to the small diameter of the sole feeding artery and lack of collateral blood supply.[41] Moreover, the clinical manifestations of ISSHL, including acute onset and unilateral symptoms, are similar to some ischemic vascular diseases, such as transient ischemic attacks, cerebral stroke and amaurosis fugax, as well the complication of vertigo in some ISSHL cases further supports the presence of an underlying vascular problem.[42] Some studies have explored that some risk factors for ischemic vascular disease, including smoking, hypertension, hyperglycemia, and hyperlipidemia, are also risk factors for the development of ISSHL.[3] Thus, inflammation-induced cochlear ischemia seems to be a common pathway in the development of SSNHL. However, the detailed mechanism of inflammation in ISSHL is still not clear, and more studies are warranted to identify it.
In recent years, there were many studies evaluating the effects of various inflammatory parameters during serologic evaluation of patients with ISSHL. According to some included studies of this meta-analysis, several single inflammatory indexes, such as WBC, neutrophil, lymphocyte, and monocyte, were much higher in the ISSHL patients groups rather than the healthy controls groups, but the use of composite parameters, including NLR and PLR, could serve to better place participants into an inflammatory profile than a single measure.[26,31,34] This superiority may be due to the stability of composite parameters, because single measures prone to be affected by various pathological and physiological conditions.[43] Furthermore, neutrophil, lymphocyte, and monocyte counts can be increased with the elevated WBC count, which are still under acceptable normal value in some cases and could not be judged for an abnormal finding in the hematologic examination. On the other hand, NLR or PLR demonstrates the ratio of 2 different WBC subtypes counts, and is considered to be more stable than the single inflammatory indexes.[44] Therefore, NLR or PLR acts as a comprehensive index represented 2 complementary immune pathways, and is more valuable than any other parameter of single cell counts on predicting the onset and development of ISSHL.
NLR is an easily measured, widely available biomarker of inflammation that is routinely measured in complete blood count tests without any additional cost. It correlates with clinical status and can aid in the risk stratification of patients with various diseases. It is also as valuable as some high-cost inflammatory markers, such as IL-6, IL-1α, IL-8, and TNF-α.[45] Unlike the total WBC count, NLR reflects the balance of 2 complementary but paradoxical pathways of the immune system: neutrophils represent the active nonspecific inflammatory mediator that initiates the first line of defense, whereas lymphocytes represent the regulatory or protective component of inflammation.[46] In addition, Seo et al[31] reported that both NLR and PLR were increased significantly for the unrecovered ISSHL patients compared with the recovered patients. But after adjustment by multivariate analysis, only NLR level was associated strongly with the recovery of ISSHL and was an independent risk factor for the improvement of pure-tone averages later. Lee et al[47] showed that the NLR levels, rather than the PLR levels, were significantly different between children ISSHL patients and healthy controls. Thus, NLR seems to be a more reliable predictor of onset and prognosis of ISSHL than PLR.
In this study, we included 10 publications with 15 studies which containing 1029 ISSHL patients and 1020 healthy individuals to assess the relationship between NLR levels and ISSHL. Our results showed that NLR value of ISSHL patients was much higher than that of healthy populations. These results indicated that neutrophils and lymphocytes might play important roles in the pathogenesis of ISSHL, and NLR level can be a potential predictor for ISSHL formation. However, this finding should be cautiously interpreted due to the extreme heterogeneity. The existence of heterogeneity affects the combined effect of the studies, and also the interpretation of the results of meta-analysis.[48] Strict inclusion and exclusion criteria are helpful to decrease the heterogeneity of the studies, but, due to the presence of some potential confounding factors, there was still extreme heterogeneity among the included studies. The most common methods to handle heterogeneity in meta-analysis are sensitivity and subgroup analyses. By performing sensitivity analysis, the pooled outcomes after stepwise removal of each study showed no observed difference on the effects of NLR on ISSHL onset. After stratifying some confounding effects, such as study country, baseline matching, and laterality, the overall results of subgroup analyses were still statistical significance, which demonstrated that none of them could completely explain the heterogeneity. Therefore, our results were stable and reliable. Instead, we used a random-effects model to calculate the consolidated results to minimize the influence of heterogeneity to some extent.
The prediction of the prognosis of a certain disease is beneficial for the selection of a therapeutic strategy and for the reasonable allocation of medical resources.[49] In this meta-analysis, we also compared the NLR levels among ISSHL patients with different recovery degrees, and found that NLR value of “unrecovered” patients was evidently higher than those of “recovered” patients. This result could be attributed to the higher inflammatory situation in “unrecovered” patients, and may help clinician scaring for ISSHL patients with higher NLR levels in terms of treatment and prognosis. Due to the extreme heterogeneity, we also conducted subgroup analyses based on study region, laterality, type of steroid, medication administration, maintenance treatment, follow-up period, and definition of “recovered,” the combined outcome of each subgroup for the prognostic value of NLR on ISSHL was not weaken and still statistical significance. Therefore, it was reasonable to think that neutrophils and lymphocytes might be contributed to the development of ISSHL, and NLR could be an independent index for prognosis of ISSHL.
Although a previous meta-analysis of NLR levels related to prognosis of ISSHL has been published by Cao et al,[50] some advances of the present meta-analysis should be highlighted. First, based on a comprehensive literature search, our meta-analysis ultimately included 12 studies with 1028 patients, which were greater than the previous study (8 studies with 843 patients), and the evidence-based power was more strong. Second, our meta-analysis did not enroll a study concerning the prognostic role of NLR in children with ISSHL, which had been included in the previous study, because it might lead to an imprecision on the overall outcome due to extreme differences of physiology, etiology, and morbidity between underage and adult patients with ISSHL. Third, because the efficacy determinations among the included studies were markedly distinct, which might be a potential confounder of the pooled results, the present meta-analysis has conducted a subgroup analysis based on it. Finally, except for prognostic value, the present meta-analysis investigated the value of NLR in development of ISSHL, and further explored the mechanism of inflammation in the pathogenesis of ISSHL.
This systematic review and meta-analysis has several limitations that need to be addressed. First, only 4 databases (PubMed, Embase, Web of Science, and China National Knowledge Infrastructure) were searched, some studies with valuable information may have been missed. But these databases are well-known comprehensive databases and no publication bias was found, which indicated that our results of meta-analysis were credible. Second, the limited number of included studies and the small sample sizes might affect the power of our meta-analysis. Third, only case-control studies were included in the meta-analysis of the association of NLR and ISSHL formation, and it is not sufficiently powerful to demonstrate a causal relationship. Fourth, the absolute values of NLR were widely different between included studies, which might cause heterogeneity to a certain degree. Fifth, because of the absence of information, this meta-analysis failed to explore the relationship between NLR and other influencing factors of the development of ISSHL which might be helpful in corroborating our findings. This part of the work should be conducted in the further studies. Sixth, all of the included researches were retrospective studies, which may have introduced reporting bias. Seventh, some potential factors, such as the duration from the onset to the beginning of treatment, age, audiogram, the severity of hearing loss, and underlying diseases, might affect the prognosis of ISSHL, but we were unable to conduct subgroup analyses to control these aforementioned variables because of a lack of sufficient information in the included studies. Finally, clinical decision-making usually cannot be made solely on one single or overmuch markers, and coincidently only NLR was involved.
In summary, the data of the current meta-analysis showed that NLR levels of ISSHL patients were significantly higher than those of healthy controls, which indicated that the NLR might exert an impact on the pathogenesis of ISSHL. Moreover, NLR was much higher in unrecovered patients with ISSHL rather than recovered cases, which suggested that NLR showed a relatively high value in the prediction of ISSHL prognosis. Therefore, we concluded that NLR could be used as a valuable biomarker to determine the onset and prognosis of ISSHL. Understanding the mechanism of NLR in ISSHL might be beneficial to understand pathogenesis and development of ISSHL. However, due to the limitation of this meta-analysis, further studies with large population of NLR should be performed to verify the results.
Author contributions
Conceptualization: Liren Hu, Jiayuan Wu.
Data curation: Gaohua Zhang, Yufeng Wang.
Formal analysis: Liquan Chen, Zhanhui Zhang.
Investigation: Zhanhui Zhang, Yufeng Wang.
Methodology: Liquan Chen, Liren Hu, Jiayuan Wu.
Software: Gaohua Zhang, Yufeng Wang, Liren Hu.
Supervision: Zhanhui Zhang.
Validation: Liquan Chen, Yufeng Wang, Liren Hu.
Visualization: Liren Hu.
Writing – original draft: Liquan Chen, Gaohua Zhang, Jiayuan Wu.
Writing – review & editing: Yufeng Wang, Jiayuan Wu.
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, ISSHL = idiopathic sudden sensorineural hearing loss, NLR = neutrophil-to-lymphocyte ratio, SMD = standardized mean deviation.
LC and GZ contributed equally to this work.
This research was supported by grants from the Financial Fund Competitive Distribution Project for Science and Technology of Zhanjiang (No. 2017A01022).
Ethical approval and patient consent are not required, because this study is a meta-analysis based on published studies, and does not contain any studies with human participants or animals performed by any of the authors.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Stachler RJ, Chanderasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neak Surg 2012;146:S1–35. [DOI] [PubMed] [Google Scholar]
- [2].Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. Lancet 2010;375:1203–11. [DOI] [PubMed] [Google Scholar]
- [3].Chau JK, Lin JR, Atashband S, et al. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 2010;120:1011–21. [DOI] [PubMed] [Google Scholar]
- [4].Lazarini PR, Camargo AC. Idiopathic sudden sensorineural hearing loss: etipathogenic aspects. Braz J Otorhinolaryngol 2006;72:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuhn M, Heman-Ackah SE, Shaikh JA, et al. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif 2011;15:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother 2015;15:523–31. [DOI] [PubMed] [Google Scholar]
- [7].Yin SK, Feng YM. Advance in pathogenesis of bilateral sudden sensorineural hearing loss. J Chin Otorhinolaryngeal Head Neck Surg 2016;30:1100–3. [DOI] [PubMed] [Google Scholar]
- [8].Quaranta N, De Ceglie V, D’Elia A. Endothelia dysfunction in idiopathic sudden sensorineural hearing loss: a review. Audiol Res 2016;6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Berjis N, Moeinimehr M, Hashemi SM, et al. Endothelia dysfunction in patients with sudden sensorineural hearing loss. Adv Biomed Res 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gates GA, Mills JH. Presbycusis. Lancet 2005;366:1111–20. [DOI] [PubMed] [Google Scholar]
- [11].Fisher ME, Schubert CR, Nondal DM, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis 2015;238:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Trune DR, Nguyen-Huynh A. Vascular pathophysiology in hearing disorders. Semin Hear 2012;33:242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Berrettini S, Seccia V, Fortunato S, et al. Analysis of the 3-dimensional fluid-attenuated inversion-recovery (3D-FLAIR) sequence in idiopathic sudden sensorineural hearing loss. JAMA Otolaryngol Head Neck Surg 2013;139:456–64. [DOI] [PubMed] [Google Scholar]
- [14].Masuda M, Kanzaki S, Minami S, et al. Correlations of inflammation biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol 2012;33:1142–50. [DOI] [PubMed] [Google Scholar]
- [15].Mosnier I, Stepanian A, Baron G, et al. Cardiovascular and thromboembolic risk factors in idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol 2011;16:55–66. [DOI] [PubMed] [Google Scholar]
- [16].Nishida Y, Hosomi S, Yamagami H, et al. Neurophil-to-lymphocyte ratio for predicting loss of response to infliximab in ulcerative colitis. PLoS One 2017;12:e0169842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Verdoia M, Schaffer A, Barbieri L, et al. Impact of diabetes on neutrophil-to-lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab 2015;41:304–11. [DOI] [PubMed] [Google Scholar]
- [18].Qi X, Li J, Deng H, et al. Neutrophil-to-lymphocyte ratio for the prognostic assessment of hepatocellular carcinoma: a systematic review and meta- analysis of observational studies. Oncotarget 2016;7:45283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [20].Wu JY, Liang CX, Chen MY, et al. Association between tumor-stroma ratio and prognosis in solid tumor patients: a systematic review and meta-analysis. Oncotarget 2016;7:68954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [22].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [23].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007;26:4544–62. [DOI] [PubMed] [Google Scholar]
- [25].Sun Y, Xia L, Wang H, et al. Is nucleate cell count and neutrophil to lymphocyte ratio related to patients with audiographically distinct sudden sensorineural hearing loss? Medicine (Baltimore) 2018;97:e10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kocak HE, Elbistali MS, Acipayam H, et al. Are neutrophil/lymphocyte and platelet/lymphocyte ratios related with formation of sudden hearing loss and its prognosis? Eur Ann Otorhinolaryngol Head Neck Dis 2017;134:383–6. [DOI] [PubMed] [Google Scholar]
- [27].Durmus K, Terzi H, Karatas TD, et al. Assessment of hematological factors involved in development and prognosis of idiopathic sudden sensorineural hearing loss. J Craniofac Surg 2016;27:e85–91. [DOI] [PubMed] [Google Scholar]
- [28].Li H, Zhao D, Diao M, et al. Hyperbaric oxygen treatments attenuate the neutrophil-to- lymphocyte ratio in patients with idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 2015;153:606–12. [DOI] [PubMed] [Google Scholar]
- [29].Guo HQ, Xu XY, Li YR, et al. Association between peripheral blood cell ratio and curative effect in patients with sudden hearing loss. J Ningxia Med Univer 2015;37:687–90. [Google Scholar]
- [30].Kum RO, Ozcan M, Baklaci D, et al. Investigation of neutrophil-to-lymphocyte ratio and mean platelet volume in sudden hearing loss. Braz J Otorhinolaryngol 2015;81:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seo YJ, Jeong JH, Choi JY, et al. Neutrophil-to-lymphocyte ratio and platelet- to-lymphocyte ratio: novel markers for diagnosis and prognosis in patients with idiopathic sudden sensorineural hearing loss. Dis Markers 2014;2014:702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ulu S, Bucak A, Ulu MS, et al. Neutrophil-to-lymphocyte ratio as a new predictive and prognostic factor at the hearing loss of diabetic patients. Eur Arch Otorhinolaryngol 2014;271:2681–6. [DOI] [PubMed] [Google Scholar]
- [33].Ozler GS. Increased neutrophil-lymphocyte ratio in patients with idiopathic sudden sensorineural hearing loss. J Craniofac Surg 2014;25:e260–3. [DOI] [PubMed] [Google Scholar]
- [34].Ulu S, Ulu MS, Bucak A, et al. Neutrophil-to-lymphocyte ratio as a new, quick, and reliable indicator for predicting diagnosis and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol 2013;34:1400–4. [DOI] [PubMed] [Google Scholar]
- [35].Nonoyama H, Tanigwa T, Shibata R, et al. Red blood cell distribution width predicts prognosis in idiopathic sudden sensorineural hearing loss. Acta Otolaryngol 2016;136:1137–40. [DOI] [PubMed] [Google Scholar]
- [36].Quaranta N, Squeo V, Sangineto M, et al. High total cholesterol in peripheral blood correlated with poorer hearing recovery in idiopathic sudden sensorineural hearing loss. PLoS One 2015;10:e0133300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wei BP, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev 2013;11:CD003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hultcrantz E, Nosrati-Zarenoe R. Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: analysis of an RCT and material drawn from the Swedish national database. Eur Arch Otorhinolaryngol 2015;272:3168–75. [DOI] [PubMed] [Google Scholar]
- [39].Crane RA, Camilon M, Nguyen S, et al. Steroids for treatment of sudden sensorineural hearing loss: a meta-analysis of randomized controlled trials. Laryngoscope 2015;125:209–17. [DOI] [PubMed] [Google Scholar]
- [40].Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009;73:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kuhn M, Heman-Ackah SE, Shaikh JA, et al. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif 2011;15:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ballesteros F, Alodid I, Tassies D, et al. Is there an overlap between sudden neurosensorial hearing loss and cardiovascular risk factors? Audiol Neurootol 2009;14:139–45. [DOI] [PubMed] [Google Scholar]
- [43].Nash SD, Cruickshanks KJ, Zhan W, et al. Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the epidemiology of hearing loss study. J Gerontol A Biol Sci Med Sci 2014;69:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yang HB, Xing M, Ma LN, et al. Prognostic significance of neutrophil- lymphocyte ratio/patelet-lymphocyte ratio in lung cancers: a meta-analysis. Oncotarget 2016;7:76769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu JY, Chen MY, Liang CX, et al. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in cervical cancer: a meta-analysis and systematic review. Oncotarget 2017;8:13400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xin-Ji Z, Yong-Gang L, Xiao-Jun S, et al. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: a meta-analysis. Int J Surg 2015;21:84–91. [DOI] [PubMed] [Google Scholar]
- [47].Lee JS, Hong SK, Kim DH, et al. The neutrophil-to-lymphocyte ratio in children with sudden sensorineural hearing loss: a retrospective study. Acta Otolaryngol 2017;137:35–8. [DOI] [PubMed] [Google Scholar]
- [48].Song J, Chen S, Liu X, et al. Relationship between C-reactive protein level and diabetic retinopathy: a systematic review and meta-analysis. PLoS One 2015;10:e0144406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu JY, Hu LR, Chen MY, et al. Pyruvate kinase M2 overexpression and poor prognosis in solid tumors of digestive system: evidence from 16 cohort studies. Onco Target Ther 2016;9:4277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cao Z, Li Z, Xiang H, et al. Prognostic role of haematological indices in sudden sensorineural hearing loss: review and meta-analysis. Clin Chim Acta 2018;483:104–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


