Abstract
Systemic inflammatory indices are correlated with poor prognosis in cancer patients. The presence of lymphocytes in and around the tumor tissue is a predictor in rectal cancer. We aimed to explore the mechanism underlying the changes in circulating lymphocyte during neoadjuvant therapy and the way in which the count correlates with tumor response.
Around 307 patients from FOWARC trial and 64 patients from FORTUNE trial were included in the training and validation group. Circulating lymphocyte count was recorded before neoadjuvant therapy and before rectal surgery. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off value of the reduction of lymphocytes. A logistic regression model was obtained in multivariate analysis.
The blood absolute number of lymphocyte before and after therapy had no correlation with tumor response. However, total lymphocyte count (TLC) reduction was significantly higher in good response group (39.81% vs 33.31% P = .032) in the FOWARC cohort. The optimal cut-off value for TLC was 24.96%. Age, tumor length, and TLC reduction (P = .005, OR = 2.009, 95%CI 1.240–3.254) were significant factors for tumor regression in multivariate analysis. In the FORTUNE cohort, TLC reduction was the only significant factor for tumor regression in both univariate (P = .032, OR = 3.434, 95%CI 1.111–10.614) and multivariate analysis (P = .046, OR = 3.361, 95%CI 1.024–11.035).
Circulating lymphocyte count decreases during neoadjuvant therapy for locally advanced rectal cancer, and it is associated with better tumor regression. It may be involved in the immune response provoked by radiotherapy and chemotherapy.
Keywords: lymphocytes, neoadjuvant therapy, rectal neoplasms, tumor response grade
1. Introduction
Colorectal cancer is the third most common cancer, and approximately 1.2 million patients are newly diagnosed every year with colorectal cancer worldwide.[1] Preoperative chemoradiotherapy is now recommended as the standard treatment for patients with locally advanced rectal cancer, as it contributes to reduced local recurrence and toxicity.[2,3] A previous study has reported that most patients achieved downstaging, and 15% to 27% of the patients achieved pathological complete response (pCR) after preoperative chemoradiotherapy.[4]
Conventionally, chemotherapy and radiotherapy can kill tumor cells in a conventional cytotoxicity manner; however, recent evidence suggests that chemotherapy and radiotherapy could provoke immune response, which also causes tumor regression.[5,6] Several studies have demonstrated that immune cells, especially T lymphocytes infiltrating in and around the tumor, are associated with improved patient survival.[7–9] Accordingly, systemic inflammatory response is considered to be associated with poor prognosis in various cancers, which can be reflected by the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR).[10–14] Some studies also pointed out that circulating lymphocyte count before treatment may be a good prognostic factor. Lower circulating lymphocyte count was correlated with a shorter overall survival (OS), and it also indicated severe toxicity due to radiotherapy. However, all these studies have focused on baseline circulating lymphocytes.[15–18] Few studies have focused on the mechanisms of lymphocyte changes during neoadjuvant therapy. Hence, we aimed to investigate the mechanism underlying the changes in circulating lymphocyte count during preoperative chemoradiotherapy and the way in which the count correlates with tumor response. For this, we chose the FOWARC study, a prospective phase III study comparing neoadjuvant FOLFOX6 chemotherapy with or without radiation in locally advanced rectal cancer as a training cohort,[19] and a separate single arm phase II study, the FORTUNE study as a validation cohort.
2. Patients and methods
2.1. The study was based on 2 prospective cohorts
2.1.1. The training cohort
The training cohort comprised patients from the FOWARC study, which aimed to investigate the addition of oxaliplatin with or without preoperative radiotherapy in locally advanced rectal cancer. Patients enrolled in the FOWARC study data available from June 2010 to February 2015 were included in this study. Patients who were infected during treatment or who did not complete neoadjuvant therapy were excluded.
2.1.2. The validation cohort
The validation cohort comprised patients from the FORTUNE study, which aimed to explore the efficacy of preoperative chemotherapy with mFOLFOXIRI in locally advanced rectal cancer. Patients diagnosed with cT4 or fixed cT3 rectal cancer evaluated by pelvic MRI between August 2014 and September 2016 were enrolled in the study. Patients with stable/progressive disease were scheduled to undergo radiation before surgery, which were excluded in this analysis.
In the FOWARC study, patients diagnosed with locally advanced stage II/III rectal cancer were randomized to 3 different treatments: 5 two-week cycles of de Gramont (leucovorin 400 mg/m2 and fluorouracil 400 mg/m2, followed by 2400 mg/m2 fluorouracil for intravenous infusion in 48 hours) plus radiotherapy (1.8–2.0 Gy for 23–28 fractions over 5–6 weeks and a total dose of 46.0–50.4 Gy), followed by total mesolectal excision (TME) and 4 to 6 cycles of mFOLFOX plus radiotherapy (treatment was similar to that for the de Gramont-radiotherapy group plus oxalipatin 85 mg/m2on the first day of each cycle), followed by TME and 6 to 8 cycles of mFOLFOX, followed by TME.[19]
In the FORTUNE study, patients received 4 to 6 cycles of mFOLFOXIRI (irinotecan 165 mg/m2, oxalipatin 85 mg/m2, leucovorin 400 mg/m2, fluorouracil 400 mg/m2, and 2400 mg/m2 fluorouracil for intravenous infusion in 48 hours). Patients who did not respond were recommended to receive radiation (1.8–2.0 Gy for 23–28 fractions over 5–6 weeks, and a total dose of 46.0–50.4 Gy with concurrent chemotherapy regimen of mFOLFOX). Subsequently, all patients received TME.
2.1.3. Pathological assessment
The tumor stage was evaluated by 2 pathologists according to the American Joint Committee on Cancer (AJCC) TNM staging category. The tumor regression grade (TRG) was also evaluated according to the guidelines of College of American Pathologists. No residual tumor was defined as complete response (grade 0), minimal residual cancer was defined as moderate response (grade 1), minimal response was grade 2, and no definite response identified was grade 3.[20] In this study, grade 0 and grade 1 were defined as good response while grade 2 and grade 3 were defined as poor response.
2.1.4. Blood sampling and enumeration of circulating lymphocyte count
Peripheral blood was collected within 7 days before the start of neoadjuvant therapy and 7 days before rectal surgery. Circulating lymphocyte count was investigated from medical records.
All patients provided written informed consent before enrolment into the study. The study was approved by the local medical ethics committee and was conducted in accordance with the Declaration of Helsinki and good clinical practices.
2.1.5. Statistical analysis
SPSS 20.0 (IBM) was used to analyze the data. Lymphocyte count decreased during chemoradiotherapy (CRT). In this study, we examined the possible relationship between lymphocyte reduction and tumor treatment response. Mann–Whitney test was used to compared lymphocyte reduction ratio between the good response group and the poor response group. A receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off value of the ratio of lymphocyte reduction after CRT in the training cohort, which was validated in another cohort. Chi-square tests and t tests were used to compare patient and tumor characteristics between the lymphopenia and nonlymphopenia groups. A logistic regression model was established in the multivariate analysis. The factors used within the multivariate model in the training cohort and the validation cohort were age, clinically T stage, clinically TNM stage, tumor length, baseline lymphocyte count before treatment, lymphocyte reduction.
3. Results
3.1. Patient characteristics
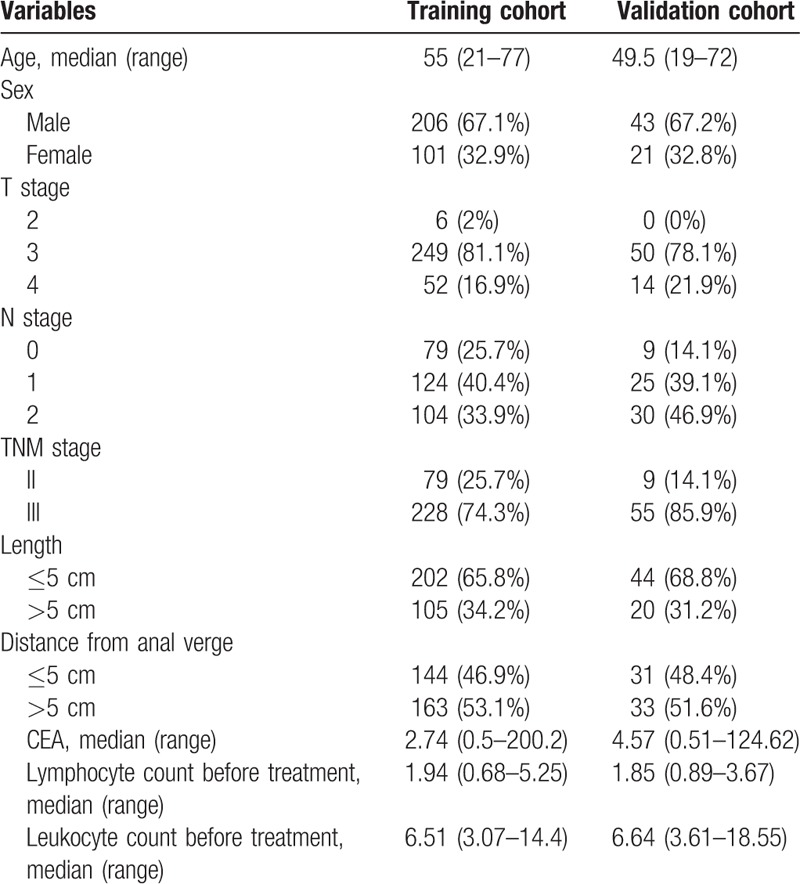
The baseline characteristics of patients in the FOWARC and FORTUNE cohorts are listed in Table 1. In the training cohort, 307 patients were included: 90 patients in the fluorouracil+ radiotherapy group, 100 patients in the mFOLFOX6+radiotherapy group, and 117 patients in the mFOLFOX6 group. Two-third of the patients were male (206, 67.1%) and one third were female (101, 32.9%). According to the TNM staging, 29 (25.7%) patients were stage II and 228 (74.3%) were stage III. The median circulating lymphocyte count at baseline before neoadjuvant therapy was 1.94. In the validation cohort, 64 patients who received only chemotherapy and not radiation were included. All 64 patients received intense neoadjuvant chemotherapy; 43 (67.2%) of these were male and 21 (32.8%) were female. Most of the patients were stage III (85.9%). The median circulating lymphocyte count before treatment was 1.85.
Table 1.
Baseline characteristic of the training cohort and the validation cohort.
3.2. High TLC reduction related to good tumor response
TLC reduction was defined as the ratio of baseline TLC minus TLC after neoadjuvant chemotherapy to baseline TLC. TLC reduction was significantly higher in good response group. In the training cohort, lymphocyte reduction was 39.81% vs 33.31% (P = .032) in the good-response and poor-response groups, respectively. In the validation cohort, lymphocyte reduction was 25.41% vs 5.34% (P = .021) in the good-response and poor-response groups, respectively. Tumor response was better as TLC further decreased during neoadjuvant therapy.
3.3. TLC reduction independently predict tumor response
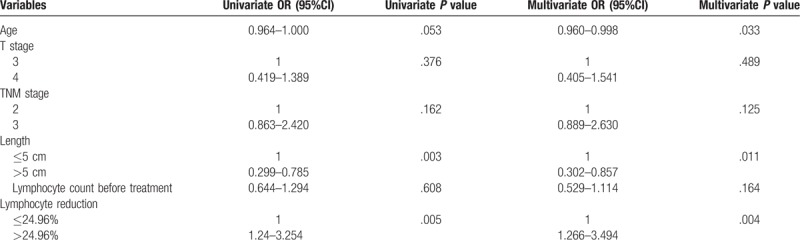
In the training cohort, an ROC curve was used, and 24.96% was the optimal cut-off value to distinguish between lymphopenia and nonlymphopenia groups for tumor response. Among 307 patients, 203 (66.1%) patients were in the lymphopenia group and 104 (33.9%) were in the nonlymphopenia group. There were no significant differences in age, sex, T stage, TNM stage, tumor length, and baseline lymphocyte number between lymphopenia and nonlymphopenia groups. Detail univariate analysis and multivariate analysis results were shown in Table 2. In the univariate analysis, tumor length (P = .003, OR = 0.485 95%CI 0.299–0.785) and TLC reduction (P = .005, OR = 2.009, 95%CI 1.240–3.254) were significant factors for tumor response. Only age (P = .033, OR = 0.979, 95%CI 0.960–0.998), tumor length (P = .011, OR = 0.509 95%CI 0.302–0.857), and TLC reduction (P = .004, OR = 2.103, 95%CI 1.266–3.494) were significant predictive factors for tumor response in multivariate analysis (Table 2).
Table 2.
Effect of prognostic factors on TRG in the training cohort.
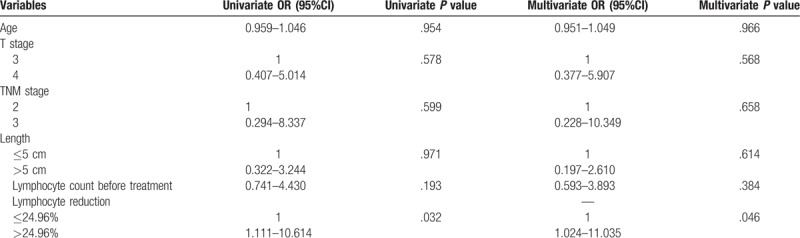
In validation cohort, there were more patients (67.2%) in the nonlymphopenia group, compared with the FOWARC cohort. There was no significant difference in baseline characteristics between lymphopenia and nonlymphopenia group. Table 3 presents the results of univariate and multivariate analysis in the FORTUNE cohort. Unexpectedly, TLC was the only significant factor for tumor regression in both univariate (P = .032, OR = 3.434, 95%CI 1.111–10.614) and multivariate analysis (P = .046, OR = 3.361, 95%CI 1.024–11.035).
Table 3.
Effect of prognostic factors on tumor response in the validation cohort.
4. Discussion
Recently, great advances have been made in immunotherapy for various cancers. For example, it was proved that the antibody of PD-1 and PD-L1 is effective and safe for melanoma, non-small-cell lung cancer, and renal-cell cancer.[21,22] However, the treatment efficacy is limited in rectal cancer. Studies have shown that mismatch repair-deficient tumors are more responsive to PD-1 blockade than mismatch repair-proficient tumors are, which account for only 5% of rectal cancers.[23,24] Several studies have reported that tumor-infiltrating lymphocyte is a marker for mismatch repair-deficient tumors in colorectal cancer.[25,26] It has been established that after inactivation of DNA mismatch repair pathway, more neoantigens are released, which are highly immunogenic, and contribute to increased infiltration of tumor tissue with activated lymphocytes.[27] Several studies have demonstrated that lymphocytes infiltrating in and around the tumor are associated with improved survival.[9,28] It has been reported that overlap of T cell receptor sequences in brain tumors and peripheral blood is a predictor of immune response and OS in patients with glioblastoma who are treated with immunotherapy, which indicated that circulating lymphocytes and tumor-infiltrating lymphocytes are correlated.[29] However, few studies have focused on the circulating lymphocytes and their impact on neoadjuvant therapy in colorectal cancer. Systemic inflammation index before treatment, for example, neutrophil-to-lymphocyte ratio, was considered to have a reverse association with the prognosis in several kinds of cancer, and to be correlated with poorer OS. All these indexes were correlated with lymphocytes.[10–12] However, few have focused on how lymphocytes change during treatment. Hence, we focused on the circulating lymphocyte and its changes during neoadjuvant therapy.
Our analysis shows that circulating lymphocyte count decreases during neoadjuvant therapy, and that lymphopenia after neoadjuvant therapy is an independent predictor in multivariate analysis for tumor regression in a prospective phase III study of neoadjuvant chemoradiotherapy of locally advanced rectal cancer. To validate its relationship with tumor regression grade, we also examined the change in circulating lymphocyte count in a prospective phase II study of intensive neoadjuvant chemotherapy of locally advanced rectal cancer. We discovered that circulating lymphocyte count decreased after neoadjuvant therapy for locally advanced rectal cancer, and that the change is correlated with tumor regression. Lymphocyte reduction is correlated with good response of the tumor.
Chemotherapy and radiation decrease the circulating lymphocyte count, which was reported to be a probable predictor of poor response to chemotherapy and immune damage caused by chemotherapy and radiation.[10–12] However, some studies have demonstrated that apoptosis of peripheral blood lymphocytes is correlated with histological regression of rectal cancer in response to preoperative chemoradiotherapy,[30] which is consistent with the results of our analysis.
We can see that in the training cohort, lymphocyte count reduction is more obvious comparing with the validation cohort, which we assume was due to the effect of radiation. Median TLC reduction of the chemotherapy only group and the other 2 chemoradiation groups were 14.29% and 52.14%. TLC reduction in the chemotherapy only group was obvious lower. It is recognized that radiation therapy targets tumor cells and releases tumor antigens, which trigger the immune system to activate tumor-specific T cells.[31] Radiation interferes directly with tumor cells and potentially breaks immune barriers between tumors and the microenvironment.[5]
With regard to chemotherapy, it has been reported that drugs used at clinically effective doses may stimulate immune effector cells or subvert immunosuppressive cells.[32] Evidence suggests that chemotherapy and radiotherapy activate dendritic cells and enhance antigen presentation, thus, selectively eliminating immunosuppressive cells.[33] Oxaliplatin, a platinum-based compound that is now currently used to treat colorectal cancer and pancreatic cancer, has been shown to increase the expression of class I human leukocyte antigen on malignant cells, which enhance antigen presentation to CD8+ T cells and selectively kill immunosuppressive cells.[34] It is still unknown why decrease of lymphocyte count is correlated with good response to neoadjuvant therapy for rectal cancer; however, it may be related to the elimination of immunosuppressive cells.[35] Also we will focus on changes of subtypes of lymphocytes.
Our study supported the notion that reduction of circulating lymphocyte count is correlated with good tumor regression grade in rectal cancer, which is based on a prospective phase III randomized clinical trial of neoadjuvant therapy of rectal cancer. We validated this finding in another prospective phase II trial of rectal cancer. However, we failed to determine the definite reason behind the correlation between the reduction of circulating lymphocyte count and good tumor regression grade, and to determine the subtype of lymphocytes that decrease the most during therapy; further studies in this regard are warranted.
5. Conclusion
Circulating lymphocyte count decreases during neoadjuvant therapy, including chemoradiotherapy and chemotherapy alone, for locally advanced rectal cancer, and TLC reduction is associated with better tumor regression and may be a predictor of tumor reponse. It may be involved in immune response provoked by radiotherapy and chemotherapy. Further studies are warranted to elucidate why the decrease of peripheral lymphocyte count is correlated with tumor response.
Author contributions
Conceptualization: Zehua Wu, Yanhong Deng.
Data curation: Zehua Wu, Jianwei Zhang, Yue Cai, Ru Deng, Liu Yang, Jianxia Li.
Formal analysis: Zehua Wu, Jianwei Zhang, Yue Cai, Ru Deng, Liu Yang, Jianxia Li, Yanhong Deng.
Project administration: Yanhong Deng.
Writing – original draft: Zehua Wu.
Writing – review & editing: Jianwei Zhang, Yue Cai, Ru Deng, Liu Yang, Jianxia Li, Yanhong Deng.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CRT = chemoradiotherapy, LMR = lymphocyte-to-monocyte ratio, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PCR = pathological complete response, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic, TME = total mesolectal excision, TRG = tumor regression grade.
This work was supported by grants from the National Natural Science Foundation of China (81472249).
The authors have no conflicts of interest to disclose.
References
- [1].Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- [2].Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- [4].Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–44. [DOI] [PubMed] [Google Scholar]
- [5].Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009;10:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ma Y, Kepp O, Ghiringhelli F, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol 2010;22:113–24. [DOI] [PubMed] [Google Scholar]
- [7].Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev 2012;38:451–66. [DOI] [PubMed] [Google Scholar]
- [8].Ferris RL, Galon J. Additional support for the introduction of immune cell quantification in colorectal cancer classification. J Natl Cancer Inst 2016;108:djw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn's-like lymphoid reaction, and survival from colorectal cancer. JNCI: J Natl Cancer Inst 2016;108: doi: 10.1093/jnci/djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen L, Zhang F, Sheng XG, et al. Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: lymphocyte. Medicine (Baltimore) 2016;95:e4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yamada S, Fujii T, Yabusaki N, et al. Clinical implication of inflammation-based prognostic score in pancreatic cancer: Glasgow prognostic score is the most reliable parameter. Medicine (Baltimore) 2016;95:e3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang GM, Zhu Y, Ma XC, et al. Pretreatment neutrophil-to-lymphocyte ratio: a predictor of advanced prostate cancer and biochemical recurrence in patients receiving radical prostatectomy. Medicine (Baltimore) 2015;94:e1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 2014;134:2403–13. [DOI] [PubMed] [Google Scholar]
- [14].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009;69:5383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kitayama J, Yasuda K, Kawai K, et al. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer 2011;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med 2012;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Joseph N, Dovedi SJ, Thompson C, et al. Pre-treatment lymphocytopaenia is an adverse prognostic biomarker in muscle-invasive and advanced bladder cancer. Ann Oncol 2016;27:294–9. [DOI] [PubMed] [Google Scholar]
- [19].Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the chinese fowarc multicenter, open-label, randomized three-arm phase iii trial. J Clin Oncol 2016;34:3300–7. [DOI] [PubMed] [Google Scholar]
- [20].Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med 2009;133:1539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bupathi M, Wu C. Biomarkers for immune therapy in colorectal cancer: mismatch-repair deficiency and others. J Gastrointest Oncol 2016;7:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jung A. Microsatellite instability and therapeutic sensitivity. Verh Dtsch Ges Pathol 2007;91:112–8. [PubMed] [Google Scholar]
- [26].Smyrk TC, Watson P, Kaul K, et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 2001;91:2417–22. [PubMed] [Google Scholar]
- [27].Westdorp H, Fennemann FL, Weren RD, et al. Opportunities for immunotherapy in microsatellite instable colorectal cancer. Cancer Immunol Immunother 2016;65:1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009;27:5944–51. [DOI] [PubMed] [Google Scholar]
- [29].Hsu M, Sedighim S, Wang T, et al. TCR sequencing can identify and track glioma-infiltrating T cells after DC vaccination. Cancer Immunol Res 2016;4:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ishihara S, Iinuma H, Fukushima Y, et al. Radiation-induced apoptosis of peripheral blood lymphocytes is correlated with histological regression of rectal cancer in response to preoperative chemoradiotherapy. Ann Surg Oncol 2012;19:1192–8. [DOI] [PubMed] [Google Scholar]
- [31].Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 2015;1:1325–32. [DOI] [PubMed] [Google Scholar]
- [32].Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013;39:74–88. [DOI] [PubMed] [Google Scholar]
- [33].Apetoh L, Locher C, Ghiringhelli F, et al. Harnessing dendritic cells in cancer. Semin Immunol 2011;23:42–9. [DOI] [PubMed] [Google Scholar]
- [34].Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012;11:215–33. [DOI] [PubMed] [Google Scholar]
- [35].Schmidt MA, Fortsch C, Schmidt M, et al. Circulating regulatory T cells of cancer patients receiving radiochemotherapy may be useful to individualize cancer treatment. Radiother Oncol 2012;104:131–8. [DOI] [PubMed] [Google Scholar]


