Abstract
The current study was to evaluate soluble ST-2 level and left ventricular ejection fraction (LVEF) in patients with breast cancer receiving doxorubicin or trastuzumab treatment for 6 months and determine whether soluble ST-2 level can be used to predictive left ventricular function impairment.
Patients who were diagnosed as having breast cancer receiving doxorubicin or trastuzumab or combined therapy were enrolled. Demographic data, prior medical history and related medical therapy, and site and stage of breast cancer information were collected from electronic health record. Fasting blood was used to detect soluble ST-2 and brain natriuretic peptide (BNP) levels before and after 6 months doxorubicin or trastuzumab therapy. Echocardiography was performed before and after 6 months of doxorubicin or trastuzumab therapy.
Participants were divided into 3 groups based on tertiary soluble ST-2 level. Compared with 1st tertiary group, patients in the 3rd tertiary group had higher proportion receiving combined therapy (14.3% vs 4.7%, P < .05). Baseline soluble ST-2 level was similar across groups. After 6 months’ therapy, soluble ST-2 level was significantly higher in the 3rd tertiary group. Pearson correlation analysis showed that soluble ST-2 level was positively correlated with left ventricular volume and E/e’ ratio while negatively correlated with LVEF. Doxorubicin, trastuzumab, combined therapy, soluble ST-2 level, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker treatment were all independently associated with LVEF change.
In breast cancer patients receiving doxorubicin or trastuzumab therapy, soluble ST-2 level can be used to predict cardiac function and structure changes.
Keywords: breast cancer, cardiac function, soluble ST-2
1. Introduction
Doxorubicin and trastuzumab are commonly used to treat patients with breast cancer.[1–3] Nevertheless, prior studies have consistently demonstrated that both drugs are significantly associated with cardiomyocytes injury, which gradually leads to congestive heart failure (CHF), and the underlying mechanisms are due to oxidative stress elicited by doxorubicin treatment.[4,5] Trastuzumab is a monoclonal antibody that targets the ErbB2 signaling pathway.[6] Notably, the ErbB2 signaling pathway is pivotal to protect cardiovascular system from injury induced by inflammation and oxidation.[7] Prior clinical studies have shown that approximately 9% to 26% of patients who had received doxorubicin treatment developed left ventricular dysfunction,[8,9] and around 13% of patients with trastuzumab therapy had incident left ventricular dysfunction.[10] In addition, the incidence of CHF with combined doxorubicin and trastuzumab therapy is up to 27%.[11] Both experimental and clinical data together strongly indicate that periodically assessing cardiac functional and structural change before and after doxorubicin or trastuzumab therapy could help detect and prevent CHF development in patients receiving doxorubicin or trastuzumab therapy.
ST-2, a member of the interleukin 1 receptor family, is a unique biomarker with pluripotent effects, which is believed to have effects on cell proliferation, inflammatory states, and oxidative stress.[12–14] Experimental studies also demonstrated that ST-2 is expressed by cardiac cells in response to myocardial stress. ST-2 that binds to interleukin-33 has been proved to be cardioprotective in experimental models, including reducing cardiomyocytes apoptosis, ameliorating cardiac fibrosis, and improving cardiac function.[12–14] Clinical studies have also shown that among patients presenting with acute dyspnea, baseline ST-2 level was positively associated with clinical markers of CHF severity, including left ventricular ejection fraction (LVEF) and NYHA functional classification.[15,16]
Despite many studies that have demonstrated the independent association between doxorubicin and trastuzumab therapy and incident CHF, few studies have been conducted in the Chinese populations. In addition, whether the novel heart failure biomarker ST-2 can be used to predict cardiac functional and structural change post doxorubicin and trastuzumab therapy is unknown. Therefore, one may anticipate that assessing the soluble ST-2 in plasma may be helpful to identify minor injury of cardiac tissue in the initial stage, which in turn may help to prevent CHF development induced by doxorubicin and trastuzumab treatment.
Thus, the objective of current study was to evaluate the change of soluble ST-2 level and LVEF in patients with breast cancer receiving doxorubicin or trastuzumab treatment for 6 months and determine whether soluble ST-2 level can be used to predict left ventricular function impairment.
2. Methods
2.1. Studied design and participants enrollment
This was an observational and single-center study, and the present study was approved by the Clinical Research Ethical Committee of The First People's Hospital of Danzhou. Patients (≥ 18 years old) who were diagnosed as having breast cancer receiving doxorubicin or trastuzumab or combined therapy were enrolled after informed consent was obtained. Those who were pregnant, had prior history of coronary heart disease, myocardial infarction, valvular heart disease, cardiomyopathy, baseline LVEF < 50%, or symptomatic CHF was excluded.
2.2. Data collection
Demographic data, prior medical history and related medical therapy, and the site and stage of breast cancer information were collected from electronic health record by 2 independent investigators. Fasting blood was used to detect soluble ST-2 and brain natriuretic peptide (BNP) levels before and after 6 months doxorubicin or trastuzumab therapy. In brief, soluble ST-2 level was measured using a sandwich double monoclonal antibody ELISA method (Quantikine1 ELISA; R&D Systems, Minneapolis, MN) in accordance to manufacturer's instructions. The inter- and intra-assay variation was 6% and 5%, respectively. Echocardiography (ECG) was performed by an experienced cardiologist who was blinded to the patients’ information before and after 6 months doxorubicin or trastuzumab therapy. Patients were placed in left lateral position, and left ventricular volumes from apical 4 and 2-chamber views were obtained through tracing the endocardia border of left ventricle using 2D transthoracic ECG and the machine automatically calculated LVEF using the biplane Simpson method.[17] Left ventricular end systolic/diastolic volume (LVESV/LVEDV), left ventricular end systolic/diastolic diameter (LVESD/LVEDD), and E/e’ ratio were collected to evaluate structural change of left ventricle.
2.3. Studied endpoints
Studied endpoints of current study were used to evaluate the association of soluble ST-2 level and changes of LVEF. In addition, cardiac structures assessed by ECG were also compared before and after 6 months of doxorubicin or trastuzumab therapy.
2.4. Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) if normal distribution otherwise presented as median (interquartile range), and categorical variables were presented as number (percentage) of cases. Differences between groups were analyzed for statistical significance with 1-way analysis of variance (ANOVA), χ2 test, or Fisher exact test as appropriate. Correlations between soluble ST-2 level and left ventricular function and volume were assessed with Pearson correlation. Independent factors for LVEF change from baseline to 6 months’ treatment were evaluated with the use of stepwise multiple linear regressions analysis. To be specific, we first used univariate regression analysis to evaluate the association between potential factors and LVEF change; thereafter, in the multivariate regression analysis, factors with P value < .2 were entered into the model. Receiver operating characteristic curve (ROC) were performed to evaluate the sensitivity and specificity of soluble ST-2 in predicting LVEF change. All the statistical analyses were performed in the SPSS software (IBM 23.0 version, IBM. Co.) and a 2-sided P value < .05 was considered as statistically significant.
3. Results
From July of 2016 to September 2017, a total of 175 patients diagnosed as having breast cancer were hospitalized and 146 patients agreed to participate in the current study. After exclusion of 20 patients, 126 patients were recruited in the current study and all of these patients finished 6 month's follow-up (Fig. 1).
Figure 1.
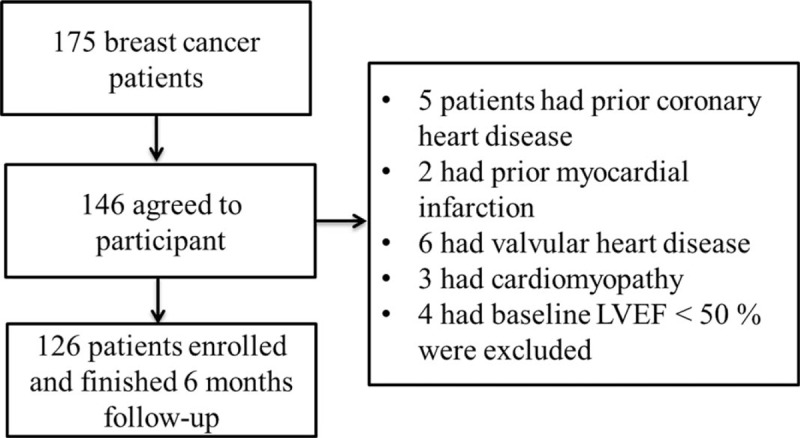
Schematic of study.
3.1. Baseline characteristics comparison
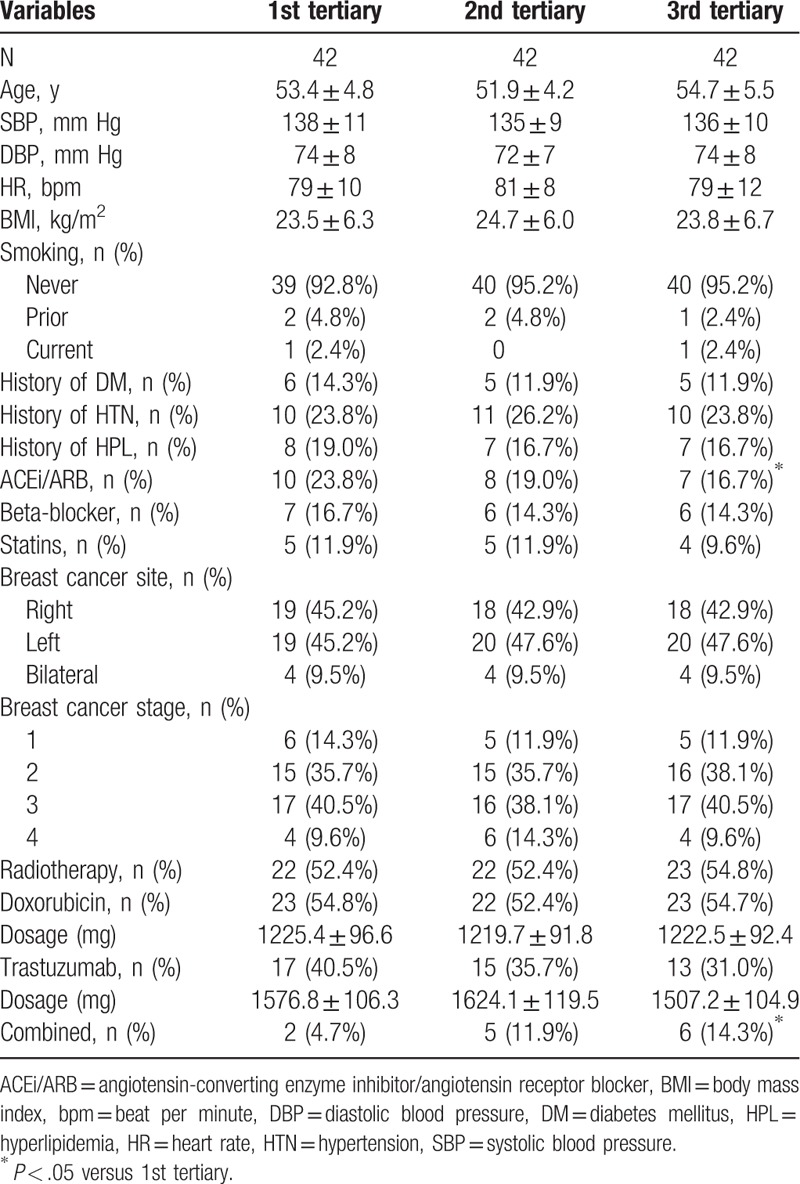
All participants were divided into 3 groups on the basis of tertiary soluble ST-2 level at the 6th month follow-up. Baseline characteristics were compared between these 3 groups. As presented in the Table 1, compared with the 1st tertiary group, patients in the 3rd tertiary group had higher proportion receiving combined therapy (14.3% vs 4.7%, P < .05) but had lower proportion receiving angiotensin-converting enzyme inhibitor/angiotensin receptor blocker [(ACEi/ARB), 16.7% vs 23.8%, P < .05]. While no significant differences in other variables between these 3 groups were observed. Nearly 9.5% patients had bilateral breast cancer and around 50% patients had stage 3 and 4 breast cancer. In addition, more than 50% patients had radiotherapy in each group.
Table 1.
Baseline characteristics comparisons.
3.2. Comparison of cardiac function and structures
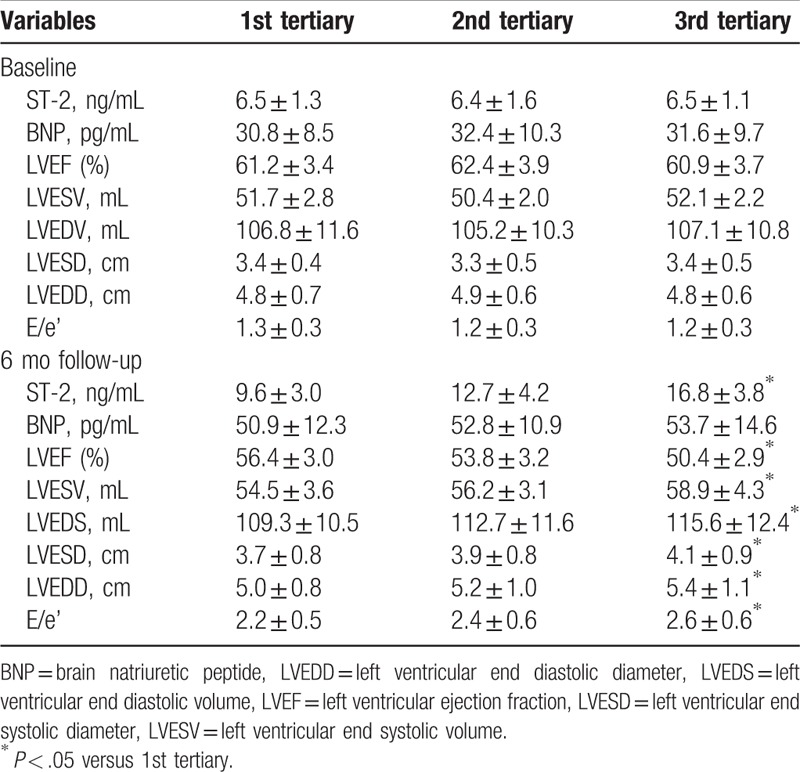
ECG was performed at baseline and 6 months follow-up to evaluate and compare the changes of cardiac function and structures. As presented in the Table 2, there was no significant difference in LVEF and cardiac structures and volume at baseline. However, after 6 months follow-up, compared with the 1st tertiary of soluble ST-2 level, LVEF was significantly reduced, while left ventricular structure and volume was significantly increased in the 3rd tertiary group (P < .05). In addition, E/e’ ratio, a marker of diastolic function,[18] was also significantly higher in the 3rd tertiary group, indicating that cardiac diastolic function in the 3rd tertiary group was significantly impaired.
Table 2.
Comparison biomarker levels and cardiac function and structure.
3.3. Correlation of soluble ST-2 level and cardiac function and structures
As presented in the Table 2, baseline soluble ST-2 level in 3 groups was similar (1st tertiary group 6.5 ± 1.3 ng/mL, 2nd tertiary group 6.4 ± 1.6 ng/mL, 3rd tertiary group 6.5 ± 1.1 ng/mL, P = .978). However, after 6 months therapy, compared with the 1st tertiary group, soluble ST-2 level was significantly higher in the 3rd tertiary group (1st tertiary group 9.6 ± 3.0 ng/mL, 2nd tertiary group 12.7 ± 4.2 ng/mL, 3rd tertiary group 16.8 ± 3.8 ng/mL, P < .05). Pearson correlation analysis showed that soluble ST-2 level was positively correlated with LVESV, LVEDV, and E/e’ ratio while negatively correlated with LVEF, with correlation efficient was 0.39, 0.41, 0.37, and -0.51, respectively [(P < .05), Fig. 2]. Although plasma BNP levels were also increased after 6 months therapy compared with BNP levels at baseline, no significant between-group differences were observed after 6 months therapy.
Figure 2.
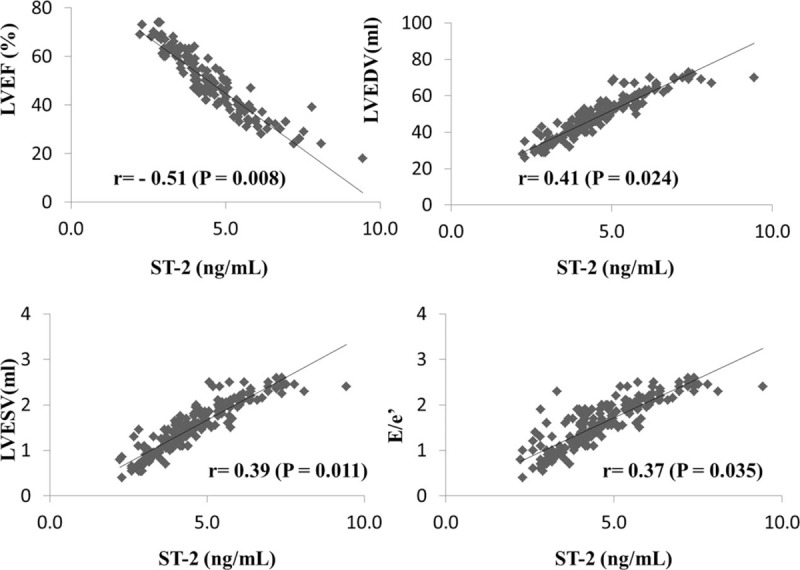
Correlation between soluble ST-2 and cardiac function and structure.
3.4. Potential independent factors for LVEF change
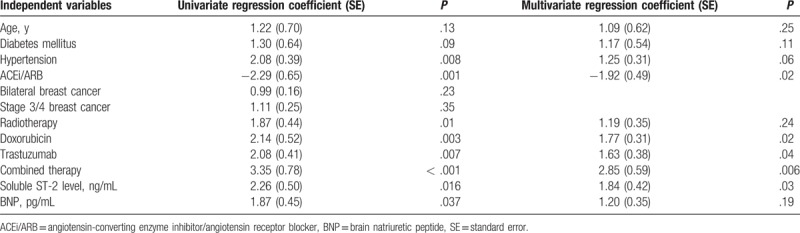
As presented in the Table 3, in the univariate regression analysis, hypertension, ACEi/ARB treatment, radiotherapy, doxorubicin, trastuzumab, combined therapy, soluble ST-2 level, and BNP level were all independently associated with LVEF change. All these variables and age and diabetes mellitus were entered into the model of multivariate regression analysis. As summarized in the Table 3 that doxorubicin, trastuzumab, combined therapy, soluble ST-2 level, and ACEi/ARB treatment remained independently associated with LVEF change.
Table 3.
Independent factors for LVEF change from baseline to 6 months’ follow-up.
3.5. Sensitivity and specificity of soluble ST-2 in predicting LVEF change
The receiver operating characteristic curve was conducted to evaluate the sensitivity and specificity of soluble ST-2 in predicting LVEF change. As revealed in Fig. 3, the area under the curve for soluble ST-2 to predict LVEF change was 0.74 (P = .002), and the cutoff value of soluble ST-2 was 13.4 ng/mL, with sensitivity was 73% and specificity was 79%, respectively.
Figure 3.
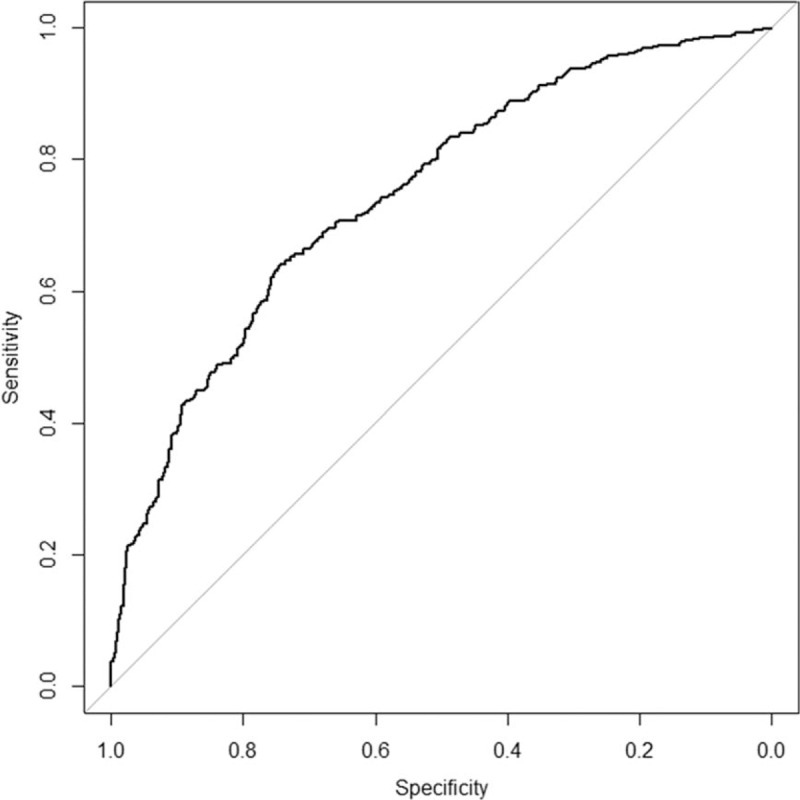
The ROC curve in predicting LVEF change.
4. Discussion
Our current study indicates that in Chinese patients with breast cancer, doxorubicin and trastuzumab therapy have adverse effects on left ventricular structure and function, which is additive by combined therapy. Baseline soluble ST-2 level can be used to predict left ventricular structure and function change, with an acceptable sensitivity and specificity. Future studies are warranted to elucidate whether soluble ST-2 level can be used to predict long-term outcome in patients with breast cancer receiving doxorubicin or trastuzumab therapy.
Notably, breast cancer is a leading cause of morbidity and mortality in women around the world.[19,20] Doxorubicin and trastuzumab are the cornerstone regimen for breast cancer. However, many studies have shown the cardiotoxicity of these 2 medications. For example, Narayan et al[21] enrolled 277 breast cancer patients receiving doxorubicin, trastuzumab, or combined therapy. After 3 years’ follow-up, they found that both doxorubicin and trastuzumab resulted in modest, persistent declines in LVEF. In addition, left ventricular volumes and diastolic function change was also significantly associated with LVEF decrease.[21] Consistent to prior study, our current study also observed that doxorubicin and trastuzumab therapy leaded to cardiac function and structure changes. Extending to the previous study, we found that even 6 months therapy could result in significant changes of cardiac function and structure. In addition, our current study has evaluated the change of serum soluble ST-2 and BNP levels, which had not been investigated in prior studies. To our best knowledge, this should be the first few studies to evaluate soluble ST-2 changes before and after doxorubicin and trastuzumab therapy. The clinical implication of these findings is that measurement of soluble ST-2 level may help to identify cardiotoxicity of doxorubicin or trastuzumab therapy in the early stage, which in turn help physician adjust medications doses and prevent CHF development.
Consistent to prior studies,[22,23] our current study also observed the addictive adverse effects of combined therapy on cardiac function and structure when compared with single therapy. Soluble ST-2 level was also significantly higher in combined group (combined 14.2 ± 6.0 ng/mL, doxorubicin 10.2 ± 3.7 ng/mL, trastuzumab 9.6 ± 3.1 ng/mL, P < .05), suggesting that physicians should be cautious of combined therapy in terms of their adverse effects on cardiac function and soluble ST-2 level might be useful for dosage adjustment.
Both our current study and prior studies have shown BNP elevation with doxorubicin and trastuzumab therapy compared with that at baseline.[24,25] However, in our current study, we did not find significant differences in BNP level between different groups, indicating that soluble ST-2 might be more sensitive than BNP in reflecting cardiac function and structure changes in an early stage. Furthermore, the ROC curve was 0.74, indicating that soluble ST-2 had a fair capacity to discriminate the change of LVEF. When the cutoff value of was 13.4 ng/mL, the soluble ST-2 had the best sensitivity and specificity to predict LVEF change. From clinical perspective, with a cutoff value, it was helpful and useful for physicians to guide therapy by monitoring soluble ST-2 change so as to predict LVEF change and prevent CHF development.
We observed that ACEi/ARB therapy appeared to have favorable effect on cardiac function. Many randomized clinical trials have demonstrated the cardioprotective effects of ACEi/ARB on preventing CHF development as well as improving cardiac function in patients with established CHF.[26,27] Our current study showed that ACEi/ARB therapy was beneficial to improve cardiac dysfunction induced by doxorubicin or trastuzumab. More clinical studies are needed to corroborate our current findings. In addition, experimental studies are needed to elucidate the underlying mechanisms.
There are some limitations of current study. First of all, this is a single center and observational study, and causal relationship could not be drawn. However, current study provides foundation for future study to corroborate our findings and elucidate the underlying mechanisms. Second, current study was subjected to a relative small sample size and short-term duration and future large sample size and long-term follow-up study is needed. Third, findings from current study should not extrapolate to other populations group. Last but not the least, although we have adjusted for potential confounding, observational design and unmeasured and undetected biases still exit.
5. Conclusion
In patients with breast cancer receiving doxorubicin or trastuzumab therapy, soluble ST-2 level can be used to predict cardiac function and structure changes.
Acknowledgment
We appreciate very much for the help Dr Fang Yang provided to us in performing statistical analysis and for the help Dr James V. Rivers provided to us in revising our paper.
Author contributions
Conceptualization: Guoding Huang, Jianfeng Zhai, Xiuzhu Huang, Dongdan Zheng.
Data curation: Guoding Huang, Jianfeng Zhai, Xiuzhu Huang.
Formal analysis: Guoding Huang, Jianfeng Zhai, Dongdan Zheng.
Funding acquisition: Guoding Huang.
Methodology: Xinting Huang.
Supervision: Dongdan Zheng.
Validation: Guoding Huang.
Writing – original draft: Guoding Huang.
Writing – review & editing: Guoding Huang.
Footnotes
Abbreviations: ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, BNP = brain natriuretic peptide, CHF = congestive heart failure, ECG = echocardiography, LVEF = left ventricular ejection fraction, LVESD/LVEDD = left ventricular end systolic/diastolic diameter, LVESV/LVEDV = left ventricular end systolic/diastolic volume.
The authors have no conflicts of interest.
The author(s) of this work have nothing to disclose.
References
- [1].Theriault RL, Carlson RW, Allred C, et al. Breast cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2013;11:753–60. quiz 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–84. [DOI] [PubMed] [Google Scholar]
- [3].Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saeed MF, Premecz S, Goyal V, et al. Catching broken hearts: pre-clinical detection of doxorubicin and trastuzumab mediated cardiac dysfunction in the breast cancer setting. Can J Physiol Pharmacol 2014;92:546–50. [DOI] [PubMed] [Google Scholar]
- [5].Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002;20:1215–21. [DOI] [PubMed] [Google Scholar]
- [6].Zheng L, Tan W, Zhang J, et al. Combining trastuzumab and cetuximab combats trastuzumab-resistant gastric cancer by effective inhibition of EGFR/ErbB2 heterodimerization and signaling. Cancer Immunol Immunother 2014;63:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Lorenzo C, Paciello R, Riccio G, et al. Cardiotoxic effects of the novel approved anti-ErbB2 agents and reverse cardioprotective effects of ranolazine. Onco Targets Ther 2018;11:2241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Drafts BC, Twomley KM, D’Agostino R, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013;6:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–8. [DOI] [PubMed] [Google Scholar]
- [10].Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy-associated cardiovascular disease. Nat Rev Clin Oncol 2016;13:172–84. [DOI] [PubMed] [Google Scholar]
- [11].Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol 2013;14:72–80. [DOI] [PubMed] [Google Scholar]
- [12].Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–90. [DOI] [PubMed] [Google Scholar]
- [13].Broch K, Ueland T, Nymo SH, et al. Soluble ST2 is associated with adverse outcome in patients with heart failure of ischaemic aetiology. Eur J Heart Fail 2012;14:268–77. [DOI] [PubMed] [Google Scholar]
- [14].Bayes-Genis A, Januzzi JL, Gaggin HK, et al. ST2 pathogenetic profile in ambulatory heart failure patients. J Card Fail 2015;21:355–61. [DOI] [PubMed] [Google Scholar]
- [15].Januzzi JL, Peacock WF, Maisel AS, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol 2007;50:607–13. [DOI] [PubMed] [Google Scholar]
- [16].Rehman SU, Mueller T, Januzzi JL. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol 2008;52:1458–65. [DOI] [PubMed] [Google Scholar]
- [17].Cai A, Zhang J, Wang R, et al. Joint effects of obstructive sleep apnea and resistant hypertension on chronic heart failure: a cross-sectional study. Int J Cardiol 2018;257:125–30. [DOI] [PubMed] [Google Scholar]
- [18].Zhou D, Huang Y, Fu M, et al. Prognostic value of tissue Doppler E/e’ ratio in hypertension patients with preserved left ventricular ejection fraction. Clin Exp Hypertens 2018;40:554–9. [DOI] [PubMed] [Google Scholar]
- [19].Abrahams HJG, Gielissen MFM, Verhagen CAHHVM, Knoop H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: a systematic review. Clin Psychol Rev 2018;63:1–1. [DOI] [PubMed] [Google Scholar]
- [20].Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015;314:1599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Narayan HK, Finkelman B, French B, et al. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation 2017;135:1397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 2010;28:3416–21. [DOI] [PubMed] [Google Scholar]
- [23].Smith JW, Vukelja S, Hoffman AD, et al. Phase II, multicenter, single-arm, feasibility study of eribulin combined with capecitabine for adjuvant treatment in estrogen receptor-positive, early-stage breast cancer. Clin Breast Cancer 2016;16:31–7. [DOI] [PubMed] [Google Scholar]
- [24].Murtagh G, Lyons T, O’Connell E, et al. Late cardiac effects of chemotherapy in breast cancer survivors treated with adjuvant doxorubicin: 10-year follow-up. Breast Cancer Res Treat 2016;156:501–6. [DOI] [PubMed] [Google Scholar]
- [25].Murtagh G, Laffin LJ, Beshai JF, et al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2016;9:e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- [27].Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med 2014;174:773–85. [DOI] [PubMed] [Google Scholar]


