Abstract
Rationale:
Familial hydatidiform mole is extremely rare while familial gestational trophoblastic neoplasia (GTN) has never been reported. Inspired by 2 biological sisters with postmolar GTN and liver toxicity, we reviewed susceptible maternal-effect genes and explored the role of possible drug transporter genes in the development of GTN.
Patient concerns:
We reported one Chinese family where the two sisters developed postmolar GTN while experiencing fast remission and significant hepatic toxicity from actinomycin D chemotherapy.
Diagnoses:
The index pregnancy was diagnosed with curettage. The following GTN was confirmed when there was a rise in beta-hCG for three consecutive weekly measurements over at least a period of 2 weeks. Computed tomography was used to identify lung metastasis. The elder sister was diagnosed with gestational trophoblastic neoplasia (III: 2) while the younger sister was diagnosed as III: 3 according to WHO scoring system.
Interventions:
Patients were treated with actinomycin D of 10 μg/kg intravenously for 5 days every 2 weeks. When hepatic toxicity was indicated, polyene phosphatidyl choline and magnesium isoglycyrrhizinate were prescribed.
Outcomes:
Both patients responded extremely well to the 5-day actinomycin D regimen. Beta-hCG remained less than 2 mIU/ml after 5 cycles while computed tomography scan showed downsized pulmonary nodules. Both experienced significant rise in ALT and AST levels that could be ameliorated with corresponding medication. Monthly followed-up showed negative beta-hCG levels and normal liver enzyme levels.
Lessons:
We speculated that the known or unknown NLRP7 and KHDC3L mutations might be correlated with drug disposition in liver while liver drug transporters such as P-glycoprotein family that are also expressed in trophoblasts might be correlated to GTN susceptibility. Future genomic profiles of large samples alike using next generation sequencing are needed to confirm our hypothesis and discover yet unknown genes.
Keywords: drug transporter, familial, gestational trophoblastic neoplasia, liver, susceptibility
1. Introduction
Gestational trophoblastic disease is a group of uncommon conditions associated with abnormal pregnancy. The benign forms include complete and partial hydatidiform moles. The malignant forms are grouped under gestational trophoblastic neoplasia (GTN), including invasive mole, choriocarcinoma, placental site trophoblastic tumor, and epithelioid trophoblastic tumor.[1,2] GTN can develop after a molar or nonmolar pregnancy. Due to lack of specific histological features, GTN is mainly diagnosed by beta-hCG surveillance.[3,4] Although most women can be cured by chemotherapy, many interesting issues arise. For example, we still do not fully understand why molar pregnancy happens and cannot predict whether it will recur or be followed by the development of GTN. We also cannot tell why some cases become drug resistant while others are not. Population studies have revealed risk factors such as maternal age, dietary habits, socioeconomic status and ethnicity difference while karyotyping and genetic investigations have discovered the androgenic nature of sporadic hydatidiform moles.[5–7] Still, these findings cannot explain the current confusions, especially for those familial cases.
Advances in molecular pathology over the last decade have brought clinicians a clearer view on the genetic aberrations of gestational trophoblastic disease. Most recently, it is now well recognized that familial biparental complete hydatidiform mole are correlated with mutations of maternal-effect genes such as NLRP7 and KHDC3L, which not only affect implantation of the embryo by altering inflammatory pathways but also impair maternal imprinting to reset at early embryogenesis.[8–10] However, these gene mutations were mainly studied in biparental complete hydatidiform mole, not mentioning any form of GTN. It is then hardly feasible to explain the disease progression or drug resistance with the current mutants.
Here, we reported one Chinese family where the 2 sisters developed postmolar GTN while experiencing fast remission and significant hepatic toxicity from actinomycin D chemotherapy. We also conducted a thorough literature review to provide a closer understanding of the existing genetic aberrations and provide a new perspective of the future genetic research on this group of diseases.
2. Case report
This study was approved by Ethical Review Committee of the West China Second Hospital of Sichuan University and written informed consent was obtained from both patients. In September 2013, the elder sister (22-year-old, gravidity-0, parity-0) visited the local hospital complaining of vaginal bleeding after 5-week period of amenorrhea. Serum beta-hCG level was 3120 mIU/mL and no mass was present in uterus or pelvic cavity according to ultrasonography. A further diagnostic laparoscopy was conducted but no pregnancy tissue was found. Methotrexate and mifepristone were prescribed but beta-hCG elevated to 3413.4 mIU/mL after 1 week. The patient was then referred to the Department of Gynecology and Obstetrics of West China Second University Hospital. She underwent a dilatation and curettage (D&C) that showed endometrial polyps and proliferative phase endometrium (Fig. 1A). During the follow-up, the beta-hCG level increased weekly to 7612.9 mIU/mL after one month. According to the FIGO recommendation, GTN can be confirmed when there is a rise in beta-hCG for 3 consecutive weekly measurements over at least a period of 2 weeks or more.[1] A further computed tomography scan of her chest revealed an isolated 2 cm nodule in the lower right lung. Therefore, the gestational trophoblastic neoplasia (III: 2) was diagnosed.
Figure 1.
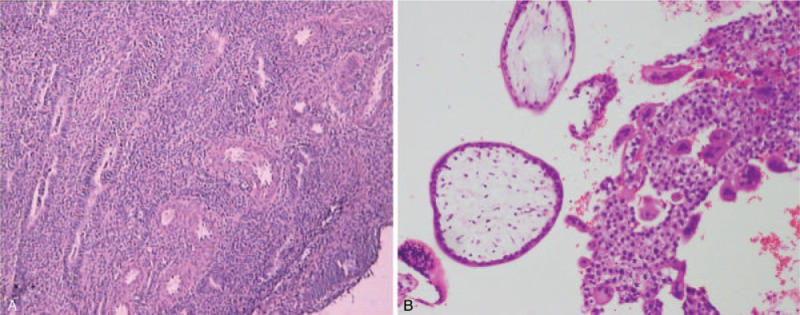
Pathologic assessment of the uterus in the index pregnancy after dilation and curettage. (A) For the elder sister, hematoxylin-eosin showed endometrial polyps and proliferative phase endometrium. The absence of fetal parts detected by curettage and laparoscopy might indicate a miscarriage of a hydatidiform mole or other deformed pregnancies. (B) For the younger sister, microscopic examination demonstrated generalized trophoblastic proliferation with hydropic degenerated villi, suggesting complete hydatidiform mole.
Pretreatment laboratory evaluation confirmed the eligibility for chemotherapy including normal aspartate transaminase (AST) and alanine transaminase (ALT) levels. The patient was then treated with actinomycin D (10 μg/kg intravenously for 5 days every 2 weeks; dosage and treatment interval were adjusted when chemotherapy contradictive adverse events were observed) starting from November 2013.[1] One week after the first cycle treatment, beta-hCG dramatically decreased to 491.9 mIU/mL. However, the liver blood test showed significant elevation of AST (116U/l) and ALT (70U/l) with no particular abnormality of liver and gallbladder in ultrasonography. Markers of hepatitis virus were detected negative to exclude latent infection. The patient was immediately treated with polyene phosphatidyl choline and magnesium isoglycyrrhizinate until both enzymes fell to acceptable concentrations for chemotherapy.
During the subsequent treatment, the patient responded extremely well to the 5-day actinomycin D regimen. Beta-hCG dropped to <2 mIU/mL after 3 cycles, which was followed by additional 2 cycles of chemotherapy for consolidation. Computed tomography scan also showed downsized pulmonary nodule. Notably, 3 to 7 days after actinomycin D injection in each cycle, AST and ALT would elevate to levels where treatment were needed even though oral polyene phosphatidyl choline and glycyrrhizinic acid were prescribed during the whole treatment process. The patient was followed up for 12 months and she maintained negative beta-hCG levels and normal liver enzyme levels (Fig. 2A).
Figure 2.
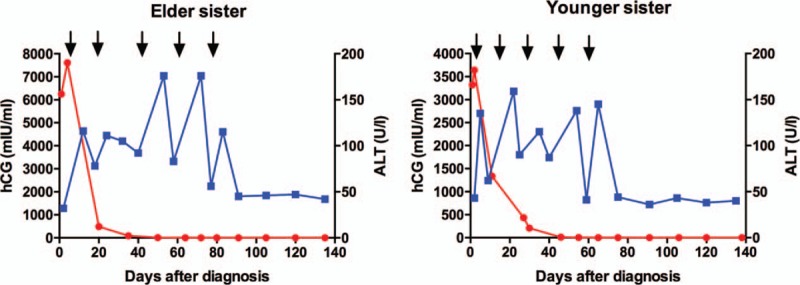
Serum beta-hCG and ALT levels during chemotherapy. Both patients responded extremely well to the 5-day actinomycin D regimen (the timing of actinomycin D treatment was shown with black arrow). Beta-hCG dropped to less than 2 mIU/mL after 2 and 3 cycles, respectively (each dot on the red line refers to each hCG value tested during surveillance). Notably, both ALT levels would elevate significantly 3 to 7 days after each cycle's actinomycin injection (each dot on the blue line refers to each ALT value tested during surveillance). Neither organic lesion could be found in ultrasonography nor hepatitis virus infection could be suspected in the serum test. We successfully ameliorated the liver condition before each new cycle by using polyene phosphatidyl choline, magnesium isoglycyrrhizinate, and ademetionine 1,4-butanedisulfonate.
Four years later, the 22-year-old, gravidity-0, parity-0 younger sister visited the local hospital in April 2017 for 5 weeks of amenorrhea. Beta-hCG was more than 10000 mIU/mL but no embryo was found in uterine cavity in ultrasonography. While suggested follow-up, she presented to our hospital for vaginal bleeding and abdominal pain after one week. Tests showed beta-hCG level of 29,000 mIU/mL and ultrasonography showed a 4 cm high echo mass with 1 cm fluid sonolucent area inside. She underwent suction evacuation under ultrasound guidance. The postoperative pathological assessment suggested complete mole (Fig. 1B). In the subsequent follow-up, the beta-hCG level dropped to 2000 mIU/mL but rose to 3641.2 mIU/mL after 3 consecutive weekly measurements. Vaginal ultrasound examination of pelvis was conducted and revealed a 2 cm high echo mass with irregular fluid area inside. Multiple nodules were displayed in both lungs according to the computed tomography but the chest x-ray only discovered 5 nodules (Fig. 3A and C). With the accumulated evidence, she was diagnosed with gestational trophoblastic neoplasia (III: 3).
Figure 3.
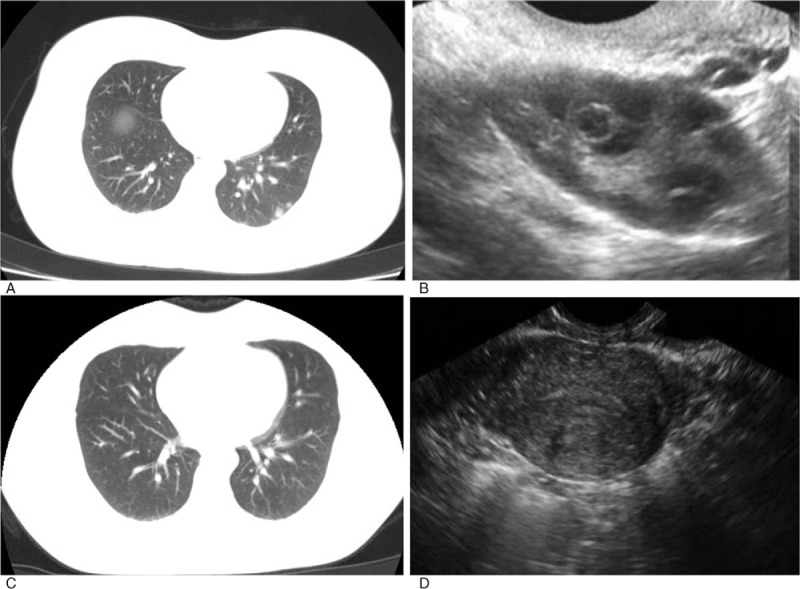
Radiographic and ultrasound images of the younger sister. (A and B) Before treatment, multiple nodules were displayed in both lungs according to the computed tomography and ultrasonography revealed a 2 cm high echo mass with irregular fluid area inside. (C and D) After 5 cycles of actinomycin D chemotherapy, computed tomography showed most of the primary pulmonary nodules were diminished and the remaining were significantly downsized, while ultrasonography detected no mass in uterine cavity.
Starting from July 2017, she was given actinomycin D chemotherapy (10 μg/kg intravenously for 5 days every 2 weeks) after a thorough pretreatment laboratory evaluation. Similar to her elder sister, she was very sensitive to actinomycin D. We conducted 5 cycles of chemotherapy, 3 of which were for consolidation. After 5 cycles, no mass could be detected in uterine cavity and the primary pulmonary nodules were significantly downsized (Fig. 3B and D). Interestingly, her ALT and AST levels were also elevated significantly after each cycle's actinomycin injection but neither organic lesion could be found in ultrasonography nor hepatitis virus infection could be suspected in the serum test. We successfully ameliorated the liver condition before each new cycle by using polyene phosphatidyl choline and magnesium isoglycyrrhizinate. The beta-hCG and ALT levels were shown in Figure 2B. After treatment, the patient has been followed-up in our clinics monthly through June 2018 and she maintains negative beta-hCG levels and normal liver enzyme levels.
3. Discussion
Gestational trophoblastic disease is a group of uncommon conditions associated with abnormal pregnancy including the premalignant complete hydatidiform moles and partial hydatidiform moles as well as the malignant gestational trophoblastic neoplasia consisting of malignant invasive moles, choriocarcinoma, placental site trophoblastic tumor, and the epitheliod trophoblastic tumor. Although most cases of GTN can now be successfully treated and cured by chemotherapy, the possibility of recurrence and fertility control afterwards greatly affect those patients in their family planning.[11,12] Thus, risks factors and potential genetic elements were explored through past years. Maternal age, dietary habits, socioeconomic status, and ethnicity difference are considered significant risk factors of GTN occurrence.[13,14] Familial molar pregnancies or gestational trophoblastic diseases are exceedingly rare. Only a few cases have been reported, most of which were familial or recurrent complete hydatidiform moles in Middle Eastern and Caucasian families.[15–17] Although little is known about the underlying pathophysiology, those rare cases illustrated possible genetic factors in GTN susceptibility such as CATERPILLER protein family gene mutations and alterations in genetic imprinting.[18] Here, we reported one Chinese family where the 2 sisters developed postmolar GTN while experiencing fast remission and significant hepatic toxicity from actinomycin D chemotherapy.
In this family, the 2 sisters shared similar history. First, they both presented with molar pregnancy in early trimester and developed stage III low-risk GTN within 4 months after curettage according to beta-hCG surveillance. Second, they responded extremely well to 5-day actinomycin D chemotherapy but experienced significant rise in ALT and AST levels indicating hepatic toxicity that could be ameliorated with corresponding medication. While this familial occurrence and drug response might be unrelated, this indicated that there might be common genetic factors that could predispose patients to gestational trophoblastic disease and at the same time affect liver transporting function of particular drugs.
Accumulating karyotype and genotype data demonstrated that familial molar pregnancies were not always separate entities. Recent studies of patients with familial recurrent hydatidiform moles identified 2 candidate genes called NLRP7 and KHDC3L that are located on chromosome 19 and 6, respectively.[19,20] To date, approximately 50 NLRP7 mutations and 4 KHDC3L mutations have been uncovered in familial molar pregnancy patients in either homozygous or compound heterozygous fashion.[21] The cytoplasmic NLRP7 protein is a member of the CATERPILLER protein family with implications in inflammation, apoptosis and innate immunity. In wild-type individuals, the NLRP7 protein can inhibit caspase-1-dependent interleukin-1beta, a proinflammatory cytokine abundantly expressed in the female reproductive tract while the KHDC3L protein co-localizes with NLRP7 to the microtubule-organizing center and the Golgi apparatus in human oocytes and preimplantation embryos.[22] It is then speculated that mutations in NLRP7 and KHDC3L can impair the implantation and development of the embryo by altering inflammatory pathways in the uterus. Most recently, several articles hypothesized that defects of NRLP7 regulation of cytokines might disrupt the global imprinting within the ovum on the basis that imprinting gene defects with silencing of maternal imprinted genes and expression of paternal imprinted counterparts were detected in molar tissues of NLRP7 or KHDC3L mutated familial molar pregnancy patients.[23–25] Whereas, no consensus has been reached as to the specific role of NRLP7 in GTN while most of the mechanisms are demonstrated on hypothesis as well as indirect evidence in vitro. Notably, it has been shown that NLRP7 transcripts are expressed in human tissues including liver, placenta, thymus, testis, and ovaries.[26] Whether specific domain mutations in NLRP7 would impair liver function is still unknown because none of the previous familial cases have reported NLRP7 mutations and hepatic toxicity at the same time. From another perspective, since the specific function of NLRP7 and KHDC3L are yet unknown, this phenomenon might unveil new mechanisms of these genes in the pathogenesis of GTN or lead to possible new mutations in different loci that are correlated with known liver drug transporters.
Actinomycin D plays a pivotal role in the treatment of gestational trophoblastic neoplasia. It binds to DNA in a guanine-dependent manner to prevent RNA synthesis and can inhibit the incorporation of nucleotide triphosphates into DNA.[27] Actinomycin D is a relatively well-tolerated drug with minimal side effects even in pediatric patients of Wilms tumor.[28] Previous pharmacokinetic studies demonstrated the extensive variation of actinomycin D exposure in different patients but the underlying mechanism is unknown.[29] It is postulated that actinomycin D transporters would affect the drug disposition and metabolism, resulting in different extents of efficacy and adverse effects. Several studies have proved that actinomycin D is a substrate for ATP-binding cassette (ABC) transporters in liver. Included within ABC transporters are members of the P-glycoprotein (ABCB) family, such as multidrug resistance protein 1 (MDR1), the bile salt export pump (BSEP) and the multidrug resistance-associated (MRP, ABCC) protein family that exhibit either influx or efflux properties.[30,31] Interestingly, studies have also explored the expression of P-glycoprotein in human placentas and hydatidiform moles. These studies revealed that P-glycoprotein expressed in trophoblasts has drug binding sites and the ability to transport vincristine, suggesting that P-glycoprotein in the placenta protects the fetus from xenobiotics and confers drug resistance on moles.[32,33] Therefore, mutation of drug transporter genes expressed in both liver and trophoblasts might inhibit efflux of drug from liver thus cause liver toxicity and increase blood drug concentration (Fig. 4). It might also change the drug affinity to these trophoblasts, thus enhance or diminish drug treatment efficacy. Yet whether the mutation of these genes is correlated with GTN susceptibility is not clarified. However, it is known that ABC transporters also mediate cell fusion, stem cell function, and vasculogenic plasticity.[34] One study stained ABCB5 in healthy placental tissues, partial moles, complete moles, choriocarcinoma and placental site trophoblastic tumor tissues and found different expressions of ABCB5 between healthy villous trophoblasts and GTN tissues.[35] Therefore, it is possible that certain mutation of these drug transporter genes might increase the risk of GTN. In consideration of our case, studying liver drug transporter genes that are expressed in trophoblasts might be helpful in further elucidating familial gestational trophoblastic diseases.
Figure 4.
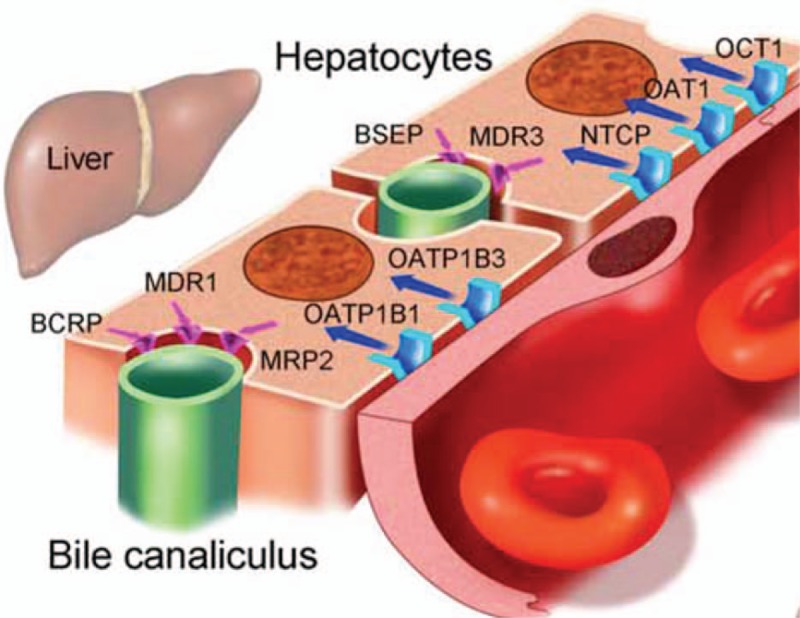
Discovered drug transporters in liver.[31] The coordinated expression and activity of uptake and efflux transporters mediates absorption of exogenous (drugs) and endogenous substrates from the bloodstream in the hepatocyte or proximal tubular cell, respectively. Drugs may undergo further biotransformation or be excreted into bile or urine for subsequent elimination from the body. Mutation of drug transporter genes that expressed in both liver and trophoblasts such as MDR1 might inhibit efflux of drug from liver thus cause liver toxicity and increase blood drug concentration.
In conclusion, this study is the first to report familial GTN in Chinese population. Unlike the recurrence of complete moles in previous literature, the gestational trophoblastic neoplasia developed after the molar pregnancy in the 2 sisters, indicating a more malignant mechanism under the scene. It is also the first to reveal the possible relationship between GTN susceptibility and liver transporters. We acknowledge that the major limitation of this study was the lack of related genetic analysis. It remains unknown if mutations of NLRP7/KHDC3L or drug transporter genes were truly existed in this family. Thus, we should not exclude the possibility that this might be an isolated case in which the familial occurrence and drug response were unrelated. However, it still uncloses another perspective of the genetic research on GTN based on the current poorly answered questions on why hydatidiform moles develop and how they become malignant. Future genomic profiles of large samples alike using next generation sequencing are needed to confirm our hypothesis.
Acknowledgment
We would like to thank Dr Gang Ning and Dr Hong Luo for helping provide patients’ radiologic and ultrasound images; we would like to thank Dr Kaixuan Yang for helping provide patients’ pathologic images.
Author contributions
Conceptualization: Qingli Li.
Data curation: Xiyan Mu, Liang Song, Yu Ma
Formal analysis: Xiyan Mu, Yu Ma
Funding acquisition: Qingli Li, Rutie Yin, Xia Zhao
Investigation: Rutie Yin, Xia Zhao.
Methodology: Xiyan Mu, Danqing Wang
Resources: Xiyan Mu, Danqing Wang, Xia Zhao
Writing – original draft: Xiyan Mu, Qingli Li
Writing – review & editing: Liang Song, Xia Zhao, Danqing Wang, Yu Ma, Rutie Yin
Conceptualization: Qingli Li.
Data curation: Xiyan Mu, Liang Song, Yu Ma.
Formal analysis: Xiyan Mu, Yu Ma.
Funding acquisition: Rutie Yin, Xia Zhao, Qingli Li.
Investigation: Xia Zhao.
Methodology: Xiyan Mu, Danqing Wang.
Resources: Xiyan Mu, Danqing Wang, Xia Zhao.
Writing – original draft: Xiyan Mu, Qingli Li.
Writing – review & editing: Rutie Yin, Danqing Wang, Liang Song, Yu Ma, Xia Zhao.
Footnotes
Abbreviations: ALT = alanine transaminase, AST = aspartate transaminase, GTN = gestational trophoblastic neoplasia, hCG = human chorionic gonadotropin.
Funders: this work was supported by West China Second Hospital, Sichuan University.
The authors have no conflicts of interest to disclose.
References
- [1].Ngan HY, Seckl MJ, Berkowitz RS, et al. Update on the diagnosis and management of vgestational trophoblastic disease. Int J Gynaecol Obstet 2015;131(suppl 2):S123–6. [DOI] [PubMed] [Google Scholar]
- [2].Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet 2010;376:717–29. [DOI] [PubMed] [Google Scholar]
- [3].Coyle C, Short D, Jackson L, et al. What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol 2018;148:254–7. [DOI] [PubMed] [Google Scholar]
- [4].Jiang F, Wan XR, Xu T, et al. Evaluation and suggestions for improving the FIGO 2000 staging criteria for gestational trophoblastic neoplasia: a ten-year review of 1420 patients. Gynecol Oncol 2018;18:30271–3. [DOI] [PubMed] [Google Scholar]
- [5].Melamed A, Gockley AA, Joseph NT, et al. Effect of race/ethnicity on risk of complete and partial molar pregnancy after adjustment for age. Gynecol Oncol 2016;143:73–6. [DOI] [PubMed] [Google Scholar]
- [6].Atrash HK, Hogue CJ, Grimes DA. Epidemiology of hydatidiform mole during early gestation. Am J Obstet Gynecol 1986;154:906–9. [DOI] [PubMed] [Google Scholar]
- [7].Jacobs PA, Hunt PA, Matsuura JS, et al. Complete and partial hydatidiform mole in Hawaii: cytogenetics, morphology and epidemiology. Br J Obstet Gynaecol 1982;89:258–66. [DOI] [PubMed] [Google Scholar]
- [8].Reddy R, Akoury E, Phuong Nguyen NM, et al. Report of four new patients with protein-truncating mutations in C6orf221/KHDC3L and colocalization with NLRP7. Eur J Hum Genet 2013;21:957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hodges MD, Rees HC, Seckl MJ, et al. Genetic refinement and physical mapping of a biparental complete hydatidiform mole locus on chromosome 19q13.4. J Med Genet 2003;40:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nguyen NM, Zhang L, Reddy R, et al. Comprehensive genotype-phenotype correlations between NLRP7 mutations and the balance between embryonic tissue differentiation and trophoblastic proliferation. J Med Genet 2014;51:623–34. [DOI] [PubMed] [Google Scholar]
- [11].Couder F, Massardier J, You B, et al. Predictive factors of relapse in low-risk gestational trophoblastic neoplasia patients successfully treated with methotrexate alone. Am J Obstet Gynecol 2016;215:80.e1–7. [DOI] [PubMed] [Google Scholar]
- [12].Ireson J, Jones G, Winter MC, et al. Systematic review of health-related quality of life and patient-reported outcome measures in gestational trophoblastic disease: a parallel synthesis approach. Lancet Oncol 2018;19:e56–64. [DOI] [PubMed] [Google Scholar]
- [13].Eagles N, Sebire NJ, Short D, et al. Risk of recurrent molar pregnancies following complete and partial hydatidiform moles. Hum Reprod 2015;30:2055–3. [DOI] [PubMed] [Google Scholar]
- [14].Altman AD, Bentley B, Murray S, et al. Maternal age-related rates of gestational trophoblastic disease. Obstet Gynecol 2008;112:244–50. [DOI] [PubMed] [Google Scholar]
- [15].Buyukkurt S, Fisher RA, Vardar MA, et al. Heterogeneity in recurrent complete hydatidiform mole: presentation of two new Turkish families with different genetic characteristics. Placenta 2010;31:1023–5. [DOI] [PubMed] [Google Scholar]
- [16].Landolsi H, Rittore C, Philibert L, et al. Screening for NLRP7 mutations in familial and sporadic recurrent hydatidiform moles: report of 2 Tunisian families. Int J Gynecol Pathol 2011;30:348–53. [DOI] [PubMed] [Google Scholar]
- [17].Murdoch S, Djuric U, Mazhar B, et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat Genet 2006;38:300–2. [DOI] [PubMed] [Google Scholar]
- [18].Sanchez-Delgado M, Martin-Trujillo A, Tayama C, et al. Absence of maternal methylation in biparental hydatidiform moles from women with NLRP7 maternal-effect mutations reveals widespread placenta-specific imprinting. PLoS Genet 2015;11:e1005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Akoury E, Zhang L, Ao A, et al. NLRP7 and KHDC3L, the two maternal-effect proteins responsible for recurrent hydatidiform moles, co-localize to the oocyte cytoskeleton. Hum Reprod 2015;30:159–69. [DOI] [PubMed] [Google Scholar]
- [20].Andreasen L, Christiansen OB, Niemann I, et al. NLRP7 or KHDC3L genes and the etiology of molar pregnancies and recurrent miscarriage. Mol Hum Reprod 2013;19:773–81. [DOI] [PubMed] [Google Scholar]
- [21].Wang CM, Dixon PH, Decordova S, et al. Identification of 13 novel NLRP7 mutations in 20 families with recurrent hydatidiform mole; missense mutations cluster in the leucine-rich region. J Med Genet 2009;46:569–75. [DOI] [PubMed] [Google Scholar]
- [22].Rezaei M, Nguyen NM, Foroughinia L, et al. Two novel mutations in the KHDC3L gene in Asian patients with recurrent hydatidiform mole. Hum Genome Var 2016;3:16027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pinheiro AS, Proell M, Eibl C, et al. Three-dimensional structure of the NLRP7 pyrin domain: insight into pyrin-pyrin-mediated effector domain signaling in innate immunity. J Biol Chem 2010;285:27402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang P, Dixon M, Zucchelli M, et al. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS One 2008;3:e2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Khare S, Dorfleutner A, Bryan NB, et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 2012;36:464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Slim R, Wallace EP. NLRP7 and the genetics of hydatidiform moles: recent advances and new challenges. Front Immunol 2013;4:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goldberg IH, Rabinowitz M, Reich E. Basis of actinomycin action. I. DNA binding and inhibition of RNA-polymerase synthetic reactions by actinomycin. Proc Natl Acad Sci USA 1962;48:2094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gommersall LM, Arya M, Mushtaq I, et al. Current challenges in Wilms’ tumor management. Nat Clin Pract Oncol 2005;2:298–304. [DOI] [PubMed] [Google Scholar]
- [29].Walsh C, Bonner JJ, Johnson TN, et al. Development of a physiologically based pharmacokinetic model of actinomycin D in children with cancer. Br J Clin Pharmacol 2016;81:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lown KS, Mayo RR, Leichtman AB, et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther 1997;62:248–60. [DOI] [PubMed] [Google Scholar]
- [31].Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther 2005;78:260–77. [DOI] [PubMed] [Google Scholar]
- [32].Nakamura Y, Ikeda S, Furukawa T, et al. Function of P-glycoprotein expressed in placenta and mole. Biochem Biophys Res Commun 1997;235:849–53. [DOI] [PubMed] [Google Scholar]
- [33].Hill CR, Jamieson D, Thomas HD, et al. Characterisation of the roles of ABCB1, ABCC1, ABCC2 and ABCG2 in the transport and pharmacokinetics of actinomycin D in vitro and in vivo. Biochem Pharmacol 2013;85:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang S, Tang L, Lin J, et al. ABCB5 promotes melanoma metastasis through enhancing NF-kappaB p65 protein stability. Biochem Biophys Res Commun 2017;492:18–26. [DOI] [PubMed] [Google Scholar]
- [35].Volpicelli ER, Lezcano C, Zhan Q, et al. The multidrug-resistance transporter ABCB5 is expressed in human placenta. Int J Gynecol Pathol 2014;33:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


