Abstract
The expression of T-cell immunoglobulin domain, mucin domain-3 (Tim-3) in unexplained recurrent spontaneous abortion (URSA) was investigated.
Tim-3 mRNA expression in peripheral blood mononuclear cells (PBMCs) of URSA and control groups was assayed by fluorescent quantitative real-time polymerase chain reaction. Tim-3 protein expression intensity and localization in placental villi and uterine decidua were determined using immunohistochemical assay. The CD4+Tim-3+/CD4+ cell ratio in PBMCs was determined by flow cytometry.
Tim-3 mRNA expression in PBMCs was significantly higher in URSA than in normal controls (1.32 ± 0.25 vs 1.20 ± 0.12, P < .05). Tim-3 was expressed in placental tissue from both URSA patients and normal pregnant females (controls); however, the expression intensity was higher in the URSA group (0.54 ± 0.31 vs 0.35 ± 0.22, P < .05). CD4+Tim-3+/CD4+ cell ratio in PBMCs was significantly higher in the URSA group than that in the control group (4.53 ± 1.66% vs 1.28 ± 0.71%, P < .05).
Increased Tim-3 expression in PBMCs and placental tissue of URSA might affect maternal-fetal immune tolerance. Tim-3 was involved in the pathogenesis of URSA, which was expected to serve as an indicator for the immune evaluation of URSA.
Keywords: PBMCs, Tim-3, URSA
1. Introduction
T-cell immunoglobulin domain and mucin domain (Tim) gene family is a class of genes involved in the immune response and was first described in 2002.[1] Engagement of TIM-3 by its ligand galectin-9 negatively regulates interferon secretion and blocks the induction of T-cell tolerance.[2] Tim molecules are a group of transmembrane glycoproteins containing a signal peptide, an immunoglobulin (Ig)-like domain, a mucin-like domain, and an intracellular domain with phosphorylation sites. Human Tim family consists of Tim-1, Tim-3, and Tim-4. Tim-1 is expressed on all activated T-cells, preferentially being expressed on the surface of T-helper (Th)-2 cells, and likely functioning as a novel and potent costimulatory molecule for CD4+ T-cells.[3] Tim-3 is expressed on differentiated Th1 cells, Th17, CD8 T-cells, monocytes, and dendritic cells which is related to autoimmune diseases and allograft rejection.[4–7] Several studies have shown that the Tim family is a critical protein in the maintaining immune homeostasis and closely associated with immunological diseases.[8]
Recurrent spontaneous abortion (RSA) is defined as 2 or more consecutive and early miscarriages occurring during the first trimester of pregnancy. RSA is a common complication of pregnancy, affecting approximately 2% women of child-bearing age.[9] Approximately 70% cases of RSA are due to unknown causes and are defined as unexplained RSA (URSA). The relationship between URSA and immune factors has become evident with the development of reproductive immunology. Wegmann et al[10] showed that normal pregnancy in mammals is a Th2-dominant phenomenon. This finding opened up a new field of study into the etiology of URSA and the mechanism of immunotherapy, triggering several human studies. Currently, one of the possible mechanisms for maintaining normal pregnancy in humans is believed to be the production of cytokines and hormones by the placenta, resulting in the inactivation of T-cells or a bias toward Th2 response.[11] Blockade of Tim-3 resulted in increment of Th1 cytokines at the maternal-fetal unit and affect maternal-fetal immune tolerance.[12] However, studies on Tim molecules in RSA are scarce.
For this study, patients with typical URSA were chosen. To investigate the role of Tim-3 in the URSA, Tim-3 mRNA expression levels in peripheral blood mononuclear cells (PBMCs) were measured by fluorescent quantitative real-time polymerase chain reaction, Tim-3 protein expression and localization were determined by immunohistochemical assay.
2. Materials and methods
2.1. URSA patients and normal controls
This study was approved by Ethics Committee of Qilu Hospital and patients voluntarily signed an informed consent. The URSA group (n = 35), aged 23 to 34 years with a history of 2 to 5 prior miscarriages were selected from our hospital. Additionally, the aged 23 to 31 years women in the normal early gravidas group (n = 25) requested for artificial abortion in our hospital and had at least one normal pregnancy before. They enrolled as the normal control group, with no adverse pregnancy history such as abortion, stillbirth, or neonatal death; no autoimmune diseases; and no evidence of previous or current TORCH infections. Villli, deciduas and peripheral blood samples (total of 8 mL) were collected from each participant and preserved respectively at the time of evacuation (8 ± 3weeks).
2.2. Quantitative real-time PCR assay for Tim-3 mRNA expression
Peripheral blood samples (5 mL) were collected from the cubital vein of each participant in the URSA and control groups. Samples were treated with an anticoagulant, acid-citrate-dextrose (ACD), and lymphocyte separation medium, followed by centrifugation at 300g for 20 minutes. Mononuclear cells were then isolated and washed. Total RNA was extracted using TRIzol and was reverse-transcribed to cDNA. Oligo (dT) was used as primer of mRNA. No amplification was seen in non-RT samples. Forward and reverse primers were designed as follows (Table 1).
Table 1.
Primers for fluorescent quantitative real-time PCR.
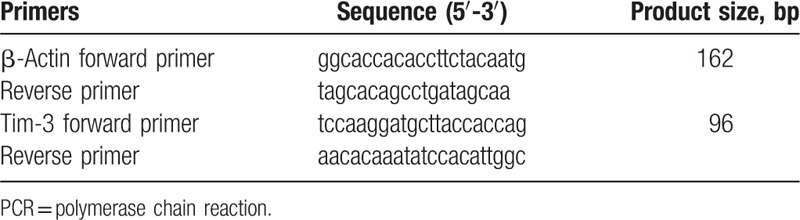
The primers were synthesized by Invitrogen. Quantitative RT-PCR was performed by in a total volume of 20 μL reaction solution containing 10 μL of 2X SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 1 μL of forward primer, 1 μL of backward primer, 6 μL of H2O and 2 μL of diluted cDNA template. β-actin as internal reference gene. The reaction conditions comprised initial denaturation at 95°C for 5 minutes, followed by 35 cycles of 95°C for 15 seconds and 60°C for 1 minutes.
The melting curve analysis involved initial denaturation at 95°C for 2 minutes followed by 2 cycles of 95°C for 15 seconds and 60°C for 1 minutes.
2.3. Flow cytometry assay of CD4+Tim-3+ T-cells
ACD-treated blood samples (100 μL) were mixed with 2 mL hemolysin, followed by centrifugation at 400g for 10 min. After washing 2 times with phosphate buffered saline (PBS), the supernatant was discarded. Cell pellet was resuspended in PBS and separately dispensed into 2 tubes with 100 μL each. One portion of the sample was treated with 20 μL FITC-conjugated anti-human CD4 antibody (eBioscience lnc, San Diego) and 5 μL PE-conjugated anti-human Tim-3 antibody (R&D systems Inc.). The other portion of the sample served as a negative control and was combined with 2 μL anti-human IgG1-FITC. The reactions were allowed to continue for 30 min at room temperature in the dark. After centrifugation and washing twice with PBS, samples were analyzed using FC 500 (Beckman Coulter Inc., Indianapolis). The data were analyzed using System II V3.0 software.
2.4. Immunohistochemistry for Tim-3 protein expression in decidua and villi
Control and URSA placental tissues were fixed with 4% formalin. Sections (4- to 6-μm thick) from paraffin-embedded tissue specimens were cut and mounted on polylysine-coated slides for immunohistochemical analysis. Briefly, tissue sections were dewaxed in xylene (2 × 10 minutes) and rehydrated through a graded series of ethanol and distilled water. After deparaffinization, antigen retrieval was performed at 98°C in a microwave oven for 10 minutes in 10 mmol/L sodium citrate buffer (pH 6.0). Then the slides were rinsed in 0.01 mol/L phosphate-buffered saline solution (PBS) (2 × 5 minutes) with gentle agitation and blocked in 10% normal serum with 1% bovine serum albumin in PBS for 2 hours at room temperature. Sections were then incubated with goat antihuman TIM-3 immunoglobulin (Ig) G (1:100, R&D Systems, Minneapolis) in a humid chamber overnight at 4°C. A 0.3% hydrogen peroxide solution (vol/vol) containing sodium azide was used at room temperature for 10 minutes to block endogenous peroxidase activity. After a thorough wash in PBS, rabbit antigoat IgG avidin-biotin peroxidase complex kit and DAB peroxidase substrate kit (Maixin Co, Fuzhou, China) were included to visualize the binding sites of the primary anti-body according to the manufacturer's instructions. Sections were faintly counterstained with hematoxylin. For negative controls normal serum or isotope IgG was included in each of the immunostaining procedures. Sections were observed under a microscope to determine the location and intensity of staining. “Positive cells” exhibited brownish yellow-stained plasma membrane or cytoplasm, whereas “negative cells” demonstrated hematoxylin-stained, blue nuclei and unstained cytoplasm. Images were analyzed using HMIAS-2000 high-definition color medical image analysis system, where 10 visual fields were randomly observed microscopically under high magnification. Mean optical density of “positive cells” was measured and semiquantitative statistical analysis was performed.
2.5. Statistical analysis
Experimental data were presented as 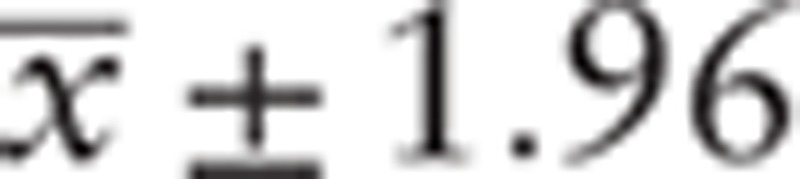 standard deviation (SD). Significant differences between samples were analyzed using paired-sample t test. P < .05 was considered statistically significant. Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL).
standard deviation (SD). Significant differences between samples were analyzed using paired-sample t test. P < .05 was considered statistically significant. Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Tim-3 mRNA expression in PBMCs
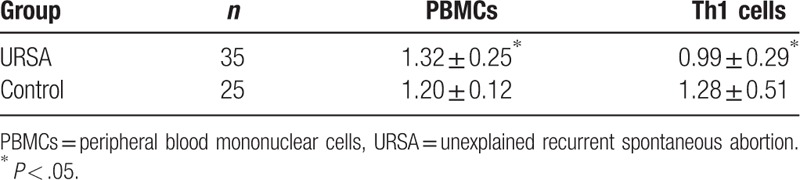
During preliminary experiments, RT-PCR amplification products of β-actin and Tim-3 were separated using 3% agarose gel electrophoresis. Both products demonstrated a single band of the desired size. The melting curve of both target genes exhibited a single peak, indicating the absence of nonspecific amplification. Based on the relative expression as to target genes, Tim-3 expression in PBMCs was significantly higher in URSA patients (1.32 ± 0.25 vs 1.20 ± 0.12, P < .05); however, Tim-3 expression in Th1 cells was significantly lower in URSA patients than that in normal controls (0.99 ± 0.29 vs 1.28 ± 0.51, P < .05). Comparison of the relative Tim-3 mRNA expression (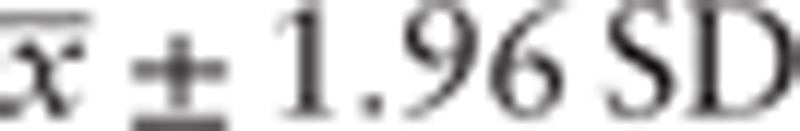 ) between groups is presented in Table 2.
) between groups is presented in Table 2.
Table 2.
Relative expression of Tim-3 mRNA in PBMCs and Th1 cells of unexplained recurrent spontaneous abortion (URSA) patients and normal controls.
3.2. CD4+ Tim3+/CD4+ ratio in PBMCs
Flow cytometry results showed that the CD4+Tim3+/CD4+ cell ratio in PBMCs was significantly higher in the URSA group than that in the control group (4.53 ± 1.66% vs 1.28 ± 0.71%, P < .05).
3.3. Tim-3 protein levels in tissues
In normal placental villi, Tim-3 protein was mainly expressed mainly on the cell membrane of syncytiotrophoblast and cytotrophoblast. Mean optical densities of Tim-3-positive signals from both groups were measured and statistically analyzed. The result showed that Tim-3 expression on the syncytiotrophoblast and cytotrophoblast membrane in placental villi of the URSA group was significantly higher than that of controls (0.54 ± 0.31 vs 0.35 ± 0.22, P < .05) (Fig. 1).
Figure 1.
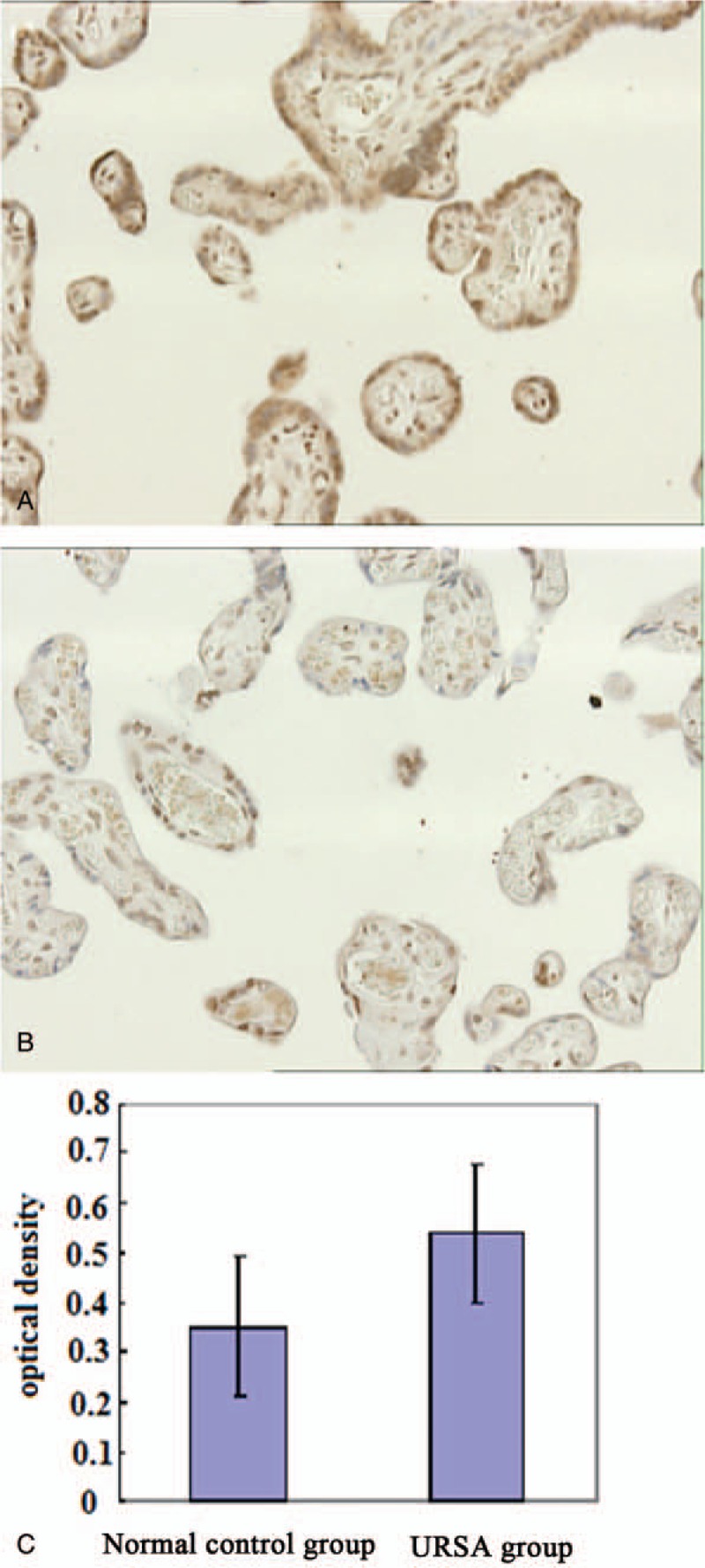
Tim-3 expression in placenta. (A) Immunohistochemical staining of Tim-3 in placenta from the URSA group 200×; (B) Immunohistochemical staining of Tim-3 in placenta from the control group 200×; and (C) Mean optical densities of positive signals of both groups were measured and statistically analyzed. Compared to the control group, significant differences were observed in URSA group, P < .05. URSA = unexplained recurrent spontaneous abortion.
4. Discussion
RSA is a common complication of pregnancy involving multiple pathogenic factors, mainly of fetal and maternal origin. The primary fetal cause is chromosomal abnormality, and maternal causes mainly refer to factors that influence the endometrium and/or embryonic development. The known maternal causes include coagulation disorders, autoimmune deficiencies, endocrine disorders, and endometrial defects.[13]
Tim-3 is an important member of the Tim family, playing important roles in regulating innate immunity and specific immune responses. Tim-3 is expressed in various types of cells, including human natural killer cells, monocytes, dendritic cells, mouse master cells, and macrophages.[14] Tim-3 is expressed in differentiated Th1 cells. It regulates Th1 cell-mediated immune response and is associated with autoimmune diseases and allograft rejection.[15] Several studies have shown that the Tim family is closely associated with a variety of immunological diseases, such as autoimmune diseases, transplantation immunity, and tumor immunity; however, few studies have been performed on maternal-fetal immunity.
The etiology of URSA is highly complicated. It is currently thought that one of the mechanisms underlying maternal-fetal tolerance involves the cytokines and hormones produced from placenta inactivating the T-cells or making a shift to Th2 bias. In this study, the role of Tim-3 in URSA was investigated at the mRNA level by RT-PCR assay of 25 normal controls and 35 URSA patients. The results showed that Tim-3 mRNA expression levels were significantly higher in PBMCs from URSA patients than those from normal controls (P < .05). This observation indicates the association between higher Tim-3 expression and URSA. Despite expression of Tim-3 increased in PBMCs from URSA patients, Tim-3 mRNA expression was decreased in Th1 cell subsets. It is showed that Tim-3/Tim-3L signaling pathway is involved in the production and immunosuppressive function of antigen-specific CD4+CD25+ regulatory T-cell populations. Therefore, downregulation of Tim-3 expression on Th1 cell surface may cause the abrogation of immunological tolerance by influencing the number and function of regulatory T-cells. As cell populations are different between peripheral blood and the fetal-placental unit, mRNA expression cannot fully reflect protein expression level. Thus, Tim-3 expression was further analyzed at the protein level to determine whether Th1/Th2 immune-balance shift exists in URSA patients, who exhibit stronger Th immune response. The results showed that Tim-3 protein expression was significantly increased in placental tissue from URSA patients than women undergoing normal pregnancy.
In summary, our results showed that as a negative regulator in immune responses, Tim3 reactively increased in URSA patients. The abnormal expression of Tim-3 in pregnant woman might be deleterious to pregnancy outcome. This finding indicated that Tim-3 was involved in the pathogenesis of URSA, which was expected to serve as an indicator to predict the risk of abortion in pregnant woman.
Author contributions
Conceptualization: Xuewei Zhuang.
Data curation: Xiyan Xia, Lingxiao Liu, Xin Zhang.
Formal analysis: Xiyan Xia, Yi Zhang, Xin Zhang.
Funding acquisition: Xuewei Zhang, Chuanxin Wang.
Validation: Xuewei Zhuang, Xiyan Xia, Lingxiao Liu, Yi Zhang, Xin Zhang, Chuanxin Wang.
Writing – original draft: Xuewei Zhuang.
Writing – review & editing: Xuewei Zhuang, Chuanxin Wang.
Footnotes
Abbreviations: ACD = acid citrate dextrose, FQ = fluorescence quantitative, PBMCs = peripheral blood mononuclear cells, PCR = polymerase chain reaction, Th = T helper cells, Tim = T cell immuglobulin domain and mucin domain, TORCH = toxoplasmosis, other (viruses), rubella, cytomegalovirus, herpes simplex virus, URSA = unexplained recurrent spontaneous abortion.
This study was supported by grants from Shandong Provincial Nature Science Foundation (2015ZRE27571), Shandong Provincial Key Research and Development Program (2016GSF201169), Shandong Provincial Medicine and Health Development Plan (QW019), and Shandong Provincial Population and Family Development Plan (200910).
The authors have no conflicts of interest to disclose.
References
- [1].Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002;415:536–41. [DOI] [PubMed] [Google Scholar]
- [2].Sabatos CA, Chakravarti S, Cha E, et al. Interaction of TIM-3 and TIM-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol 2003;4:1102–10. [DOI] [PubMed] [Google Scholar]
- [3].Kuchroo VK, Umetsu DT, DeKruyff RH, et al. The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol 2003;3:454–62. [DOI] [PubMed] [Google Scholar]
- [4].Hastings WD, Anderson DE, Kassam N, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol 2009;39:2492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sanchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 2003;4:1093–101. [DOI] [PubMed] [Google Scholar]
- [6].Oikawa T, Kamimura Y, Akiba H, et al. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol 2006;177:4281–7. [DOI] [PubMed] [Google Scholar]
- [7].Nakayama M, Akiba H, Takeda K, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 2009;113:3821–30. [DOI] [PubMed] [Google Scholar]
- [8].Baghdadi M, Jinushi M. The impact of the TIM gene family on tumor immunity and immunosuppression. Cell Mol Immunol 2014;11:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baek KH, Lee EJ, Kim YS. Recurrent pregnancy loss: the key potential mechanisms. Trends Mol Med 2007;13:310–7. [DOI] [PubMed] [Google Scholar]
- [10].Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal-fetal relationship; is successful pregnancy a Th2 phenomenon? Immunol Today 1993;14:353–6. [DOI] [PubMed] [Google Scholar]
- [11].Paul WE. Fundamental Immunology. 2003;Philadelphia: Lippincott Williams & Wilkins, 802–803. [Google Scholar]
- [12].Chabtini L, Mfarrej B, Mounayar M, et al. TIM-3 regulates innate immune cells to induce fetomatarnal tolerance. J Immunol 2013;190:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li TC, Makris M, Tomsu M, et al. Recurrent miscarriage, aetiology, management and prognosis. Hum Reprod 2002;8:463–81. [DOI] [PubMed] [Google Scholar]
- [14].Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005;6:1245–52. [DOI] [PubMed] [Google Scholar]
- [15].Umetsu SE, Lee WL, McIntire JJ, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol 2005;6:447–54. [DOI] [PubMed] [Google Scholar]


