Abstract
The application of methotrexate (MTX) in the treatment of autoimmune diseases has been gradually increasing, but reports of MTX treatment for advanced ocular cicatricial pemphigoid (OCP) are extremely rare. This study investigated the efficacy and adverse reactions of low-dose MTX in patients with OCP.
This was a retrospective, noncontrolled, case series study. Eleven patients diagnosed with advanced OCP (4 cases in stage III and 7 cases in stage IV) were enrolled. Treatment by oral administration of MTX (7.5 ± 2.5 mg) alone was performed. Visual acuity of the patients, conjunctival inflammation, cicatrization, ocular surface keratinization, and toxic side effects of drugs were evaluated.
All patients enrolled in this study were females aged 32 to 83 years. Patients were followed up for 4 to 33 months. Low-dose MTX improved visual acuity of 3 cases (6 eyes, 27.3%). Conjunctival inflammation of 5 patients (10 eyes) rested after treatment, and conjunctival inflammation of 3 cases (6 eyes, 27.3%) was decreased with an effective rate of 72.7%. Cicatrices of 8 cases (15 eyes) showed degeneration after treatment with an effective rate of 71.4% (15/21). Ocular surface keratinization receded in 4 cases with an effective rate of 66.7%. None of the patients discontinued the treatment due to severe toxic side effects. All patients tolerated mild drug-induced gastrointestinal reactions. Three patients terminated the treatment in advance after 4 to 6 months due to no improvement in the disease condition.
Observation of clinical efficacy and safety findings demonstrated that low-dose MTX can be used to treat patients with advanced OCP.
Keywords: cicatricial conjunctival inflammation, low dose, methotrexate, ocular cicatricial pemphigoid
1. Introduction
Ocular cicatricial pemphigoid (OCP) is an autoimmune subepithelial blistering disease accompanied by visual impairment. It is characterized by binocular asymmetry, chronic progressive, or recurrent conjunctivitis combined with conjunctival cicatrization, as well as secondary corneal vascularization and corneal opacity.[1] Advanced OCP might lead to symblepharon, blepharelosis and ankyloblepharon, resulting in corneal opacity, ocular surface keratinization, and severe Sjogren syndrome. If timely treatment of OCP was not performed due to corneal neovascularization, it finally leads to blindness.[2,3] Epidemiological data showed that the blindness rate of OCP can be as high as 27%.[4] OCP mainly affects the elderly population and is commonly seen in population aged 50 to 80 years. OCP remains more aggressive in younger patients, and immunomodulatory treatment is considered to be ineffective in approximately 30% of OCP patients.[5,6] OCP is an autoimmune chronic cicatricial conjunctivitis, and abnormal regulation of immune system is an important feature of OCP.[7] The true incidence rate of occult OCP in patients is unknown. This is due to that the early clinical manifestations of OCP are not obvious, the sensitivity of immunopathological diagnosis is poor, differential diagnosis is difficult, and 40% of patients suffer from progressive conjunctival fibrosis caused by delayed treatment due to lack of clinically visible inflammation of the ocular surface, thereby most of the patients are diagnosed at an advanced stage. Therefore, diagnosis and treatment of OCP remains difficult.[4,5] Foster has proposed a 4-stage clinical classification based on its clinical manifestations, as follows: stage I: conjunctivitis and subconjunctival cicatrization, Stage II: shortening of conjunctival fornix, Stage III: progressive subconjunctival fibrosis and symblepharon, Stage IV: advanced OCP, manifesting as ocular surface keratinization, disappearance of conjunctival fornix, ankyloblepharon as well as fixation of eyelid and eyeball.[8]
OCP is a systemic disease and treatment of this should aim to control systemic immune disorders, inhibit the processes of conjunctival inflammation and cicatrization, as well as maintain the normal relationship between eyelid and ocular surface.[8] Clinically, long-term treatment of combined multiple immunosuppressive agents is usually applied to control the ocular inflammations in patients with OCP.[9] This treatment greatly increases the possibility of potential side effects, and drug reduction causes disease exacerbation, thereby affecting its widespread use clinically to a certain extent.[1,10–12] Moreover, in spite of the use of systemic chemotherapy intervention, there are patients who suffer from blindness due to disease progression. In addition, for cicatrization caused by advanced OCP, drug therapy can only control its progression, but remains difficult to be reversed.[7] Studies have reported that surgical treatment, such as eyelid surgery, amniotic membrane transplantation (AMT), corneal transplantation, etc. may be conducive to ocular surface reconstruction and visual rehabilitation for patients with advanced OCP.[1] Nevertheless, the success rate of surgical treatment was low, leading to mechanical damage, and aggravation of the disease. Thus, it is not suitable for elderly patients. If ocular inflammations are not well controlled in perioperative patients, it might lead to ulcerative keratitis after surgery.[13,14]
Methotrexate (MTX) is a folic acid analog that has antiinflammatory and cytostatic effects. Low-dose methotrexate (MTX, less than 15 mg/week) is considered safe and effective for long-term use in the treatment of autoimmune diseases such as rheumatoid arthritis,[15,16] systemic lupus erythematosus,[17] psoriatic arthritis,[18] etc. MTX is also used for the treatment of scleritis, noninfectious uveitis, inflammatory pseudotumor, orbital myopathy, etc.[19–22] Some scholars[23] have suggested that MTX alone can be used as a first-line drug for treating rapid progressive or mid-term OCP. However, few clinical reports reported the efficacy and safety of MTX in the treatment of advanced OCP in China and at abroad.[11] Therefore, our study investigated advanced OCP patients treated by oral administration of low-dose MTX alone to explore the impact on the prognosis.
2. Methods
A total of 11 patients diagnosed with advanced OCP were collected from Xi’an No. 4 Hospital, the Second Affiliated Hospital of Xi’an Medical University and Xi’an Aier Eye Hospital from December 2011 to April 2017. All the patients were orally administrated with low-dose MTX after signing the informed consent. A retrospective case series study was performed, which was approved by the Ethics Committee of the Hospital (Table 1).
Table 1.
Demographics, medical history, efficacy, and adverse effects of MTX treatment.
The diagnosis was mainly based on specific clinical manifestations of OCP, including binocular symmetrical or asymmetric conjunctival inflammation, symblepharon, narrowing of conjunctival fornix, cicatrization, ocular surface keratinization, and ankyloblepharon.[3] The disease course was staged for all OCP patients based on the Foster classification. In this study, all the 11 OCP patients underwent best-corrected visual acuity examination and slit-lamp examination. Some patients previously underwent ANA (antinuclear antibody) examination. Other systemic diseases other than OCP were excluded with the help of internists. At each visit, all the patients underwent examinations by internists, dermatologists, and ophthalmologists. The patients were followed up for at least 6 months after study initiation.
Each patient was orally administrated with low-dose MTX (7.5 ± 2.5 mg) once every week after OCP and drug introduction was clearly explained to the patients. Based on the body weight of the patients, the maximum dose administered was 10 mg/week, and the minimum dose was 5 mg/week. Folic acid, 5 mg was orally administrated on the day after MTX oral administration, which is used to reduce adverse reactions of MTX and the dose was adjusted according to the clinical manifestations and side effects of the patients. Meanwhile, artificial tears were used locally to relieve from the symptoms, and 1% cyclosporine was used in patients with ocular surface keratinization. All patients underwent routine blood tests, liver and renal function tests as well as chest X-ray examination before MTX treatment. No obvious abnormality was noted in all patients.
Visual acuity of the patients, conjunctival inflammation, cicatrization, ocular surface keratinization, toxic side effects of drug, etc., were again assessed 1 month after administration. Review was performed after every 1 to 2 months. The degree of ocular surface keratinization and progression of cicatrization were independently evaluated using semi-quantitative grading (0 to +++) by 2 doctors who had more than 20 years experience in keratosis. Classification of ocular surface keratinization was as follows: Grade 0: both cornea and conjunctiva were normal, Grade I: mild dryness of cornea and conjunctiva, tarnish of bulbar conjunctiva and darkness of cornea, Grade II: dryness of the cornea and conjunctiva, opacity of superficial cornea, deep and superficial neovascularization, keratoconjunctival tarnish and visible anterior-chamber iris under slip lamp, Grade III: cornea and conjunctiva remain dry as skin, the cornea and conjunctiva were opacity, small scales on the surface that cannot be wet by eye drops, and the anterior chamber along with iris were opacity. The progression of cicatrization was classified as follows: Grade 0: no hyperemia in cicatricial fibers, small amount of blood vessels in the cicatrization, and stopped cicatricial progression, Grade I: mild hyperemia in cicatricial fibers, a small amount of blood vessels in the cicatrization and nonobvious cicatricial progression, Grade II: hyperemia in cicatrization and mild edema. Observation and comparison after 4 months of follow-up showed mild progression in ankyloblepharon or symblepharon. Grade III represented hyperemia and edema in cicatrization, which was bright red in color. Moreover, observation after 4 months of follow-up indicated a definitive progression in ankyloblepharon or symblepharon. The time standards identifying clinically effective or ineffective were whether there were changes in subjective symptoms of the patients and objective signs after 4 months of treatment.
After medication, routine blood was tested every month, while liver and renal function tests were performed every 8 weeks. If routine blood as well as liver and renal functions during follow-up showed abnormalities, the internists held a consultation to decide whether the drug dose should be adjusted or the treatment should be terminated. All patients were followed up for 4 to 33 months (14.9 months on average) cumulatively.
3. Results
All patients included in this study were females, which showed significant gender differences and were in line with the characteristics of the onset of OCP. The age of the patients was 32 to 83 years (the average age was 63.4 years). Among the 11 patients, 5 cases (45%) had mucosal lesions involved in other parts. Furthermore, among these 5 cases, 3 cases developed oral mucosal ulcer, tongue mucosal ulcer and oral adhesion, 1 case had epiglottitis and posterior hypopharyngeal wall ulcer combined with nasopharyngeal adhesion, and 1 case suffered from desquamative gingivitis. Before the visit, 2 cases (case 6 and case 8) were diagnosed with symblepharon in other hospitals due to decreased visual acuity 1 month before the visit, and thus they underwent separation of symblepharon combined with AMT. Postoperative visual acuity was decreased to the light sense from preoperative CF/50CM, and a wide range of adhesion, and ocular surface keratinization combined with fixation of eyeball were observed. Two cases (case 3 and case 6) were previously treated for 1 month with prednisone combined with CTX, but the treatment was terminated due to acute liver damage. One case (case 5) underwent treatment of intravenous immunoglobin (IVIg) for 3 cycles (IVIg 2 g/kg/cycle), then short-term treatment with prednisone was performed, but the efficacy was poor. Cataract surgery was performed for 4 eyes in 3 cases (cases 1, 5, and 6) where prednisone (0.5 mg/kg) was orally administered 2 weeks before the operation. Treatment with prednisone was continuously performed after the operation, and conjunctival inflammation was well stabilized. Four of the 11 cases had never undergone systemic immunosuppressive therapy before visit (Table 1).
Disease course was staged based on the Foster classification. There were 4 cases (36.4%) in stage III and 7 cases (63.6%) in stage IV. All patients were followed up for 4 to 33 months (14.9 months on average), cumulatively. After oral administration of low-dose MTX, visual acuity was improved in 3 cases (6 eyes, 27.3%). Conjunctival inflammation rested in 5 cases (10 eyes), and receded in 3 cases (6 eyes, 27.3%), with an effective rate of 72.7%. Cicatrization in 8 cases (15 eyes) was degenerated with an effective rate of 71.4% (15/21). Obvious improvement of ocular surface keratinization was noted in 4 cases with an effective rate of 66.7% (Figs. 1–3 and Table 1). Among these 4 cases, 3 patients (cases 1, 7, and 8) had stopped the medication after 6, 4, and 4 months of treatment, respectively. This is because the conjunctival inflammation did not recede and ocular surface keratinization showed no change. Moreover, the ineffective rate of treatment was 27% (3/11) (Fig. 4).
Figure 1.
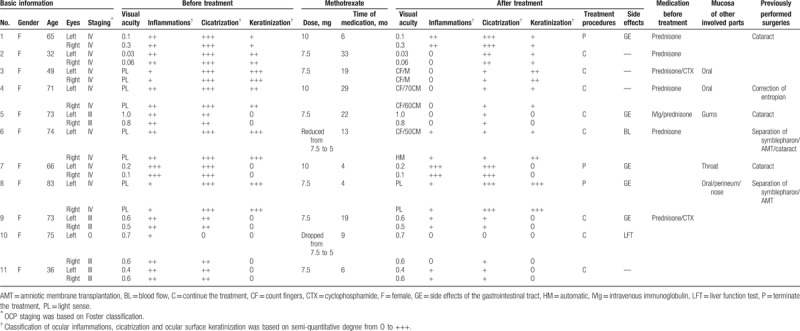
(Case 3) Before the treatment, we could obviously see the keratinization (A arrow) and conjunctivialization (B arrow) on the corneal. Conjunctiva appeared proliferation (C arrow). The light cannot reach anterior chamber through the corneal. After the treatment for 19 months, keratinization of corneal was partly reversed (A arrow), conjunctivialization had faded (B arrow). The proliferation on the conjunctiva was improved and angle of sclera edge could be identified (D arrow). And the iris was visible through corneal.
Figure 3.
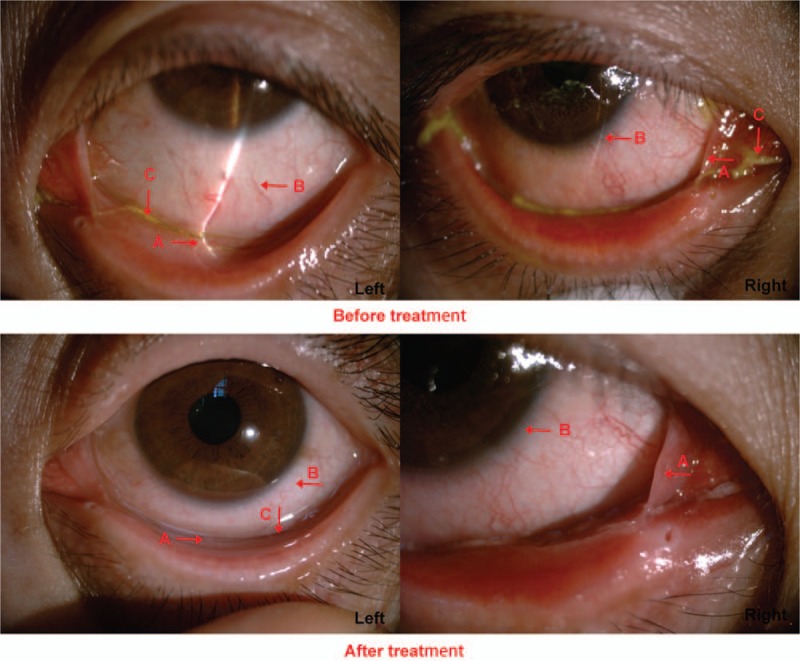
(Case 11) Before the treatment, we could obviously see the conjunctival adhesion (A arrow), conjunctival hyperemia (B arrow) and lots of viscous secretions (C arrow). After the treatment for 6 months, conjunctival adhesion was released (A arrow), conjunctival hyperemia was alleviated (B arrow), and secretions were decreased (C arrow).
Figure 4.
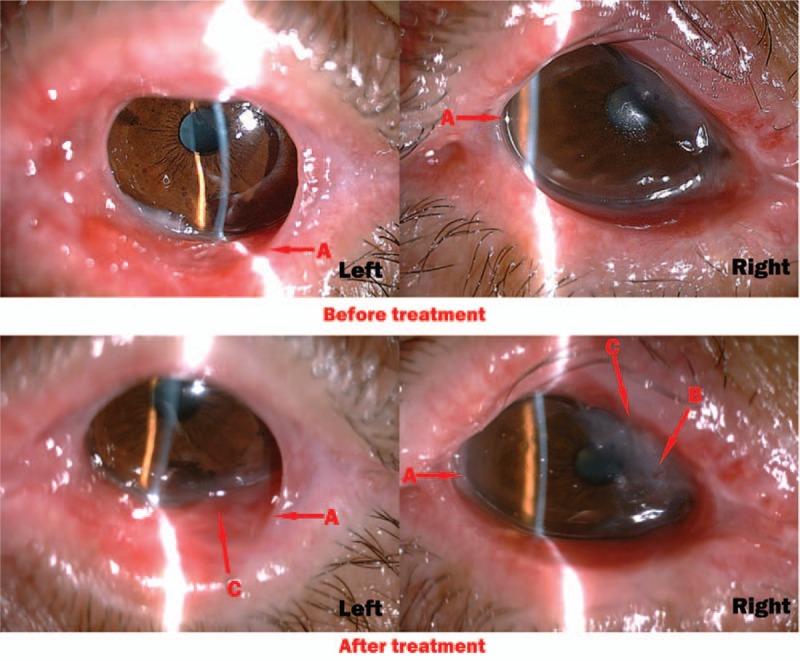
(Case 7) Before the treatment, it showed that the patient suffered from severe conjunctival adhesion (A arrow). After the treatment for 4 months, conjunctival hyperplasia became more serious (A arrow). The right corneal appeared obviously conjunctivialization (B arrow) and conjunctival sac constriction (C arrow).
Figure 2.
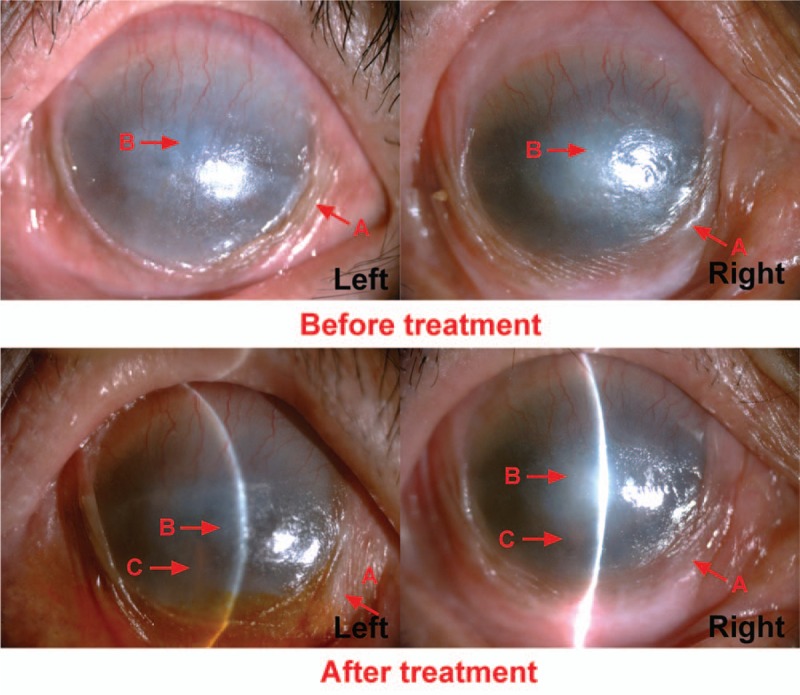
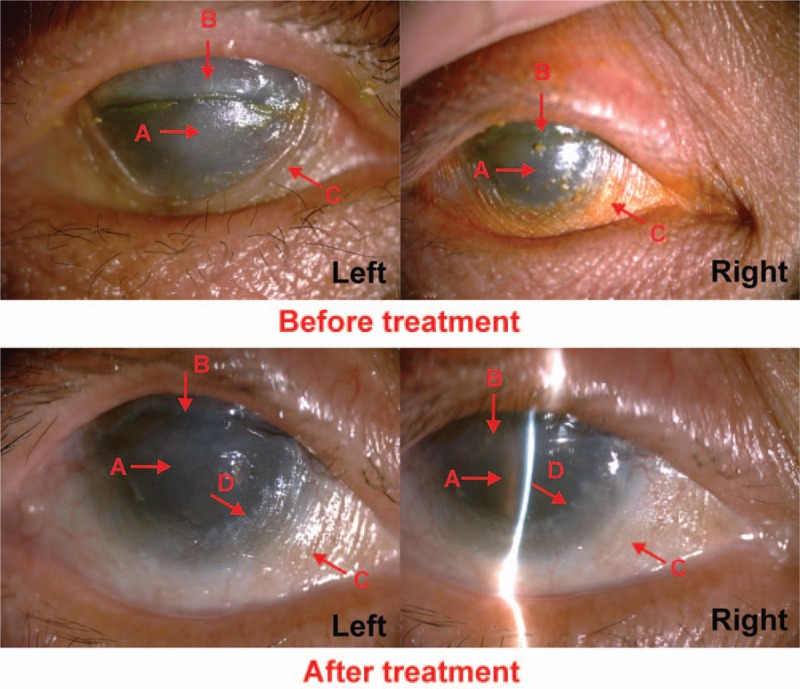
(Case 4) Before the treatment, conjunctiva appeared proliferation (A arrow). We could obviously see the keratinization (B arrow) on the corneal. The light cannot reach anterior chamber through the corneal. After the treatment for 29 months, conjunctivialization had faded (A arrow), keratinization of corneal was partly reversed (B arrow). And the iris was visible through corneal (C arrow).
One patient (case 10) had mild elevation of alanine aminotransferase (46u/l) after 2 months of MTX administration. The patient was orally administrated with 35 mg silybin capsule 3 times/day after consultation by internists, and the treatment was continued by lowering the MTX dose to 5 mg/week. Alanine aminotransferase level was dropped to 24u/l after 1 month, and oral administration of MTX at 5 mg/week was continued until the end of the treatment. Routine blood examination was performed in 1 patient (case 6) at 6 months after administration of MTX. The results indicated that RBC was 3.0 × 109/L, and hemoglobin was 90 g/L. After consultation by internists, the patient was orally administrated with sodium sulfate and inosine. Moreover, the treatment was continued by lowering the MTX dose to 5 mg/week. Review after 1 month showed that the routine blood was normal, and oral administration of MTX at 5 mg/week was continued until the end of the treatment. After treatment, 5 patients suffered from gastrointestinal reactions such as nausea, but the treatment was not discontinued because the patients were able to tolerate. The incidence rate of all toxic side effects was 64% (7/11). None of the patients discontinued the treatment due to severe adverse drug reactions (Table 1).
4. Discussion
OCP is a rare ophthalmological disease, which is seen mostly in the elderly population. In recent years, with the improvement of our understanding regarding OCP, there is an increasing trend in the number of clinically diagnosed cases, the stage at diagnosis of OCP is advanced, and can also occur in younger population. Among the 11 cases enrolled in this study, the age of onset was less than 40 years for 2 cases, and the incidence in young people accounted for 18% (2/11). This disease is considered as more common than our current understanding. In this study, diagnosis of all the 11 patients were in line with OCP clinical diagnostic criteria accepted internationally,[7] and there was only 1 young patient (case 2) whose clinical manifestations were consistent with the clinical diagnostic criteria. However, since the patient was young, it is necessary to further diagnose. Conjunctival biopsy and direct immunofluorescence prompted linear deposition of IgG on epithelial basement membrane, which was consistent with the pathological diagnostic criteria of OCP, thus confirming OCP in this patient. Scars on the ocular surface caused by drugs, thermal burns, and chemical burns were excluded by medical history. Cicatricial conjunctivitis caused by lichen planus, Stevens–Johnson syndrome, erythema multiforme, pemphigus vulgaris, bullous pemphigoid, and acquired epidemolysis bullosa were excluded by consultation with the dermatologist.[7] After diagnosis, immunosuppressive therapy was performed by oral administration of low-dose MTX in all patients.
MTX is a traditional antimetabolite drug that has been in clinical use for more than 40 years, and can effectively control mild to moderate OCP inflammation by regulating immune function of the patients. In this study, 11 patients with advanced OCP were treated by oral administration of low-dose MTX. The average follow-up time of the patients was 14.9 months, and a total of 8 cases obtained full control or inhibition of conjunctival inflammation and cicatrization with an effective rate of up to 72.7% (8/11). The effective rate obtained in our study was a bit lower than the effective rate reported by McCluskey et al study [83% (10/12)].[11] This may be due to that our study mainly included stage IV OCP patients (63.3%), while McCluskey et al study included stage II OCP patients (58.3%). Findings of this study proved that the treatment was more difficult and efficacy remained poorer for progressive diseases such as OCP at advanced stages. However, our study showed some encouraging results where binocular visual acuity of 3 cases (27.3%) was improved. Moreover, ocular surface keratinization was reversed after treatment in 4 of the 6 patients with advanced OCP (the effective rate was 66.7%). Reversal of ocular surface keratinization may be due to the partial recovery of the ocular surface functions after conjunctival inflammation was inhibited. Hervas Ontiveros et al[23] also reported that 1 patient with active OCP at medium stage underwent subcutaneous injection of MTX of 15 mg/week for 2 years. Symptoms of discomfort were completely alleviated after 6 months of treatment, resulting in the significant improvement of the disease condition and enhancement of binocular visual acuity to 0.9. Furthermore, conjunctival hyperemia significantly receded and ocular surface keratinization disappeared, shortening of conjunctival fornix was stable, and no adverse reactions were observed. After 2 years of treatment, disease condition of the patient was stable without any recurrence or occurrence of new diseases, which were consistent with the results of our study. Therefore, we considered that visual acuity can be saved partially after administration of MTX clinically even for advanced OCP patients with completely keratinized ocular surface. We thought that majority of clinical ophthalmological diagnosis should be immediately treated only if they met the clinical diagnostic criteria of OCP. Pathological diagnosis, cellular immune detection, and cytokine detection should be performed by some medical or research teams with better equipment. It was worth noticing that repeated conjunctival biopsy may provoke severe conjunctival inflammation. In this study, 3 patients terminated the treatment because the disease condition showed no improvement. Treatment failure in this study may be because the disease condition of the 3 cases was severe, conjunctival inflammation and cicatrization progressed rapidly, and ocular surface keratinization was difficult to recover.
Adverse reactions of MTX treatment included nausea, vomiting, and hair loss. However, the most severe side effects include liver and pulmonary fibrosis during long-term treatment.[24] Study of McCluskey et al[11] has demonstrated that the incidence of MTX side effects remained low. Study of Kjellman et al[25] also found that there are rare patients who have terminated MTX treatment due to side effects, and most of the side effects are mild. In addition, MTX did not reduce the life expectancy of patients. On the contrary, the survival rate of patients undergoing low-dose MTX treatment seems higher compared to patients undergoing other treatments. In this study, no severe adverse reactions such as liver and pulmonary fibrosis were observed, and none of the patients terminated the treatment due to adverse drug reactions.
Meanwhile, administration of MTX is convenient and usually administrated orally once every week. If gastrointestinal absorption weakens, intramuscular injection can be applied to improve the condition. Subcutaneous administration can be performed if the patients could not tolerate gastrointestinal side effects. In our study, the method of administration of once a week reduced the possibility of the occurrence of potential side effects of the drug. Although the experiment was not randomly controlled, other studies also confirmed these findings. Similar significant effects were obtained by injecting the same dose of MTX intramuscularly.[26]
In addition, as a surgeon, the author noted that 2 of the 11 patients who underwent separation of symblepharon combined with AMT in other hospitals before the treatment for uncontrolled conjunctival inflammations and cicatrization accelerated the disease progression after treatment. Similar type of cases has been reported in foreign literature.[27,28] Therefore, author of this study believed that controlling and stabilizing systemic diseases were necessary conditions for surgical treatment.
In view of the rarity of OCP condition, slow and variable progression, as well as tendency of its occurrence in the elderly, design of this study and difficulty in accomplishing the goals increased. A retrospective, uncontrolled case series similar to our study was limited by its design and small sample size, which inherently involved significant recruitment bias. Advantages of these studies (as our study) include complete records of information, and each patient was evaluated and tracked by the same researcher. We expected to perform prospective comparative study using larger sample size and long-term follow-up to evaluate the efficacy and safety of MTX and other systemic agents on OCP.
Early diagnosis and timely treatment are important factors to fully control the development of OCP. In this study, initial therapy using MTX effectively controlled the activity of conjunctival inflammation and inhibited the progression of cicatrization. Therefore, even for patients with advanced OCP, this regimen can still effectively reverse the ocular surface keratinization preventing rapid progression of the disease. Present study results suggested that oral administration of low-dose MTX each week effectively treated OCP patients and easy to tolerate. Thus, it can be considered in the treatment of patients with advanced OCP.
Author contributions
Conceptualization: Chen Xie, Yuan He, Huifeng Liu, Binliang Zhu, Jiang Zhu.
Data curation: Chen Xie, Yuan He, Huifeng Liu, Jiang Zhu.
Formal analysis: Chen Xie, Yewen Shi, Yuan He, Huifeng Liu.
Investigation: Binliang Zhu, Jiang Zhu.
Methodology: Yuan He, Huifeng Liu.
Project administration: Binliang Zhu, Jiang Zhu.
Resources: Jiang Zhu.
Software: Chen Xie.
Supervision: Chen Xie, Yuan He, Huifeng Liu, Binliang Zhu.
Validation: Yuan He, Huifeng Liu, Binliang Zhu, Jiang Zhu.
Visualization: Yuan He, Huifeng Liu, Binliang Zhu, Jiang Zhu.
Writing – original draft: Chen Xie.
Writing – review & editing: Chen Xie, Yewen Shi, Yuan He, Jiang Zhu.
Footnotes
Abbreviations: AMT = amniotic membrane transplantation, BL = blood flow, CF = count fingers, CTX = cyclophosphamide, GE = side effects of the gastrointestinal tract, HM = automatic, IVIg = intravenous immunoglobulin, LFT = liver function test, MTX = methotrexate, OCP = ocular cicatricial pemphigoid, PL = light sense.
YS and CX contributed equally to this work as the first author.
The authors have no conflicts of interest to disclose.
References
- [1].Sobolewska B, Deuter C, Zierhut M. Current medical treatment of ocular mucous membrane pemphigoid. Ocul Surf 2013;11:259–66. [DOI] [PubMed] [Google Scholar]
- [2].Levine MR. Black EH, Nesi FA, Calvano CJ, Gladstone GJ, Levine MR. Ocular Cicatricial Pemphigoid. Smith and Nesi's Ophthalmic Plastic and Reconstructive Surgery. New York, NY: Springer New York; 2012. 355–7. [Google Scholar]
- [3].Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 2002;138:370–9. [DOI] [PubMed] [Google Scholar]
- [4].Saw VP, Dart JK. Ocular mucous membrane pemphigoid: diagnosis and management strategies. Ocul Surf 2008;6:128–42. [DOI] [PubMed] [Google Scholar]
- [5].Williams GP, Nightingale P, Southworth S, et al. Conjunctival neutrophils predict progressive scarring in ocular mucous membrane pemphigoid. Invest Ophthalmol Vis Sci 2016;57:5457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Taylor J, McMillan R, Shephard M, et al. World Workshop on Oral Medicine VI: a systematic review of the treatment of mucous membrane pemphigoid. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:161.e20–71.e20. [DOI] [PubMed] [Google Scholar]
- [7].Ahmed M, Zein G, Khawaja F, et al. Ocular cicatricial pemphigoid: pathogenesis, diagnosis and treatment. Prog Retin Eye Res 2004;23:579–92. [DOI] [PubMed] [Google Scholar]
- [8].Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc 1986;84:527–663. [PMC free article] [PubMed] [Google Scholar]
- [9].Kempen JH, Gangaputra S, Daniel E, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol 2008;146:802.e1–12.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alavi A, Lowe J, Walsh S, et al. Corticosteroid-induced hyperglycemia is increased 10-fold in patients with pemphigus. Int J Dermatol 2012;51:1248–52. [DOI] [PubMed] [Google Scholar]
- [11].McCluskey P, Chang JH, Singh R, et al. Methotrexate therapy for ocular cicatricial pemphigoid. Ophthalmology 2004;111:796–801. [DOI] [PubMed] [Google Scholar]
- [12].Saw VP, Dart JK, Rauz S, et al. Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes. Ophthalmology 2008;115:253.e1–61.e1. [DOI] [PubMed] [Google Scholar]
- [13].Kiire CA, Srinivasan S, Inglis A. Peripheral ulcerative keratitis after cataract surgery in a patient with ocular cicatricial pemphigoid. Cornea 2011;30:1176–8. [DOI] [PubMed] [Google Scholar]
- [14].Geerling G, Dart JK. Management and outcome of cataract surgery in ocular cicatricial pemphigoid. Graefes Arch Clin Exp Ophthalmol 2000;238:112–8. [DOI] [PubMed] [Google Scholar]
- [15].Weinblatt ME, Trentham DE, Fraser PA, et al. Long-term prospective trial of low-dose methotrexate in rheumatoid arthritis. Arthritis Rheum 1988;31:167–75. [DOI] [PubMed] [Google Scholar]
- [16].Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 1985;312:818–22. [DOI] [PubMed] [Google Scholar]
- [17].Sato EI. Methotrexate therapy in systemic lupus erythematosus. Lupus 2001;10:162–4. [DOI] [PubMed] [Google Scholar]
- [18].Willkens RF, Williams HJ, Ward JR, et al. Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis Rheum 1984;27:376–81. [DOI] [PubMed] [Google Scholar]
- [19].Samson CM, Waheed N, Baltatzis S, et al. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology 2001;108:1134–9. [DOI] [PubMed] [Google Scholar]
- [20].Dev S, McCallum RM, Jaffe GJ. Methotrexate treatment for sarcoid-associated panuveitis. Ophthalmology 1999;106:111–8. [DOI] [PubMed] [Google Scholar]
- [21].Shah SS, Lowder CY, Schmitt MA, et al. Low-dose methotrexate therapy for ocular inflammatory disease. Ophthalmology 1992;99:1419–23. [DOI] [PubMed] [Google Scholar]
- [22].Holz FG, Krastel H, Breitbart A, et al. Low-dose methotrexate treatment in noninfectious uveitis resistant to corticosteroids. Ger J Ophthalmol 1992;1:142–4. [PubMed] [Google Scholar]
- [23].Hervas Ontiveros A, Salom D, Espana Gregori E, et al. Methotrexate as a treatment in ocular cicatricial moderate pemphigoid. Arch Soc Esp Oftalmol 2014;89:447–9. [DOI] [PubMed] [Google Scholar]
- [24].Queisi MM, Zein M, Lamba N, et al. Update on ocular cicatricial pemphigoid and emerging treatments. Surv Ophthalmol 2016;61:314–7. [DOI] [PubMed] [Google Scholar]
- [25].Kjellman P, Eriksson H, Berg P. A retrospective analysis of patients with bullous pemphigoid treated with methotrexate. Arch Dermatol 2008;144:612–6. [DOI] [PubMed] [Google Scholar]
- [26].Culton DA, Diaz LA. Treatment of subepidermal immunobullous diseases. Clin Dermatol 2012;30:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Foster CS, Sainz De La Maza M. Ocular cicatricial pemphigoid review. Curr Opin Allergy Clin Immunol 2004;4:435–9. [DOI] [PubMed] [Google Scholar]
- [28].Trigui A, Kammoun B, Ghodhbane M, et al. [Penetrating keratoplasty in ocular cicatricial pemphigoid]. J Fr Ophtalmol 2002;25:48–51. [PubMed] [Google Scholar]


