Abstract
This study aimed to analyze the clinical manifestations of patients with pyogenic liver abscess and characteristics of pathogenic that caused their infections, in order to provide guidance for the identification of the pathogens that cause liver abscess and selection of antibiotics for treatment of this disease.
In the present study, the clinical characteristics, laboratory results, as well as the species and drug resistance of pathogens in patients with bacterial liver abscesses admitted to our hospital from January 2013 to December 2015 were retrospectively analyzed. The patients were treated by ultrasound or CT-guided percutaneous portal vein catheterization and drainage combined with intravenous infusion of antibiotics (the third-generation cephalosporins, the coformulation of carbapenem and dehydropeptidase-I inhibitors, or the coformulation of tazobactam and piperacillin).
A total of 178 patients were diagnosed with liver abscess by B ultrasound or CT. The abscesses mostly occurred in elderly male patients and patients with diabetes mellitus. The major clinical and hematological features were fever (163/178, 91.2%), single focal abscess (146/178, 82.0%), elevated white blood cell count, and percentage of neutrophils (136/178, 76.4%). A total of 102 nonrepetitive strains of bacteria were isolated, including Klebsiella pneumoniae (82 strains, 80.3%), Escherichia coli (8 strains), Pseudomonas aeruginosa (2 strains), Acinetobacter baumannii (1 strain), and Gram-positive cocci (9 strains). Susceptibility to antimicrobial drugs was determined by analyzing the minimum inhibitory concentration, and among the 8 cultured E coli strains, 5 strains that could produce extended-spectrum β-lactamase (ESBLs) were among the most commonly seen nosocomial infections. In the present study, bacterial liver abscesses were mostly community-acquired, and K pneumoniae was highly susceptive to the commonly used antibiotics. Five patients had poor outcomes due to infectious shock or the accompanying liver cancer. In other patients, after treatment, the body temperature and the inflammatory indices, such as the total white blood cell count and C-reactive protein, returned to normal levels, and the area of abscess decreased.
Most of the bacterial liver abscesses were caused by K pneumoniae, in which only a few strains exhibited resistance to the commonly used antibiotics. The use of ultrasound- or CT-guided percutaneous drainage combined with antibiotics was an appropriate way to treat the liver abscesses of these patients.
Keywords: clinical manifestation, drug resistance rate, Klebsiella pneumoniae, liver abscess
1. Introduction
Bacterial liver abscess ranked second in hepatic infectious disease and can be life-threatening. It is caused by various organisms, including Escherichia coli, Klebsiella pneumoniae, Streptococcus anginosus, and anaerobes such as Bacteroides.[1–3] Bacterial liver abscess is commonly seen in patients with liver diseases, biliary diseases, diabetes, and in patients who have undergone invasive operations.[4] Previous population-based study of pyogenic liver abscess in North America reported that the most commonly recorded bacterial infections were caused by Streptococcus species (29.5%) and E coli (18.1%).[5] However, in recent years, the incidence of bacterial liver abscess has continuously increased from 1.1/100,000 to 17.6/100,000 individuals, especially liver abscess caused by K pneumoniae.[5–8] In the United States, the incidence of bacterial liver abscess increased from 2.7 per 100,000 individuals in 1994 to 4.1 per 100,000 individuals in 2005, with an annual increase of 4.1%.[5] In Taiwan, the incidence was higher than that in the United States, and also increased from 10.83 per 100,000 individuals in 2000 to 15.45 per 100,000 individuals in 2011.[6] In Denmark, between 1977 and 2002, the incidence increased from 0.6 to 1.8 per 100,000 individuals.[7] In China, the annual incidence of bacterial liver abscess and liver abscess caused by K pneumoniae increased from 0.266% and 0.155% during the 10-year period of 1994 to 2003, to 0.448% and 0.290% in the next 11 years from 2004 to 2015, respectively.[8] Community-acquired pyogenic liver abscess caused by K pneumoniae has emerged in Taiwan and other Asian countries, although it was rarely reported in China.[9] Therefore, clinical manifestations of patients with pyogenic liver abscess and pathogenic characteristics of their infections were analyzed in this study, in order to provide suggestions for early and accurate diagnose of bacterial liver abscess, and to find the effective therapeutic methods, especially the use of the appropriate antibiotics. In this study, we analyzed the clinical and etiological characteristics of patients with bacterial liver abscess treated in our hospital from January 2013 to December 2015. We aimed to explore the clinical and pathogenic features of bacterial liver abscess more deeply and provide basic information for the prevention and treatment.
2. Methods
2.1. Patients
This retrospective study was conducted with the approval from the Ethics Committee of Nanjing First Hospital, Nanjing Medical University. Written informed consent was obtained from all participants.
A total of 178 patients with fever, chills, abdominal pain, as well as other clinical manifestations, were diagnosed with liver abscess lesions by abdominal ultrasound or CT, and positive puncture fluid culture.
The patients were treated by ultrasound or CT-guided percutaneous portal vein catheterization and drainage combined with intravenous infusion of antibiotics (the third-generation cephalosporins, the coformulation of carbapenem and dehydropeptidase-I inhibitors, or the coformulation of tazobactam and piperacillin). The course of treatment ranged within 2 to 4 weeks.
2.2. Analysis of the clinical data of the patients
Medical records of the 178 patients were acquired from the medical record inquiry system. Clinical data, laboratory data, and imaging data obtained from the medical records of these patients were summarized and analyzed.
The outcomes of the patients were categorized into 2 groups, that is, “Cure and improvement,” which meant favorable outcomes, and “Death, infectious shock or the accompanying liver cancer,” which meant that the patients had poor outcomes.
2.3. Analysis of the bacteria and their drug sensitivity
A total of 102 etiological samples isolated from the pyogenic liver abscesses of the patients were retrospectively analyzed, and drug sensitivity rates of the pathogens were also calculated.
The pus culture and drug sensitivity test were routine laboratory work to identify the pathogens that caused the liver abscess. Pus was directly inoculated on blood agar and China blue plate culture, and bacteria were identified using the VITEK II microbial identification system (bioMérieux, Rhône-Alpes, France).
The drug sensitivity of the bacteria was determined by analyzing the minimum inhibitory concentration using the drug sensitive card (bioMérieux). The selection of antibiotics and the interpretation of the drug sensitivity results was based on American Clinical and Laboratory Standards Institute recommendations.[10,11] These results were analyzed using Whonet 5.6.
2.4. Statistical analysis
Statistical analysis was performed using the SPSS (version 22.0) statistical software package. The χ2 test was used to evaluate the differences in the categorical variables. The Student t test was used to evaluate the differences in the continuous variables. P < .05 was considered statistically significant in all analyses.
3. Results
3.1. Clinical features of the patients
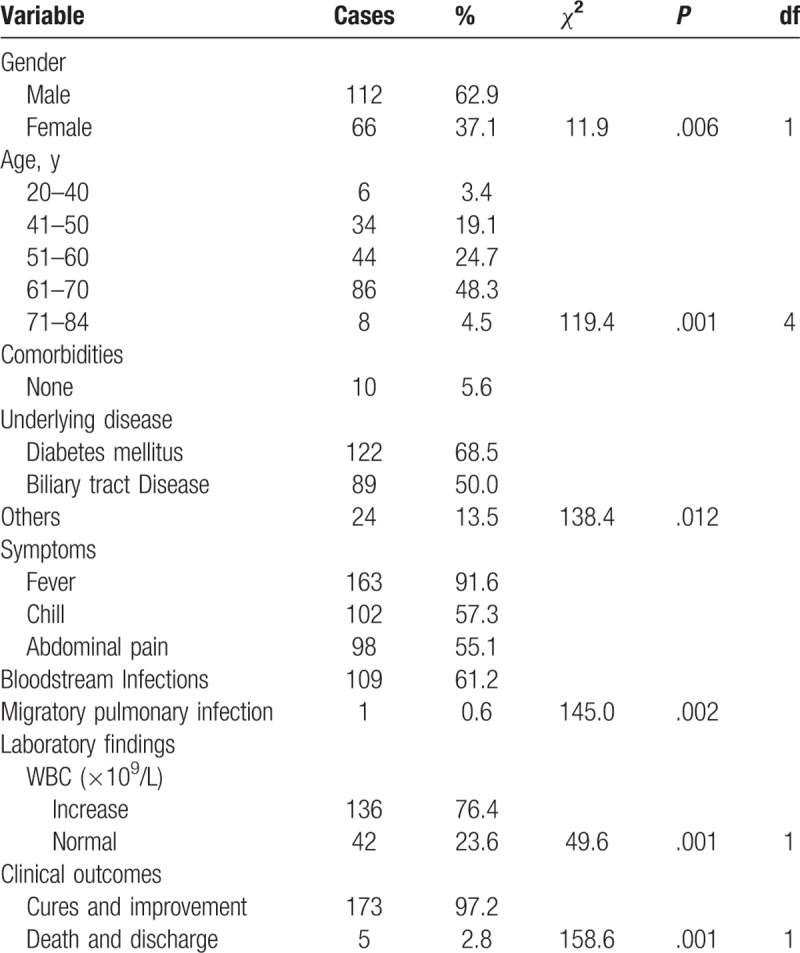
In this retrospective study, among the 178 cases, 112 cases were male, 66 cases were female. The age of these patients ranged from 22 to 84 years (average age: 52.1 ± 15.4 years), and the ages of most patients were within 40 to 70 years (164/178, 92.1%). Furthermore, the samples of 102 patients were positive for bacteria in the pus culture.
The most common clinical and hematological features were fever (163/178, 91.2%), single abscess (146/178, 82.0%), elevated white blood cell (WBC) count and increased percentage of neutrophils (136/178, 76.4%). Among these patients, a total of 145 patients (81.5%) had diabetes mellitus. The proportion of patients with diabetes mellitus differed significantly from the patients without diabetes mellitus (Chi-square value 24.472, P < .05).
Five cases had aggravated conditions, including death, infectious shock and the accompanying liver cancer, while others were getting better or were discharged from the hospital (Table 1). Migratory pulmonary lesions were found in 1 patient.
Table 1.
Demographic characteristics and clinical features of 102 patients with PLA.
3.2. Outcomes of the patients
Among the 178 patients, 5 patients had poor outcomes due to infectious shock or the accompanying liver cancer (the “Death, infectious shock or accompanying liver cancer” group). After appropriate antimicrobial treatment (the third-generation cephalosporins, the coformulation of carbapenem and dehydropeptidase-I inhibitors, or the coformulation of tazobactam and piperacillin), assisted by puncture drainage or surgical treatment guided by ultrasound, the body temperature of the 173 patients (the “Cure and improvement” group) returned to normal levels. Ultrasonic reexamination revealed that the area of abscess decreased, and the inflammatory indices, such as the total white blood cells count and C-reactive protein, returned to normal levels, and the outcome was thus good.
3.3. Analysis of the pathogens and results
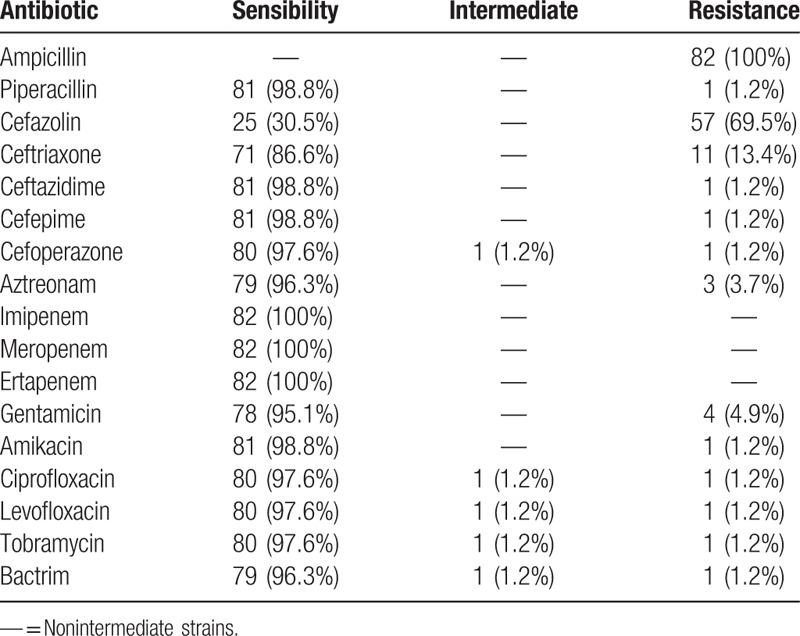
A total of 102 nonrepetitive strains were isolated from 178 cases, including 82 strains of K pneumoniae (80.3%), 8 strains of E coli, 2 strains of Pseudomonas aeruginosa, one strain of Acinetobacter baumannii, and 9 strains of Gram-positive cocci. Susceptibility of these bacteria to antibiotics was determined by analyzing the minimal inhibitory concentration of the antibiotics, and the results revealed that 5 strains of E coli produced extended-spectrum β-lactamases (ESBLs), which were among the most commonly seen nosocomial infections. These results indicate that the most common organism identified from the liver abscess aspiration cultures was K pneumoniae. The drug resistance of the 82 strains of K pneumoniae is shown in Table 2. In addition, in 78 patients (95.1%), the empirical use of antibiotics matched with the sensibility of the bacteria to the antibiotic, which proved that the empirical treatment was adequate in most patients.
Table 2.
The drug resistance of 82 strains of Klebsiella pneumoniae.
4. Discussion
Bacterial liver abscess is a disease that is potentially life-threatening. Since the liver has double blood supplies, that is, the hepatic artery and the portal vein, the portal vein, and the gastrointestinal tract are connected, leading to increased chance of pathogenic invasion of the liver and the subsequent inflammation of the liver parenchyma as well as necrosis of the hepatic tissue. With the use of antibiotics and immunosuppressants, the aging of the population, as well as the increasing kinds and the frequent use of medical equipment, the incidence of bacterial liver abscess continues to increase.[12] This retrospective study suggests that compared with other patients, patients with diabetes mellitus may be more prone to get bacterial liver abscess, as the proportion of these patients differed significantly from the patients without diabetes mellitus. In addition, we found that K pneumoniae was the main pathogen that caused the bacterial liver abscess, compared with E. coli. The result was similar to previous studies.[4,12] The major manifestations of patients with bacterial liver abscess included fever, chills and pain caused by the abscess. In addition, one case of metastatic infection to the lung was found in this study. It has been reported that the invasion by the highly pathogenic K pneumoniae could lead to poor outcomes, such as endophthalmitis, meningitis, and necrotizing fasciitis.[13–15] In our research, 5 patients had poor outcomes due to septic shock or the accompanying liver cancer, while other patients had good outcomes and were cured after antibiotics treatment combined with percutaneous drainage or surgery, as shown by the indicators that became normal after treatment, such as normal body temperature, reduced area of abscess, normal levels of WBC and C-reactive protein. Ultrasound-guided percutaneous portal vein catheterization is a simple and effective treatment for bacterial liver abscess with minimal invasiveness.[16] With the use of ultrasound-guided puncture technique in the treatment of liver abscess, very few patients need surgical resection of the hepatic abscesses. This study found that the K pneumoniae that caused the bacterial liver abscess was less resistant to antibiotics than E coli. However, in a case reported in China, the enterobacteriaceae that caused the bacterial liver abscess was resistant to carbapenems and was also capable of producing ESBLs.[17,18] Clinically, immediately after collecting the specimens from the patients, we should properly preserve them, and isolate the pathogenic bacteria and carry out drug sensitivity tests as soon as possible, in order to obtain the best drug sensitivity result and thus provide correct guidance for choosing the appropriate antibiotics. In the present study, the empirical treatment was proved to be adequate to most patients, which was due to the correct decision of the physicians and the accurate etiological identification and susceptibility results. But there are still some limitations in our study. First, in this retrospective study, we failed to carry out further molecular analysis of the pathogens. Second, we did not explore the virulence of the K pneumoniae, as well as the relationship between the pathogens and the clinical manifestations.
Author contributions
Conceptualization: Wen-Jing Wang, Zhen Tao.
Data curation: Wen-Jing Wang, Hui-Ling Wu.
Formal analysis: Wen-Jing Wang.
Investigation: Wen-Jing Wang, Hui-Ling Wu.
Methodology: Wen-Jing Wang, Zhen Tao.
Project administration: Zhen Tao.
Resources: Hui-Ling Wu.
Software: Hui-Ling Wu.
Supervision: Zhen Tao.
Writing – original draft: Wen-Jing Wang.
Writing – review & editing: Zhen Tao, Hui-Ling Wu.
Footnotes
Abbreviation: ESBLs = extended-spectrum β-lactamase.
Ethic statement: This study complied with ethical standards.
The authors have no conflicts of interest to disclose.
References
- [1].Chan DS, Archuleta S, Llorin RM, et al. Standardized outpatient management of Klebsiella pneumoniae liver abscesses. Int J Infect Dis 2013;17:e185–8. [DOI] [PubMed] [Google Scholar]
- [2].Rahimian J, Wilson T, Oram V, et al. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004;39:1654–9. [DOI] [PubMed] [Google Scholar]
- [3].Tsai FC, Huang YT, Chang LY, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 2008;14:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol 2005;100:322–31. [DOI] [PubMed] [Google Scholar]
- [5].Meddings L, Myers RP, Hubbard J, et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol 2010;105:117–24. [DOI] [PubMed] [Google Scholar]
- [6].Tseng CW, Chen YT, Lin CL, et al. Association between chronic pancreatitis and pyogenic liver abscess: a nationwide population study. Curr Med Res Opin 2017;33:505–10. [DOI] [PubMed] [Google Scholar]
- [7].Jepsen P, Vilstrup H, Schønheyder HC, et al. A nationwide study of the incidence and 30-day mortality rate of pyogenic liver abscess in Denmark, 1977–2002. Aliment Pharmacol Ther 2005;21:1185–8. [DOI] [PubMed] [Google Scholar]
- [8].Qian Y, Wong CC, Lai S, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep 2016;6:38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fung CP, Chang FY, Lee SC, et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 2002;50:420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fourth Informational Supplement. Wayne, PA: CLSI; 2013. [Google Scholar]
- [11].Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fourth Informational Supplement. Wayne, PA: CLSI; 2015. [Google Scholar]
- [12].Liu Y, Wang JY, Jiang W. An increasing prominent disease of Klebsiella pneumoniae liver abscess: etiology, diagnosis, and treatment. Gastroenterol Res Pract 2013;2013:258514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Siu LK, Yeh KM, Lin JC, et al. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012;12:881–7. [DOI] [PubMed] [Google Scholar]
- [14].Keynan Y, Karlowsky JA, Walus T, et al. Pyogenic liver abscess caused by hypermucoviscous Klebsiella pneumoniae. Scand J Infect Dis 2007;39:828–30. [DOI] [PubMed] [Google Scholar]
- [15].Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 2013;4:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu S, Wang Y, Chen J, et al. Application of ultrasound-guided percutaneous intrahepatic portal vein catheterization with antibiotic injection for treating unliquefied bacterial liver abscess. Hepatol Res? 2017;47:E187–92. [DOI] [PubMed] [Google Scholar]
- [17].Li W, Sun G, Yu Y, et al. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis 2014;58:225–32. [DOI] [PubMed] [Google Scholar]
- [18].Decré D, Verdet C, Emirian A, et al. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J Clin Microbiol 2011;49:3012–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


