Abstract
High expression of programmed death receptor 1 (PD-1) and its ligand (PD-L1) by leukocytes in primary classical Hodgkin lymphoma (cHL) is associated with inferior outcome. However, it is unclear how expression varies during disease progression, and in the event of relapse. Our aim was to study PD-1 and PD-L1 in consecutive biopsies from untreated and treated cHL patients. We screened pathology registries from 3500 cHL patients. Eleven patients had a diagnostic cHL biopsy and a previous benign lymph node biopsy reclassified as cHL when reviewed and designated as the untreated. Thirty patients had a primary and a relapse biopsy, designated as the treated. Biopsies were immunostained to detect PD-1+ and PD-L1+ leukocytes, and PD-L1+ tumor cells. In the untreated, none of the markers were statistically significantly different when biopsies 1 and 2 were compared. In the treated, 19, 22, and 18 of 30 cases had increased proportions of PD-1+ leukocytes, PD-L1+ leukocytes and PD-L1+ tumor cells, respectively, and were all statistically significantly increased when primary and relapse biopsies were compared. PD-1 and PD-L1 most likely increase due to primary treatment with chemotherapy and radiotherapy, which could have implications regarding treatment with PD-1 inhibitors.
Introduction
Classical Hodgkin lymphoma (cHL) has an overall good prognosis, but a proportion of patients suffer from refractory disease or relapse, with an increased risk of early death[1]. Programmed death receptor 1 (PD-1) and its ligand (PD-L1) are expressed to variable degrees in inflammatory conditions[2, 3] and malignancies[4–6]. Checkpoint inhibitors targeting the PD-1 pathway are effective treatment options in relapsed and refractory cHL[7, 8], but in first line the efficacy of these drugs is unknown. Consequently, the biological dynamicity of the PD-1 pathway in cHL during disease progression needs to be described. Patients with primary cHL with high proportions of both PD-1+ and PD-L1+ leukocytes have inferior outcome[9], but nothing has been published about how expression of PD-1 and PD-L1 evolves over time and when primary and relapse biopsies are compared.
cHL is characterized by a tumor microenvironment that consists of a variable infiltration of different leukocytes and few malignant Hodgkin and Reed-Sternberg (HRS) cells[10, 11]. This is a defining feature[12], which distinguishes cHL from other malignancies. Only a few studies have investigated how PD-1 and PD-L1 changes over time in other untreated malignant tumors. Higher proportions of PD-1+ macrophages in colorectal carcinoma have been associated with a longer disease duration[13]. In a previous study from our research group, there was a tendency for patients with cHL with high proportions of PD-L1+ leukocytes to present with advanced tumor stage[9]. It has never been studied whether PD-1 and PD-L1 are heterogeneously expressed in paired biopsies from patients with untreated cHL.
The PD-1 pathway may also become upregulated due to longer disease duration and following anti-cancer treatment in relapsed malignancies[13, 14]. Increased proportions of PD-L1+ tumor cells in non-small cell lung cancer (NSCLC)[15], and PD-1+ lymphocytes in multiple myeloma[16] were observed at relapse compared to primary diagnosis. It is unclear to what extent the PD-1 pathway changes in cHL when primary and relapse samples are compared.
Our aim was to determine how expression of PD-1 and PD-L1 develops both during disease progression in a unique material of untreated patients, and in primary treated patients that relapsed. Additionally, we wanted to explore whether expression of PD-1 and PD-L1 in relapse samples was related to survival after relapse. Our hypothesis was that higher proportions of PD-1+ and PD-L1+ leukocytes and PD-L1+ HRS cells would be associated with longer disease duration and relapse.
Materials and methods
Patients and tissue samples
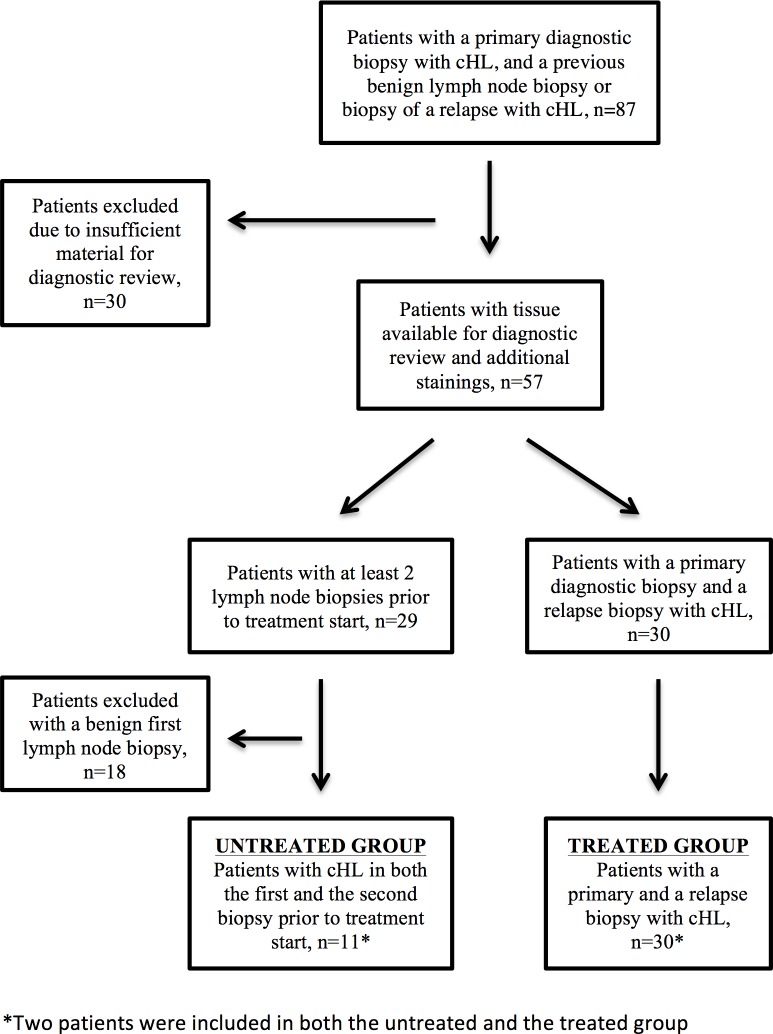
Pathology records during the years 1980–2015 in three Swedish referral pathology departments (Uppsala, Umeå and Lund) were screened for all cHL cases (n = 3500). Among them, 87 patients diagnosed with primary cHL that had at least one lymph node biopsy taken prior to the cHL diagnostic date were identified. Sections and formalin-fixed, paraffin embedded (FFPE) tissue blocks from these patients were collected and cases with remaining tissue were investigated (Fig 1). The diagnosis of cHL requires the microscopic evaluation of the tumor-engaged tissue[17]. Following primary diagnosis, patients are promptly treated[1]. It is therefore difficult to identify untreated patients with more than one biopsy with primary cHL. To address how PD-1 and PD-L1 varies in repeated biopsies in untreated patients with cHL, we reviewed the lymph node biopsies originally classified as non-malignant in patients that later developed cHL, with the intent that some non-malignant biopsies would be reclassified as cHL. Patients with at least two lymph node biopsies with cHL prior to start of treatment provided 11 paired cases. Patients with a biopsy at the primary diagnosis and a biopsy after primary treatment at relapse provided 30 paired cases (Fig 1).
Fig 1. Flowchart of patients included in the study for the untreated and the treated group.
Treatment
Patients were treated according to national guidelines, which generally included chemotherapy with or without radiotherapy (Table 1). Since the material included patients diagnosed with primary cHL over a long time period (1980–2015), chemotherapy regimens differed (Table 1). Relapsed disease was treated with high-dose chemotherapy and autologous stem cell transplantation (ASCT) in young patients with limited comorbidity.
Table 1. Clinical characteristics of patients in the treated and the untreated group.
| Untreated group, n (%) | Treated group, n (%) | |
|---|---|---|
| All patients | 11 (100) | 30 (100) |
| Age* | ||
| ≥45 years | 5 (45) | 11 (37) |
| <45 years | 6 (55) | 19 (63) |
| Sex | ||
| Male | 8 (73) | 18 (60) |
| Female | 3 (27) | 12 (40) |
| Histologic subtype | ||
| Nodular sclerosis | 6 (55) | 22 (73) |
| Mixed cellularity | 5 (45) | 8 (27) |
| Stage** | ||
| IA-IIA | 5 (45) | 11 (37) |
| IIB-IVB | 6 (55) | 17 (57) |
| Missing | 0 (0) | 2 (6) |
| Primary treatment received | ||
| Radiotherapy only | § | 3 (10) |
| 2–4 ABVD or MOPP/A(B)VD + RT | § | 5 (17) |
| 6–8 ABVD or MOPP/A(B)VD ± RT | § | 10 (33) |
| 6–8 BEACOPP | § | 3 (10) |
| Other*** | § | 7 (23) |
| Missing | § | 2 (7) |
| Autologous stem cell transplantation at relapse | ||
| Yes | § | 14 |
| No | § | 12 |
| Missing | § | 4 |
*Age at diagnosis of relapse in the treated group, and age at primary diagnosis in the untreated group.
**Stage at primary diagnosis.
***Other chemotherapy regimens were: OEPA (3 children and 1 elderly), one patient each received VACOP-B, Bendamustine and CHOP.
§Not applicable.
ABVD = Doxorubicine, Bleomycin, Vinblastine, Dacarbazine, BEACOPP = Bleomycin, Etoposide, Doxorubicine, Cyclophosphamide, Vincristine, Procarbazine, Prednisolone, CHOP = Cyclophosphamide, Hydroxydaunorubicin, Oncovin, Prednisolone, MOPP = Mustargen, Oncovin, Procarbazine, Prednisolone, OEPA = Oncovin, Etoposide, Prednisolone, Doxorubicine, VACOP-B = Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Prednisolone, Bleomycin
Histological reclassification
Examination of the cases was performed by two of the authors. Of the 87 cases, 30 had insufficient FFPE material, and were excluded from the study. Twenty-nine cases had a lymph node biopsy classified as benign prior to a biopsy with cHL, and 11 (38%) of these were re-classified as cHL in biopsy 1 and confirmed to have cHL in biopsy 2. Since these patients received no treatment after biopsy 1, this group is referred to as the untreated group. Thirty paired samples with primary and relapsed cHL were confirmed during the diagnostic review. Since these patients received treatment after their primary diagnostic biopsy, this group is referred to as the treated group. Two patients were included in both the untreated and the treated group since they had tumor material available in biopsy 1 and biopsy 2, and also a biopsy of a subsequent relapse (Fig 1).
Immunohistochemistry
As part of the diagnostic review, complementary immunohistochemical double-stainings for CD30 and paired box 5 protein (PAX5) were performed when needed. Cases were immunohistochemically stained with the Intellipath FLX automated staining system (Biocare Medical, Pacheco, CA). Single staining for PD-1 and double stainings for PD-L1/PAX5, and CD30/PAX5 were performed using 4 μm FFPE whole tumor sections. PD-1 was visualized with the mouse monoclonal antibody (mAb) NAT105/ab52587 (Abcam, Cambridge, UK), PD-L1 with the rabbit mAb E1L3N/13684 (Cell Signaling Technology, Danvers, MA), CD30 with the mouse mAb Ber-H2/IR602 (Dako, Santa Clara, CA). Two antibodies were used for PAX5 depending on whether it was double stained for PD-L1 or CD30, because the anti-PD-L1 was a rabbit antibody and the anti-CD30 was a mouse antibody. With PD-L1, PAX5 was visualized with the mouse mAb M7307/DAK-Pax5 (Dako), and with CD30, PAX5 was visualized with the rabbit polyclonal antibody ab140341 (Abcam). All antibodies were diluted 1:50. Antigen for PD-1 was retrieved in citrate buffer, and PD-L1, CD30 and both PAX5s were retrieved in TE buffer in a pressure cooker. PD-1 and CD30 were envisioned with MACH3 mouse HRP reagents (brown) (Biocare Medical), PD-L1 was envisioned with the Betazoid DAB detection kit (brown) (Biocare Medical), and both PAX5s were envisioned with the Warp Red chromogen (Biocare Medical). Finally, the sections were counterstained with Intellipath FLX´s hematoxylin.
Evaluation of PD-1 and PD-L1
The proportions of PD-1+ and PD-L1+ leukocytes, and PD-L1+ HRS cells were manually quantified in 5 high power fields (HPF) at 400x (0.0625mm2). HPFs with visible HRS cells and most prevalent infiltration of PD-1+ or PD-L1+ cells were chosen and fibrosis was avoided. Cells with at least weak membranous staining for each marker were designated as positive, while other cells were designated as negative. The proportions were calculated by dividing the number of positive leukocytes by the total number of leukocytes (positive and negative), and the number of positive HRS cells by the total number of HRS cells. To investigate whether longer disease duration affected expression of PD-1 and PD-L1 in the untreated group, cases were grouped into short vs long duration between biopsy 1 and biopsy 2. The cut-off was set at the median time between biopsy 1 and biopsy 2, which was 5 months. To elucidate the prognostic impact regarding expression of PD-1 and PD-L1 in relapsed tumor samples, we applied cut-offs for the relapsed cases in the treated group, set at the 75th percentile in order to divide patients into high vs low expression. High vs low proportion of PD-1+ leukocytes was defined as ≥5% vs <5% PD-1+ leukocytes. High vs low proportion of PD-L1+ leukocytes was defined as ≥46% vs <46% PD-L1+ leukocytes. High vs low proportion of PD-L1+ HRS cells was defined as ≥94% vs <94% PD-L1+ HRS cells.
Epstein-Barr virus status
Biopsies with available FFPE material were stained with in situ hybridization technique to detect Epstein-Barr virus (EBV)-encoded small RNAs (EBER) in HRS cells in the untreated group.
Statistical methods
The median was chosen to describe the average value for PD-1+ and PD-L1+ leukocytes, and PD-L1+ HRS cells, since we could not assume that the material was normally distributed. Wilcoxon signed-rank test was used to determine differences between continuous variables, i.e. proportions of PD-1+ and PD-L1+ leukocytes, and PD-L1+ HRS cells compared between biopsy 1 and biopsy 2 in the untreated group, and primary and relapse biopsy in the treated group. The survival outcome analyzed was time from relapse to death, defined as time from diagnosis of relapse confirmed by biopsy, to death from any cause. Survival differences between groups were calculated with Cox proportional hazards regression. A simple multivariate model included adjustment for age at relapse. Age was treated as a continuous variable. Statistics were analyzed with the R software and P values <0.05 were considered significant.
Ethics
Ethical approval was obtained by the regional ethics committee in Uppsala, Sweden according to the declaration of Helsinki (2014/020, 2014/020/1, and 2014/233). Informed consent from participants was not required according to the ethics committee. All data were fully anonymized before accessed.
Results
Untreated group
In the untreated group, the median age was 43 years. Histologically, 6 (55%) cases had nodular sclerosis (NS) and 5 (45%) cases had mixed cellularity (MC) (Table 1).
Expression of PD-1 and PD-L1 in biopsy 1 and biopsy 2 in the untreated group
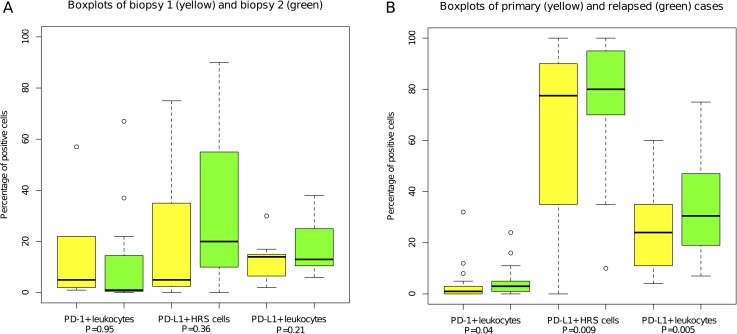
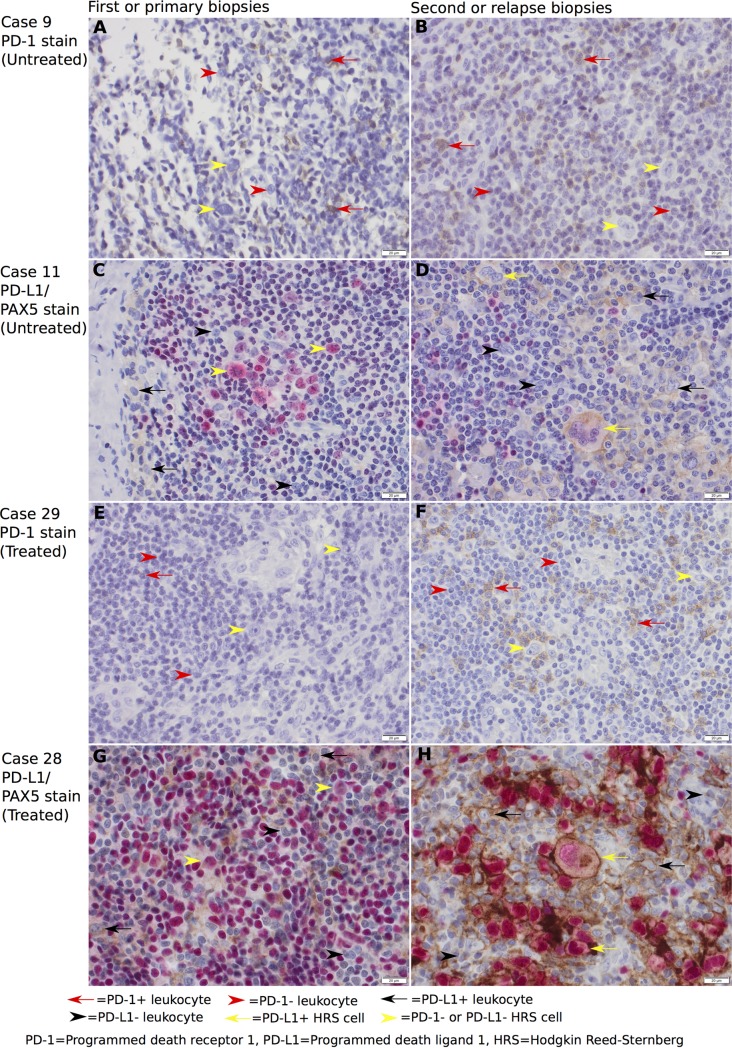
For PD-1+ leukocytes, 4 (36%) cases had an increased expression, 5 (45%) a decreased expression, and 2 (18%) remained unchanged in biopsy 2 compared to biopsy 1 (Fig 2A), median proportion was 5% in biopsy 1 and 1% in biopsy 2. For PD-L1+ leukocytes, 8 (73%) had an increased and 3 (27%) had a decreased expression in biopsy 2 compared to biopsy 1, median proportion was 14% in biopsy 1 and 13% in biopsy 2 (Fig 2A). For PD-L1+ HRS cells, 6 (55%) cases had an increased expression, 2 (18%) cases a decreased expression, and 3 (27%) remained unchanged in biopsy 2 compared to biopsy 1, median proportion was 5% in biopsy 1 and 20% in biopsy 2 (Fig 2A). With Wilcoxon signed-rank test, there were no statistically significant differences considering the median difference between biopsy 1 and biopsy 2 considering PD-1+ leukocytes (p = 0.95), PD-L1+ leukocytes (p = 0.21), or PD-L1+ HRS cells (p = 0.36). Examples of immunohistochemically stained slides with PD-1 for case 9 (Fig 3A and 3B), and with PD-L1/PAX5 for case 11 (Fig 3C and 3D) are shown in Fig 3.
Fig 2.
Boxplots of distributions of percentage of positive cells for (A) the untreated group and (B) the treated group. Wilcoxon signed-rank test P values are included comparing biopsy 1 and 2 in the untreated group, and primary and relapse biopsies in the treated group.
Fig 3.
Representative PD-1 and PD-L1/PAX5 immunohistochemical stainings at 400x magnification with untreated (A-D) and treated (E-H) patients. Brown membranous staining indicates PD-1+ or PD-L1+ cells, while red nuclear staining indicates PAX5+ cells. Untreated: (A) Case 9 biopsy 1 (average 4% PD-1+ leukocytes), (B) case 9 biopsy 2 (average 7% PD-1+ leukocytes), (C) case 11 biopsy 1 (average 5% PD-L1+ leukocytes and 0% PD-L1+ HRS cells), and (D) case 11 biopsy 2 (average 10% PD-L1+ leukocytes and 100% PD-L1+ HRS cells). Treated: (E) Case 29 primary biopsy (average 1% PD-1+ leukocytes), (F) case 29 relapse biopsy (average 16% PD-1+ leukocytes), (G) case 28 primary biopsy (average 10% PD-L1+ leukocytes and 0% PD-L1+ HRS cells), and (H) case 28 relapse biopsy (average 19% PD-L1+ leukocytes and 100% PD-L1+ HRS cells).
Differences in expression of PD-1 and PD-L1 with disease duration in the untreated group
In most cases, less than a year elapsed between biopsy 1 and biopsy 2 (median 5 months). However, case 11 showed an exceptionally long time (66 months) between the biopsies, with a marked elevation in the proportion of PD-L1+ HRS cells, from 0% in biopsy 1 to 80% in biopsy 2. All cases that expressed PD-L1 in the HRS cells in biopsy 1 also expressed PD-L1 in the HRS cells in biopsy 2 (Table 2).
Table 2. Clinicopathological characteristics and proportion of PD-1+ leukocytes, PD-L1+ leukocytes and PD-L1+ HRS cells in the untreated group.
| Case | Calendar yeara |
Time between biopsies (months)b |
Agec | EBV status | Locationd | Original diagnosise | Histologyf | PD-1+ leukocytes (%) | PD-L1+ leukocytes (%) | PD-L1 +HRS (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biopsy 1 | Biopsy 2 | Biopsy 1 | Biopsy 2 | Biopsy 1 | Biopsy 2 | ||||||||
| 1 | 1991 | 1 | 43 | Neg | Inguinal | Suspected lymphoma | MC | 57 | 67 | 30 | 9 | 5 | 20 |
| 2 | 1986 | 1 | 75 | Pos | Neck | Infectious disease | MC | 22 | 0 | 14 | 11 | 5 | 10 |
| 3 | 1990 | 2 | 42 | Pos | Abdomen | Suspected lymphoma | MC | 7 | 1 | 2 | 11 | 0 | 0 |
| 4 | 1993 | 2 | 33 | Neg | NA | Suspected lymphoma | NS | 22 | 37 | 8 | 25 | 0 | 0 |
| 5 | 2010 | 4 | 6 | Neg | Axilla | Infectious disease | NS | 2 | 1 | 15 | 33 | 5 | 20 |
| 6 | 1991 | 5 | 24 | Pos | Inguinal | Reactive lymphadenopathy | NS | 1 | 0 | 15 | 25 | 35 | 90 |
| 7 | 1997 | 7 | 32 | Neg | Neck | Pathologic, no malignancy | NS | 5 | 7 | 5 | 13 | 50 | 70 |
| 8 | 2001 | 8 | 66 | Neg | Neck | Pathologic, no malignancy | MC | 1 | 1 | 10 | 13 | 10 | 10 |
| 9 | 2013 | 8 | 47 | Neg | Abdomen | Suspected lymphoma | NS | 4 | 7 | 15 | 38 | 75 | 40 |
| 10 | 1998 | 13 | 66 | Neg | Inguinal | Reactive lymphadenopathy | MC | 22 | 22 | 17 | 6 | 35 | 10 |
| 11 | 2000 | 66 | 63 | Neg | Abdomen | Reactive lymphadenopathy | NS | 2 | 0 | 5 | 10 | 0 | 80 |
aYear of initial diagnosis of cHL.
b Time elapsed between biopsy 1 and biopsy 2 in months.
cAge at initial diagnosis of cHL.
dBiopsy 1 and biopsy 2 were taken from the same location in all cases.
eAt biopsy 1.
fAt biopsy 2.
PD-1 = Programmed death receptor 1, PD-L1 = Programmed death ligand 1, HRS = Hodgkin Reed-Sternberg, MC = Mixed cellularity, NS = Nodular sclerosis, EBV = Epstein-Barr virus, NA = Not available.
EBV is constantly expressed in the HRS cells in biopsy 1 and biopsy 2 in the untreated group
Three of 11 cases (27%) were EBV positive in the HRS cells in both biopsy 1 and 2 (Table 2). If biopsy 1 was negative or positive, the corresponding biopsy 2 was also negative or positive, respectively.
Treated group
In the treated group, the median age at primary diagnosis was 32.5 years, the median age at relapse was 36.5 years and the median time to relapse was 3 years. Histologically, 73% had NS and 27% had MC at primary diagnosis (Table 1). One had NS histology at primary diagnosis and MC at relapse, while all others had the same histology at relapse as in the primary biopsy. Following relapse, 8 (27%) patients died and median follow-up was 6.5 years (range 0.3–30.9 years).
PD-1 and PD-L1 are upregulated in the relapse biopsies compared to the primary biopsies in the treated group
More cases showed an upregulation rather than a downregulation in the relapse biopsy compared to the primary biopsy for all markers (Fig 2B). For PD-1+ leukocytes, 19 cases (63%) showed a proportional increase, 7 (23%) a decreased expression, and 4 (13%) remained unchanged in the relapse biopsy compared to the primary biopsy, median proportion was 1% in the primary biopsy and 3% in the relapse biopsy (Fig 2B). For PD-L1+ leukocytes, 22 cases (73%) had an increased expression, 7 (23%) a decreased expression, and 1 (3%) remained unchanged in the relapse biopsy compared to the primary biopsy, median proportion was 24% in the primary biopsy and 30.5% in the relapse biopsy (Fig 2B). For PD-L1+ HRS cells, 18 cases (60%) had an increased expression, 3 (10%) a decreased expression, and 9 (30%) remained unchanged in the relapse biopsy compared to the primary biopsy, median proportion was 77.5% in the primary biopsy and 80% in the relapse biopsy (Fig 2B). The increases between the primary and relapse biopsies were statistically significantly different considering PD-1+ leukocytes (p = 0.04), PD-L1+ leukocytes (p = 0.005), and PD-L1+ HRS cells (p = 0.009) (Fig 2B). In addition, we observed that all 30 (100%) relapse biopsies expressed PD-L1 on the HRS cells to some degree (>0%), while it was expressed to some degree in 26 (87%) of the primary biopsies (Table 3). Examples of immunohistochemically stained slides with PD-1 for case 29 (Fig 3E and 3F) and with PD-L1/PAX5 for case 28 (Fig 3G and 3H) are shown in Fig 3.
Table 3. Clinicopathological characteristics and proportion of PD-1+ leukocytes, PD-L1+ leukocytes and PD-L1+ HRS cells in the treated group*.
| Case | Calendar yeara |
Time between biopsiesb | Agec | EBV statusd | Biopsy location (primary/relapse) | Diagnosis (primary/relapse) | PD-1+ leukocytes (%) | PD-L1+ leukocytes (%) | PD-L1 +HRS (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary | Relapse | Primary | Relapse | Primary | Relapse | |||||||
| 1 | 2005 | 20 | 26 | NA | abdomen/abdomen | MC/MC | 1 | 3 | 19 | 19 | 100 | 100 |
| 2 | 2006 | 112 | 41 | NA | neck/neck | NS/NS | 0 | 1 | 27 | 40 | 100 | 100 |
| 3 | 2010 | 40 | 35 | NA | neck/mediastinum | NS/NS | 0 | 3 | 37 | 75 | 100 | 100 |
| 4 | 2010 | 76 | 12 | NA | neck/mediastinum | MC/MC | 0 | 8 | 41 | 48 | 100 | 70 |
| 5 | 2015 | 8 | 26 | negative | neck/neck | NS/NS | 5 | 4 | 45 | 57 | 100 | 100 |
| 6 | 1997 | 31 | 31 | NA | neck/neck | NS/NS | 0 | 1 | 40 | 31 | 95 | 100 |
| 7 | 2001 | 23 | 37 | NA | neck/neck | NS/NS | 0 | 2 | 16 | 20 | 90 | 90 |
| 8 | 1991 | 17 | 24 | NA | inguinal/NA | NS/NS | 0 | 1 | 25 | 30 | 90 | 70 |
| 9 | 2000 | 10 | 16 | NA | neck/neck | NS/NS | 1 | 1 | 31 | 29 | 90 | 90 |
| 10 | 2009 | 41 | 25 | NA | neck/neck | NS/NS | 0 | 3 | 34 | 50 | 90 | 95 |
| 11 | 2015 | 7 | 32 | negative | neck/neck | NS/NS | 3 | 5 | 38 | 12 | 90 | 90 |
| 12 | 1998 | 45 | 46 | NA | neck/neck | NS/MC | 0 | 3 | 60 | 43 | 85 | 50 |
| 13 | 2003 | 36 | 45 | NA | neck/neck | NS/NS | 1 | 0 | 26 | 29 | 80 | 80 |
| 14 | 1984 | 136 | 32 | NA | neck/paraaortal | MC/MC | 2 | 1 | 33 | 34 | 80 | 80 |
| 15 | 2007 | 15 | 55 | NA | inguinal/inguinal | MC/MC | 1 | 1 | 35 | 43 | 80 | 85 |
| 16 | 1980 | 146 | 36 | negative | neck/neck | NS/NS | 3 | 11 | 22 | 53 | 75 | 90 |
| 17 | 1981 | 72 | 27 | NA | neck/neck | NS/NS | 1 | 3 | 4 | 17 | 70 | 90 |
| 18 | 2009 | 8 | 65 | NA | axilla/NA | NS/NS | 0 | 1 | 21 | 26 | 65 | 75 |
| 19 | 2014 | 12 | 16 | NA | axilla/inguinal | NS/NS | 3 | 7 | 25 | 58 | 60 | 70 |
| 20 | 2011 | 8 | 33 | NA | neck/neck | NS/NS | 4 | 1 | 10 | 37 | 50 | 80 |
| 21 | 1981 | 64 | 16 | NA | NA/NA | MC/MC | 0 | 0 | 18 | 47 | 45 | 100 |
| 22 | 1989 | 43 | 75 | NA | inguinal/inguinal | NS/NS | 3 | 0 | 10 | 18 | 40 | 70 |
| 23 | 2014 | 17 | 64 | negative | inguinal/inguinal | NS/NS | 32 | 8 | 18 | 20 | 35 | 70 |
| 24 | 2005 | 7 | 76 | positive | neck/neck | MC/MC | 0 | 1 | 46 | 34 | 30 | 35 |
| 25 | 1986 | 36 | 29 | NA | mediastinum/neck | NS/NS | 8 | 24 | 8 | 17 | 15 | 40 |
| 26 | 1994 | 49 | 13 | NA | neck/lung | NS/NS | 12 | 2 | 8 | 7 | 10 | 40 |
| 27 | 1989 | 20 | 12 | NA | axilla/axilla | NS/NS | 0 | 5 | 4 | 11 | 0 | 80 |
| 28 | 2011 | 68 | 68 | NA | neck/neck | NS/NS | 4 | 6 | 10 | 19 | 0 | 100 |
| 29 | 1990 | 53 | 42 | NA | abdomen/NA | MC/MC | 1 | 16 | 11 | 9 | 0 | 10 |
| 30 | 1981 | 48 | 39 | NA | NA/NA | MC/MC | 3 | 3 | 23 | 73 | 0 | 80 |
aYear of primary diagnosis of cHL.
bTime elapsed between biopsy 1 and biopsy 2 in months.
cAge at primary diagnosis of cHL.
dEBV status determined with EBER at primary biopsy.
PD-1 = Programmed death receptor 1, PD-L1 = Programmed death ligand 1, HRS = Hodgkin Reed-Sternberg, MC = Mixed cellularity, NS = Nodular sclerosis, EBV = Epstein-Barr virus, NA = Not available.
*Sorted according to expression of PD-L1 by HRS cells.
PD-1 and PD-L1 in the relapse biopsy in relation to prognosis in the treated group
High proportions of PD-1+ and PD-L1+ leukocytes, and PD-L1+ HRS cells did not affect time to death after relapse in univariate or age-adjusted analyses. The only prognostic discriminator was age at relapse, which was associated with a shorter time to death after relapse, hazard ratio: 1.05 (95% confidence interval, 1.002–1.09, p = 0.04).
Discussion
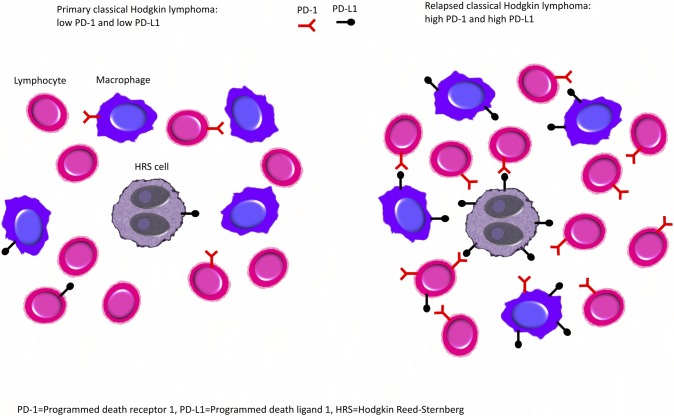
This study describes the biologic variation over time of the PD-1 pathway in cHL. In paired biopsies from previously untreated and previously treated patients with primary and relapsed cHL, respectively, we found that PD-1 and PD-L1 were upregulated in the relapsed (treated) group, and a tendency that PD-L1+ leukocytes were upregulated in the untreated group (Fig 4).
Fig 4. Visual summary of the main findings in the study.
Possible clinical applications
Upregulation of the PD-1 pathway is primarily of biologic interest, but could also be relevant when PD-1 blockade is tested as front-line treatment[18]. PD-1 blockade is only currently routinely used in refractory and relapsed cHL, with high treatment response rates[19], particularly in those with high expression of PD-L1 on HRS cells[20, 21]. In other malignancies, conventional chemotherapy upregulates expression of PD-L1 on tumor cells in cases of primary head and neck cancer[22] and lung adenocarcinoma[15]. It is unknown whether conventional front-line chemotherapy and radiotherapy upregulates expression of PD-L1 on HRS cells in cHL, however our findings with a higher expression of PD-L1 in relapsed than in primary biopsies indicate such an association. In chronic lymphocytic leukemia patients with Richter transformation, high overall expression of PD-1 and PD-L1, and prior treatment with ibrutinib resulted in a more favorable response to PD-1 inhibition[23]. The most suitable chemotherapy and radiotherapy in combination with PD-1 inhibition, and optimal sequences between chemotherapy and radiotherapy, and PD-1 inhibition should be further explored in clinical trials.
Expression of PD-1 and PD-L1 in the treated and the untreated group
Most (60–73%) patients in the treated group had a higher expression of each marker in their relapse biopsy compared to their primary biopsy, demonstrating that the PD-1 pathway appears to be upregulated at relapse in cHL. We also found that all relapse biopsies expressed PD-L1 in the HRS cells at least to some degree, which is compatible with previous studies where PD-L1 was expressed by HRS cells in all relapsed cases with cHL[7, 8]. The findings were less convincing for the untreated group; nevertheless 73% of the patients had an increased proportion of PD-L1+ leukocytes in biopsy 2 compared to biopsy 1, indicating that also disease progression itself, and not only treatment might upregulate PD-L1 expression in leukocytes. EBV was present in the HRS cells in 3 cases in the untreated group, but there was no change in EBV status when biopsy 1 and biopsy 2 were compared.
Mechanism for upregulation of PD-L1 on HRS cells
An upregulation of PD-L1 on HRS cells at relapse might include several mechanisms, mainly genetic alterations following anti-cancer treatment, but may also reflect longer disease duration. As mentioned previously, how contemporary front-line chemotherapy regimens alter the PD-1 pathway in cHL has never been investigated earlier. However, treatment has been associated with an increased expression of PD-L1 on tumor cells in other malignancies. In a mouse breast cancer model, radiotherapy upregulated expression of PD-L1 on tumor cells[24]. In a colorectal cancer cell line, 5-fluorouacil upregulated expression of PD-L1 on tumor cells[25] in primary tumors. HRS cells frequently harbor copy gains in the gene 9p24.1, which is associated with expression of the PD-1 ligands on the HRS cells[26]. It is known that chemotherapy might affect gene status in NSCLC[27]. Primary treatment might induce genetic alterations in the 9p24.1 gene, which could explain the higher proportion of PD-L1+ HRS cells at relapse.
Mechanism for upregulation of PD-L1 on leukocytes
cHL is characterized by an extensive inflammatory infiltrate consisting of various leukocytes[28]. In malignant diseases, PD-1 ligands become upregulated on leukocytes in the tumor microenvironment by various pro-inflammatory cytokines, e.g. interferon γ, interleukin 10, and tumor necrosis factor α, produced by tumor cells and other leukocytes such as T cells[3, 29–31]. Thus, a more pro-inflammatory tumor milieu is a plausible explanation for our findings, i.e. a higher proportion of PD-L1+ leukocytes in the microenvironment in relapse biopsies in the treated group, and an indication of a higher proportion of PD-L1+ leukocytes in the untreated group. In addition, in the mouse breast cancer model referred to above, radiotherapy upregulated expression of PD-L1 on dendritic cells[24]. Thus, treatment also likely induces a higher proportion of PD-L1+ leukocytes, as we noted in the treated group.
Mechanism for upregulation of PD-1 on leukocytes
PD-1 is expressed by different activated leukocytes (e.g. lymphocytes, dendritic cells, and macrophages), and is upregulated on T cells following persistent exposure to antigens[3, 32]. This was also seen in the study on the colorectal cancer mouse model[13], where PD-1+ macrophages were upregulated following longer disease duration without treatment. In addition, patients with relapsed multiple myeloma had a more pronounced infiltration of PD-1+ lymphocytes after than before treatment[16]. Increased proportions of PD-1+ leukocytes in relapsed cases in the treated group might thus be a result of persistent or previous exposure to antigens.
Altered expression of PD-1 and PD-L1 related to treatment or time?
To summarize the previous paragraphs: upregulation of PD-1 and PD-L1 can be explained by a more pronounced inflammatory microenvironment following longer disease duration, changed microenvironmental milieu in the relapsed setting, or the primary treatment. The median time between the primary and the relapse biopsy in the treated group was considerably longer than the median time between biopsy 1 and biopsy 2 in the untreated group, 36 and 5 months, respectively. Thus, it is difficult to determine whether it is the treatment, disease progression, altered conditions in the relapsed microenvironment, or all combined that are most important for the upregulation of PD-1 and PD-L1.
PD-1 and PD-L1 importance for survival in relapsed cases
In a previous study, we found that high proportions of PD-1+ and PD-L1+ leukocytes in primary tumors with cHL were associated with inferior outcome, while expression of PD-L1 on HRS cells had no impact on outcome[9]. In the current study, no association with survival according to high vs low expression of PD-1+ and PD-L1+ leukocytes, and PD-L1+ HRS cells was found. However, only 27% of the patients in our cohort died, thus a considerably lower proportion in comparison with a large retrospective Swedish cohort where approximately 50% of the patients died following relapse[1]. Re-biopsied patients are probably more prone to receive treatment with curative intent, than patients for whom a new biopsy of a suspected relapse is not performed. Thus, our material likely favored to mainly include young and healthy individuals with little comorbidity.
Methodological considerations for immunohistochemical stainings
Both lymphocytes and antigen presenting cells (i.e. macrophages, monocytes and dendritic cells) are able to express both PD-1 and PD-L1 in malignancies[32] Since it is troublesome to differentiate large lymphocytes from small macrophages and monocytes based solely on morphologic examination, we decided to adapt a summary measure and term them as leukocytes. However, it is known that PD-1 is mostly expressed by T-cells, and PD-L1 is mostly expressed by macrophages in cHL[29]. This seemed true in our material in general when manually evaluated, which is also exemplified in Fig 3 where PD-1 is mainly expressed by smaller lymphocytes, and PD-L1 is mainly expressed by larger macrophages. Hence, our PD-1 findings are mostly based on expression by lymphocytes while PD-L1 findings are mostly based on expression by macrophages.
Methodological considerations for the untreated group
Core needle biopsies may contain insufficient material to diagnose lymphomas[33]. However, for the untreated group, only biopsy 1 for case 3 and 4, and biopsy 2 for case 10 (Table 2) were core needle biopsies, while the rest were whole lymph node excisions. Hence, in most cases the original misclassification is probably not explained by inadequate material provided in biopsy 1. HRS cells have a distinct immunohistochemical profile, being positive for CD30 and PAX5[17]. For biopsy 1, only 2 cases were examined with immunohistochemical markers in the original diagnostic process. Most of the cases not examined with immunohistochemistry were diagnosed prior to the year 2000 (Table 2), when immunohistochemistry was less commonly used. We reclassified 38% of the first biopsies as cHL, likely explained by our utilization of immunohistochemical markers in combination with a confirmatory biopsy with cHL in biopsy 2, which simplified our review procedure compared to the original diagnostic procedure.
Strengths and limitations
To our knowledge, no previous publication has investigated the expression of PD-1 and PD-L1 in repeated biopsies in patients with cHL. However, due to treatment recommendations advocating immediate treatment in HL, only 11 cases had consecutive biopsies where patients remained untreated, despite screening 3500 cases. This limited the statistical power and our results should be viewed as hypothesis generating. For all patients in the untreated group, both biopsy 1 and biopsy 2 were taken from the same location, minimizing the bias of possibly different composition of the microenvironment in the same patient between different localizations. The unique material and design of our study make an important knowledge contribution regarding immune checkpoint regulation in cHL.
Conclusions
This is the first study to describe that PD-1 and PD-L1 are upregulated in relapsed cHL compared to primary diagnosis, and the tendency that PD-L1+ leukocytes are upregulated with time in untreated cHL. This further adds knowledge about the complex inflammatory infiltrate orchestrated by the PD-1 pathway in cHL. Future studies are warranted to elucidate whether it is longer disease duration, primary treatment, or altered conditions in the relapse microenvironment that upregulate the PD-1 pathway. However, based on numerous previous studies[15, 16, 23–25], PD-1 and PD-L1 are most likely upregulated due to previous treatment with chemotherapy and radiotherapy, which could have implications regarding treatment with PD-1 inhibition in cHL.
Supporting information
(XLSX)
(XLSX)
Acknowledgments
We want to thank Justina Damjanovic Vesterlund and Svetlana Popova for help with immunohistochemical stainings, and Åsa Håkansson for her help with the EBER stainings, all at Uppsala University Hospital, Uppsala, Sweden. We also want to thank all of the participating pathology departments for providing the tissue material.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Lions Cancer Research Fund, Uppsala, Sweden and the Swedish Cancer Society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Glimelius I, Diepstra A. Novel treatment concepts in Hodgkin lymphoma. Journal of internal medicine. 2017;281(3):247–60. 10.1111/joim.12582 . [DOI] [PubMed] [Google Scholar]
- 2.Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. The Journal of experimental medicine. 2007;204(10):2321–33. 10.1084/jem.20062150 ; PubMed Central PMCID: PMCPMC2118444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. The New England journal of medicine. 2016;375(18):1767–78. 10.1056/NEJMra1514296 ; PubMed Central PMCID: PMCPMC5575761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muenst S, Soysal SD, Tzankov A, Hoeller S. The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Targets. 2015;19(2):201–11. 10.1517/14728222.2014.980235 . [DOI] [PubMed] [Google Scholar]
- 5.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer research. 2015;75(11):2139–45. 10.1158/0008-5472.CAN-15-0255 ; PubMed Central PMCID: PMCPMC4452411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14(4):203–20. 10.1038/nrclinonc.2016.168 . [DOI] [PubMed] [Google Scholar]
- 7.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England journal of medicine. 2015;372(4):311–9. 10.1056/NEJMoa1411087 ; PubMed Central PMCID: PMC4348009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;(31):3733–9. 10.1200/JCO.2016.67.3467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollander P, Kamper P, Smedby K-E, Enblad G, Ludvigsen M, Mortensen J, et al. High proportions of PD-1+ and PD-L1+ leukocytes in classical Hodgkin lymphoma microenvironment are associated with inferior outcome. Blood advances. 2017;1(18):1427–39. 10.1182/bloodadvances.2017006346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu R, Sattarzadeh A, Rutgers B, Diepstra A, van den Berg A, Visser L. The microenvironment of classical Hodgkin lymphoma: heterogeneity by Epstein-Barr virus presence and location within the tumor. Blood Cancer J. 2018;8(1):e622 10.1038/bcj.2017.102 ; PubMed Central PMCID: PMCPMC5819328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollander P, Rostgaard K, Smedby KE, Molin D, Loskog A, de Nully Brown P, et al. An anergic immune signature in the tumor microenvironment of classical Hodgkin lymphoma is associated with inferior outcome. European journal of haematology. 2018;100(1):88–97. 10.1111/ejh.12987 . [DOI] [PubMed] [Google Scholar]
- 12.Kuppers R, Engert A, Hansmann ML. Hodgkin lymphoma. J Clin Invest. 2012;122(10):3439–47. 10.1172/JCI61245 ; PubMed Central PMCID: PMCPMC3534167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–9. 10.1038/nature22396 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21(4):687–92. 10.1158/1078-0432.CCR-14-1860 ; PubMed Central PMCID: PMCPMC4334715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Li H, Pang R, Huang J. Altered status of programmed death-ligand 1 after recurrence in resected lung adenocarcinoma patients. Onco Targets Ther. 2017;10:2003–7. 10.2147/OTT.S127498 ; PubMed Central PMCID: PMCPMC5388238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paiva B, Azpilikueta A, Puig N, Ocio EM, Sharma R, Oyajobi BO, et al. PD-L1/PD-1 presence in the tumor microenvironment and activity of PD-1 blockade in multiple myeloma. Leukemia. 2015;29(10):2110–3. 10.1038/leu.2015.79 . [DOI] [PubMed] [Google Scholar]
- 17.Poppema S PS, Delsol G, Stein H, Weiss LM, Swerdlow SH, Warnke RA, Jaffe ES. Hodgkin Lymphoma In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. World Health Organization. Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4 th ed. 2007, Lyon, France: IARC Press; 2008. 321–334. [Google Scholar]
- 18.Gay ND, Okada CY, Chen AI, Scott EC. Targeting the programmed cell death 1 pathway in Hodgkin lymphoma: the place of nivolumab. Ther Adv Hematol. 2017;8(5):175–80. 10.1177/2040620717695723 ; PubMed Central PMCID: PMCPMC5407507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansell SM. Nivolumab in the Treatment of Hodgkin Lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23(7):1623–6. 10.1158/1078-0432.CCR-16-1387 . [DOI] [PubMed] [Google Scholar]
- 20.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–94. 10.1016/S1470-2045(16)30167-X ; PubMed Central PMCID: PMCPMC5541855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roemer MGM, Redd RA, Cader FZ, Pak CJ, Abdelrahman S, Ouyang J, et al. Major Histocompatibility Complex Class II and Programmed Death Ligand 1 Expression Predict Outcome After Programmed Death 1 Blockade in Classic Hodgkin Lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2018;36(10):942–50. 10.1200/JCO.2017.77.3994 ; PubMed Central PMCID: PMCPMC5877802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leduc C, Adam J, Louvet E, Sourisseau T, Dorvault N, Bernard M, et al. TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open. 2018;3(1):e000257 10.1136/esmoopen-2017-000257 ; PubMed Central PMCID: PMCPMC5761289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129(26):3419–27. 10.1182/blood-2017-02-765685 ; PubMed Central PMCID: PMCPMC5492091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95. 10.1172/JCI67313 ; PubMed Central PMCID: PMCPMC3904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Der Kraak L, Goel G, Ramanan K, Kaltenmeier C, Zhang L, Normolle DP, et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. J Immunother Cancer. 2016;4:65 10.1186/s40425-016-0163-8 ; PubMed Central PMCID: PMCPMC5067917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(23):2690–7. 10.1200/JCO.2016.66.4482 ; PubMed Central PMCID: PMCPMC5019753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai H, Wang Z, Chen K, Zhao J, Lee JJ, Wang S, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(25):3077–83. 10.1200/JCO.2011.39.3744 ; PubMed Central PMCID: PMCPMC5321076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vardhana S, Younes A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica. 2016;101(7):794–802. 10.3324/haematol.2015.132761 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey CD, Gusenleitner D, Lipschitz M, Roemer MGM, Stack EC, Gjini E, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood. 2017;130(22):2420–30. 10.1182/blood-2017-03-770719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61–73. 10.1016/j.immuni.2013.07.005 ; PubMed Central PMCID: PMCPMC3782392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8(2):2171–86. doi: 10.18632/oncotarget.13895 ; PubMed Central PMCID: PMCPMC5356790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdulla M, Laszlo S, Triumf J, Hedstrom G, Berglund M, Enblad G, et al. Core needle biopsies for the diagnosis of diffuse large B-cell lymphoma—a great concern for research. Acta oncologica. 2017;56(1):106–9. 10.1080/0284186X.2016.1245863 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.