Abstract
Ambulatory blood pressure monitoring (ABPM) correlates more closely to organ damages than clinic blood pressure (BP). In the current study we aimed to investigate the association between micro- and macrovascular complications of diabetes and both diurnal and nocturnal variability in BP.
A total of 192 patients with type 2 diabetes (T2DM) who had complete data on ABPM were selected. BP categories were defined based on 2017 ACC/American Heart Association BP guideline. The cross-sectional association between different BP phenotypes and diabetes complications including cardiovascular disease (CVD), nephropathy, retinopathy, and neuropathy was assessed using multiple logistic regression models adjusted for age, sex, body mass index, hypertension (HTN), hemoglobin A1c, fasting blood glucose (FBG), triglyceride (TG), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and total cholesterol.
Approximately 48.9% of participants with T2DM had 24-hour HTN. The prevalence of daytime, nighttime, and clinic HTN were 35.9%, 96.3%, and 53.1%, respectively. Approximately 54.2% of participants had nondipping nocturnal pattern and 28.6% were risers. Nondipping nocturnal BP was associated with CVD, neuropathy, and retinopathy (P = .05, .05, and .014, respectively). Sleep trough morning blood pressure surge (MBPS) was associated with neuropathy (P = .023). Neuropathy was also associated with other components of MBPS (P < .05).
We demonstrated that diabetic neuropathy was associated with all the components of MBPS and abnormal dipping status. Our results indicated loss of nocturnal BP dipping but not MBPS as a risk factor for CVD and retinopathy in patients with T2DM. Our findings once again highlighted the importance of ambulatory BP monitoring and targeted antihypertensive therapy directed toward to restore normal circadian BP in patients with T2DM.
Keywords: ambulatory blood pressure monitoring, diabetes complications, morning surge, nocturnal dipping, type 2 diabetes
1. Introduction
Ideal 24-hour blood pressure (BP) control not only consists of control of average daily BP, but also its diurnal and nocturnal variability.[1] Most hypertensive patients are monitored by clinic-based BP measurement and their daily BP variability is not available. However, studies have shown that office BP measurement is not an ideal method for representing 24-hour BP compared with ambulatory blood pressure monitoring (ABPM).[2] For example, office monitoring has less sensitivity to predict organ damage associated with hypertension (HTN).[3–5]
Research in BP variability has led to the discovery of the normal nocturnal dipping pattern of BP. In healthy subjects, BP at night (sleep) is 10% to 20% less than diurnal (waking) BP.[6] However, abnormal BP dipping patterns either by the having less nocturnal dipping (0%–10% decrease) or even rise in BP in sleep or by having exaggerated dipping of BP at nights (>20% decrease) are associated with higher cardiovascular morbidity and mortality and damages to organs such as brain and kidney.[7–9]
Morning blood pressure surge (MBPS) is another component of BP variability, which high values have been shown to independently increase risk of cardiovascular diseases (CVDs).[10,11] There are 3 definitions for MBPS including sleep-trough surge, prewaking surge, and rising surge. Sleep trough surge is one of the dynamic diurnal surges during the specific period from sleep to early morning. Prewaking surge is the BP change occurring 4 hours before and after arising. The rising surge may detect the morning risk just after arising.[12]
Type 2 diabetes mellitus (T2DM) is a disease involving multiple organs and is known to expose patients to HTN. Approximately 35% to 75% of diabetes complications is estimated to be attributed to HTN.[13] It has been demonstrated that diabetic patients tend to have higher nocturnal BP and higher MBPS.[14] Thus, high-risk hypertensive diabetic patients are indicated for ABPM.[15]
It is known that BP variability is partly controlled by genetics and geographical temporality adjusting the body's circadian rhythm.[16] It highlights the importance of studying different populations residing different geographical latitudes to have a better understanding of underlying mechanisms. At the time of this study, there has been no report of ABPM parameters in an Iranian population.
Therefore, in this work we aimed to study different BP parameters in patients with T2DM from an Iranian population. As the impact of abnormal nocturnal BP dipping pattern and exaggerated MBPS on organs damages is emphasized numerously, here for the first time we studied the association between different BP phenotypes and presence of diabetes complications including CVD, nephropathy, retinopathy, and neuropathy in patients with T2DM.
2. Materials and methods
2.1. Study population
This is a cross-sectional study conducted in diabetes clinic of Vali-Asr Hospital affiliated with Tehran University of medical sciences. Participants with incomplete data on ABPM, missing clinic BP, and missing data on other covariates were excluded, leaving 192 participants for the final sample size. The diagnosis of diabetes was made by an internist or endocrinologist based on American Diabetes Association 2015 guideline.[17] All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Before enrollment written informed consents were taken from all participants. The ethic committee of the Tehran University of Medical Sciences approved the study protocol. The number of ethic committee approval was 9211402002.
2.2. Data collection
Demographic and anthropometric characteristics including age, weight, waist circumference, hip circumference, and height were obtained from the participants through the interview and baseline measurements. Weight was measured using Seca portable manual scale with patients wearing light clothing and was recorded to the nearest 0.1 kg. A stable stadiometer (Seca trademark) was used to measure height while the patient standing still, with no shoes or socks on, and was recorded with a precision of 0.1 cm. Waist and hip circumferences were measured using a flexible measurement tape.
Body mass index (BMI) was computed as weight in kilograms divided by height per square meter (kg/m2).
Systolic and diastolic BP measurement was performed on the arm of seated participants after 10 minutes of resting using ERKAmeter 3000 mercury sphygmomanometer at clinic. The measurement was repeated after 15 minutes and the average was reported. ABPM was performed using Tiba Ambulo 2400 BP monitor with gas-power cuff inflation. The device used oscillometric method to measure BP. The measurements were taken every 30 minutes during a 24-hour period.
Patients were instructed to fast overnight for 12 to 14 hours before blood sampling. The following morning at the diabetes clinic 10 mL of venous blood was drawn and collected from each patient while seated, sampled in cold biochemistry tubes (4°C–8°C), and sent within 4 hours to representative collaborating laboratories. Samples were then immediately centrifuged (1500 rmp for 10 minutes at standard room temperature: 21°C) and the extracted serum was used for laboratory evaluations.
Fasting blood glucose (FBG) was measured by the glucose oxidase method. Hemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography. Measurement of serum creatinine was performed by Jaffe method.[18] Plasma total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were determined using direct enzymatic method (Parsazmun, Karaj, Iran).
2.3. Outcome definitions
BP categories were defined based on 2017 American Heart Association guideline. Twenty-four-hour ABPM HTN was defined as mean systolic blood pressure (SBP) during a 24-hour period ≥125 mm Hg or mean diastolic blood pressure (DBP) ≥75 mm Hg. Daytime and nighttime HTN was defined as mean daytime SBP ≥130 mm Hg or DBP ≥80 mm Hg and mean nighttime SBP ≥110 mm Hg or DBP ≥65 mm Hg, respectively. Clinic HTN was defined as mean clinic SBP ≥130 mm Hg or DBP ≥80 mm Hg. Individuals with SBP and DBP in 2 categories were designated to the higher BP category.[19]
History of HTN was defined as SBP >140 mm Hg or DBP >90 mm Hg in at least 2 visits before the study.
2.4. Nighttime BP dipping parameters
Nighttime BP dipping (%) was calculated as (1 − SBP/average daytime SBP) × 100. Subgroup classification based on nighttime dipping was defined as extreme dipper: >20%; dipper: ≤20%, >10%; nondipper: ≤10%, >0%; riser: ≤0%.
2.5. Morning BP surge parameters
Sleep trough MBPS: morning SBP (2-hour average after arising) − lowest nighttime SBP (1-hour moving average).
Prewaking MBPS: morning SBP − preawake SBP (2-hour average before awakening).
Rising MBPS: morning SBP on rising − SBP on supine <30 minutes before arising.
2.6. Diabetes complication
History of CVD was defined as an episode of CCU admission, myocardial infarction, history of coronary artery bypass graft surgery, or percutaneous intervention.
Diagnosis of diabetic retinopathy was made based on chart review results and ophthalmologic funduscopic examination.
Diabetic nephropathy was defined by the presence of albuminuria, as an albumin excretion rate ≥30 mg/24 hour on at least 2 out of 3 consecutive occasions during the past 6 months or estimated glomerular filtration rate <60 mL/min/1.73 m2.
Diabetic neuropathy was defined based on the diabetic neuropathy symptom (DNS) scoring system.[20] Those with DNS score of >1 were examined by expert physicians to assess the diabetic neuropathy examination score adopted from Mythili et al.[21] Briefly, muscle strength, tendon reflex, and sensation examinations were performed. Those with examination score of >3 according to Mythili scoring system were identified as cases with diabetic neuropathy.
2.7. Statistical analyses
Continuous variables were summarized as mean [standard deviation (SD)] and categorical variables were presented as number (percentage). Continuous variables among groups were examined using a 1-way analysis of variance and independent sample t test. Chi square test was used for group comparison of categorical variables. The cross-sectional association between different BP phenotypes and diabetes complications including, nephropathy, retinopathy, and neuropathy was assessed using multiple logistic regression models adjusted for age, sex, BMI, HTN, using antihypertensive medication, HbA1c , FBG, TG, HDL-C, LDL-C, and total cholesterol. Regarding CVD penalized logistic regression via data augmentation with a weekly informative prior (f 1 1) for odds ratio (OR; implying the prior belief that OR is between 1/648, 648) with 95% probability was used to reduce sparse data bias and increase the precision of the estimate. All analyses were performed using Stata version 12. A P value <.05 was considered necessary to reject the null hypothesis.
3. Results
3.1. Baseline characteristics
There were a total of 192 participants (mean age 58.1) including 87 men and 105 women. The prevalence of neuropathy, nephropathy, retinopathy, and CVD were 33.8%, 24.4%, 56.2%, and 21.8%, respectively. Baseline characteristics including demographic and laboratory measurements of the study population according to presence of diabetes complications are shown in Table 1. Table 2 demonstrates data obtained from 24-hour BP measurements based on presence of diabetes complications. Patients with neuropathy had higher prewaking and rising MBPS and lower dipping fraction, LDL-C, and total cholesterol.
Table 1.
Baseline characteristics of the study population.
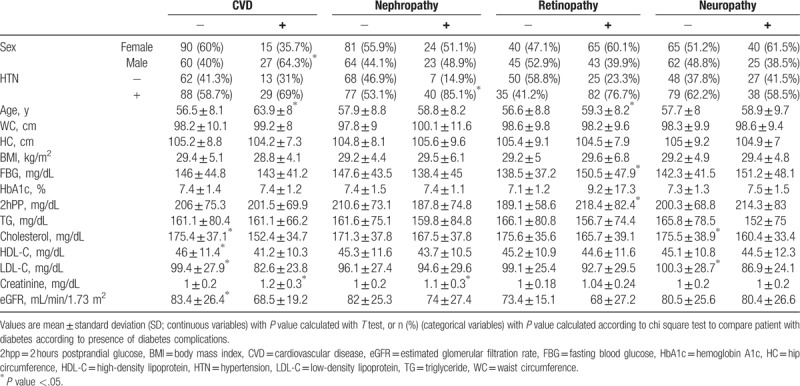
Table 2.
Ambulatory blood pressure monitoring parameters according to presence of diabetes complications.
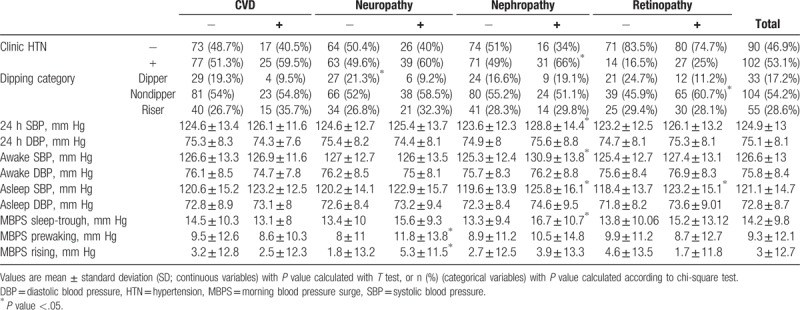
Creatinine, mean 24-hour SBP, mean awake SBP, mean awake DBP, mean asleep SBP, sleep trough MBPS, and clinic HTN were significantly higher in patients with nephropathy. Age, FBG, 2 hours post prandial glucose, Nondipping status and mean asleep SBP were higher in patients with retinopathy. Patients with CVD were older. They had lower HDL-C, LDL-C, total cholesterol, and glomerular filtration rate compared to patients without CVD (Tables 1–2).
3.2. BP phenotypes and diabetes complications
Approximately 48.9% of participants with T2DM had 24-hour HTN. The prevalence of daytime, nighttime, and clinic HTN were 35.9%, 96.3%, and 53.1%, respectively, and 54.2% of participants had nondipping nocturnal pattern and 28.6% were risers (Table 2).
Table 3 shows ORs and 95% confidence intervals (CIs) for CVD, nephropathy, retinopathy, and neuropathy according to nighttime dipping status, MBPS, and other BP phenotypes.
Table 3.
Association of different hypertension phenotypes and diabetes complications.
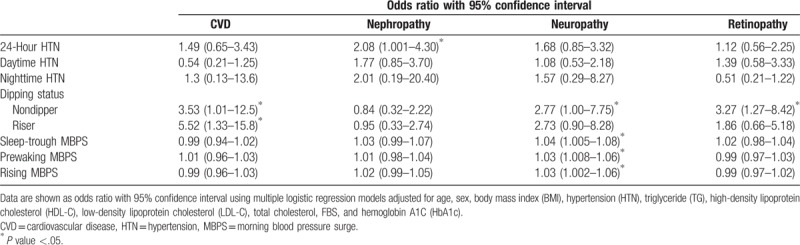
After adjusting for multiple confounders, in the fully adjusted model, the risk of CVD in nondipper and riser patients was at least 3 and 5 times, respectively, more likely compared to patients with normal dipping status (P = .05, P = .018, respectively). There was no significant association between CVD and other phenotypes of BP.
Nephropathy was associated with 24-hour HTN (P = .05). No association was observed for other BP parameters.
Increase in MBPS according to all definitions including sleep-trough, prewaking, and rising was associated with neuropathy (0.023, 0.010, 0.032, respectively). Nondipping status had a positive correlation with neuropathy (P = .05).
Nondipping status was significantly associated with higher risk of retinopathy (P = .014). There was not a significant association between retinopathy and other parameters of BP.
Figure 1 shows prevalence of diabetes complications including nephropathy, neuropathy, retinopathy, and CVD based on nocturnal dipping pattern. Percentage of patients with retinopathy and neuropathy was significantly higher in patients with abnormal dipping pattern in the crude analysis. Figure 2 demonstrates mean MBPS according to all definitions in patients with and without diabetes complications. Prewaking and rising MBPS was higher in patients with diabetic neuropathy. No difference was seen in other diabetes complication in the crude analysis.
Figure 1.
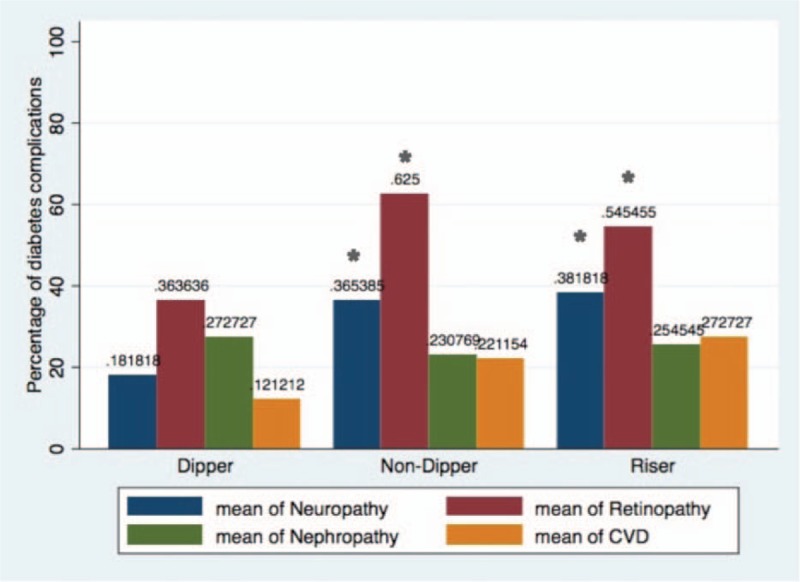
Comparison of prevalence (%) of CVD, nephropathy, neuropathy, and retinopathy among patients with type 2 diabetes (T2DM), categorized into dipper, nondipper, and risers are illustrated. Percentage of patients with retinopathy and neuropathy was significantly higher in patients with abnormal dipping pattern. Comparison was done using logistic regression models. (∗) shows significance difference with dipper group at the level of P < .05. CVD = cardiovascular disease.
Figure 2.
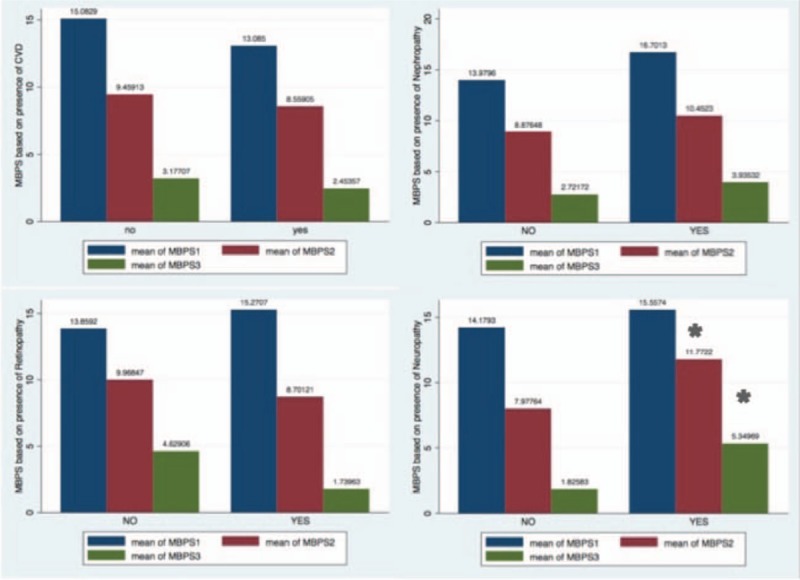
Morning blood pressure surge (mean) according to all definitions based on presence of diabetes complications are illustrated; prewaking and rising MBPS was higher in patients with diabetic neuropathy. No difference was seen in other diabetes complication. Comparison was done using T test. (∗) shows significance difference at the level of P < .05. MBPS1 = sleep-trough morning blood pressure surge, MBPS2 = prewaking morning blood pressure surge, MBPS3 = rising morning blood pressure surge.
4. Discussion
The aim of this study was to determine the association between different BP phenotypes and presence of diabetes complications including CVD, nephropathy, retinopathy, and neuropathy in patients with T2DM.
We found that diabetic neuropathy was positively associated with sleep-trough, prewaking, and rising MBPS and abnormal dipping status. These associations were independent of HTN, glycemic control, and lipid profile.
Although HTN has been indicated as an important risk factor of diabetic neuropathy in several studies,[22,23] there are few studies on the association of different phenotypes of HTN with neuropathy, which are mainly limited to autonomic neuropathy. Several observations have linked cardiac autonomic neuropathy to nondipping status by means of disruption in sympathovagal interactions and relative sympathetic prevalence during the nights in patients with diabetes.[24,25]
Regarding peripheral neuropathy, Sommerfield et al studied 100 patients with T2DM categorized to dipper and nondipper to investigate the clinical variables associated with dipping status using different cutoffs for dipping status compared to our study. No significant difference was seen between these 2 groups in degree of neuropathy.[26] Stella et al[27] also demonstrated that despite the significant impact of nondipping status on cardiac autonomic neuropathy there was no difference in Confirmed distal symmetric polyneuropathy between dipper and nondipper patients with type 1 diabetes. In the current study we for the first time examined how circadian BP variability can be related to peripheral neuropathy using both diurnal and nocturnal parameters. We showed that 1 SD increase in sleep-trough, prewaking, and rising morning surge was associated with 3% to 4% and abnormal dipping status with almost 2- to 3-fold increase in risk of diabetic neuropathy.
The effect of HTN upon peripheral nerve in patients with diabetes can be due to metalloproteinase upregulation at the sites of myelin at sensory nerve fibers. This mechanism results in loss of myelin thickness and contributes to diabetic peripheral neuropathy.[28] Reduced red blood cell Na/K ATPase is another factor, reported to be involved in pathophysiology of HTN and its link with neuropathy in insulin-dependent diabetes.[29]
Data on cardiovascular complications are more expanded compared with other complications. ABPM can disclose early cardiovascular organ damage in patients with T2DM.[30] Left ventricular hypertrophy and functional vascular organ damage were reported in diabetic patients with nondipping nocturnal patterns.[30] Eguchi et al[31] demonstrated riser pattern was associated with 150% increase in CVDs in both patients with and without diabetes. In Sommerfield et al[26] study history of macrovascular complications was significantly higher in nondipper patients, but this association disappeared after adjustment for age.
Our results indicate that CVDs are higher in nondipper and also riser patients after adjustment for potential risk factors.
Data concerning morning BP surge as a risk factor of CVD are controversial. Although it has been shown that sleep-trough MBPS affects left ventricular mass index as a marker of structural cardiac organ damage in normotensive patients[32] and the rate of morning surge in SBP was an independent determinant of myocardial infarction,[33] Ayala et al[34] documented that a greater prewaking MBPS was associated with significantly lower risk of CVD in general population. Blunted prewaking and sleep-trough MBPS was again reported to be associated with excessive risk of cardiovascular events in patients with essential HTN elsewhere.[10] Another study on newly diagnosed T2DM examined the association of sleep trough and prewaking MBPS with markers of early vascular target organ damage including pulse wave velocity, the results did not show any significant association.[35] Our findings also suggest that MBPS by any definition is not associated with CVD in patients with T2DM.
HTN and nephropathy often coexist in patients with diabetes. It has been hypothesized that microalbuminuria usually develops when HTN is established.[36] In the current study mean SBP and mean asleep DBP and clinic HTN were higher in patients with nephropathy. Furthermore we found 24-hour HTN as an independent risk factor of nephropathy in patients with T2DM. We also demonstrated that sleep-trough MBPS was higher in patients with nephropathy, though after adjustment for potential risk factors the association was not significant anymore. The association of sleep-trough MBPS with deterioration of kidney function and development of CKD was observed in hypertensive[37] and normotensive subjects[32] previously, although in a population-based observational study this association was not reported to be significant among African Americans.[38] This heterogeneity in results may be due to the racial differences. Barbieri et al[39] showed sleep-trough MBPS is positively associated with microalbuminuria in patients with T2DM. There are some hypotheses suggesting that inflammation and endothelial dysfunction resulting from elevated plasma levels of E-selectin and intercellular adhesion molecule 1 may be the link between MBPS and renal function.[40,41]
Previous studies on nocturnal dipping status mostly revealed a positive association between absence of nocturnal dipping pattern and nephropathy.[26,30,42] In contrast we did not observe any association between dipping status and nephropathy in Iranian patients with T2DM. We assumed this discrepancy might be a result of genetic and geographical differences, which can affect the circadian BP rhythm.[16] Racial impact was shown previously in the association of diurnal variation of BP and CVD in chronic kidney disease.[43]
None-dipping pattern and nocturnal HTN have been reported to be associated with retinopathy in patients with essential HTN and type 1 diabetes.[27,44,45] There are few studies examining diabetic retinopathy and ABPM parameters in patients with T2DM. Sommerfield et al[26] did not find any association between dipping status and retinopathy in patients with T2DM. On the contrary, Knudsen et al[46] stated that presence of retinopathy was associated with blunted nocturnal BP reduction. Here we found a positive association between nondipping status and prevalent retinopathy but MBPS was not related to retinopathy. Likewise, more extended increase in night BP values compared to day BP in patients with diabetic retinopathy was reported previously.[46] Blunted nocturnal BP reduction inflicts a higher 24-hour BP load to the vasculature; hence, this abnormality in circadian BP leads to endothelial dysfunction and inflammation due to enhancement of vascular wall shear stress. All these mechanisms result in development and progression of microvascular complications.[47]
There are some limitations in our study to note. One is the cross-sectional design of the study. Prospective studies are needed to help described directionality of association, reduce possible reverse causation. Second, the results are based on one-time ABPM. This may affect the accuracy of the results. Another limitation of our study is that we did not have information about the quality of sleep. Since sleep apnea and degree of desaturation during sleep may influence the dipping pattern.[48] One important limitation of this study is the small sample size in some combination of exposure and outcome for example the number of patients with CVD and normal dipping status was 4, which resulted in imprecise OR estimate with wide CI as well sparse data bias (OR inflation). We note that the magnitude of sparse data bias is not expected to be high as there is no huge OR estimate in our study and the number of events per variable is >4 for all models. Moreover no table cell count is <4. However we used penalized log regression via data augmentation with a weakly informative prior, which reduces spars data bias and increase the precision of the estimate.[49–51] However some 95% CI are still wide and studies with larger sample size are needed in future.
In conclusion, we demonstrated that diabetic neuropathy was associated with MBPS according to all definitions and abnormal dipping status. Our results indicated loss of nocturnal BP dipping but not MBPS as a risk factor for CVD and retinopathy in patients with T2DM. Our findings once again highlighted the importance of ambulatory BP monitoring and targeted antihypertensive therapy directed toward to restore normal circadian BP in patients with T2DM.
Acknowledgment
Authors wish to thank patients for their participation and kind cooperation.
Author contributions
Data curation: Mohammad Taghi Najafi, Asma Jaafarinia.
Methodology: Mohammad Taghi Najafi, Asma Jaafarinia, Sadaf Esteghamati.
Writing – original draft: Pegah Khaloo.
Formal analysis: Hamid Alemi, Mohsen Afarideh.
Conceptualization: Asma Jaafarinia, Alireza Esteghamati.
Writing – review and editing: Michael J Blaha, Mohammadhassan Mirbolouk, Mohammad Ali Mansournia, Manouchehr Nakhjavani, Alireza Esteghamati.
Footnotes
Abbreviations: ABPM = ambulatory blood pressure monitoring, BMI = body mass index, BP = blood pressure, CABG = coronary artery bypass graft, CI = confidence interval, CVD = cardiovascular disease, DBP = diastolic blood pressure, DNS = diabetic neuropathy symptom, FBG = fasting blood glucose, HbA1c = hemoglobin A1c, HDL-C = high-density lipoprotein cholesterol, HTN = hypertension, LDL-C = low-density lipoprotein cholesterol, MBPS = morning blood pressure surge, OR = odds ratio, SBP = systolic blood pressure, T2DM = type 2 diabetes, TG = triglyceride.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Parati G. Blood pressure variability: its measurement and significance in hypertension. J Hypertens Suppl 2005;23:S19–25. [DOI] [PubMed] [Google Scholar]
- [2].Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ 2011;342:d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mancia G, Zanchetti A, Agabiti-Rosei E, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation 1997;95:1464–70. [DOI] [PubMed] [Google Scholar]
- [4].Fagard RH, Staessen JA, Thijs L. Prediction of cardiac structure and function by repeated clinic and ambulatory blood pressure. Hypertension 1997;29(1 pt 1):22–9. [DOI] [PubMed] [Google Scholar]
- [5].Ohkubo T, Hozawa A, Nagai K, et al. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama study. J Hypertens 2000;18:847–54. [DOI] [PubMed] [Google Scholar]
- [6].Fagard RH. Dipping pattern of nocturnal blood pressure in patients with hypertension. Expert Rev Cardiovasc Ther 2009;7:599–605. [DOI] [PubMed] [Google Scholar]
- [7].Wang C, Zhang J, Liu X, et al. Reversed dipper blood-pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One 2013;8:e55419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pierdomenico SD, Pierdomenico AM, Cuccurullo F. Morning blood pressure surge, dipping, and risk of ischemic stroke in elderly patients treated for hypertension. Am J Hypertens 2014;27:564–70. [DOI] [PubMed] [Google Scholar]
- [9].Hoshide S, Kario K, Hoshide Y, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens 2003;16:434–8. [DOI] [PubMed] [Google Scholar]
- [10].Verdecchia P, Angeli F, Mazzotta G, et al. Day-night dip and early-morning surge in blood pressure in hypertension: prognostic implications. Hypertension 2012;60:34–42. [DOI] [PubMed] [Google Scholar]
- [11].Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003;107:1401–6. [DOI] [PubMed] [Google Scholar]
- [12].Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010;56:765–73. [DOI] [PubMed] [Google Scholar]
- [13].Epstein M, Sowers JR. Diabetes mellitus and hypertension. Hypertension 1992;19:403–18. [DOI] [PubMed] [Google Scholar]
- [14].Sun L, Yan B, Gao Y, et al. Relationship between blood pressure reverse dipping and type 2 diabetes in hypertensive patients. Sci Rep 2016;6:25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].John Wiley & Sons, Ltd, Kario K. First, Focusing on “Morning Hypertension”, in Essential Manual of 24 Hour Blood Pressure Management. 2015;1-14. [Google Scholar]
- [16].Agarwal R. Regulation of circadian blood pressure: from mice to astronauts. Curr Opin Nephrol Hypertens 2010;19:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care 2015;38(suppl):S8–s16. [DOI] [PubMed] [Google Scholar]
- [18].Toora BD, Rajagopal G. Measurement of creatinine by Jaffe's reaction--determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian J Exp Biol 2002;40:352–4. [PubMed] [Google Scholar]
- [19].Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 2018;71:1269–324. [DOI] [PubMed] [Google Scholar]
- [20].Meijer JW, Smit AJ, Sonderen EV, et al. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med 2002;19:962–5. [DOI] [PubMed] [Google Scholar]
- [21].Mythili A, Kumar KD, Subrahmanyam KA, et al. A comparative study of examination scores and quantitative sensory testing in diagnosis of diabetic polyneuropathy. Int J Diabetes Dev Ctries 2010;30:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Forrest KY, Maser RE, Pambianco G, et al. Hypertension as a risk factor for diabetic neuropathy: a prospective study. Diabetes 1997;46:665–70. [DOI] [PubMed] [Google Scholar]
- [23].Jarmuzewska EA, Ghidoni A, Mangoni AA. Hypertension and sensorimotor peripheral neuropathy in type 2 diabetes. Eur Neurol 2007;57:91–5. [DOI] [PubMed] [Google Scholar]
- [24].Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011;27:639–53. [DOI] [PubMed] [Google Scholar]
- [25].Spallone V, Bernardi L, Ricordi L, et al. Relationship between the circadian rhythms of blood pressure and sympathovagal balance in diabetic autonomic neuropathy. Diabetes 1993;42:1745–52. [DOI] [PubMed] [Google Scholar]
- [26].Sommerfield AJ, Robinson L, Padfield PL, et al. Clinical variables associated with non-dipping of nocturnal blood pressure in type 2 diabetes. Br J Diabet Vasc Dis 2008;8:236–40. [Google Scholar]
- [27].Stella P, Tabak AG, Zgibor JC, et al. Late diabetes complications and non-dipping phenomenon in patients with type 1 diabetes. Diabetes Res Clin Pract 2006;71:14–20. [DOI] [PubMed] [Google Scholar]
- [28].De Visser A, Hemming A, Yang C, et al. The adjuvant effect of hypertension upon diabetic peripheral neuropathy in experimental type 2 diabetes. Neurobiol Dis 2014;62:18–30. [DOI] [PubMed] [Google Scholar]
- [29].Jannot MF, Raccah D, Dufayet de la Tour D, et al. Relationship between neuropathy, hypertension and red blood cell Na/K ATPase in patients with insulin-dependent diabetes mellitus. Diabetes Metab 1999;25:35–42. [PubMed] [Google Scholar]
- [30].Jennersjo PE, Wijkman M, Wiréhn AB, et al. Circadian blood pressure variation in patients with type 2 diabetes—relationship to macro- and microvascular subclinical organ damage. Prim Care Diabetes 2011;5:167–73. [DOI] [PubMed] [Google Scholar]
- [31].Eguchi K, Pickering TG, Hoshide S, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens 2008;21:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Soylu A, Yazici M, Duzenli MA, et al. Relation between abnormalities in circadian blood pressure rhythm and target organ damage in normotensives. Circ J 2009;73:899–904. [DOI] [PubMed] [Google Scholar]
- [33].Luo Y, Wang YL, Wu YB, et al. Association between the rate of the morning surge in blood pressure and cardiovascular events and stroke. Chin Med J (Engl) 2013;126:510–4. [PubMed] [Google Scholar]
- [34].Ayala DE, Dominguez-Sardiña M, Otero A, et al. 1C.05: morning surge and sleep-time blood pressure as prognostic markers of cardiovascular risk: the Hygia Project. J Hypertens 2015;33(suppl 1):e10. [Google Scholar]
- [35].Lyhne JM, Laugesen E, Hoyem P, et al. Morning blood pressure surge and target organ damage in newly diagnosed type 2 diabetic patients: a cross sectional study. BMC Endocr Disord 2015;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 1999;341:1127–33. [DOI] [PubMed] [Google Scholar]
- [37].Turak O, Afsar B, Siriopol D, et al. Morning blood pressure surge as a predictor of development of chronic kidney disease. J Clin Hypertens (Greenwich) 2016;18:444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McMullan CJ, Hickson DA, Taylor HA, et al. Prospective analysis of the association of ambulatory blood pressure characteristics with incident chronic kidney disease. J Hypertens 2015;33:1939–46. discussion 1946. [DOI] [PubMed] [Google Scholar]
- [39].Barbieri M, Rizzo MR, Fava I, et al. Awaking blood pressure surge and progression to microalbuminuria in type 2 normotensive diabetic patients. J Diabetes Res 2016;2016:5876792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Knudsen ST, Jeppesen P, Frederiksen CA, et al. Endothelial dysfunction, ambulatory pulse pressure and albuminuria are associated in type 2 diabetic subjects. Diabet Med 2007;24:911–5. [DOI] [PubMed] [Google Scholar]
- [41].Amar J, Ruidavets JB, Bal Dit Sollier C, et al. Relationship between C reactive protein and pulse pressure is not mediated by atherosclerosis or aortic stiffness. J Hypertens 2004;22:349–55. [DOI] [PubMed] [Google Scholar]
- [42].Knudsen ST, Laugesen E, Hansen KW, et al. Ambulatory pulse pressure, decreased nocturnal blood pressure reduction and progression of nephropathy in type 2 diabetic patients. Diabetologia 2009;52:698–704. [DOI] [PubMed] [Google Scholar]
- [43].McMullan CJ, Yano Y, Bakris GL, et al. Racial impact of diurnal variations in blood pressure on cardiovascular events in chronic kidney disease. J Am Soc Hypertens 2015;9:299–306. [DOI] [PubMed] [Google Scholar]
- [44].Rodrigues TC, Canani LH, Viatroski RS, et al. Masked hypertension, nocturnal blood pressure and retinopathy in normotensive patients with type 1 diabetes. Diabetes Res Clin Pract 2010;87:240–5. [DOI] [PubMed] [Google Scholar]
- [45].Nakano Y, Oshima T, Ozono R, et al. Non-dipper phenomenon in essential hypertension is related to blunted nocturnal rise and fall of sympatho-vagal nervous activity and progress in retinopathy. Auton Neurosci 2001;88:181–6. [DOI] [PubMed] [Google Scholar]
- [46].Knudsen ST, Poulsen PL, Hansen KW, et al. Pulse pressure and diurnal blood pressure variation: association with micro- and macrovascular complications in type 2 diabetes. Am J Hypertens 2002;15:244–50. [DOI] [PubMed] [Google Scholar]
- [47].Knudsen ST. Ambulatory blood pressure, endothelial perturbation, and microvascular complications in type 2 diabetes. Dan Med Bull 2010;57:B4145. [PubMed] [Google Scholar]
- [48].Sekizuka H, Osada N, Akashi YJ. The factors affecting the non-dipper pattern in Japanese patients with severe obstructive sleep apnea. Intern Med 2018;57:1553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ 2016;352:i1981. [DOI] [PubMed] [Google Scholar]
- [50].Greenland S, Mansournia MA. Penalization, bias reduction, and default priors in logistic and related categorical and survival regressions. Stat Med 2015;34:3133–43. [DOI] [PubMed] [Google Scholar]
- [51].Mansournia MA, Geroldinger A, Greenland S, et al. Separation in logistic regression: causes, consequences, and control. Am J Epidemiol 2018;187:864–70. [DOI] [PubMed] [Google Scholar]


