Abstract
The aim of this study was to identify risk factors for extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (E coli) bloodstream infection (BSI) among carriers hospitalized between March 2011 and June 2016 at the ICU of the West China Hospital.
The cases were patients with at least 1 episode of ESBL-producing E coli BSI within 1 week after a positive rectal swab. Controls were selected randomly 1:2 among ESBL-producing E coli rectal carriers who did not develop BSI.
Among 19,429 ICU patients, 9015 (46.4%) had a positive rectal swab for ESBL-producing E coli. Of them, 42 (0.5%) were diagnosed with ESBL-producing E coli BSI. The in-hospital mortality was higher for the BSI patients compared with controls (19.1% vs. 6.0%, P = .031). In the past 72 hours, patients in case group were more likely to use penicillin (odds ratio [OR] = 12.076; 95% confidence interval [CI]: 1.397–104.251, P = .02), cephalosporin (OR = 6.900; 95% CI: 1.493–31.852, P = .01), and carbapenem (OR = 5.422; 95% CI: 1.228–23.907, P = .03) as compared to patients in control group. Also, when compared to patients in control group, patients in case group were likely to stay for a longer time in ICU before positive rectal swab test (OR = 1.041, 95% CI: 1.009–1.075, P = .01) and have higher maximum body temperature before positive rectal swab (OR = 8.014; 95% CI: 2.408–26.620, P = .001).
Bacteremia owing to ESBL-producing E coli was associated with high antimicrobial exposure, hospital stay, and maximum body temperature.
Keywords: Bacteremia, BSI, ESBL-producing E coli, rectal
1. Introduction
Extended-spectrum β-lactamase (ESBL) bacteria are rapidly emerging worldwide.[1–6] Recent reports from the CHINET surveillance network for bacterial resistance showed that the prevalence of ESBL-producing Escherichia coli (E coli) was 53.6% in China,[7] posing a serious threat to public health.[8] ESBL-producing E coli is resistant to penicillin, cephalosporin, and monobactam, and is also commonly resistant to fluoroquinolone and cotrimoxazole.[9–11]
E coli is one of the most common ESBL-producing species and the common most cause of bloodstream infection (BSI).[12–14] The patients admitted to the intensive care unit (ICU) are in a poor health condition and often they will have lowered immunity. Therefore, this results in high rates of nosocomial infections.[15] ESBL-producing E coli BSIs are associated with higher mortality, longer ICU stay, and higher costs compared with BSI caused by non-ESBL-producing bacteria.[16,17]
There are 2 steps in the development of BSI (colonization and infection) and few clinical studies have investigated the 2 steps separately, leaving obscure the infection step of already colonized patients.[18] Unfortunately, the epidemiology of ESBL-producing E coli is complex because it is difficult to identify bacterial colonization before infection. Indeed, most previous studies used non-ESBL-producing E coli carriers as controls.[19–21] Therefore, identifying patients with ESBL-producing E coli as well as the factors associated with infection are of paramount importance for the patients and physicians.[19–21]
There are some studies about ESBL-producing E coli colonization and risk factors for ESBL-producing E coli BSI[8–11], but the factors associated with ESBL-producing E coli BSI among rectal ESBL-producing E coli carriers in the ICU are poorly known. Therefore, this study aimed to identify risk factors for ESBL-producing E coli BSI among rectal ESBL-producing E coli carriers in the ICU. Rectal carriers were selected because rectal cultures are routinely used at our institution for patients in the ICU. This study may provide physicians with useful information to improve therapeutic approaches and improve the control rate of nosocomial infections in the ICU.
2. Materials and methods
2.1. Study design and population
This was a retrospective nested case-control study performed at the ICU of West China Hospital, a 4800-bed tertiary teaching hospital that has a 220-bed adult ICU and a 20-bed pediatric ICU. Both adult and pediatric patients hospitalized from March 2011 to June 2016 and with a ESBL-producing E coli-positive rectal swab were included. Before receiving the results of drug sensitivity, the patients were treated based on the physicians’ experience. The treatment approaches were then changed according to the results of antibiotics sensitivity.
The cases consisted of patients with at least 1 episode of ESBL-producing E coli BSI within 1 week after a positive rectal swab (which is a routine test at our ICU), to reduce the probability that the infection came from a different source than the rectum. Each BSI patient was included only once at the time of first ESBL-producing E coli isolation from BCs. Control samples were selected randomly at a ratio 1:2 among ESBL-producing E coli rectal carriers who did not develop BSI. The 2 controls were selected with a time window of ±1 week from each case of BSI to minimize seasonal variations of E coli infection.[22]
This study was approved by the review board of the West China Hospital, Sichuan University. All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The need for individual consent was waived by the committee because of the retrospective nature of the study.
2.2. Data collection
Data were collected from the Hospital Information System, an electronic medical chart system. Demographic data, underlying condition, body temperature for 1 week before positive rectal swab, ICU stay before the studied event period, history of hospitalization within the last 3 months, blood analysis within 2 weeks before the studied event period, previous antimicrobial exposure (within 3 months and 72 hours before the positive rectal swab), antibiotics prescription during the studied event period, invasive procedures or devices (including central vein catheterization, urinary catheter, gastric catheter, tracheotomy, invasive ventilation, surgery, thoracentesis, and abdominocentesis) before the studied event period, and in-hospital mortality were analyzed.
2.3. Definitions
Rectal carriers were defined as patients with ESBL-producing E coli isolated from a rectal swab but without symptoms and signs of invasive infection. ESBL-producing E coli BSI was defined as BSI documented by at least one BC positive for an ESBL-producing E coli strain and clinical signs of systemic inflammatory response syndrome.[23] The studied event period was considered from the first positive rectal swab to death or hospital discharge. Antibiotics use’ density, as defined by the World Health Organization, was calculated as the value of number of patients using antibiotics per 100 days-person.
2.4. Microbiology
Rectal swabs were inoculated on a chromogenic agar plate (ChromID ESBL plate; bioMerieux, Marcy l’Étoile, France) containing cefpodoxime as a selective agent. BCs were incubated using the BacTAlert automated system (Alert 3D; BioMerieux, Hazelwood, MO). The Vitek 2 Compact automated system (BioMerieux, Hazelwood, MO) was used for the identification of E coli isolates.
2.5. Statistical analysis
Continuous data were expressed as mean ± standard deviation if normally distributed, or as median and interparticle ranges (IQR) if non-normally distributed, based on the Kolmogorov-Smirnov test. The t test or the Mann-Whitney U test was used to analyze the continuous variables, as appropriate, whereas the χ2 or the Fisher exact test was used for categorical variables. Binary logistic regression (backward conditional) was used to select the factors associated with BSI, and the enter method was used to correct the final model. Variables with P values <.05 in the univariate analyses were included in the multivariate model. The goodness of fit of the regression equation was assessed by the Hosmer-Lemeshow test. ROC curves were used to verify whether the regression model could discriminate low- versus high-risk BSI patients among ESBL-producing E coli carriers. Statistical analysis was performed using SPSS 21.0 (IBM, Armonk, NY). Two-sided P values <.05 were considered statistically significant.
2.6. Data availability
All data generated or analyzed during this study are included in this article.
3. Results
3.1. Characteristics of the patients
During the study period, 19,429 ICU patients were screened for multidrug-resistant bacteria. Among them, 9015 (46.4%) had a positive rectal swab for ESBL-producing E coli. Of them, 42 (0.5%) were diagnosed with ESBL-producing E coli BSI. The BSI patients were compared with 84 randomly selected ESBL-producing E coli rectal carriers without documented BSI during hospitalization (Fig. 1).
Figure 1.
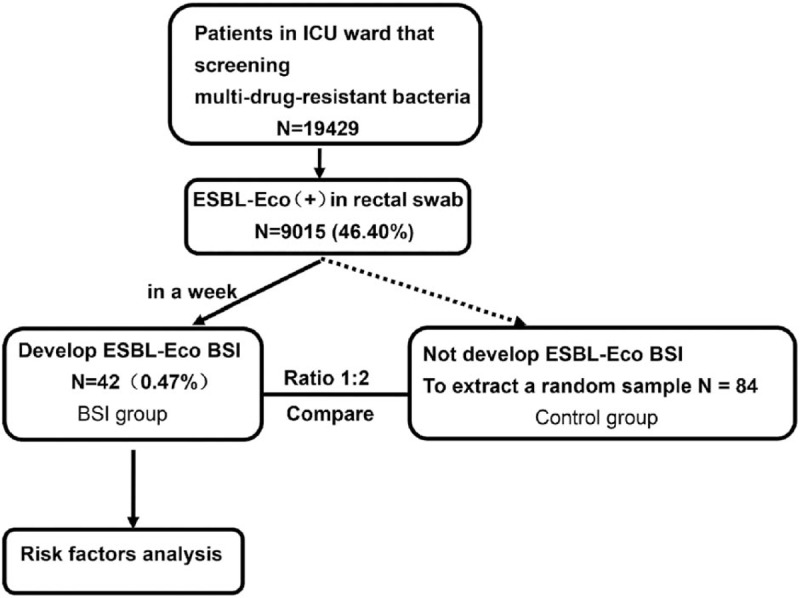
Patients flowchart.
In the BSI group, blood culture (BC) revealed that 17 (40.5%) patients had multipathogen infection in addition to ESBL-producing E coli, including non-ESBL-producing E coli bacteria (n = 13, 31.0%), fungus (n = 2, 4.8%), and non-ESBL-producing E coli bacteria concomitant with fungus (n = 2, 4.8%).
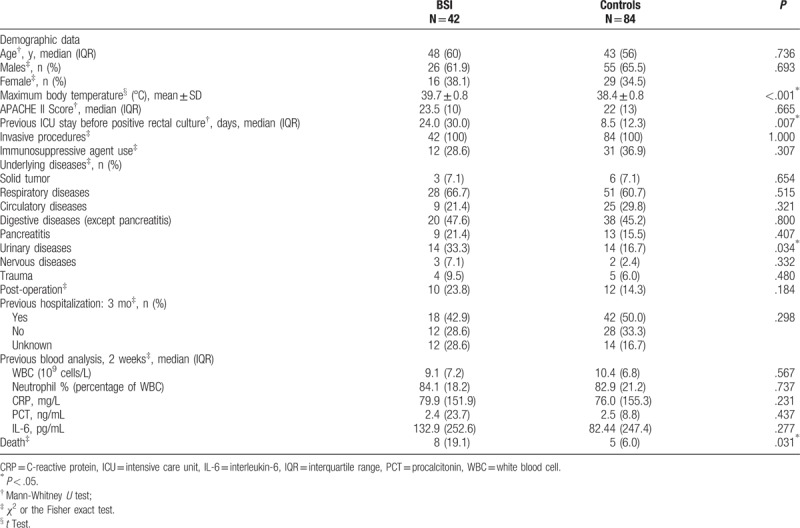
Table 1 shows that there were no significant differences for age, sex, previous 3-month hospitalization, and previous 2-week blood analysis between the patients with BSI and controls (all P > .05). BSI patients had higher maximum body temperature (39.7 ± 0.8 vs. 38.4 ± 0.8°C, P < .001) 1 week before positive rectal swab, longer stay in the ICU before positive rectal swab test (median of 24 vs. 8.5 days, P = .007), and higher frequency of urinary infection (33.3% vs. 16.7%, P = .034). Among the patients with urinary infection, the BSI group included 5 patients with positive E coli urine cultures (UCs) within 1 week before positive BC, and one had a positive E coli UC 5 months after BSI.
Table 1.
Characteristics of the patients.
3.2. Mortality
The in-hospital mortality rate was higher for the patients with BSI compared to controls (19.1% vs. 6.0%, P = .031) (Table 1).
3.3. Antibiotics exposure
Patients with ESBL-producing E coli BSI had been more frequently exposed to previous β-lactam antibiotics than controls (P = .001 for previous 3 months and P = .002 for previous 72 hours). After ESBL-producing E coli BSI diagnosis, 35 of 42 (83.3%) patients were administrated carbapenem for therapy since 38 of 42 (90.5%) ESBL E coli strains from case group patients were sensitive to carbapenems (Table 2). Within the 72 hours before positive rectal swab and during their hospitalization for BSI, the antibiotics use’ density of the patients with BSI was higher than in controls (Fig. 2).
Table 2.
Antibiotic use between the case and control groups†.
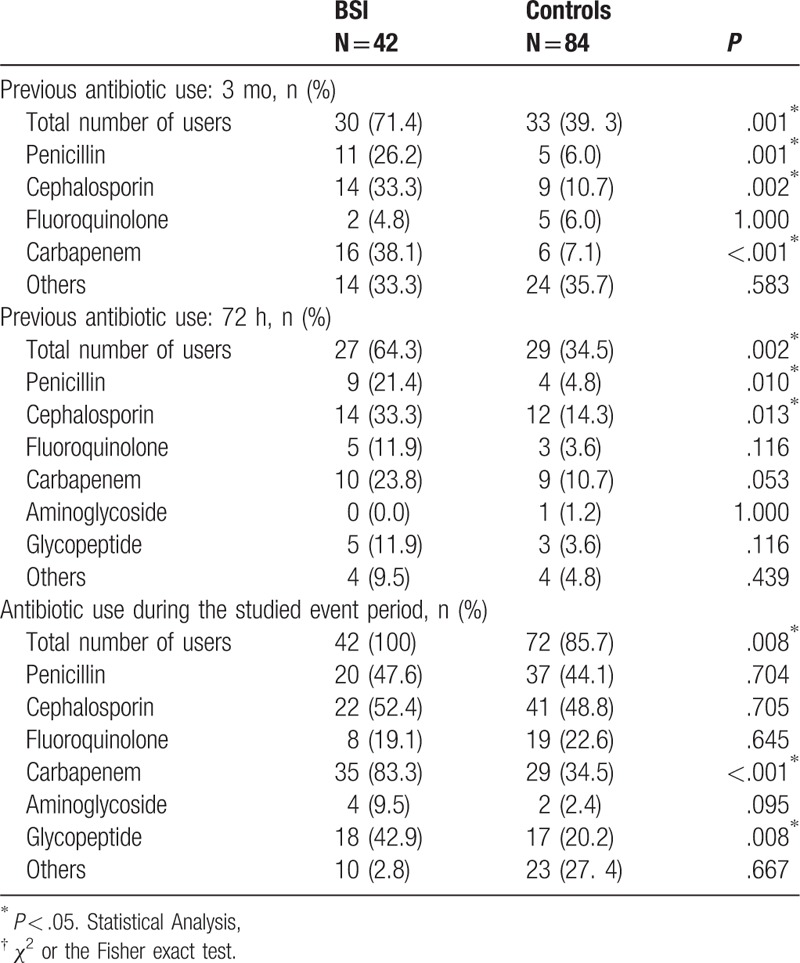
Figure 2.
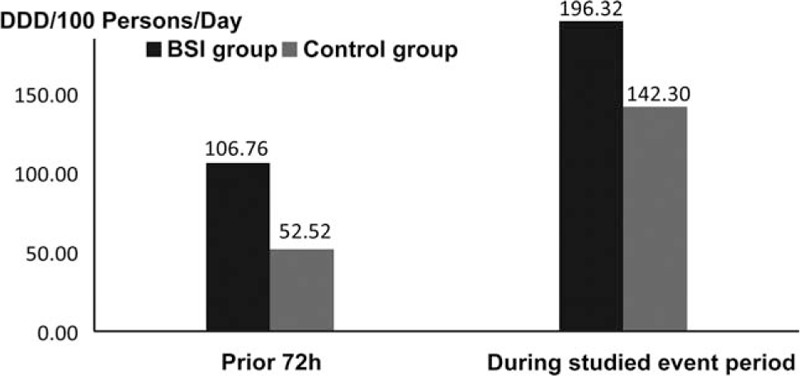
Antibiotics use’ density in the previous 72-hour period and during the studied event period between the case and control groups. DDD = defined daily dose, as defined by WHO.
3.4. Multivariate analysis
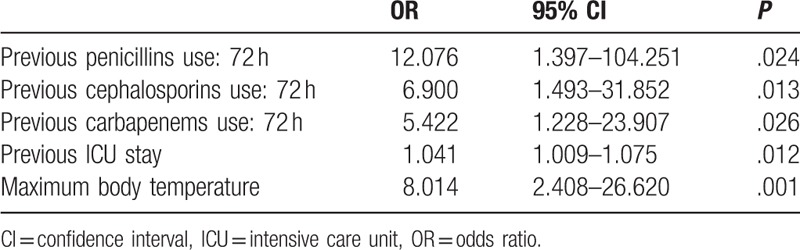
The factors with P values <.05 in univariate analyses including maximum body temperature, urinary diseases, previous ICU stay, previous 3 months’ penicillins use, previous 3 months’ cephalosporins use, previous 3 months’ carbapenems use, previous 72 hours’ penicillins use, previous 72 hours’ cephalosporins use, previous 72 hours’ carbapenems were added into the multivariate logistic regression analysis. As compared to patients in control group, patients in case group were more likely to use penicillin (odds ratio [OR] = 12.076; 95% confidence interval [CI]: 1.397–104.251, P = .02), cephalosporin (OR = 6.900; 95% CI: 1.493–31.852, P = .01), and carbapenem (OR = 5.422; 95% CI: 1.228–23.907, P = .03) in the past 72 hours. Also, patients in case group were likely to stay for a longer time in ICU before positive rectal swab test (OR = 1.041, 95% CI: 1.009–1.075, P = .01) and have higher maximum body temperature before positive rectal swab (OR = 8.014; 95% CI: 2.408–26.620, P = .001) when compared with patients in control group (Table 3). The Hosmer-Lemeshow test (P = .375) showed that the model displayed acceptable goodness of fit. The pseudo-R2 (Nagelkere) was 0.675.
Table 3.
Multivariate logistic regression analysis.
3.5. Receiver-operating characteristic curve analysis
The receiver-operating characteristics (ROC) curve analysis suggested that the predictive value of the regression model to discriminate low-risk from high-risk patients had an area under the curve of 0.933 (95% CI: 0.882–0.983, P < .0001).
4. Discussion
The factors associated with ESBL-producing E coli BSI among ESBL-producing E coli rectal carriers in the ICU are poorly known. The aim of the present study was to identify factors associated with ESBL-producing E coli BSI among ESBL-producing E coli carriers in the ICU. The use of β-lactam antibiotics and high body temperature were independently associated with BSI occurrence in ESBL-producing E coli rectal carriers. Bacteremia owing to ESBL-producing E coli was associated with high mortality rates.
This retrospective nested case-control study reports for the first time in a Chinese population that 0.5% of the ESBL-producing E coli rectal carriers developed BSI. This rate is smaller than that of a Spanish study that showed a rate of ESBL-producing E coli BSI of 3.5%.[24] The in-hospital mortality rate of patients with ESBL-producing E coli BSI among ESBL-producing E coli rectal carriers in the ICU was 19.1%, which was much higher than the mortality reported by a previous study on other ESBL-producing bacteria (9.6%),[25] but lower than that of the Spanish study, which showed a mortality of 50.9% for ESBL-producing E coli and ESBL-producing Klebsiella pneumoniae, taken together.[24]
In the present study, the subjects were ESBL-producing E coli rectal carriers hospitalized in the ICU and were therefore high-risk patients because of their severe condition and compromised immune status. Previous studies showed that procalcitonin (PCT) levels had high predictive value for bacteremia,[26,27] but this factor showed no association with BSI in the present study. A meta-analysis suggested that the diagnostic performance of PCT for bacteremia in emergency departments is moderate,[28] possibly because PCT is also associated with non-infectious diseases.[29,30]
In the present study, among all ESBL-producing E coli rectal carriers, the antibiotics use’ density of patients with BSI was much higher than that in non-BSI patients. In addition, BSI patients among ESBL-producing E coli rectal carriers in case group are more likely to use β-lactam antibiotics in the past 72 hours as compared to patients in control group. These results are supported by a Spanish study that showed that the previous use (within 3 months) of >2 different classes of antimicrobials was associated with a higher risk of BSI by ESBL-producing E coli.[24] Another Spanish study showed that a previous use of cephalosporin and carbapenem was associated with ESBL-producing E coli BSI.[31] Rodriguez-Bano et al[32] showed that ESBL-related BSI patients were more exposed to β-lactam antibiotics or fluoroquinolone. Tumbarello et al[33] showed that previous exposure to antimicrobials in general was associated to ESBL-producing strains. Therefore, we can hypothesize that under the pressure of large-spectrum β-lactam antibiotics and antibiotics in general, enteric normal flora becomes imbalanced and may cause ESBL-producing E coli BSI. There is a possibility that β-lactam antibiotics were not able to kill ESBL-producing E coli effectively, but they might have killed other β-lactam-sensitive bacteria. Consequently, the dysbiosis resulted in ESBL-producing E coli entering the blood stream and causing infection. Nevertheless, additional studies are necessary to address this point. In addition, it must be stressed that the different classification systems of antibiotics use may lead to different results and risk factors, as previously suggested.[34]
The factors observed in the present study were consistent with some published studies of BSI caused by ESBL-producing bacteria.[33,35–38] Nevertheless, the present study focused on factors of ESBL-producing E coli infection among patients already colonized by the bacteria, while many previous studies focused on the colonization step, which does not necessarily progress to infection. Previous studies reported that the presence of invasive devices, older age, and longer stay in hospital were risk factors for BSI caused by ESBL-producing bacteria,[37–39] but the presence of invasive devices and older age were not found to be associated with BSI in the present study. At the West China Hospital, invasive devices are routinely used in practically all patients in the ICU wards and this factor could not be analyzed. Regarding age and hospital stay, all patients in the ICU are usually weak and with low immunity, not only the oldest ones. In addition, all ICU patients are with severe conditions at the start. Therefore, there may be some differences in BSI occurrence between ICU and general wards. On the other hand, the present study showed that ICU stay before positive rectal swab was associated with BSI caused by ESBL-producing bacteria, as supported by previous studies, albeit in various bacteria, patient populations, and sites of infections (blood, pneumonia, among others).[37–41] Longer hospital stay increases the likelihood of being exposed to contamination and the risk of infection.
In the present study, 5 patients with urinary infection owing to E coli ultimately developed ESBL-producing E coli BSI (11.9% of the patients with ESBL-producing E coli BSI), but this number was too small to perform any reliable statistical analysis. Nevertheless, urinary infection could be a risk factor for BSI, as previously observed.[42] As is well known, high temperature is a consequence of infections and a mean to combat them. In the present study, high temperature was independently associated with ESBL-producing E coli BSI, but the retrospective nature of the study prevents any case-to-effect analysis.
There are some limitations to the present retrospective study. First, the number of BSI patients was small, representing only a small proportion of all ESBL-producing E coli carriers. These results should be validated with prospective multi-center studies. Second, antibiotic records may not completely represent the outpatient antibiotics use, antibiotics use in other hospitals, or life-time history of antibiotics use, which might bias the data. Third, homology between ESBL-producing E coli in the rectum and ESBL-producing E coli in the blood was not tested and it is unknown whether BSIs were caused by the same strain of ESBL-producing E coli. Fourth, the selection plates for ESBL selection contained cefpodoxime, which has been reported to be a highly efficient screening antibiotic, which could bias the population of bacteria.[43,44] Finally, in some patients, BSI was caused by non-ESBL-producing E coli pathogens; whether this was caused by contamination of the samples or weaken state of the patients leading to opportunistic infections warrants further study. Unfortunately, because of the small sample size, reliable subgroup analyses were not possible.
In conclusion, BSI patients among ESBL-producing E coli rectal carriers were more likely to use β-lactam antibiotics and had higher body temperature as well as longer hospital stay as compared to the patients in control group. The mortality rates increased when ESBL-producing E coli rectal carriers developed bacteremia. A predictive model with these variables may be useful to identify patients at low- versus high risk of ESBL-producing E coli BSI and avoid overuse of broad-spectrum antibiotics or pay special attention to the ICU patients who have a high body temperature. Nevertheless, further studies are needed to validate those results.
Acknowledgements
The authors thank the West China Hospital of Sichuan University and all personnel who assisted us in the study.
Author contributions
Formal analysis: Yi Xie.
Investigation: Minxue Liu, Mengjiao Li, Lijuan Wu, Qifei Song, Dan Zhao, Zhixing Chen, Mei Kang, Yi Xie.
Methodology: Minxue Liu, Mengjiao Li, Lijuan Wu, Qifei Song, Dan Zhao, Zhixing Chen, Mei Kang, Yi Xie.
Resources: Yi Xie.
Validation: Yi Xie.
Visualization: Yi Xie.
Writing – original draft: Minxue Liu, Mengjiao Li, Lijuan Wu, Mei Kang, Yi Xie.
Writing – review & editing: Minxue Liu, Mengjiao Li, Lijuan Wu, Qifei Song, Dan Zhao, Mei Kang, Yi Xie.
Footnotes
Abbreviations: BC = blood culture, BSI = bloodstream infection, CI = confidence interval, E coli = Escherichia coli, ESBL = extended-spectrum β-lactamase, ICU = intensive care unit, IQR = interquartile range, ROC = receiver-operating characteristics, UCs = urine cultures.
The authors report no conflicts of interest.
References
- [1].Pena C, Gudiol C, Tubau F, et al. Risk-factors for acquisition of extended-spectrum beta-lactamase-producing Escherichia coli among hospitalised patients. Clin Microbiol Infect 2006;12:279–84. [DOI] [PubMed] [Google Scholar]
- [2].Canton R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol 2006;9:466–75. [DOI] [PubMed] [Google Scholar]
- [3].Pasricha J, Koessler T, Harbarth S, et al. Carriage of extended-spectrum beta-lactamase-producing enterobacteriacae among internal medicine patients in Switzerland. Antimicrob Resist Infect Control 2013;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cheong HS, Ko KS, Kang CI, et al. Clinical significance of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae blood isolates with inducible AmpC beta-lactamase. Microb Drug Resist 2012;18:446–52. [DOI] [PubMed] [Google Scholar]
- [5].Stuart JC, Diederen B, Al Naiemi N, et al. Method for phenotypic detection of extended-spectrum beta-lactamases in enterobacter species in the routine clinical setting. J Clin Microbiol 2011;49:2711–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kanamori H, Yano H, Hirakata Y, et al. Molecular characteristics of extended-spectrum beta-lactamases and qnr determinants in Enterobacter species from Japan. PLoS One 2012;7:e37967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect 2016;22(suppl 1):S9–14. [DOI] [PubMed] [Google Scholar]
- [8].Stewardson A, Fankhauser C, De Angelis G, et al. Burden of bloodstream infection caused by extended-spectrum beta-lactamase-producing enterobacteriaceae determined using multistate modeling at a Swiss University Hospital and a nationwide predictive model. Infect Control Hosp Epidemiol 2013;34:133–43. [DOI] [PubMed] [Google Scholar]
- [9].Chen Y, Shoichet B, Bonnet R. Structure, function, and inhibition along the reaction coordinate of CTX-M beta-lactamases. J Am Chem Soc 2005;127:5423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen Y, Delmas J, Sirot J, et al. Atomic resolution structures of CTX-M beta-lactamases: extended spectrum activities from increased mobility and decreased stability. J Mol Biol 2005;348:349–62. [DOI] [PubMed] [Google Scholar]
- [11].Malloy AM, Campos JM. Extended-spectrum beta-lactamases: a brief clinical update. Pediatr Infect Dis J 2011;30:1092–3. [DOI] [PubMed] [Google Scholar]
- [12].Hilali F, Ruimy R, Saulnier P, et al. Prevalence of virulence genes and clonality in Escherichia coli strains that cause bacteremia in cancer patients. Infect Immun 2000;68:3983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Laupland KB, Gregson DB, Church DL, et al. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect 2008;14:1041–7. [DOI] [PubMed] [Google Scholar]
- [14].Gray J. Epidemiology of Escherichia coli bloodstream infections in children. J Hosp Infect 2017;95:383–4. [DOI] [PubMed] [Google Scholar]
- [15].Marshall JC, Marchall KAM. ICU-acquired infection: mortality, morbidity, and costs. Infection control in the intensive care unit. Milan: Springer; 2012. [Google Scholar]
- [16].Schwaber MJ, Navon-Venezia S, Kaye KS, et al. Clinical and economic impact of bacteremia with extended- spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2006;50:1257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tumbarello M, Spanu T, Di Bidino R, et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother 2010;54:4085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Biehl LM, Schmidt-Hieber M, Liss B, et al. Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients - Review of the literature from a clinical perspective. Crit Rev Microbiol 2016;42:1–6. [DOI] [PubMed] [Google Scholar]
- [19].Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Infect Control 2006;34(5 suppl 1):S20–28. discussion S64-73. [DOI] [PubMed] [Google Scholar]
- [20].Ramphal R, Ambrose PG. Extended-spectrum beta-lactamases and clinical outcomes: current data. Clin Infect Dis 2006;42(suppl 4):S164–172. [DOI] [PubMed] [Google Scholar]
- [21].Tansarli GS, Poulikakos P, Kapaskelis A, et al. Proportion of extended-spectrum beta-lactamase (ESBL)-producing isolates among Enterobacteriaceae in Africa: evaluation of the evidence—systematic review. J Antimicrob Chemother 2014;69:1177–84. [DOI] [PubMed] [Google Scholar]
- [22].Al-Hasan MN, Lahr BD, Eckel-Passow JE, et al. Seasonal variation in Escherichia coli bloodstream infection: a population-based study. Clin Microbiol Infect 2009;15:947–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Giannella M, Trecarichi EM, De Rosa FG, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect 2014;20:1357–62. [DOI] [PubMed] [Google Scholar]
- [24].Quirante OF, Cerrato SG, Pardos SL. Risk factors for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Braz J Infect Dis 2011;15:370–6. [DOI] [PubMed] [Google Scholar]
- [25].Nasa P, Juneja D, Singh O, et al. An observational study on bloodstream extended-spectrum beta-lactamase infection in critical care unit: incidence, risk factors and its impact on outcome. Eur J Intern Med 2012;23:192–5. [DOI] [PubMed] [Google Scholar]
- [26].Theodorou VP, Papaioannou VE, Tripsianis GA, et al. Procalcitonin and procalcitonin kinetics for diagnosis and prognosis of intravascular catheter-related bloodstream infections in selected critically ill patients: a prospective observational study. BMC Infect Dis 2012;12:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Albrich WC, Mueller B. Predicting bacteremia by procalcitonin levels in patients evaluated for sepsis in the emergency department. Expert Rev Anti Infect Ther 2011;9:653–6. [DOI] [PubMed] [Google Scholar]
- [28].Jones AE, Fiechtl JF, Brown MD, et al. Procalcitonin test in the diagnosis of bacteremia: a meta-analysis. Ann Emerg Med 2007;50:34–41. [DOI] [PubMed] [Google Scholar]
- [29].Fazili T, Endy T, Javaid W, et al. Role of procalcitonin in guiding antibiotic therapy. Am J Health Syst Pharm 2012;69:2057–61. [DOI] [PubMed] [Google Scholar]
- [30].Liu HH, Guo JB, Geng Y, et al. Procalcitonin: present and future. Ir J Med Sci 2015;184:597–605. [DOI] [PubMed] [Google Scholar]
- [31].Martinez JA, Aguilar J, Almela M, et al. Prior use of carbapenems may be a significant risk factor for extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella spp. in patients with bacteraemia. J Antimicrob Chemother 2006;58:1082–5. [DOI] [PubMed] [Google Scholar]
- [32].Rodriguez-Bano J, Navarro MD, Romero L, et al. Risk-factors for emerging bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Clin Microbiol Infect 2008;14:180–3. [DOI] [PubMed] [Google Scholar]
- [33].Tumbarello M, Spanu T, Sanguinetti M, et al. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother 2006;50:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].MacAdam H, Zaoutis TE, Gasink LB, et al. Investigating the association between antibiotic use and antibiotic resistance: impact of different methods of categorising prior antibiotic use. Int J Antimicrob Agents 2006;28:325–32. [DOI] [PubMed] [Google Scholar]
- [35].Mosqueda-Gomez JL, Montano-Loza A, Rolon AL, et al. Molecular epidemiology and risk factors of bloodstream infections caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae A case-control study. Int J Infect Dis 2008;12:653–9. [DOI] [PubMed] [Google Scholar]
- [36].Muro S, Garza-Gonzalez E, Camacho-Ortiz A, et al. Risk factors associated with extended-spectrum beta-lactamase-producing Enterobacteriaceae nosocomial bloodstream infections in a tertiary care hospital: a clinical and molecular analysis. Chemotherapy 2012;58:217–24. [DOI] [PubMed] [Google Scholar]
- [37].Wu UI, Yang CS, Chen WC, et al. Risk factors for bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli. J Microbiol Immunol Infect 2010;43:310–6. [DOI] [PubMed] [Google Scholar]
- [38].Rodriguez-Bano J, Picon E, Gijon P, et al. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis 2010;50:40–8. [DOI] [PubMed] [Google Scholar]
- [39].Kang CI, Kim SH, Kim DM, et al. Risk factors for and clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2004;25:860–7. [DOI] [PubMed] [Google Scholar]
- [40].Seligman R, Ramos-Lima LF, Oliveira Vdo A, et al. Risk factors for infection with multidrug-resistant bacteria in non-ventilated patients with hospital-acquired pneumonia. J Bras Pneumol 2013;39:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yallew WW, Kumie A, Yehuala FM. Risk factors for hospital-acquired infections in teaching hospitals of Amhara regional state, Ethiopia: a matched-case control study. PLoS One 2017;12:e0181145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rodriguez-Bano J, Navarro MD, Romero L, et al. Bacteremia due to extended-spectrum beta -lactamase-producing Escherichia coli in the CTX-M era: a new clinical challenge. Clin Infect Dis 2006;43:1407–14. [DOI] [PubMed] [Google Scholar]
- [43].Livermore DM, Hawkey PM. CTX-M: changing the face of ESBLs in the UK. J Antimicrob Chemother 2005;56:451–4. [DOI] [PubMed] [Google Scholar]
- [44].Glupczynski Y, Berhin C, Bauraing C, et al. Evaluation of a new selective chromogenic agar medium for detection of extended-spectrum beta-lactamase-producing Enterobacteriaceae. J Clin Microbiol 2007;45:501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


