Fact 1: Infection with Giardia lamblia is one of the most common causes of waterborne nonbacterial and nonviral diarrheal disease
G. lamblia (syn. intestinalis, duodenalis) is a zoonotic enteroparasite. It proliferates in an extracellular and noninvasive fashion in the small intestine of vertebrate hosts, causing the diarrheal disease known as giardiasis. Virtually all mammals can be infected with G. lamblia, and epidemiological data point to giardiasis as a zoonosis [1]. Infections in humans may be asymptomatic or associated with diarrhea, malabsorption, bloating, abdominal pain, fatigue, and weight loss. Based on the latest figures provided by WHO, G. lamblia is the third most common agent of diarrheal disease worldwide with over 300 million reported cases per annum, preceded only by rotavirus and Cryptosporidium parvum and hominis in the most vulnerable target group of children under five years of age [2]. The prevalence of giardiasis in humans ranges from 2%–3% in industrialized countries, up to 30% in low-income and developing countries [3]. Giardiasis was formerly included in the WHO neglected diseases initiative and is directly associated with poverty and poor quality of drinking water [4]. Acute infection develops over a period of three weeks, peaking at eight days post infection. Generally, healthy hosts clear the infection within 2–3 weeks, whereas the occasional chronically infected host shows signs of villus and crypt atrophy, enterocyte apoptosis, and ultimately severe disruption of epithelial barrier function [5]. Infection with G. lamblia has also been linked to the development of irritable bowel syndrome and chronic fatigue [6].
Fact 2: G. lamblia presents a simplified subcellular organization but is not a primitive eukaryote
To date, four types of endomembrane compartments have been identified in the Giardia trophozoite, namely: the endoplasmic reticulum (ER), the nuclei, terminally-differentiated mitochondrial remnants named mitosomes, and peripheral vacuoles (PVs) [7]. Encystation-specific vesicles (ESVs) constitute a fifth compartment present only in encysting cells. Extreme genomic divergence has led to frequent artefacts such as long-branch attraction in earlier phylogenetic studies [8]. Combined with observations of elements of prokaryotic metabolism and the absence of bona fide eukaryotic organelles such as the Golgi apparatus, endosomes, and mitochondria, this resulted in a misclassification of G. lamblia as a primitive eukaryote and a concomitant misinterpretation of its evolutionary history [9]. However, molecular paleontology approaches aimed at identifying machinery present in the last eukaryotic common ancestor (LECA) indicated that this organism likely possessed all extant eukaryotic organelles and corresponding trafficking pathways. This supports the notion that species with a simplified cellular organization such as the ancestor of G. lamblia evolved via a reduction of complexity, likely linked to adoption of a parasitic lifestyle, and that G. lamblia is therefore most likely not a primary primitive eukaryote but secondarily reduced. Interestingly, recent efforts aimed at rooting the eukaryotic tree place this root between the Excavata supergroup, to which G. lamblia belongs, and all other eukaryotes [10].
Fact 3: G. lamblia feeds using specialized organelles called PVs
Due to streamlining of most anabolic pathways, G. lamblia cells are highly dependent on nutrient uptake from the host’s gut by means of an array of organelles, i.e., PVs (Fig 1A). This active host–pathogen interface is at the crossroads of both endo- and exocytic trafficking in G. lamblia trophozoites (Fig 1B). The main function of PVs is to periodically endocytose fluid-phase extracellular material and to expel harmful or unusable substances into the environment again. This is in contrast to the unidirectional endocytic uptake of fluid-phase material via cytostome-like structures in many protozoa as well as in Spironucleus spp., the closest known relatives of Giardia [11]. PVs effectively act as “safety-lock" compartments for efficient environmental sampling, i.e., the uptake and intracellular sorting of gut content. The “kiss and flush” working model (Fig 1C) developed in our group is based on experimental data suggesting that PV membranes and the plasma membrane (PM) transiently fuse (the “kiss” phase) and become continuous, thereby generating an opening to the extracellular space allowing exchange of fluid-phase material between the PV lumen and the environment [12]. This PV–PM connection is then resolved, and sorting of usable nutrients occurs within the acidifying PV lumen. Any material that is not retained would then be released back into the extracellular space in a new round of PV–PM connection (the “flush” phase). The flushing of the PV lumen makes exchange of fluid-phase material bidirectional and likely compensates for the lack of bona fide lysosomes as endpoints of endocytic transport. Endocytosis through PVs is likely the main route of nutrient uptake into the Giardia cell, although there are isolated reports on receptor-mediated uptake of lipid particles. The giardial putative low-density lipoprotein (LDL) receptor (GILRP; purple ribbon in Fig 1C) in G. lamblia was shown to interact with AP2 components [13], although its exact trafficking mechanism remains uncharacterized (Fig 1C). Short actin filaments were also shown to be involved in LDL uptake and were localized in close proximity to PVs [14].
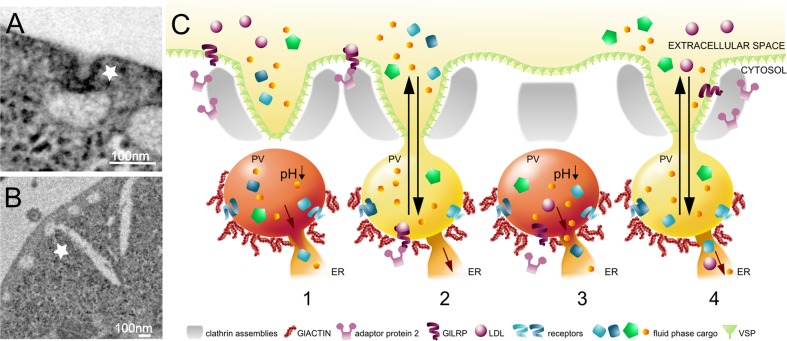
Fig 1. A working model for fluid-phase and receptor-mediated nutrient uptake through the PVs of G. lamblia.
(A) A TEM image of a PV making contact with a PM-derived invagination (white star). (B) Connections between PVs and ER membranes (white star) are frequently detected in TEM tomograms. (C) The “kiss and flush” working model for fluid-phase and receptor-mediated endocytosis in PVs is based on previously published data [12,13,14,27] and is represented as a continuum of nutrient entry, retention, release, and transfer. (1) Acidifying PVs contain fluid-phase cargo, which is either free or retained by PV-resident receptors (blue ribbons). Released cargo travels further to the lumen of connecting ER tubules. Some cargoes (green pentagon) can be excluded from further passage to the ER. (2) Uptake is mediated by fusion between the VSP-coated PM and the PV membrane through PM-derived invaginations surrounded by clathrin arrays associated to AP2 complexes. This event corresponds to the “kiss” phase in which formation of a channel allows exchange of fluid-phase material between the PV lumen and the extracellular space. Usable nutrients may move freely or be bound by receptors lining the organelle lumen, whereas useless or harmful molecules may be released back in the extracellular space during the “flush” phase. LDL receptor (GILRP) traffics in an AP2-dependent manner from the PM (1) to the PV membrane. (3) PV–PM luminal continuity terminates and a new round of receptor-bound nutrient release mediated by intralumenal acidification ends with further passage to connected ER. (4) The PV is now ready for another round of endocytosis and exchange with the extracellular environment and GILRP is recycled back to the cell’s surface. ER, endoplasmic reticulum; GILRP, giardial putative low-density lipoprotein receptor; LDL, low-density lipoprotein; PM, plasma membrane; PV, peripheral vacuole; TEM, transmission electron microscopy; VSP, variant surface protein.
Fact 4: G. lamblia survives in the environment as infectious cysts
Completion of the life cycle by transmission of G. lamblia to a new host requires no vectors and is based on the alternation of a vegetative stage, the trophozoite, and an environmentally resistant infectious stage—the cyst. The cyst is the only stage of G. lamblia able to survive outside of the host and is responsible for the initiation of a new infectious cycle. Cyst development may already begin in the small intestine of parasitized hosts when a variable fraction of proliferating trophozoites initiates a cellular differentiation program called encystation [15]. Laboratory protocols for inducing encystation include lipid depletion and an increase in culturing medium pH over a period of ca. 20–24 hours [16]. The assumption is that these conditions mimic decreasing lipid availability and ascending pH gradients naturally present along the gastrointestinal tract. During encystation, flagellated pear-shaped binucleated trophozoites undergo dramatic morphological and biochemical cellular remodeling, culminating in the formation of nonflagellated oval quadrinucleated cysts, surrounded by a cyst wall (CW). The CW is composed of a thick mesh of cyst wall proteins (CWPs) complexed to a unique sugar polymer of β1,3-linked-N-acetylgalactosamine [17] and virtually shields the cyst’s interior from any solvent. Deposition of the CW is a tightly-regulated event that occurs exclusively in encysting trophozoites and requires neogenesis of specialized secretory organelles called ESVs [18]. ESVs traffic, sort, and modify mainly CWPs from their initial site of deposition at the ER en route to the parasite cell’s surface. Elimination of a single CWP by complete gene disruption was shown to abolish CW formation altogether [19]. The exact in vivo stimuli for this process are not yet well known, although recent studies on the dynamics of encystation in animal models point towards a link between high-density focal trophozoite populations in the proximal small intestine and encystation [15]. These findings argue against the natural pH and lipid gradients being the sole external triggers for differentiation. In turn, this raises the interesting possibility that trophozoites can sufficiently alter the local environment to generate conditions favorable for triggering differentiation.
Cysts are then shed through host feces and were reported to remain viable for several months in water at temperatures below 10 °C and several weeks at room temperature [20]. Based on experimental gerbil (Meriones ungulatus) infections, the minimal infectious dose is less than 10 cysts [21]. Once viable cysts are ingested, passage through the stomach and physical stimuli (temperature, pH) initiate a cellular program termed excystation in which both host and parasite proteases collaborate to degrade the CW, allowing the short-lived excyzoite to escape and rapidly divide twice to give rise to four trophozoites [22]. In vitro (and most likely in vivo) this process is completed within minutes [23].
Fact 5: Novel rational design-based vaccination strategies against G. lamblia are yielding encouraging results
Currently, treatment of giardiasis in humans is based almost exclusively on administration of antiprotozoals belonging to the family of 5-nitroimidazoles, whereas infected animals are treated with benzimidazoles. A crude veterinary vaccine called GiardiaVax for nonchronically infected dogs and cats was already licensed [24]. However, it does not inhibit trophozoite proliferation and in many cases has to be combined with additional drug treatment. In recent years, work done especially by the Lujan group at the Universidad Católica de Córdoba in Argentina has uncovered the potential for variant surface proteins (VSPs) as effective vaccination antigens in companion animals. The entire trophozoite surface is covered by a dense coat of VSP anchored in the plasma membrane with a C-terminal hydrophobic transmembrane anchor sequence. From a repertoire of over 200 homologous genes encoded in the parasite genome, only one VSP is expressed on the surface of every single trophozoite at any given moment. Antigenic switching and regulation of VSP expression were shown to occur by RNAi-like mechanisms that could be perturbed to deregulate VSP production, leading to trophozoites exposing the full VSP panel on their surface [25]. Oral vaccination trials on gerbils showed that animals initially infected with deregulated cells expressing all of the VSPs encoded in their genome are largely protected from challenge infections by Giardia clones that express a unique VSP on their surface or by cysts obtained from infected individuals [25]. The same vaccine was tested on cats and dogs, showing high efficiency in preventing new infections and reducing chronic giardiasis in domestic animals both in experimental and natural infections [26]. These data are based on the rational design of vaccination strategies underpinned by a deep understanding of G. lamblia’s molecular and cell biology and hold great promise for eradication of giardiasis in both animals and humans.
Funding Statement
This work was supported by grant number 31003A_166437 awarded to ABH by the Swiss National Science Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ryan U, Caccio SM (2013) Zoonotic potential of Giardia. International journal for parasitology 43: 943–956. 10.1016/j.ijpara.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 2.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, et al. (2013) Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS ONE 8: e72788 10.1371/journal.pone.0072788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Xiao L (2011) Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clinical microbiology reviews 24: 110–140. 10.1128/CMR.00033-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends in parasitology 22: 203–208. 10.1016/j.pt.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 5.Halliez MC, Buret AG (2013) Extra-intestinal and long term consequences of Giardia duodenalis infections. World journal of gastroenterology 19: 8974–8985. 10.3748/wjg.v19.i47.8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litleskare S, Rortveit G, Eide GE, Hanevik K, Langeland N, et al. (2018) Prevalence of Irritable Bowel Syndrome and Chronic Fatigue 10 Years After Giardia Infection. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 16: 1064–1072.e1064. [DOI] [PubMed] [Google Scholar]
- 7.Faso C, Hehl AB (2011) Membrane trafficking and organelle biogenesis in Giardia lamblia: use it or lose it. International journal for parasitology 41: 471–480. 10.1016/j.ijpara.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 8.Roger AJ, Svard SG, Tovar J, Clark CG, Smith MW, et al. (1998) A mitochondrial-like chaperonin 60 gene in Giardia lamblia: Evidence that diplomonads once harbored an endosymbiont related to the progenitor of mitochondria. Proceedings of the National Academy of Sciences of the United States of America 95: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Embley TM, Hirt RP (1998) Early branching eukaryotes? Current opinion in genetics & development 8: 624–629. [DOI] [PubMed] [Google Scholar]
- 10.He D, Fiz-Palacios O, Fu CJ, Fehling J, Tsai CC, et al. (2014) An alternative root for the eukaryote tree of life. Current biology: CB 24: 465–470. 10.1016/j.cub.2014.01.036 [DOI] [PubMed] [Google Scholar]
- 11.Williams CF, Lloyd D, Poynton SL, Jorgensen A, Millet CO, et al. (2011) Spironucleus species: Economically-Important Fish Pathogens and Enigmatic Single-Celled Eukaryotes. Journal of Aquaculture Research & Development. [Google Scholar]
- 12.Zumthor JP, Cernikova L, Rout S, Kaech A, Faso C, et al. (2016) Static Clathrin Assemblies at the Peripheral Vacuole-Plasma Membrane Interface of the Parasitic Protozoan Giardia lamblia. PLoS Pathog 12: e1005756 10.1371/journal.ppat.1005756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivero MR, Jausoro I, Bisbal M, Feliziani C, Lanfredi-Rangel A, et al. (2013) Receptor-mediated endocytosis and trafficking between endosomal-lysosomal vacuoles in Giardia lamblia. Parasitology research 112: 1813–1818. 10.1007/s00436-012-3253-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paredez AR, Nayeri A, Xu JW, Krtkova J, Cande WZ (2014) Identification of obscure yet conserved actin-associated proteins in Giardia lamblia. Eukaryotic cell 13: 776–784. 10.1128/EC.00041-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barash NR, Nosala C, Pham JK, McInally SG, Gourguechon S, et al. (2017) Giardia Colonizes and Encysts in High-Density Foci in the Murine Small Intestine. mSphere 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morf L, Spycher C, Rehrauer H, Fournier CA, Morrison HG, et al. (2010) The transcriptional response to encystation stimuli in Giardia lamblia is restricted to a small set of genes. Eukaryotic cell 9: 1566–1576. 10.1128/EC.00100-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuelson J, Robbins P (2011) A simple fibril and lectin model for cyst walls of Entamoeba and perhaps Giardia. Trends in parasitology 27: 17–22. 10.1016/j.pt.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konrad C, Spycher C, Hehl AB (2010) Selective condensation drives partitioning and sequential secretion of cyst wall proteins in differentiating Giardia lamblia. PLoS Pathog 6: e1000835 10.1371/journal.ppat.1000835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebneter JA, Heusser SD, Schraner EM, Hehl AB, Faso C (2016) Cyst-Wall-Protein-1 is fundamental for Golgi-like organelle neogenesis and cyst-wall biosynthesis in Giardia lamblia. Nature communications 7: 13859 10.1038/ncomms13859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bingham AK, Jarroll EL Jr., Meyer EA, Radulescu S (1979) Giardia sp.: physical factors of excystation in vitro, and excystation vs eosin exclusion as determinants of viability. Experimental parasitology 47: 284–291. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer FW 3rd, Johnson CH, Hsu CH, Rice EW (1991) Determination of Giardia lamblia cyst infective dose for the Mongolian gerbil (Meriones unguiculatus). Applied and environmental microbiology 57: 2408–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernander R, Palm JE, Svard SG (2001) Genome ploidy in different stages of the Giardia lamblia life cycle. Cellular microbiology 3: 55–62. [DOI] [PubMed] [Google Scholar]
- 23.Hetsko ML, McCaffery JM, Svard SG, Meng TC, Que XC, et al. (1998) Cellular and transcriptional changes during excystation of Giardia lamblia in vitro. Experimental parasitology 88: 172–183. 10.1006/expr.1998.4246 [DOI] [PubMed] [Google Scholar]
- 24.Olson ME, Ceri H, Morck DW (2000) Giardia vaccination. Parasitology today 16: 213–217. [DOI] [PubMed] [Google Scholar]
- 25.Rivero FD, Saura A, Prucca CG, Carranza PG, Torri A, et al. (2010) Disruption of antigenic variation is crucial for effective parasite vaccine. Nature medicine 16: 551–557, 551p following 557. 10.1038/nm.2141 [DOI] [PubMed] [Google Scholar]
- 26.Serradell MC, Saura A, Rupil LL, Gargantini PR, Faya MI, et al. (2016) Vaccination of domestic animals with a novel oral vaccine prevents Giardia infections, alleviates signs of giardiasis and reduces transmission to humans. NPJ vaccines 1: 16018 10.1038/npjvaccines.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abodeely M, DuBois KN, Hehl A, Stefanic S, Sajid M, et al. (2009) A Contiguous Compartment Functions as Endoplasmic Reticulum and Endosome/Lysosome in Giardia lamblia. Eukaryotic cell 8: 1665–1676. 10.1128/EC.00123-09 [DOI] [PMC free article] [PubMed] [Google Scholar]