Abstract
Background:
The aim of this study was to systematically evaluate the prognostic role of pretreatment lactate dehydrogenase (LDH) concentration for survival in patients with lung cancer through performing a meta-analysis.
Methods:
PubMed, EMBASE, Cochrane Library, Web of Science, and China National Knowledge Infrastructure were searched for potentially relevant literature. The study and patients’ characteristics were extracted. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were pooled to estimate the prognostic role of LDH in patients with lung cancer.
Results:
Fourteen studies with 4084 patients were included. Higher pretreatment LDH concentration was significantly associated with an increased risk of overall mortality in patients with lung cancer (HR = 1.49, 95% CI, 1.38–1.59). Subgroup analysis of studies also resulted in a significantly increased risk of mortality in patients with small cell lung cancer (SCLC, HR = 1.54, 95% CI, 1.43–1.67) or nonsmall cell lung cancer (NSCLC, HR = 1.25, 95% CI, 1.06–1.46), with high pretreatment LDH concentration. No significant between-study heterogeneity was observed (I2 = 12.0%, P = .321). No significant publication bias was found (P = .352) in the meta-analysis.
Conclusion:
The results suggested that higher pretreatment LDH concentration was associated with worse overall survival in patients with lung cancer. The findings may assist future research on anticancer therapy by targeting LDH and help predict prognosis in lung cancer patients. However, high-quality studies are required to further research and support these associations. Moreover, confounding factors such as patient ethnicity and tumor type should be considered in future studies.
Keywords: lactate dehydrogenase, lung cancer, prognosis, survival
1. Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide.[1] Among patients with lung cancer, approximately 85% are nonsmall cell lung cancer (NSCLC).[2] About 15% are small cell lung cancer (SCLC), which is the most aggressive type of lung cancer.[3] Treatments for lung cancer patients mainly include surgery, chemotherapy, radiotherapy, and target therapy in recent years.[3,4] With improvement in diagnosis and treatment, the prognosis of lung cancer remains poor. The 5-year survival rates of NSCLC and SCLC were lower than 15% [1] and only 1% to 3%,[4] respectively. Also, the prognosis widely varies, so it is worthwhile to explore prognostic biomarkers for patients with lung cancer.
In recent years, serum lactate dehydrogenase (LDH) concentration was found to play a prognostic role in many tumors, such as Ewing sarcoma, urologic cancers, malignant mesothelioma, and nasopharyngeal carcinoma, and so on.[5–9] Some researchers also investigated the prognostic role of LDH in patients with lung cancer, but the results were not conclusive. Some studies demonstrated that higher LDH was associated with poorer prognosis in lung cancer patients.[10–14] However, some researchers found that this association was not significant.[4,15,16] Due to the controversy, we aimed to perform a meta-analysis to systematically evaluate the prognostic role of LDH in patients with lung cancer.
2. Methods
2.1. Search strategy
As this is a meta-analysis, ethical approval was not necessary. PubMed, EMBASE, Cochrane Library, Web of Science, and China National Knowledge Infrastructure (CNKI) were searched for potentially eligible studies (last update ran on April 5, 2018). The following keywords were used: (“lung neoplasms” OR “lung cancer” OR “lung carcinoma”) AND (“lactate dehydrogenase”) AND (“prognosis” OR “outcome” OR “survival” OR “mortality”) (Table 1). Additional literature was located through screening reference lists of previous systematic reviews and included studies. No language restrictions were adopted.
Table 1.
Search strategies.

2.2. Study selection
Two investigators performed the study selection process independently, and disagreements were resolved by consensus. Studies were considered eligible if they met all of the following inclusion criteria: the patients were diagnosed with lung cancer by histopathological examination; the LDH of the patients were measured; patients were followed up for survival outcomes; and enough data were reported to estimate the prognostic value of LDH in lung cancer patients. Study types were not restricted: retrospective/prospective or random clinical studies/observational studies. However, reviews, case reports, conference abstracts, unrelated articles, and studies without enough data were excluded.
2.3. Data extraction
The data extraction process was also performed by 2 authors independently, with any disagreements being discussed. The primary data included hazard ratio (HR) for overall survival (OS) with 95% confidence interval (95% CI). Multivariate analyses data were extracted over univariate analyses data. The basic characteristics of the studies and patients were also extracted, including first author, publication year, country, the number of patients, sex of patients, age of patients, tumor subtype, and so on.
2.4. Study quality assessment
The Newcastle–Ottawa Scale (NOS) criteria were used to assess the quality of the included studies.[17] The NOS scale assessed 3 perspectives of the study: subject selection; comparability of subject; and exposure (for case–control studies)/outcome (for cohort studies). The NOS scores ranged from 0 to 9, and studies with 7 scores or more were considered as high-quality studies.
2.5. Statistical analysis
Forest plots were constructed to estimate the pooled prognostic value of LDH in patients with lung cancer. The pooled HR was considered significant if the P value was less than .05. The between-study heterogeneity was also assessed, with I2 > 50% or P < .10 indicating significant heterogeneity. Random effect models were used and sensitivity analysis was performed if significant heterogeneity existed. Subgroup analyses were also performed according to patient source and tumor type. Publication bias was assessed by Begg [18] and Egger tests,[19] with P > .10 implying no significant publication bias. The statistical analyses were performed by STATA 11.0 (STATA Corporation, College Station, TX).
3. Results
3.1. Literature research
The initial literature search identified 2127 citations. Among them, 589 were duplicated and were removed. The rest 1538 studies were screened by titles and abstracts, and 1492 were excluded according to the inclusion and exclusion criteria. The rest 46 studies were assessed in full text and 32 were further excluded. Eventually, 14 articles [4,10–16,20–25] met the inclusion criteria and were included. The study selection process is shown in Fig. 1.
Figure 1.
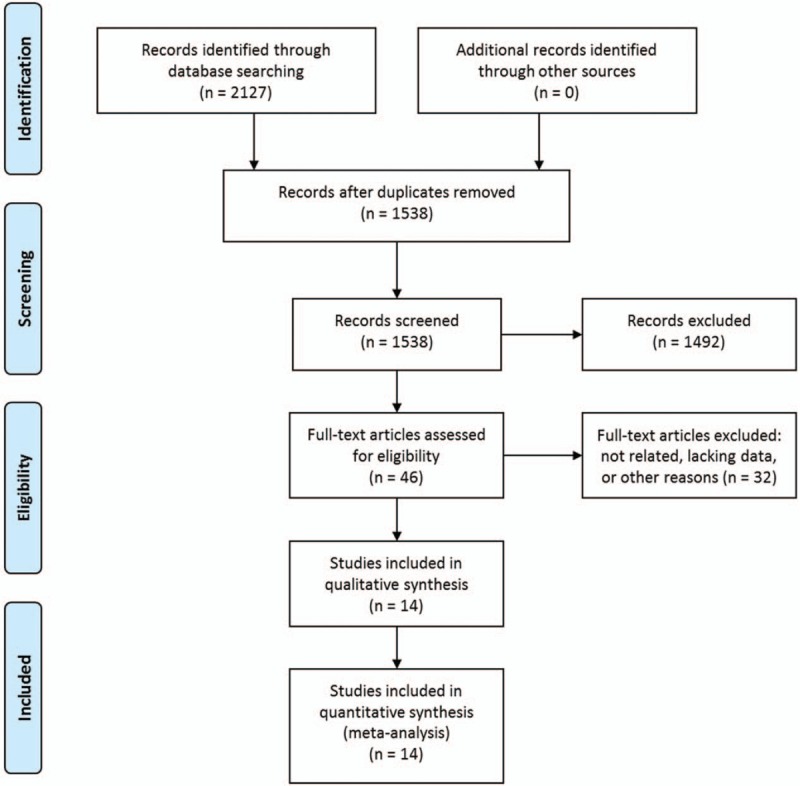
Selection process of studies.
3.2. Study characteristics
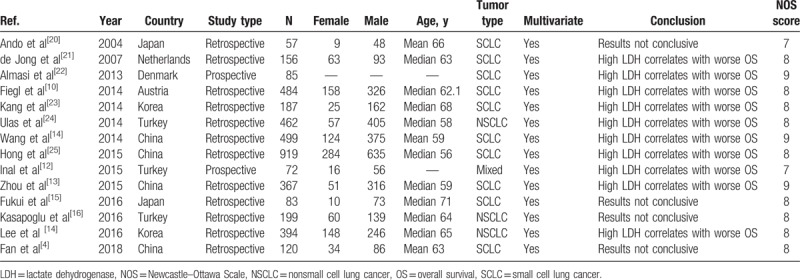
The basic characteristics of the 14 included studies are summarized in Table 2. The studies were published between 2004 and 2018, and 12 of them were published in the last 5 years. The studies were conducted in 7 different countries. Two of them were prospective studies. A total of 4084 patients were included. In each study, the number of male patients was higher than that of female patients. The tumor types mainly included NSCLC and SCLC. The survival outcomes were all OS, and the HRs were all from multivariate analyses. Among the 14 studies, 10 studies concluded that higher LDH was associated with worse survival in lung cancer patients. The NOS scores were all above 7, suggesting the qualities of the studies were good.
Table 2.
Characteristics of the included studies.
3.3. Association between LDH concentration and overall mortality risk
After pooling the results of the 14 studies together, higher pretreatment LDH concentration was significantly associated with an increased risk of overall mortality in patients with lung cancer (HR = 1.49, 95% CI, 1.38–1.59) (Fig. 2). No significant between-study heterogeneity was observed (I2 = 12.0%, P = .321).
Figure 2.
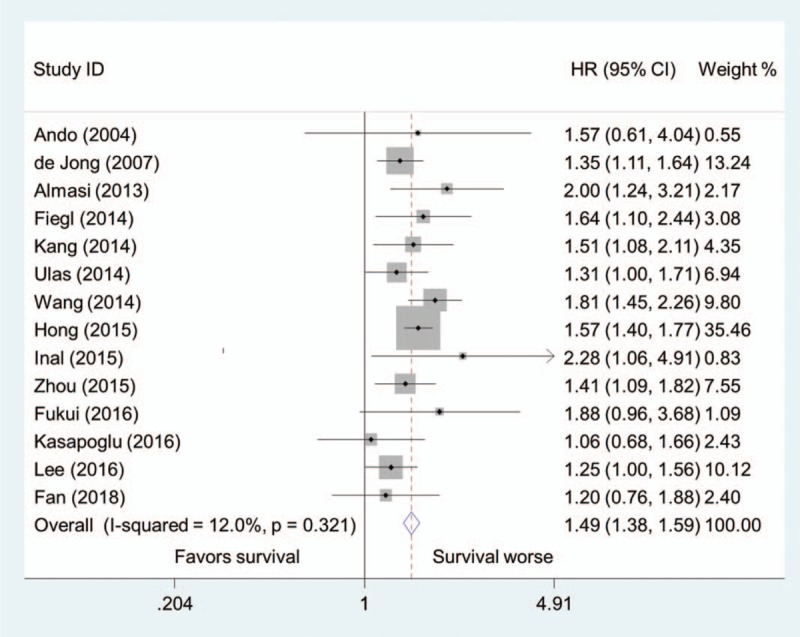
Pooled hazard ratio of higher pretreatment LDH concentration for overall survival in patients with lung cancer.
3.4. Subgroup analysis
3.4.1. Patient source
The 14 studies were divided into Asian group (8 studies) and Caucasian group (6 studies) according to patients’ source. Subgroup analysis showed that patients with higher pretreatment LDH concentration had a significantly increased risk of mortality in both Asian group (HR = 1.52, 95% CI, 1.40–1.65; I2 = 7.0%, P = .376) and Caucasian group (HR = 1.40, 95% CI, 1.23–1.60; I2 = 19.4%, P = .287) (Fig. 3).
Figure 3.
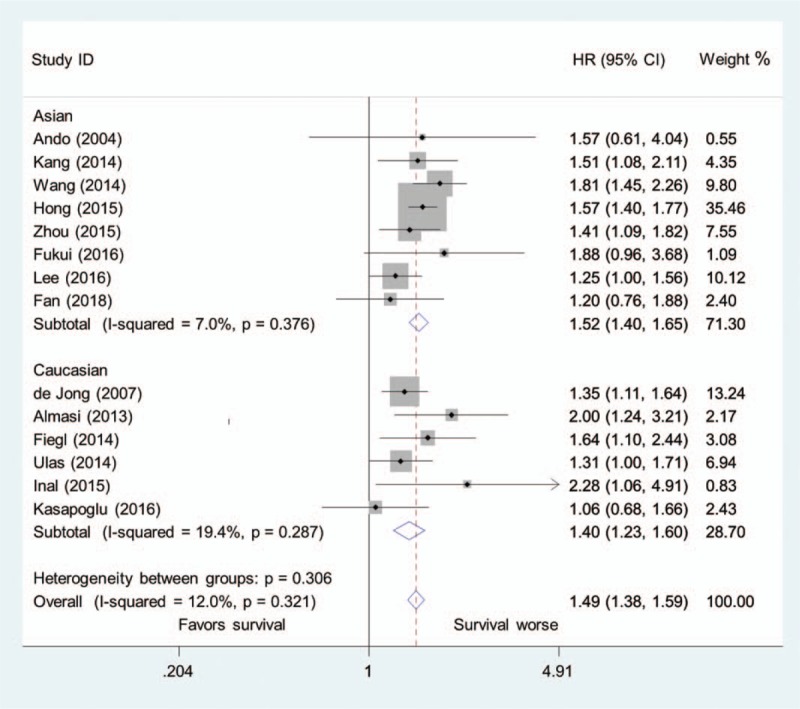
Pooled hazard ratio of higher pretreatment LDH concentration for overall survival in patients with lung cancer in the Asian group and Caucasian group.
3.4.2. Tumor type
Of the 14 studies, 10 examined SCLC, 3 examined NSCLC, and 1 examined mixed lung cancers. Subgroup analysis of studies also resulted in a significantly increased risk of mortality in patients with SCLC (HR = 1.54, 95% CI, 1.43–1.67; I2 = 0.0%, P = .615) or NSCLC (HR = 1.25, 95% CI, 1.06–1.46; I2 = 0.0%, P = .723), with high pretreatment LDH concentration (Fig. 4).
Figure 4.
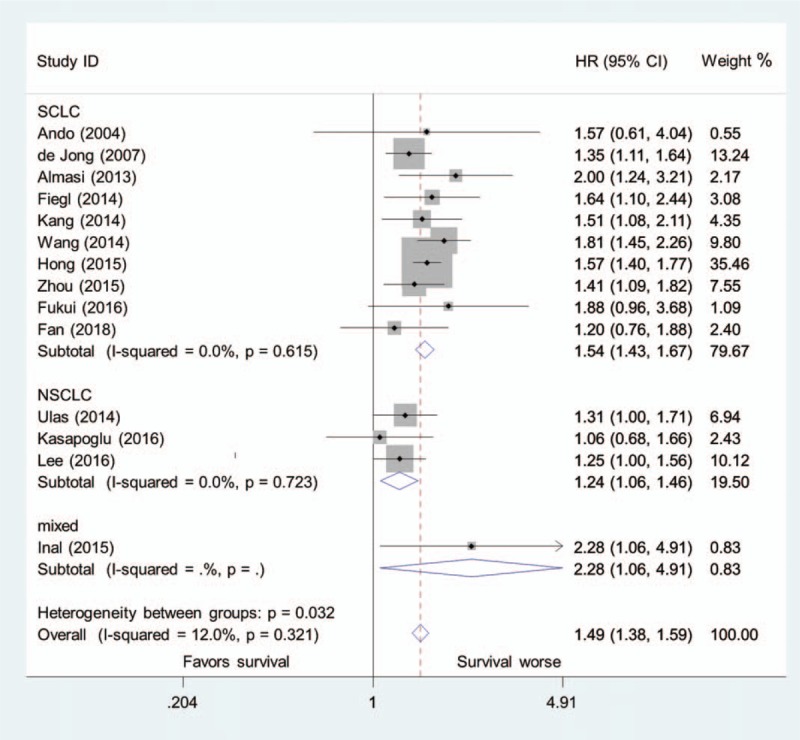
Pooled hazard ratio of higher pretreatment LDH concentration for overall survival in patients with SCLC, NSCLC, and mixed lung cancers.
All the meta-analyses results above are summarized in Table 3.
Table 3.
Summary of meta-analysis results.
3.4.3. Publication bias
No significant publication bias was found in the meta-analysis (P = .352 for Begg test and .951 for Egger test). The Begg plot of publication bias of the 14 studies is shown in Fig. 5.
Figure 5.
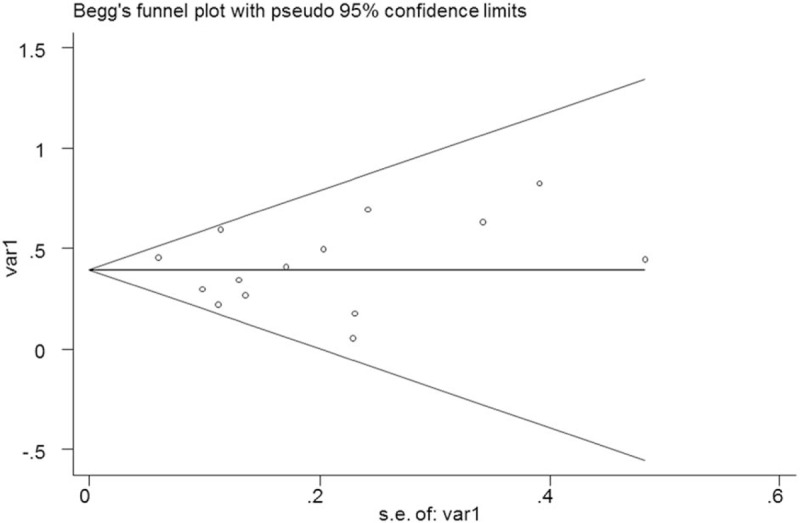
The Begg publication bias plot of the 14 included studies.
4. Discussion
This study aimed to evaluate the prognostic role of LDH in patients with lung cancer. We performed a meta-analysis to summarize the evidence, and 14 studies were included. Our results suggested that higher LDH was associated with poorer OS in patients with lung cancer.
Subgroup analyses were also performed to further explore the role of LDH in patients with lung cancer. In both Asian group and Caucasian group, higher LDH was found to be associated with worse OS in patients with lung cancer. The pooled HR in the Asian group was slightly higher than that in the Caucasian group, suggesting that LDH might better predict the prognosis in Asian populations. As to different tumor types, the pooled HRs were all significant, and the pooled HR was the highest in patients with mixed lung cancer and the lowest in patients with NSCLC. These findings suggested that LDH might not be a good prognostic marker in patients with NSCLC. However, due to the limited number of studies in the subgroups, caution should be applied as to the subgroup analyses results, and more studies are needed to verify these findings.
LDH is distributed in many tissues of the human body. LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid. The reaction between lactic acid and pyruvic acid usually occurs under anaerobic conditions, such as intratumoral environment. The concentration of serum LDH is elevated in various diseases, including many malignant tumors. High concentrations of LDH reflex anaerobic glycolytic metabolism in the tumor environment.[14] Lee et al[14] also found that, compared with the low metastatic score group, the patients in the high metastatic score group had significantly higher concentrations of serum LDH. Therefore, the concentrations of LDH reflect the extent of many tumors and could serve as a nonspecific marker,[14] which might be an underlying mechanism why high concentrations of LDH are associated with worse OS of lung cancer patients.
The findings of this study may imply potential therapeutic implications (targeting LDH) in patients with lung cancer. Some researchers have investigated the effect of lowering LDH concentration in cancer patients. Yang et al [26] investigated the effects of oxamate, a classic inhibitor of LDH-A, in both NSCLC cells and normal lung epithelial cells. They found that oxamate significantly suppressed the proliferation of NSCLC cells, and it had a much lower toxicity in normal cells. Their results suggested the potential use of targeting LDH-A in NSCLC treatment. Koukourakis et al[27] examined the role of the addition of vatalanib, a VEGF-receptor inhibitor, to FOLFOX 4 in colorectal cancer patients with high serum LDH concentrations. Their results showed that the addition of vatalanib diminished the effect of LDH expression on the prognosis of patients. Thus, our results further support the therapeutic potential of targeting LDH in patients with lung cancer.
Although most studies concluded that higher pretreatment LDH concentration was associated with worse OS in patients with lung cancer,[10–14,21–25] some studies did not come to this conclusion.[4,15,16,20] LDH is increased not only in cancer patients but also in other diseases, for example, infections, heart failure, acute pancreatitis and anemia, and so on.[4,28] Therefore, other factors that influence LDH concentrations may result in the insignificant association between LDH concentrations and survival.[4] In future studies, patients with other diseases that may influence the LDH concentrations should be excluded.
Kasapoglu et al[16] investigated the factors influencing survival in NSCLC patients with malignant pleural effusions. Interestingly, they found that the survival time was significantly longer in patients with blood LDH concentrations less than 250 U/L than those with more than 250 U/L; however, the survival time was not significantly different between the patients with pleural fluid LDH concentrations less than 250 U/L and those with more than 250 U/L.[16] These results may suggest that blood LDH concentrations could better predict survival than pleural fluid LDH concentrations, but more research is needed to verify this.
Apart from LDH, many other factors have been found in the prognosis of lung cancer, such as plasma fibrinogen, serum hemoglobin concentration, D-dimer plasma concentration, white blood cell count, platelet count, serum albumin concentration, C-reactive protein/albumin ratio, and so on.[4,12,13,15,16,25] The assessment of LDH is easy and inexpensive. This readily accessible and cheap prognostic factor may serve as a promising tool in clinical work. In the future, serum LDH concentrations could be used to predict the survival of lung cancer along with other well-established biomarkers through performing multidimensional assessment of the known markers to improve prognostic algorithms.[14]
There are some limitations in our meta-analysis. First, the number of included studies in our meta-analysis was limited. And the numbers of studies in the subgroups were even smaller. Therefore, the results of this meta-analysis should be treated with caution. More studies are needed to verify our findings. Second, the characteristics of the studies and patients between the included articles varied. For example, the ethnicity and tumor type of the patients were not the same. Furthermore, although no significant between-study heterogeneity was observed in our meta-analysis, the I2 was not 0%, suggesting difference between the results of the studies. Besides, publication bias should not be completely excluded, although no significant publication bias was found in our meta-analysis, as it was a major concern for all meta-analyses.
In conclusion, our results suggested that higher pretreatment LDH concentration was associated with worse OS in patients with lung cancer. The findings may assist future research on anticancer therapy by targeting LDH and help predict prognosis in lung cancer patients. However, due to the limited number of included studies, more well-designed studies are warranted to further verify our results.
Author contributions
Conceptualization: Taibing Deng, Yu Meng, Weimin Li.
Data curation: Taibing Deng, Jing Zhang, Yu Meng, Yong-Zhao Zhou.
Formal analysis: Taibing Deng, Jing Zhang, Yu Meng.
Funding acquisition: Taibing Deng, Yu Meng, Weimin Li.
Investigation: Yu Meng.
Methodology: Taibing Deng, Jing Zhang, Yu Meng.
Project administration: Weimin Li.
Resources: Yong-Zhao Zhou.
Software: Taibing Deng, Jing Zhang, Yu Meng, Yong-Zhao Zhou.
Supervision: Weimin Li.
Validation: Jing Zhang, Yu Meng.
Writing – original draft: Taibing Deng, Jing Zhang, Yu Meng.
Writing – review & editing: Taibing Deng, Jing Zhang, Yu Meng, Yong-Zhao Zhou, Weimin Li.
Footnotes
Abbreviations: CI = confidence interval, CNKI = China National Knowledge Infrastructure, HR = hazard ratio, LDH = lactate dehydrogenase, NOS = Newcastle–Ottawa Scale, NSCLC = nonsmall cell lung cancer, OS = overall survival, SCLC = small cell lung cancer.
TD, JZ, and YM contributed equally to this work.
Funding/support: This work was supported by the Transformation Projects of Sci-Tech Achievements of Sichuan Province (2016CZYD0001) and the Sci-Tech Support Program of Science and Technology Department of Sichuan Province (2016SZ0073).
The authors declare no conflict of interest in preparing this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: new biological insights and recent therapeutic advances. CA Cancer J Clin 2011;61:91–112. [DOI] [PubMed] [Google Scholar]
- [3].Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fan S, Guan Y, Zhao G, et al. Association between plasma fibrinogen and survival in patients with small-cell lung carcinoma. Thorac Cancer 2018;9:146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li S, Yang Q, Wang H, et al. Prognostic significance of serum lactate dehydrogenase levels in Ewing's sarcoma: a meta-analysis. Mol Clin Oncol 2016;5:832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shen J, Chen Z, Zhuang Q, et al. Prognostic value of serum lactate dehydrogenase in renal cell carcinoma: a systematic review and meta-analysis. PLoS One 2016;11:e0166482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang Y, Xu T, Wang Y, et al. Prognostic role of lactate dehydrogenase expression in urologic cancers: a systematic review and meta-analysis. Oncol Res Treat 2016;39:592–604. [DOI] [PubMed] [Google Scholar]
- [8].Zhuo Y, Lin L, Wei S, et al. Pretreatment elevated serum lactate dehydrogenase as a significant prognostic factor in malignant mesothelioma: a meta-analysis. Medicine (Baltimore) 2016;95:e5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhai C, Gu K, Zhai X, et al. Prognostic value of serum lactate dehydrogenase in patients with nasopharyngeal carcinoma: a meta-analysis. Clin Lab 2017;63:1777–85. [DOI] [PubMed] [Google Scholar]
- [10].Fiegl M, Pircher A, Waldthaler C, et al. Small steps of improvement in small-cell lung cancer (SCLC) within two decades: a comprehensive analysis of 484 patients. Lung Cancer 2014;84:168–74. [DOI] [PubMed] [Google Scholar]
- [11].Wang X, Jiang R, Li K. Prognostic significance of pretreatment laboratory parameters in combined small-cell lung cancer. Cell Biochem Biophys 2014;69:633–40. [DOI] [PubMed] [Google Scholar]
- [12].Inal T, Anar C, Polat G, et al. The prognostic value of D-dimer in lung cancer. Clin Respir J 2015;9:305–13. [DOI] [PubMed] [Google Scholar]
- [13].Zhou T, Zhan JH, Hong SD, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep 2015;5:10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee DS, Park KR, Kim SJ, et al. Serum lactate dehydrogenase levels at presentation in stage IV non-small cell lung cancer: predictive value of metastases and relation to survival outcomes. Tumour Biol 2016;37:619–25. [DOI] [PubMed] [Google Scholar]
- [15].Fukui T, Itabashi M, Ishihara M, et al. Prognostic factors affecting the risk of thoracic progression in extensive-stage small cell lung cancer. BMC Cancer 2016;16:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kasapoglu US, Arinc S, Gungor S, et al. Prognostic factors affecting survival in non-small cell lung carcinoma patients with malignant pleural effusions. Clin Respir J 2016;10:791–9. [DOI] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [19].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ando S, Suzuki M, Yamamoto N, et al. The prognostic value of both neuron-specific enolase (NSE) and Cyfra21-1 in small cell lung cancer. Anticancer Res 2004;24:1941–6. [PubMed] [Google Scholar]
- [21].de Jong WK, Fidler V, Groen HJ. Prognostic classification with laboratory parameters or imaging techniques in small-cell lung cancer. Clin Lung Cancer 2007;8:376–81. [DOI] [PubMed] [Google Scholar]
- [22].Almasi CE, Drivsholm L, Pappot H, et al. The liberated domain I of urokinase plasminogen activator receptor: a new tumour marker in small cell lung cancer. APMIS 2013;121:189–96. [DOI] [PubMed] [Google Scholar]
- [23].Kang MH, Go SI, Song HN, et al. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer 2014;111:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ulas A, Turkoz FP, Silay K, et al. A laboratory prognostic index model for patients with advanced non-small cell lung cancer. PLoS One 2014;9:e114471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015;236:297–304. [DOI] [PubMed] [Google Scholar]
- [26].Yang Y, Su D, Zhao L, et al. Different effects of LDH-A inhibition by oxamate in non-small cell lung cancer cells. Oncotarget 2014;5:11886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res 2011;17:4892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ackers C, Rustin GJ. Lactate dehydrogenase is not a useful marker for relapse in patients on surveillance for stage I germ cell tumours. Br J Cancer 2006;94:1231–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


