Abstract
Rationale:
Basiliximab and etanercept have achieved promising responses in steroid-refractory graft versus host disease (SR-GVHD). However, the in vivo immune changes following the treatment have not been elucidated.
Patient concerns:
A 14-year-old boy presented with skin rash and diarrhea 20 days after haploidentical hemotopoietic stem cell transplantation.
Diagnoses:
We made the diagnose of grade 3 acute GVHD with skin and gastrointestinal involvement.
Interventions:
After the failure of the first-line treatment with methylprednisolone, combined anti-cytokine therapies with basiliximab and etanercept were prescibed.
Outcomes:
He achieved complete remission by basiliximab and etanercept. Furthermore, we detected that donor CD3+CD56+ Natural killer T(NKT)-like cells expanded gradually after the period of lymphocytopenia caused by GVHD and anti-cytokine therapy. The expansion of NKT-like cells was in association with high serum IFN-γ. NKT-like cells showed preferred proliferation in response to IFN-γ and potent cytotoxicity against leukemia cells. The expansion persisted > 2 years and the patient had a leukemia-free survival of 66 months.
Lessons:
Our case indicated that combined anti-cytokine treatment may reset the immune system and cause NKT-like cells to exhibit a predilection for expansion.
Keywords: basiliximab, etanercept, interferon gamma, natural killer T cells, steroid-refractory acute GVHD
1. Introduction
Treatment options for steroid-refractory graft versus host disease (SR-GVHD) are limited and the prognosis is poor.[1] Inflammatory cytokines are important mediators of GVHD, and may be critical targets for therapy.[2] Our previous study showed that the combination of a CD25 monoclonal antibody (basiliximab) with a recombinant tumor necrosis factor (TNF) receptor/immunoglobulin G fusion protein (etanercept) induced a high response rate in patients with severe SR-GVHD;[3] however, the in vivo changes following the treatment with basiliximab and etanercept have not been elucidated. We herein report an unusual case of long-lasting expansion of donor-derived CD3+CD56+ natural killer T (NKT)-like cells after treatment of SR-GVHD with basiliximab and etanercept.
2. Case presentation
A 14-year-old boy was diagnosed with high-risk acute lymphocytic leukemia (ALL) and relapsed after 3 cycles of intensive chemotherapy on the Chinese ALL 2005 protocol.[4] He achieved complete remission after chemotherapy, which consisted of idarubicin, vincristine, dexamethasone, and pegaspargase. He then underwent a peripheral blood stem cell transplantation from his human leukocyte antigen-haploidentical mother. The conditioning regimen consisted of cytarabine (4 g/m2/d) for 2 days, busulfan (3.2 mg/kg2/d) for 3 days, cyclophosphamide (1.8 g/m2/d) for 2 days, semustine (MeCCNU [250 mg/m2/d]) for 1 day, and anti-T-lymphocyte globulin ([ATG-F], 2.5 mg/kg/d; Fresenius, Bad Homburg, Germany) for 4 days. GVHD prophylaxis consisted of cyclosporin A, methotrexate, and mycophenolate mofetil. Twenty days after transplantation, he developed grade 3 acute GVHD with skin and gastrointestinal involvement that was refractory to first-line treatment with methylprednisolone (2 mg/kg). Therefore, he received second-line treatment with basiliximab (20 mg/d, days 1, 4, 8, and 15) and etanercept (25 mg/d, days 1, 4, 8, 11, 15, and 22). The GVHD symptoms were well controlled after the combination treatment. We routinely detected the lymphocyte phenotype before and after therapy by flow cytometry. Lymphocytopenia developed in the first 4 weeks and resolved 8 weeks after the first dose of treatment. The first restored subgroup of lymphocytes was CD3−CD56+ natural killer (NK) cells. We subsequently detected a gradual increase in the number of CD3+CD56+ NKT-like cells beginning 6 weeks after the initiation of combination treatment, along with a rise in the number of CD3+CD56− T cells and a decrease in the number of CD3−CD56+NK cells. At 12 weeks after the treatment, the percentage of CD3+CD56+ NKT-like cells reached a peak value of 37%, and decreased slightly thereafter (Fig. 1, A and B). Chimerism analysis using short tandem repeats after cell sorting showed that the expanded NKT cells were complete donor chimerism and a PCR assay showed no evidence of clonal T-cell antigen receptor (TCR) gene rearrangement.
Figure 1.
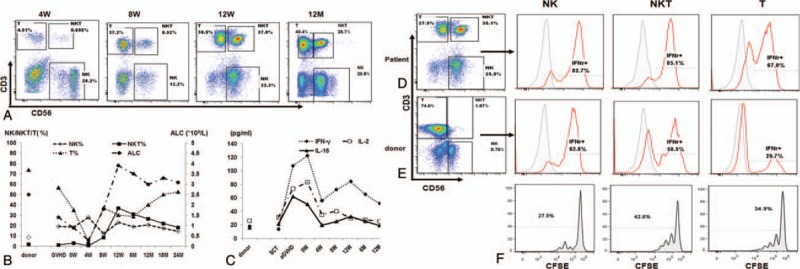
Expansion of CD3+CD56+ NKT cells in peripheral blood (PB) after treatment with basiliximab and etanercept and its association with elevated serum IFN-γ. A, Representative flow cytometry dot plot diagrams of PB cells stained with APC-conjugated anti-CD56 monoclonal antibodies (mAbs) and PE-CY7-conjugated anti-CD3 mAbs at the indicated time∗. B, The absolute lymphocyte count (ALC) and the percentage of NKT cells, NK cells, and T cells in the maternal PB and in the patient's PB at the indicated time∗. C, The serum levels of IFN-γ, IL-2, and IL-15 at the indicated time points. D and E, After activation of by PMA/ionomycin, IFN-γ production by NK, NKT, and T cells collected from the patient at 8 weeks (D) and his mother (donor; E) were detected by flow cytometry. F, Carboxyfluorescein succinimidyl ester (CFSE)-labeled NKT, T, and NK cells were cultured with 500 IU/mL IFN- γ for 96 hours. The CFSE dilution was detected using flow cytometry. The percentage of CFSElow cells means the percentage of proliferated cells in each subgroup. ∗SCT indicates the time of stem cell transplantation, 4 weeks (W), 8 W, 12 W, 6 months (M), 12 M, 18 M, and 24 M mean time points after the first dose of basiliximab and etanercept. CFSE = carboxyfluorescein succinimidyl ester, IFN = interferon, IL = interleukin, NK = natural killer cells, NKT = natural killer T cells, PMA = phorbol 12-myristate 13-acetate.
CD3+CD56+ NKT-like cells are a broad group of CD3+ T cells coexpressing TCR and NK-cell markers, sharing both NK and T cell characteristics. Studies have shown an altered quantity and quality of CD3+CD56+NKT-like cells in patients with leukemia or other cancers;[5,6] however, the function and clinical relevance of CD3+CD56+ NKT-like cells in recipients of hemotopoietic stem cell transplantation (HSCT) remains largely unexplored. Given the important roles of cytokines in the generation of CD3+CD56+ NKT cells, CD3+CD56+ NKT cells are also referred to as cytokine-induced killer cells. Thus, we are of the opinion that several cytokines induced the predominant expansion of this subgroup of CD3+CD56+ cells in this patient, so we analyzed the serum levels of interferon gamma (IFN-γ), interleukin 2(IL-2), and IL-15 at several time points. The serum levels of IFN-γ, IL-2, and IL-15 significantly increased at the time of GVHD. After GVHD was controlled, serum IL-2 and IL-15 levels decreased to baseline; however, serum IFN-γ levels decreased slightly after the treatment, but increased again and reached a second peak of 72.3 pg/mL 8 weeks after treatment, which coincided with the maximum number of CD3+CD56+ NKT-like cells (Fig. 1, B and C). Simultaneously, we collected lymphocytes from the patient and his mother (the donor) 8 weeks after the anticytokine treatment for IFN-γ production assay and noted a completely different model of IFN-γ production by the 3 subgroups of lymphocytes from the patient and his mother (Fig. 1, D and E). After activation by phorbol 12-myristate 13-acetate +ionomycin, the patient NK, NKT-like, and T cells produced a high-level of IFN-γ; however, when maternal lymphocytes were analyzed, only NK cells produced a high-level of IFN-γ, while T cells and NKT cells produced much less IFN-γ than the same subgroup from the patient. Furthermore, CD3+CD56+ NKT-like cells from the patient proliferated more potently after culture with IFN-γ than CD3+CD56− T cells and CD3−CD56+ NK cells (Fig. 1F).
Further phenotype analysis showed the expanded CD3+CD56+ NKT-like cells were predominately positive for CD8; expressed killer-cell immunoglobulin-like receptors (KIRs), NKG2D, DNAM-1, NKP46, CD27, CD69, and PD-1, and were negative for NKG2A, CTLA-4, and TIM-3 (Fig. 2, A and B). At 12 weeks after the treatment, we sorted the CD3+CD56+ NKT-like cells, CD3+CD56− T cells, and CD3−CD56+NK cells by fluorescence-activated cell sorting (FACS; Fig. 2C) and compared the cytotoxicity against an acute lymphocytic leukemia cell line (Reh cells) by a flow cytometric cytotoxicity assay.[7] NKT-like cells showed more potent cytotoxicity against Reh cells than T cells, and nearly matched the most potent killer NK cells (Fig. 2D). Similar results were observed using a degranulation assay (Fig. 2E).
Figure 2.
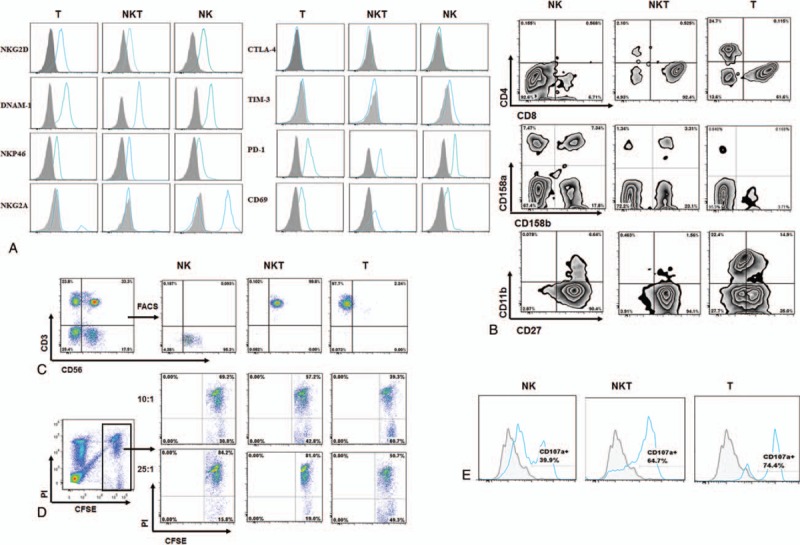
Phenotype and cytotoxicity of the expanded NKT-like cells. A, B, The phenotype of T, NKT, and NK cells was measured by flow cytometry. C, CD3+CD56+ NKT cells, CD3-CD56+NK cells, and CD3+CD56-T cells were sorted by FACS. D, Cytotoxicity of NK, NKT, and T cells against Reh cells was evaluated in a 4 hours CFSE/PI flow cytometry assay. Reh cells were labeled with CFSE and cocultured with NK, NKT, and T cells for 4 hours at 37°C at the ratios indicated above. At the end of the experiment, dead cells were labeled with PI. The percentage of Reh cell death (PIpositive in CFSEhigh) was analyzed by a flow cytometer. E, Degranulation of NK, NKT, and T cells was measured by CD107a expression following stimulation with Reh cells. CFSE = carboxyfluorescein succinimidyl ester, FACS = fluorescence-activated cell sorting, NK = natural killer cells, NKT = natural killer T cells.
Given that the expansion of functional CD3+CD56+ NKT-like cells lasted > 2 years and the patient had a leukemia-free survival of 66 months after HSCT at the time reported, we propose that the long-lasting expansion of CD3+CD56+ NKT cells contributes to the control of lymphocytic leukemia.
This study was approved by the Ethics Committee of The First Affiliated Hospital, Zhejiang University, School of Medicine and registered at the Chinese Clinical Trial Registry (www.chictr.org.cn) (Identifier: ChiCTR-OCH-12002890). The patient also provided informed consent.
3. Discussion
Kim et al[8] reported that large granular lymphocytosis could be detected in approximately 20% of recipients after allogeneic HSCT(allo-HSCT), and might lead to a favorable outcome and low risk of relapse; however, expansion of CD3+CD56+ NKT cells is rarely reported in recipients of allo-HSCT,[9] and to our knowledge, this is the first report of long-lasting expansion of CD3+CD56+ NKT cells after control of SR-GVHD with anticytokine treatment. In this case, NKT cell expansion generated gradually after the period of lymphocytopenia caused by GVHD and anticytokine therapy in association with fluctuating serum cytokines. Although the underlying mechanism could not be determined, we presume that the fluctuating serum cytokines and the contraction of donor-derived T-cell proliferation by basiliximab and etanercept contributes to NKT cell expansion.
CD3+CD56+ NKT cells represent only 2.94% ± 1.49% of lymphocytes in the peripheral blood of healthy adults and approximately 5.63% ± 2.93% of peripheral blood lymphocytes of allo-HSCT recipients 12 weeks after transplantation (unpublished data). Numerous studies have confirmed that CD3+CD56+ NKT cells can be generated in vitro using exogenous IL-2, IL-15, IFN-γ, and anti-CD3,[10] while several reports have detected in vivo expansion of CD3+CD56+ NKT cells in special situations.[9,11] Kishi et al[9] reported a case of peripheral CD3+CD56+ T-cell expansion in hyperacute GVHD and presumed the cytokine storm in hyperacute GVHD might promote the generation of CD3+CD56+ NKT cells. We found that the serum levels of IL-2, IFN-γ, and IL-15, which played important roles in the proliferation and cytotoxicity of NKT cells, were elevated significantly during GVHD and decreased after the control of GVHD. Interestingly, serum levels of IFN-γ did not return to baseline. IFN-γ has been shown to play critical roles in the proliferation and cytotoxicity of NKT cells in several studies.[12,13] Okita et al[14] reported that basiliximab augments IFN-γ production by activated peripheral blood mononuclear cells. Similarly, we found a higher level of IFN-γ production by NKT and T cells in the current patient than his healthy mother, which might contribute to the persistent high serum IFN-γ level and supported the persistent proliferation and potent cytotoxicity of NKT cells.
The combined treatment with basiliximab and etanercept might restrict the proliferation of allogeneic reactive and normal T cells, while taking advantage of the proliferation of NKT cells responding to the increased serum cytokines. Basiliximab, also known as anti-CD25, binds the high-affinity α-chain of the IL-2 receptor (IL-2R), thereby inhibiting high-affinity IL-2R signaling, but not intermediate-affinity IL-2R signaling.[15] Therefore, we reasoned that basiliximab might deprive IL-2 from high-affinity IL-2R signaling in activated T cells, while increasing IL-2 availability for intermediate-affinity IL-2R signaling in NK and NKT cells, warranting the priority of innate immune cell proliferation. It has been reported that anti-CD25 therapy by daclizumab leads to expansion of CD56 bright NK cells in patients with multiple sclerosis;[16,17] however, similar results have not been reported in GVHD patients.
Taken together, we propose 3 essential factors, as follows: the cytokine storm induced by GVHD; the persistent high serum IFN-γ levels by autocrine NKT and paracrine NK and T cells; and the potent inhibition of adaptive T-cell immunity by combined anticytokine treatment, might have led to the predilection for functional NKT cell expansion in this case. Therefore, this preliminary data might open the door to explore the hypotheses generated in this single case. Further studies are needed to explore the underlining mechanism of cytokine surges and lymphocytic subsets changes after the combination of basiliximab and etanercept for GVHD patients in the context of allo-HSCT.
4. Conclusions
We described the first time that long-lasting expansion of CD3 + CD56 + NKT was in association with fluctuating serum cytokines after anticytokine therapy in a case of SR-GVHD. Although the underlying mechanism could not be determined in a single case, our case warrants further studies to explore the roles of cytokines and NKT-like cells after anticytokine treatments for GVHD patients.
Author contributions
Conceptualization: Lixia Sheng, Yamin Tan, Yongxian Hu, Yi Luo, Guifang Ouyang.
Data curation: Lixia Sheng, Yamin Tan, He Huang.
Formal analysis: Lixia Sheng, Yamin Tan, Yongxian Hu, He Huang.
Funding acquisition: Lixia Sheng, Yongxian Hu, Qitian Mu, He Huang.
Investigation: Lixia Sheng, Huarui Fu, Jimin Shi, Guifang Ouyang.
Methodology: Lixia Sheng, Huarui Fu, Yamin Tan, Yongxian Hu, Qitian Mu, Guifang Ouyang, He Huang.
Project administration: Lixia Sheng, Huarui Fu, Yamin Tan, Guifang Ouyang, He Huang.
Resources: Huarui Fu, Yamin Tan, Yi Luo, Jimin Shi, Zhen Cai.
Software: Lixia Sheng, Huarui Fu, Yamin Tan, Yongxian Hu, Qitian Mu.
Supervision: Yi Luo, Jimin Shi, Zhen Cai, Guifang Ouyang, He Huang.
Validation: Lixia Sheng, Yamin Tan, Yi Luo, Jimin Shi, Zhen Cai, Guifang Ouyang, He Huang.
Visualization: Lixia Sheng.
Writing – original draft: Lixia Sheng, Huarui Fu, Yamin Tan.
Writing – review & editing: Lixia Sheng, Qitian Mu, Zhen Cai, Guifang Ouyang, He Huang.
Footnotes
Abbreviations: ALL = acute lymphocytic leukemia, allo-HSCT = allogeneic hemotopoietic stem cell transplantation, CFSE = carboxyfluorescein succinimidyl ester, FACS = fluorescence-activated cell sorting, HSCT = hemotopoietic stem cell transplantation, IFN = interferon, IL = interleukin, KIR = killer-cell immunoglobulin-like receptors, NK = natural killer cells, NKT = natural killer T cells, SR-GVHD = steroid-refractory graft versus host disease, TCR = T-cell antigen receptor, TNF = tumor necrosis factor.
LS, YT, and HF contributed equally to this manuscript.
This work was funded by the National Natural Science Foundation of China (81401321) and the Science Research Project of Medicine and Hygiene of Zhejiang province (2018PY052).
The authors have no conflicts of interest to disclose.
References
- [1].Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet 2009;373:1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jacobsohn DA, Vogelsang GB. Anti-cytokine therapy for the treatment of graft-versus-host disease. Curr Pharm Des 2004;10:1195–205. [DOI] [PubMed] [Google Scholar]
- [3].Tan Y, Xiao H, Wu D, et al. Combining therapeutic antibodies using basiliximab and etanercept for severe steroid-refractory acute graft-versus-host disease: a multi-center prospective study. Oncoimmunology 2017;6:e1277307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu Y, Chen J, Tang J, et al. Cost of childhood acute lymphoblastic leukemia care in Shanghai, China. Pediatr Blood Cancer 2009;53:557–62. [DOI] [PubMed] [Google Scholar]
- [5].Peng LS, Mao FY, Zhao YL, et al. Altered phenotypic and functional characteristics of CD3 + CD56 + NKT-like cells in human gastric cancer. Oncotarget 2016;7:55222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Guo W, Xing C, Dong A, et al. Numbers and cytotoxicities of CD3 + CD56 + T lymphocytes in peripheral blood of patients with acute myeloid leukemia and acute lymphocytic leukemia. Cancer Biol Ther 2013;14:916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marcusson-Stahl M, Cederbrant K. A flow-cytometric NK-cytotoxicity assay adapted for use in rat repeated dose toxicity studies. Toxicology 2003;193:269–79. [DOI] [PubMed] [Google Scholar]
- [8].Kim D, Al-Dawsari G, Chang H, et al. Large granular lymphocytosis and its impact on long-term clinical outcomes following allo-SCT. Bone Marrow Transplant 2013;48:1104–11. [DOI] [PubMed] [Google Scholar]
- [9].Kishi Y, Kami M, Murashige N, et al. Hyperacute GVHD and emergence of peripheral CD3 + CD56+ T cells and activated natural killer cells are useful markers for early diagnosis of post-transplant hemophagocytic syndrome. Bone Marrow Transplant 2005;35:415–7. [DOI] [PubMed] [Google Scholar]
- [10].Pievani A, Borleri G, Pende D, et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood 2011;118:3301–10. [DOI] [PubMed] [Google Scholar]
- [11].Costello RT, Sivori S, Mallet F, et al. A novel mechanism of antitumor response involving the expansion of CD3+/CD56+ large granular lymphocytes triggered by a tumor-expressed activating ligand. Leukemia 2002;16:855–60. [DOI] [PubMed] [Google Scholar]
- [12].Yu J, Ren X, Li H, et al. Synergistic effect of CH-296 and interferon gamma on cytokine-induced killer cells expansion for patients with advanced-stage malignant solid tumors. Cancer Biother Radiopharm 2011;26:485–94. [DOI] [PubMed] [Google Scholar]
- [13].Bonanno G, Iudicone P, Mariotti A, et al. Thymoglobulin, interferon-gamma and interleukin-2 efficiently expand cytokine-induced killer (CIK) cells in clinical-grade cultures. J Transl Med 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Okita R, Yamaguchi Y, Ohara M, et al. Targeting of CD4+CD25high cells while preserving CD4+CD25low cells with low-dose chimeric anti-CD25 antibody in adoptive immunotherapy of cancer. Int J Oncol 2009;34:563–72. [PubMed] [Google Scholar]
- [15].Chakupurakal G, Garcia-Marquez MA, Shimabukuro-Vornhagen A, et al. Immunological effects in patients with steroid-refractory graft-versus-host disease following treatment with basiliximab, a CD25 monoclonal antibody. Eur J Haematol 2016;97:121–7. [DOI] [PubMed] [Google Scholar]
- [16].Hao J, Campagnolo D, Liu R, et al. Interleukin-2/interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann Neurol 2011;69:721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sheridan JP, Zhang Y, Riester K, et al. Intermediate-affinity interleukin-2 receptor expression predicts CD56(bright) natural killer cell expansion after daclizumab treatment in the CHOICE study of patients with multiple sclerosis. Mult Scler 2011;17:1441–8. [DOI] [PubMed] [Google Scholar]


