Abstract
The aim of this retrospective cross-sectional study was to assess the usefulness of phosphase and tensin homolog deleted on chromosome 10 (PTEN) and p53 protein immunoexpression in predicting the risk of malignancy in endometrial polyps. The study was conducted at tertiary public hospital, university teaching center, and private practice clinic.
A total of 159 patients with endometrial polyps who underwent hysteroscopic polypectomy between January 2010 to December 2014 were included. p53 and PTEN immunoexpression were assessed in histologic endometrial polyp samples. Patients were allocated into 2 groups: group A, endometrial polyps without atypia (120), and group B, endometrial polyps with atypia (39), which were subdivided into A1 (80) and B1 (21) = p53−/PTEN+ immunostaining; A2 (20) and B2 (11) = p53+/PTEN+; A3 (14) and B3 (4) = p53+/PTEN−; A4 (6) and B4 (3) = p53−/PTEN−.
There was no significant difference between groups regarding clinical and epidemiologic parameters, except for age. Neoplasia incidence within groups was higher when at least 1 marker was abnormally stained (in group A, P = .0089, odds ratio [OR] = 13.94 [1.62; 120.27]; in group B, P = .0255, OR 12.73 [1.38; 117.27]). Overall neoplasia incidence was higher in group B than in group A (20.5% vs 5.8%; P = .0113). Malignant neoplasia was found more frequently in patients with p53+ (P = .0006, OR = 7.67 [2.30; 25.54]) and PTEN− (P = .0043; OR = 5.43 [1.77; 16.61]).
Immunohistochemical analysis using p53 and PTEN as markers, either alone or concomitantly, can be useful to predict malignant transformation in cases of endometrial polyps.
Keywords: hysteroscopy, immunohistochemistry, polyps, PTEN phosphohydrolase, tumor suppressor p53 protein
1. Introduction
The endometrium undergoes a series of changes throughout the menstrual cycle. The histopathologic patterns of endometrial diseases widely range from atrophy to cancer, with polyps being very common.[1] Endometrial polyps are focal circumscribed overgrowths of the endometrial mucosa, usually of the basal portion, which protude into the uterine cavity.[2,3] The etiopathogenesis of polyps is still unclear, and their incidence is increased among women aged 40 to 60 years.[4–7]
Polyps can be diagnosed using imaging technology (ultrasonography, hysterosonography, hysteroscopy, and magnetic resonance), with hysteroscopy being the main procedure used for diagnosis and treatment.[8–11] Signs and symptoms of endometrial polyps include abdominal pain, irregular menses, dysmenorrhea, abnormal uterine bleeding (abnormally heavy or prolonged flow, intermenstrual bleeding, and spotting), leucorrhea, and bleeding during intercourse.[12]
Adenocarcinoma, with a background of atypical hyperplasia in various degrees, is the most common form of malignancy found in endometrial polyps.[13–18] Other risk factors for malignancy in endometrial polyps include systemic hypertension, type 2 diabetes mellitus (DM), obesity, and prolonged time since menopause.[19–22] Uterine polyps have gained considerable attention in immunohistochemical studies, especially from those aiming at assessing their malignant potential.[14,23–26] Several studies using markers for cell cycle control in carcinogenesis, particularly p53 and phosphase and tensin homolog deleted on chromosome 10 (PTEN) have been conducted.
The objective of this study was to assess the usefulness of PTEN and p53 protein immunoexpression in predicting the risk of malignant transformation in endometrial polyps, and thus contribute for the development of new therapies for treatment.
2. Methods
This cross-sectional study was based on chart data from a convenience sample of patients diagnosed with endometrial polyps who underwent hysteroscopy followed by polypectomy at the Gynecologic Endoscopy and Family Planning Unit of the Gynecology Discipline of Botucatu Medical School, São Paulo State University-SP, and Abrão Clinic in Marília-SP, Brazil, between January 2010 and December 2014. Approval from the institutional Committee of Research Ethics was obtained on October 6, 2014 under number 820.385.
A total of 159 patients were allocated into 2 groups: Group A, 120 patients with endometrial polyps without atypia; and Group B, 39 patients with endometrial polyps with atypia restricted to the polyp, who received conservative treatment.
Data collected included age, body mass index (BMI), number of gestations, smoking status, systemic arterial hypertension (SAH), presence of type 2 DM, and time since menopause.
Paraffin blocks with the most representative fragments of endometrial polyps were selected for the assessment of p53 and PTEN immunoexpression. The primary antibodies used were a PTEN mouse monoclonal antibody (Clone 28H6, NCL-PTEN, Novocastra, at 1:150) and mouse monoclonal p53 protein (clone DO-7 mouse, M7001, DAKO, at 1:3000). Both were detected using Envision FLEX (DAKO) (Table 1). Deparaffinization at 65°C for 20 minutes, and antigen retrieval at 97°C for 20 minutes in high pH solution (Tris-EDTA pH 9.0) were performed using an automated system (PTLink, DAKO). Slides were then allowed to cool to 65°C and washed in Tris-buffered saline solution containing Tween 20, pH 7.6 (Envision Flex Wash Buffer) for 5 minutes. Peroxidase block was performed for 5 minutes in Envision Flex peroxidase block solution. After overnight incubation with primary antibodies, slides were washed for 5 minutes, incubated with EnVision FLEX/HRP (dextran polymer conjugated with horseradish peroxidase and secondary antibodies against mouse and rabbit immunoglobulins) for 20 minutes, and washed for another 5 minutes. Immunostaining was developed in a DAB plus chromogen solution (Envision Flex Substrate Working Solution - DAB plus hydrogen peroxide buffer solution) for 10 minutes. Finally, after rinsing in washing solution for 5 minutes, the slides were counterstained with Mayer hematoxylin, dehydrated in three xylol baths (2 minutes each), and mounted permanently (DAKO CS703).
Table 1.
Immunohistochemistry: antibodies, concentration, and incubation time.

Appropriate positive and negative controls were used for each staining. The expression of each marker was determined by counting 500 cells over randomly selected high-power fields. Nuclear brown staining indicated positive expression when the percentage of cells stained was >10% and negative when the percentage of cells stained was <10% (Figs. 1 and 2).
Figure 1.

Immunohistochemical analysis with p53+ (A) and p53− (B) marker (200×).
Figure 2.
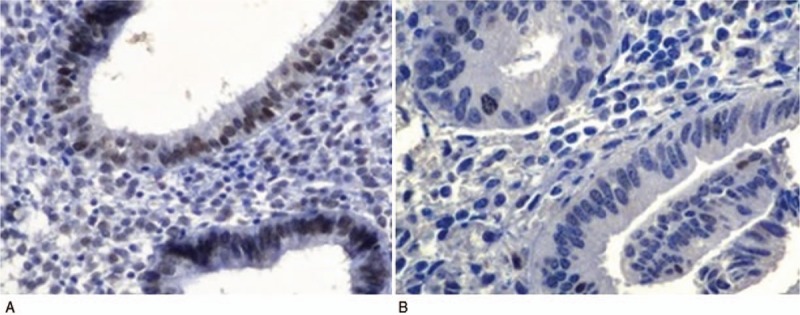
Immunohistochemical analysis with phosphase and tensin homolog deleted on chromosome 10 positive (PTEN+) (A) and PTEN− (B) marker (400×).
For data analysis, quantitative variables were expressed as mean, standard deviation, median, and minimum/maximum values, whereas qualitative variables were described by absolute frequency and percentage. Qualitative variables were analyzed by the test of Goodman for contrasts among multinomial populations. Normally distributed quantitative variables were compared using parametric tests, namely the Student t test and 1-way analysis of variance. Not normally distributed quantitative variables were assessed using the nonparametric tests of Mann–Whitney and Kruskal–Wallis. Odds ratio (OR) and relative risk were calculated considering a 95% confidence interval and P < .05. Significance level for data analysis was set at 5%.[27,28]
3. Results
Groups A and B were divided into 4 subgroups each as follows: A1 (80 patients, 66.7%) p53−/PTEN+ immunostaining, A2 (20 patients, 16.7%) p53+/PTEN+ immunostaining, A3 (14 patients, 11.7%) p53+/PTEN− immunostaining, A4 (6 patients, 5.0%) p53−/PTEN− immunostaining; and B1 (21 patients, 53.8%) p53−/PTEN+ immunostaining, B2 (11 patients, 28.2%) p53+/PTEN+ immunostaining, B3 (4 patients, 10.3%) p53+/PTEN− immunostaining, and B4 (3 patients, 7.7%) p53−/PTEN− immunostaining.
No significant differences were observed in clinical and epidemiologic parameters (BMI, number of gestations, smoking, SAH, type 2 DM, and time since menopause), indicating sample homogeneity, except for age. Mean age in group A (57.5, range of 40–89 years) significantly differed from that in group B (61, range of 40–82 years) (P = .0186). The incidence of malignant endometrial neoplasia was higher in group B than in group A (20.5% and 5.8%, respectively, P = .0113) (Table 2).
Table 2.
Clinical and epidemiologic data from 159 patients with endometrial polyps.

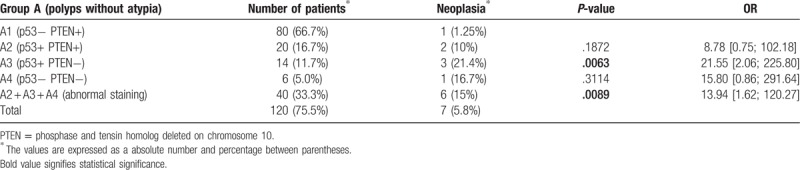
Among patients of subgroup A1 (normal staining patterns), only 1 showed neoplasia (1.25%), whereas in subgroups A2, A3, and A4 (at least 1 marker abnormally stained), 6 patients had neoplasia (15%): 2 in subgroup A2, 3 in subgroup A3, and 1 in subgroup A4 (Table 3). Neoplasia was more frequent in patients with abnormal staining than in those with normal staining patterns (15% and 1.25%, respectively; P = .0089, OR = 13.94 [1.62; 120.27]).
Table 3.
Immunostaining, neoplasia rate, P-value, and odds ratio (OR) in subgroups A1, A2, A3, and A4.
Among patients of subgroup B1 (normal staining patterns), there was only 1 with neoplasia (4.8%), while in subgroups B2, B3, and B4 (at least 1 marker abnormally stained), there were 7 patients with neoplasia (38.9%): 4 in subgroup B2, 2 in subgroup B3, and 1 in subgroup B4 (Table 4). The incidence of malignant neoplasia in patients with abnormally stained markers was higher than in those normally stained (38.9% and 4.8%, respectively, P = .0255, OR = 12.73 [1.38; 117.27]).
Table 4.
Immunostaining, neoplasia rate, P-value, and odds ratio (OR) in subgroups B1, B2, B3, and B4.

In group A, p53 expression was negative in 86 patients, of whom 2 had neoplasia, and positive in 34 patients, of whom 5 showed neoplasia (P = .0296, OR = 7.24 [1.33; 39.38]) (Table 5). In contrast, neoplasia was seen in 3 out of 100 patients with PTEN+ expression, and in 4 out of 20 with PTEN− expression (P = .0147, OR = 8.08 [1.65; 39.55]) (Table 6).
Table 5.
p53 expression, P-value, and odds ratio (OR) in subgroups A and B.

Table 6.
PTEN expression, P-value, and odds ratio (OR) in subgroups A and B.

In group B, endometrial neoplasia was observed in 2 out of 24 patients with p53− expression, and in 6 out of 15 patients with p53+ (P = .0483, OR = 7.33 [1.24; 43.41]) (Table 5). In patients with PTEN+ expression, 5 out of 32 patients had neoplasia, whereas of 7 patients with PTEN− expression, 3 showed neoplasia (P = .2715, OR = 4.05 [0.68; 23.90]) (Table 6).
4. Discussion
Endometrial polyps remain among the most enigmatic and poorly understood gynecologic diseases. The optimal treatment of these polyps remains controversial. Management with systematic polypectomy is still debatable as no consensus has been reached. Polyps usually occur in postmenopausal women, and their malignant potential is unknown. Thus, the purpose of this study was to assess the risk of malignant transformation in women with endometrial polyps.
As immunohistochemical markers can be useful to determine the risk of malignant transformation in endometrial polyps, both p53 and PTEN were used in this study. These markers were chosen because of the promising results obtained in prior studies evaluating each one of them alone. Furthermore, both p53 and PTEN mutations have been found in 50% to 70% of human malignant tumors in the bladder, brain, breast, uterine cervix, colon, rectum, esophagus, and thyroid.[29,30] To our knowledge, the use of concomitant p53 and PTEN to assess malignancy risk in endometrial polyps remains unreported. This study compared the findings obtained with concomitant p53 and PTEN with those obtained separately to determine whether the assessment of malignancy risk can be thus improved.
The TP53 gene, named for the molecular mass of its protein product (a 53-kDa nuclear phosphoprotein containing 393 amino acids), was the first tumor suppressor gene to be identified in 1979. For about 10 years, TP53 was believed to be an oncogene, a cell cycle promoter.[31,32]TP53 mutations (punctual or not) significantly alter the p53 protein. As a result, p53 loses the ability to stimulate cell cycle arrest and apoptosis.[33] Jia et al (2008), in a total of 139 endometrial samples, found p53 mutations in 0%, 43%, 72%, and 96% in resting endometrium, endometrial glandular dysplasia, serous endometrial intraepithelial carcinoma, and endometrial serous carcinoma (ESC), respectively. Most of the lesions showed overexpression of p53 protein that was significantly correlated with TP53 gene mutation. They concluded that mutation of the TP53 gene is probably one of the most important factors to initiate the ESC.[29] Trahan et al (2005), evaluating the behavior of serous papillary carcinoma in endometrial polyps and the overexpression of p53 protein, observed a high p53 mutation rate in endometrial polyps. According to these authors, the high rate of protein p53 overexpression suggests that a TP53 gene mutation occurs early in the disease and might explain the rapid growth of the tumor.[34]
The PTEN is another tumor suppressor that was isolated and sequenced in 1997.[30] PTEN is a 403-amino acid and dual lipid/protein phosphatase which can modulate cell proliferation, cell cycle arrest, and cell apoptosis, migration, and adhesion.[35] Janiec-Jankowska et al (2010), in DNA isolated from 81 endometrial cancers, found mutations in TP53 and/or PTEN genes in 64.2% of the 81 endometrial cancers: in 16.1%, mutations occurred only in TP53; in 33.3%, only in PTEN; and in 14.8%, in both TP53 and PTEN genes.[36] Their results demonstrated that TP53 gene mutations occur in some of endometrioid endometrial cancers in the presence of PTEN gene mutations, suggesting that both these genes participate in the development of these tumors, with PTEN inactivation being one of the earliest events in endometrial carcinogenesis.[37]
In this study, the group with endometrial polyps without atypia (group A), malignant endometrial neoplasia was found in 5.8% of patients, in agreement with prior reports.[34] The incidence of neoplasia significantly differed between patients with normal staining patterns and those with at least 1 marker abnormally stained (P = .0089, OR = 13.94 [1.62; 120.27]). Whereas in subgroup A1 (normal staining patterns), malignant endometrial neoplasia was seen in only 1.25%, in the remaining A subgroups (A2 + A3 + A4), which showed abnormal staining, it was found in 15% of patients. Moreover, when both markers were abnormally stained (subgroup A3), 21.4% of patients showed neoplasia (P = .0063, OR = 21.55 [2.06; 225.80]). The rates of advanced age, hypertension, and diabetes in all A subgroups were high, consistently with data reported in the literature.[4,19–22]
In group B (endometrial polyps with atypia), 20.5% of patients showed malignant endometrial neoplasia. In subgroup B1 (normal staining pattern), malignant neoplasia was seen in 4.8% of patients, similarly to previous reports.[6,36] Among the patients of the remaining B subgroups (B2 + B3 + B4), neoplasia was found in 38.9%, which significantly differs from the rate observed in subgroup B1 (P = .0255, OR = 12.73 [1.38; 117.27]). In subgroup B3 (both markers abnormally stained), malignant neoplasia was present in 50% of cases, but statistical significance was not reached (P = .0868, OR = 20.00 [1.21; 330.97]) probably due to the size of the sample.
Our results suggest that immunohistochemical analysis can be useful in cases of endometrial polyps, especially when associated with risk factors such as advanced age, high BMI, SAH, DM, and prolonged time since menopause. Comparison of most of our findings with those from previous research was limited by the lack of prior studies using p53 and PTEN concomitantly.[29,36,37]
In brief, patients with endometrial polyps showing abnormal p53 and PTEN immunohistochemistry in the presence of the aforementioned risk factors were more likely to have malignant endometrial neoplasia requiring closer follow-up.
5. Conclusion
-
1.
Immunohistochemical analysis can be useful to predict malignant transformation in cases of endometrial polyps.
-
2.
Further larger studies to confirm the data obtained are warranted.
-
3.
The risk of malignant endometrial neoplasia is higher in patients with endometrial polyps showing abnormal p53 and PTEN immunohistochemistry in the presence of advanced age.
-
4.
The incidence of malignant endometrial neoplasia was higher in women of more advance age with polyps with atypia.
Acknowledgment
The authors thank Prof Carlos E. Bacchi, pathologist, for performing the immunohistochemical evaluations with excellent quality.
Author contributions
Conceptualization: Féres Abrão, Daniel Spadoto-Dias.
Data curation: Féres Abrão, Leonardo Vieira Elias, Maria Aparecida Custódio Domingues.
Investigation: Féres Abrão, Nilton José Leite, Gustavo Filipov Peres.
Methodology: Féres Abrão.
Supervision: Rogério Dias.
Writing – original draft: Féres Abrão.
Writing – review & editing: Waldir Pereira Modotti, Daniel Spadoto-Dias, Flávia Neves Bueloni-Dias.
Footnotes
Abbreviations: BMI = body mass index, DM = diabetes mellitus, DNA = desoxyribonucleic acid, ESC = endometrial serous carcinoma, PTEN = phosphase and tensin homolog deleted on chromosome 10, SAH = systemic arterial hypertension, TP53 gene = gene encoding the p53 protein.
Postgraduation Program in Gynecology, Obstetrics and Mastology, Botucatu Medical School, São Paulo State University - FMB/UNESP, Botucatu, São Paulo, Brazil.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Baracat EC, Haidar MA, Nunes MG. Baracat EC, Lima GR, et al. Climacteric. Guide of Medicine to Ambulatory and Hospital Care. São Paulo: Manole; 2004. 339–45. [Google Scholar]
- [2].Buckley CH, Fox H. Biopsy Pathology of the Endometrium. London: Chapman an Hall Medical; 1989. 145–8. [Google Scholar]
- [3].Davis B. Endometrial stromal polyps in rodents: biology, etiology, and relevance to disease in women. Toxicol Pathol 2012;40:419–24. [DOI] [PubMed] [Google Scholar]
- [4].Van Bogaert LJ. Clinicopathologic findings in endometrial polyps. Obstet Gynecol 1988;71:771–3. [PubMed] [Google Scholar]
- [5].Dal Cin P, Vanni R, Marras S, et al. Four cytogenetic subgroups can be identified in endometrial polyps. Cancer Res 1995;55:1565–8. [PubMed] [Google Scholar]
- [6].Lopes RG, Baracat EC, de Albuquerque Neto LC, et al. Analysis of estrogen- and progesterone-receptor expression in endometrial polyps. J Minim Invasive Gynecol 2007;14:300–3. [DOI] [PubMed] [Google Scholar]
- [7].de Carvalho S, Campaner AB, Lima SM, et al. Differential expression of estrogen and progesterone receptors in endometrial polyps and adjacent endometrium in postmenopausal women. Anal Quant Cytol Histol 2011;33:61–7. [PubMed] [Google Scholar]
- [8].Aslam M, Ijaz L, Tariq S, et al. Comparison of transvaginal sonography and saline contrast sonohysterography in women with abnormal uterine bleeding: correlation with hysteroscopy and histopathology. Int J Health Sci (Qassim) 2007;1:17–24. [PMC free article] [PubMed] [Google Scholar]
- [9].Yela DA, Ravacci SH, Monteiro IM, et al. Comparative study of transvaginal sonography and outpatient hysteroscopy for detection of pathologic endometrial lesions in postmenopausal women [in Portuguese]. Rev Assoc Med Bras 2009;55:553–6. [DOI] [PubMed] [Google Scholar]
- [10].Gambadauro P, Martinez-Maestre MA, Schneider J, et al. Malignant and premalignant changes in the endometrium of women with an ultrasound diagnosis of endometrial polyp. J Obstet Gynaecol 2014;34:611–5. [DOI] [PubMed] [Google Scholar]
- [11].Gambadauro P, Martinez-Maestre MA, Schneider J, et al. Endometrial polyp or neoplasia? A case-control study in women with polyps at ultrasound. Climacteric 2015;18:399–404. [DOI] [PubMed] [Google Scholar]
- [12].Abrão F. Study of endometrial polyps: the importance of polypectomy: Botucatu Medical School, Sao Paulo State University - FBM/UNESP; 2006. Available at: http://hdl.handle.net/11449/103067. Accessed February 15, 2007. [Google Scholar]
- [13].Mittal K, Da Costa D. Endometrial hyperplasia and carcinoma in endometrial polyps: clinicopathologic and follow-up findings. Int J Gynecol Pathol 2008;27:45–8. [DOI] [PubMed] [Google Scholar]
- [14].Yasuda M, Katoh T, Hori S, et al. Endometrial intraepithelial carcinoma in association with polyp: review of eight cases. Diagn Pathol 2013;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lenci MA, Nascimento VA, Grandini AB, et al. Premalignant and malignant lesions in endometrial polyps in patients undergoing hysteroscopic polypectomy. Einstein (Sao Paulo) 2014;12:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tang Z, Zhou R, Bao D, et al. Clinical characteristics of 42 cases of malignant endometrial polyps. Zhonghua Fu Chan Ke Za Zhi 2014;49:204–7. [PubMed] [Google Scholar]
- [17].Naaman Y, Diment J, Perlman S, et al. Can malignant potential of endometrial polyps be determined by incorporating the endometrial intraepithelial neoplasia (EIN) classification? Gynecol Oncol 2015;136:254–7. [DOI] [PubMed] [Google Scholar]
- [18].Elfayomy AK, Soliman BS. Risk factors associated with the malignant changes of symptomatic and asymptomatic endometrial polyps in premenopausal women. J Obstet Gynaecol India 2015;65:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gregoriou O, Konidaris S, Vrachnis N, et al. Clinical parameters linked with malignancy in endometrial polyps. Climacteric 2009;12:454–8. [DOI] [PubMed] [Google Scholar]
- [20].Baiocchi G, Manci N, Pazzaglia M, et al. Malignancy in endometrial polyps: a 12-year experience. Am J Obstet Gynecol 2009;201:462.e1–4. [DOI] [PubMed] [Google Scholar]
- [21].Bueloni-Dias FN, Spadoto-Dias D, Nahas Neto J, et al. Predictive factors for occurrence of endometrial polyps in postmenopausal women [in Portuguese]. Rev Bras Ginecol Obstet 2014;36:489–96. [DOI] [PubMed] [Google Scholar]
- [22].Serhat E, Cogendez E, Selcuk S, et al. Is there a relationship between endometrial polyps and obesity, diabetes mellitus, hypertension? Arch Gynecol Obstet 2014;290:937–41. [DOI] [PubMed] [Google Scholar]
- [23].Athanassiadou P, Athanassiades P, Grapsa D, et al. The prognostic value of PTEN, p53, and beta-catenin in endometrial carcinoma: a prospective immunocytochemical study. Int J Gynecol Cancer 2007;17:697–704. [DOI] [PubMed] [Google Scholar]
- [24].Jarboe EA, Pizer ES, Miron A, et al. Evidence for a latent precursor (p53 signature) that may precede serous endometrial intraepithelial carcinoma. Mod Pathol 2009;22:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Miranda SP, Traiman P, Candido EB, et al. Expression of p53, Ki-67, and CD31 proteins in endometrial polyps of postmenopausal women treated with tamoxifen. Int J Gynecol Cancer 2010;20:1525–30. [DOI] [PubMed] [Google Scholar]
- [26].Su T, Sui L. Expression and significance of p63, aromatase P450 and steroidogenic factor-1 in endometrial polyp [in Chinese]. Zhonghua Fu Chan Ke Za Zhi 2014;49:604–8. [PubMed] [Google Scholar]
- [27].Zar JH. Biostatistical Analysis. 5th ed2010;Upper Saddle River, NJ: Prentice-Hall/Pearson, xiii, 944 pp. [Google Scholar]
- [28].Blair RC, Taylor RA. Biostatistics for Health Sciences. São Paulo: Pearson Education do Brasil; 2013. [Google Scholar]
- [29].Jia L, Liu Y, Yi X, et al. Endometrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clin Cancer Res 2008;14:2263–9. [DOI] [PubMed] [Google Scholar]
- [30].Gil A, Rodriguez-Escudero I, Stumpf M, et al. A functional dissection of PTEN N-terminus: implications in PTEN subcellular targeting and tumor suppressor activity. PLoS One 2015;10:e0119287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roger L, Gadea G, Roux P. Control of cell migration: a tumour suppressor function for p53? Biol Cell 2006;98:141–52. [DOI] [PubMed] [Google Scholar]
- [32].Appel ML, Edelweiss MI, Fleck J, et al. P53 and BCL-2 as prognostic markers in endometrial carcinoma. Pathol Oncol Res 2008;14:23–30. [DOI] [PubMed] [Google Scholar]
- [33].Follis AV, Llambi F, Ou L, et al. The DNA-binding domain mediates both nuclear and cytosolic functions of p53. Nat Struct Mol Biol 2014;21:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Trahan S, Tetu B, Raymond PE. Serous papillary carcinoma of the endometrium arising from endometrial polyps: a clinical, histological, and immunohistochemical study of 13 cases. Hum Pathol 2005;36:1316–21. [DOI] [PubMed] [Google Scholar]
- [35].Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene 2008;27:5443–53. [DOI] [PubMed] [Google Scholar]
- [36].Janiec-Jankowska A, Konopka B, Goluda C, et al. TP53 mutations in endometrial cancers: relation to PTEN gene defects. Int J Gynecol Cancer 2010;20:196–202. [DOI] [PubMed] [Google Scholar]
- [37].Daniilidou K, Frangou-Plemenou M, Grammatikakis J, et al. Prognostic significance and diagnostic value of PTEN and p53 expression in endometrial carcinoma. A retrospective clinicopathological and immunohistochemical study. J BUON 2013;18:195–201. [PubMed] [Google Scholar]


