Supplemental Digital Content is available in the text
Keywords: carcinoid tumor, endoscopic resection, multicenter study, neuroendocrine tumor, stomach
Abstract
Gastric neuroendocrine tumors (GNETs) are a heterogeneous group of neoplasm with varying biological characteristics. This study aimed to investigate the clinical features and outcomes of GNET patients after endoscopic diagnosis and treatment in a multicenter registry. Patients with GNETs confirmed histologically were recruited from 17 hospitals between January 2010 and April 2016 in Taiwan. Clinical, laboratory, radiological, endoscopic, pathological data, treatment strategies, follow-up periods, and survivals were collected retrospectively. Totally 187 (107 female, 80 male) patients were recruited. Mean ( ± standard deviation [SD]) age and size of tumors were 63.2-year-old ( ± 14.6) and 2.3-cm ( ± 3.0). World Health Organization (WHO) grading were 93 (49.7%) G1, 26 (13.9%) G2, 40 (21.4%) G3, and 28 (15.0%) unknown. G3 patients were older (mean ± SD, 71.6 ± 12.4 vs. 60.9 ± 14.3/56.7 ± 15.4 years), larger (6.1 ± 4.0 vs.1.2 ± 1.3/2.4 ± 2.5 cm), more distally located (35.0% vs. 7.6%/15.4%), lower proportion of superficial lesions (17.5% vs. 61.9%/53.8%) and higher rates of lymphovascular invasion (32.5% vs. 3.2%/7.7%) than G1/G2. There was no nodal or distant organ metastases despite different grading of lesions≦10 mm and those <20 mm limited to mucosa and submucosa layers. GNETs larger than 20 mm with G1, G2, and G3 had lymph node (LN) metastatic rates of 21.4%, 30.0%, and 59.3%, respectively. Survivals were different between grading for those >20 mm (log-rank test P = .02). Male gender (P = .01), deeper invasion (P = .0001), nodal (P < .0001), and distant organ metastases (P = .0001) were associated with worse outcome. In conclusion, treatment strategies for GNET should be decided by grading, size, invasiveness, and LN metastasis risk. Curative endoscopic resection is feasible for G1/2 lesions less than 20 mm and limited to mucosa/submucosa layers without lymphovascular invasion.
1. Introduction
In the early twentieth century, neuroendocrine tumors (NETs), which arise from cells of diffuse neuroendocrine system in many organs of human body, have been noted to behave differently as compared to carcinoma by Oberndorfer with the first description of a “karzinoid”.[1] Due to improvements in diagnostic technology, including endoscopy, radiological modalities, and an increased awareness, the incidence of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) has increased gradually. Over the past 4 decades, the age-adjusted incidence of GEP-NETs has increased steadily with approximately 3 to 5-fold change in western countries, especially for gastrointestinal (GI) NETs with the greatest increase in rectal and gastric neuroendocrine tumors (GNETs) patients.[2,3] The ratio of GNET to all GI-NETs, ranging from 11.9% to 23% among different countries, has increased nearly 10-fold in the USA, and 23-fold in men and 47-fold in women in the UK.[2,3] In Taiwan, the analysis of nationwide cancer registry database revealed that the stomach was ranked the third most common site among newly-diagnosed NETs of the whole body.[4] Moreover, GNETs are among the top 5 prevalent histology of gastric polypoid lesions.[5,6] Nowadays, more and more GNETs are being diagnosed incidentally by endoscopy in the absence of clinical symptoms. Therefore, to understand the clinical manifestation and management of this emerging disease is very important.
GNETs are classified into 3 subgroups with differences in clinical manifestations and prognosis.[7,8] Type I and II GNETs are often female preponderant, multiple, small size (<10∼20 mm), proximal located, well-differentiated [World Health Organization (WHO) grade (G) 1 and 2], limited to mucosa and submucosa layers, and associated with hypergastrinemia.[7,9,10] Type I, which is the major subtype of GNETs, may be associated with chronic atrophic gastritis/autoimmune gastritis with pernicious anemia, Helicobacter pylori infection, and higher intragastric pH level, whereas type II may be associated with multiple endocrine neoplasia (MEN)-I, Zollinger–Ellison syndrome (ZES) and intragastric hyperacidity.[7–10] The prognosis of type I and II are favorable with tumor related death rate less than 10%.[7,9,11] Type III GNETs represent the second common type, which behaves aggressively with rates of metastasis higher than 50% and tumor related death over approximately 25% to 30%.[7,9,11] Some advocate to subdivide Type III tumors into 2 groups (Type III and IV), where Type III GNETs are sporadic nonfunctioning and Type IV tumors are those that are poorly-differentiated non-enterochromaffin cell (ECL) origin.[12] Both type III and IV tumors are often solitary, larger than 20 mm, located at any region of stomach, invading any depth of gastric wall, gastrin-independent and male preponderance.[7,9,11] Clinical trials are rare for GNETs and the international guidelines are largely based on epidemiological and pathological aspects. Creating large registries will be an important method to improve our understanding of these tumors in different regions around the world.
The aims of this study were to investigate the clinical features and evaluate the outcomes of GNET patients according to the different management approaches in a multicenter registry in Taiwan.
2. Materials and methods
Between January 2010 and April 2016, patients with diagnosis of GNETs confirmed histologically were recruited from 17 hospitals in Taiwan. Data were collected retrospectively by doctors who filled out the uniform case record files. WHO 2010 classification system was used for grading of differentiation.[13] Clinical classification was based on the following criteria:[14] type I GNETs were those with (i) no evidence of MEN-ZES; and (ii) positive anti-parietal cell antibodies; or (iii) presence of ECL cell hyperplasia or gastric atrophy based on endoscopy or histology, or (iv) evidence of hypergastrinemia (>450 pg/mL) in the case of no evidence of autoantibodies or hyperplasia of ECL cells; type II GNETs were those with presence of type I multiple endocrine neoplasia (MEN-I) or ZES; and type III were patients not fulfilling criteria for type I and II.
Clinical, laboratory, radiological and endoscopic data were collected at the time of initial diagnosis of GNETs at each institution. All the pathologists in each study institution used WHO 2010 criteria for NET diagnosis. Data analyzed included demographic characters, tumor size, location and number, depth of tumor invasion, lymphovascular invasion, tumor stages, management approach, and presence of H pylori infection (histology or rapid urease test), serum gastrin level, follow-up periods and survivals. The depth of tumor invasion was recorded by using resected specimens for histology or endoscopic ultrasonography. Radiological examinations included computed tomography or magnetic resonance imaging to evaluate nodal or distant organ metastases. Each participating institution approved this retrospective multicenter study by the ethics committee and informed consent from individual patients was not required because of observational study design.
2.1. Statistical analysis
Continuous data were expressed as means or medians [ ± SD and ranges] as appropriate, and category data were expressed as number (percentage). The overall survival of patients was plotted in Kaplan–Meier survival plots. Univariate and multivariate analyses were performed to evaluate the risk factor for disease-related mortality. All statistical analyses were accomplished using Stata software, version 12.0 (StataCorp LP, College Station, TX).
3. Results
Demographic data of patients are shown in Table 1. Totally 187 GNET patients were recruited from 17 hospitals. There were 107 female (57.2%) and 80 male patients (42.8%). The mean ( ± SD, range) age and size were 63.2-year-old ( ± 14.6, 21∼95) and 2.4-cm ( ± 3.0, 0.1∼15), respectively. The majority were multiple in number (75.4%) and proximal located (71.1%) at corpus, fundus or cardia of stomach. The invasive depth of most GNETs was mucosa (28.9%) and submucosa (20.9%) layers and 67.4% did not have lymphovascular invasion on histology. Grading of these lesions were 93 (49.7%) G1, 26 (13.9%) G2, 40 (21.4%) G3, and 28 (15.0%) unknown grading lesions. GNET patients with G3 were more males (72.5% vs. 31.2%/30.8%, P = .0001/.0022), older (mean ± SD, 71.6 ± 12.4 vs. 60.9 ± 14.3/56.7 ± 15.4 years, P = .0002/.0001) and larger (6.1 ± 4.0 vs.1.2 ± 1.3/2.4 ± 2.5 cm, P = .0001/.0002) than G1/G2 patients. More G3 lesions were located at antrum than G1/G2 lesions (35.0% vs. 7.6%/15.4%, P = .003/.04). Proportion of lesions limited to mucosa and submucosa layers in G1/G2 lesions were higher than G3 tumors (61.9%/53.8% vs. 17.5%, P = .0003/.0012). G3 GNETs presented more lymphovascular invasion than G1/G2 lesions (32.5% vs. 3.2%/7.7%, P < .0001/.0057). The mean gastrin level for G1, G2, and G3 lesions were 792.3, 1000 and 40.4 pg/mL, respectively.
Table 1.
Demographic data of enrolled patients by WHO grading.

Demographic data of patients with different clinical classifications are shown in Supplementary Table 1. Type I GNET was the most common 1 (142/187 = 75.9%), followed by type III (44/187 = 23.5%) and type II (1 patient) tumors in this study. More female patients were noted in type I/II (64.8%/100% vs. 31.8%, P = .0002/NA) than in type III GNETs. There were no tumors with G3 in type I and II GNETs, but 90.9% of type III GNETs were grading G3. Higher proportion of type III GNETs were distally-located (37.5% vs. 12.0%/0% at antrum, P = .032/NA), deeper invasion (35.0% vs. 4.9%/0% beyond serosa, P = .003/NA), and lymph node (LN) metastasized (45.5% vs. 7.7%/0%, P = .0002/ NA) than type I/II. The H pylori positive rates in type I, II and III GNETs were 35.9%, 0%, and 9.1%, respectively.
Table 2 discloses the metastatic rates of LN and distant organ. In GNETs less than 10 mm, there was no patient with nodal or distant organ metastasis despite different grading. Among tumors with a size between approximately 11 and 20 mm, there was 1 (7.7%) G1 patient presenting lymphovascular invasion and LN metastasis with invasion of muscularis propria. There were 4 G2 lesions with size between approximately 11 and 20 mm and all of them underwent surgery. One involving mucosa (stage I), 1 invading submucosa (stage IIA) and 2 with invasion to muscularis propria (stage IIA and IIIB) were found. Among 2 G2 patients with involvement of muscularis propria, 1 (50%) had LN metastasis. GNET patients with tumors larger than 20 mm with G1, G2, and G3 had LN and distant organ metastatic rates of 21.4% and 14.3%, 30.0% and 50.0%, and 59.3% and 66.7%, respectively.
Table 2.
Status of nodal and distant organ metastasis.

The rate of clear resection margin according to different management approaches are shown in Table 3. Among GENTs≦10 mm, removal of tumors by piecemeal biopsy, polypectomy, endoscopic mucosal resection (EMR), and endoscopic submucosal dissection (ESD) achieved histological resection margin free rates of 45.5%, 45.5%, 68.8%, and 75%, respectively for G1, and 33.0%, 50.0%, 100%, and 100%, respectively for G2 tumors. For G1 tumors with size between approximately 11 and 20 mm, the resection margin free rates were 0%, 100%, and 100% for polypectomy, EMR, and ESD, respectively. Univariate and multivariate analyses of risk factors for disease-related mortality were shown in Table 4. In the univariate analysis, male sex (P = .025), greater size, more advanced grading/staging, presence of lymphovascular invasion, and distant metastasis (all with P < .001) were associated with higher mortality risk. In the multivariate analysis, more advanced stage (P = .034) was the independent risk factors for disease-related mortality. Supplementary Table 2 and Figures 1 and 2 revealed the survivals of GNETs with different grading and size. Survivals were best in G1 and worst in G3 lesions (log-rank test P < .001). Patients with tumor larger than 20 mm had worst outcome. Among GNETS larger than 20 mm, overall mortality rates for G1, G2, and G3 lesions were 28.6%, 11.1%, and 55.6%, respectively, whereas disease-related mortality rates were 21.4%, 0%, and 44.4%, respectively. Survivals were not different between lesions less than 20 mm (log-rank test P = .94 for lesions ≦10 mm, and P = .65 for those between 11∼20 mm) with different grading, but statistically significant difference for those larger than 20 mm (log-rank test P = .002). Male gender (log-rank test P = .01), deeper invasion (P = .0001), nodal (P < .0001) and distant organ metastases (P = .0001) were associated with worse outcome.
Table 3.
Resection margin free rates by different endoscopic resection and surgical management.
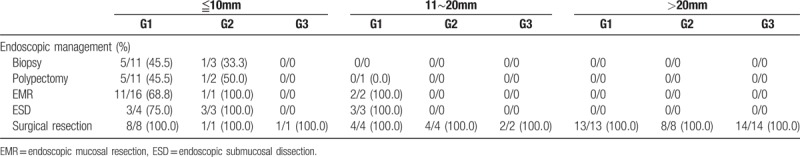
Table 4.
Univariate and multivariate analyses to evaluate risk factors for disease-related mortality.
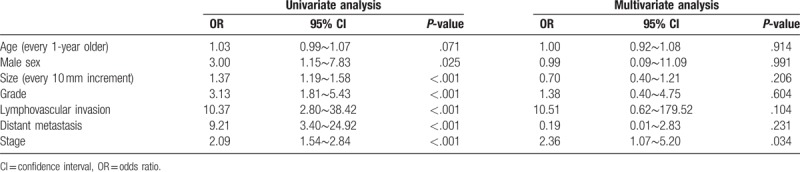
Figure 1.

Comparison of survivals according to different grading and size. A)-overall, B) ≦1 cm, C) >1 cm and ≦2 cm, D) >2 cm.
Figure 2.
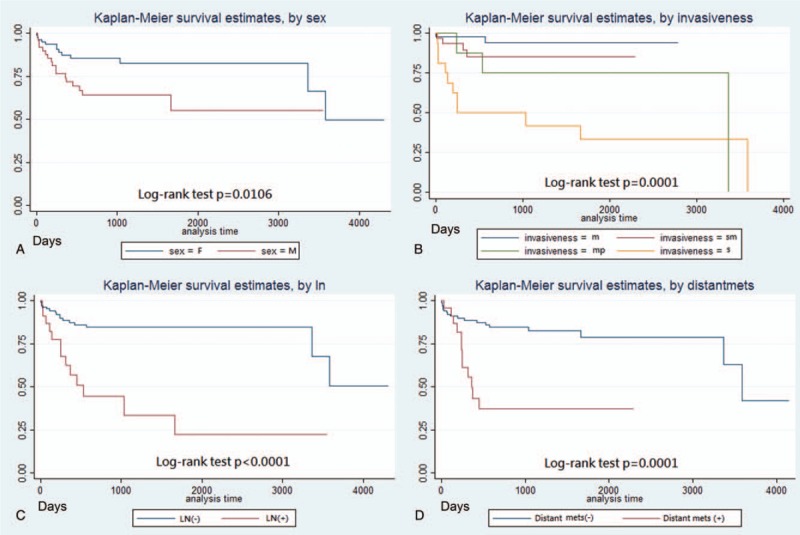
Comparison of survivals according to different factors. A) sex, B) invasiveness, C) nodal metastasis, D) distant organ metastasis.
4. Discussion
In this retrospective multicenter study conducted in Taiwan, we characterized the clinical course, management approaches and outcomes of GNETs. The majority of GNETs belong to G1 and clinical classification type I, which are associated with female predominance, smaller size, proximal location in stomach and relatively optimal outcomes. GNETs with G3 or type III were predominantly male, larger size, possibly with distal location, and poor prognosis. Endoscopic resection, including modified EMR or ESD, could be used for curative treatment of GNETs less than 10 mm despite of grading and G1/G2 tumors with size between approximately 11 and 20 mm without lymphovascular invasion and limited to mucosa and submucosa layers. Tumors with G3 larger than 20 mm had the worst survival and aggressive treatment should be considered. Male gender, deep invasiveness, nodal, and distant organ metastases were associated with worse prognosis.
With the widespread use of endoscopy examination for many GI diseases, more and more GNETs, which have been deemed as rare disease, are being detected. According to studies from large number of national histopathology or endoscopy database, about 3.3% of polypoid lesions in stomach belonged to NETs with prevalence about 0.06% in general population.[5,6] It is sometimes difficult to be differentiated from non-neoplastic gastric polyps, although some typical magnifying endoscopic features under narrow-band imaging system could be found (central depression with absent pits, and dilated darkish-brown subepithelial vessels with cork-screw capillaries) (Fig. 3). Additionally, GNETs are more associated with younger age, female and gastric atrophy than other histology types of gastric polypoid lesions.[5,6,15] Another report from nationwide cancer registry also found that the proportion of GNETs among all gastric malignancies and GI tract NETs has increased from 0.3% to 1.77% and from 2.4% to 8.7%, respectively.[15] Among epidemiological data from different countries, the ratio of GNET to all GI tract NET range from approximately 11.9% to 23%.[2,3,10] In Taiwan, the cancer registry data showed that the stomach is the third (7.4%) most common primary site of NET among other organs of human body. Therefore, facing this emerging disease entity, our nationwide multicenter study is of significance to provide information about the clinical manifestations, management approaches and outcomes for GNETs in Taiwan.
Figure 3.
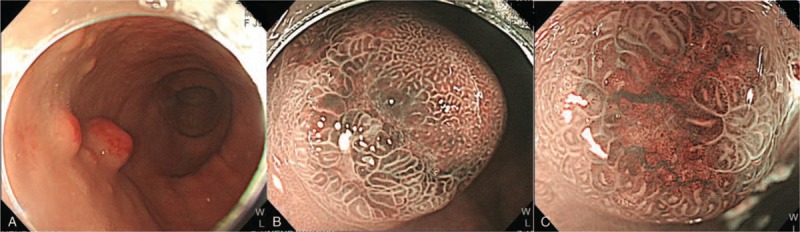
Endoscopic features of gastric neuroendocrine tumors. A) Hyperemic surface mucosa of gastric NET under white-light imaging endoscopy. B) Brownish discoloration of central part of polypoid tumor. C) Central depression with absent pits, blackish-brown subepithelial vessels with cork screw pattern of capillaries under magnifying endoscopy with narrow-band imaging system. NET = neuroendocrine tumor.
GNETs can be categorized into well and poorly differentiated tumors and subdivided into those arising from ECL cell hyperplasia secondary to excess gastrin stimulation (types I and II, primarily well-differentiated) and sporadic tumors (type III, well or poorly differentiated).[11,16] Some studies advocate the subdivision of type III GNETs into 2 groups (type III and IV) which both are gastrin-independent non-ECL cell origin, where type III tumors are sporadic nonfunctioning and type IV tumors are poorly differentiated or arise from adrenocorticotropic hormone, serotonin-cells or are of mixed endocrine–exocrine etiology.[12] However, to subdivide type III into 2 subtypes is of little clinical relevance, since the outcomes are poor and the managements are similar for both type III and IV GNETs. Type I tumors account for approximately 70% to 80% of GNETs and might be associated with H pylori related gastritis, chronic atrophic gastritis, autoimmune gastritis, and pernicious anemia.[10–12,16] More females are found in type I GNETs in most studies, including ours (Supplementary Table 1, female 64.8%), but 1 Asian multicenter study conducted in Japan showed a male preponderance (male 53.7%).[14] Usually, type I GENTs are multiple with proximal location in stomach and associated with H pylori infection.[11,12,16] But from Asian data, a Japanese retrospective multicenter study showed higher proportion (53.7%) of solitary in type I GNETs, which was similar to our study (72.5%), whereas higher positive rate for H pylori infection in type I GNETs than other types (Japanese study 24.4%, ours 35.9%).[14] It seems that the epidemiologic data vary between different countries and to understand the difference in clinical manifestation in different areas is very crucial. In our study (Supplementary Table 1), we also found that type III GNETs were more commonly male, older, larger size, more often distal location in stomach, with deeper invasion of the gastric wall, and higher risk of nodal metastasis than type I and II tumors. There was only 1 type II case with MEN-I in our study. This young female patient with type II GNET had multiple polypoid tumors with size less than 10 mm at the whole stomach with grading G1, invasion of submucosa but without metastasis. Her serum gastrin level was 986 pg/mL and she received multiple sessions of polypectomy combining somatostatin analogue therapy with stable disease. Therefore, type I and II GNETs have better outcomes than type III tumors.
NETs are considered to be slow-growing and lower malignant potential than traditional carcinomas.[1,17] Thus, conservative management strategies rather than surgical intervention are to be preferred. Previously, the European Neuroendocrine Tumor Society (ENETS) and National Comprehensive Cancer Network (NCCN) guidelines recommended simple surveillance for lesions less than 10 mm limited to mucosa and submucosa layers.[18,19] For locoregional lesions between approximately 10 and 20 mm with hypergastrinemia, observation and survey every 6 to 12 months to 10 years has also been proposed by NCCN guidelines.[19] However, some investigators advocated resecting all visible lesions despite of size using endoscopic resection techniques, including removal by biopsy forceps, EMR, ESD, or full-thickness resection device.[14,20–22] To date, there are no randomized data comparing an aggressive endoscopic approach to more conservative strategy, and we believe that the malignant potential cannot be predicted by size and invasiveness only, but clinical classification (hypergastrinemia present or not) and WHO grading are also crucial for decision of treatment strategy.[23,24] Even in type III GNETs, which have higher risk for metastatic disease, endoscopic resection can only be performed for those with G1 grading confined to submucosa, less than 10 mm and without lymphovascular invasion in resected specimens.[24] The LN metastatic rate of GNETs in different size and grading in our study are shown in Table 2. There was no patient with a tumor less than 10 mm that had nodal or distant organ metastasis despite of different grading. Among tumors with size between approximately 11 and 20 mm, the LN metastatic rates were closely associated with the depth of invasion. There was 1 (7.7%) G1 patient and 1 (25.0%) G2 patient with a tumor size between approximately 11 and 20 mm had LN metastasis, with invasion of muscularis propria. For those larger than 20 mm, the LN and distant organ metastatic rates increased in proportion of the severity of grading. Therefore, we recommend that endoscopic resection be used for curative treatment of GNETs less than 10 mm despite of grading and G1/G2 tumors with size between approximately 11 and 20 mm on the premise that lymphovascular invasion is not found in resected specimen and invasion is confined to mucosa and submucosa layers.
There are some limitations in this study. First, there are many missing data and the diagnostic workup and management approach, especially endoscopic resection methods, were not standardized because of retrospective study design. Some diminutive lesions were endoscopically biopsied without suspicion of histopathologic diagnosis of NET and some of those without R0 could not be identified at surveillance endoscopy. Second, all the pathologic data was based on medical records review and there was no centralized pathologic review of each specimen. However, pathologists in each study hospital used WHO 2010 criteria for NET diagnosis. Finally, the case number is small despite multicenter enrollment, especially when there was only 1 case classified as type II GNET. Moreover, the follow-up period was short and long-term outcome could not be revealed by this study.
In conclusion, this study was the first multicenter study in Taiwan to evaluate the clinical manifestations, management approaches and outcomes of GNET. We proposed a standardized work-up and treatment recommendations, which is illustrated in Figure 4. Further prospective analysis of a cohort with standardized diagnostic and management protocols are warranted to further our understandings of GNETs.
Figure 4.

Algorithm for diagnosis and treatment strategy for gastric NET. Dotted lines depict alternative treatment strategy. ∗Surveillance endoscopy every 6 to 12 months. APA = anti-parietal cell antibodies, anti-IFAb = anti-intrinsic factor antibodies, CAG = chronic atrophic gastritis, CgA = chromogranin A, CECT = contrast-enhanced computed tomography, EUS = endoscopic ultrasonography, EMR = endoscopic mucosal resection, ESD = endoscopic submucosal dissection, FTR = full-thickness resection, GC = genetic counselling, GNET = gastric neuroendocrine tumor, 5-HIAA = 5-hydroxyindoleacetic acid, H&E = hematoxylin and eosin stain, iPTH = intact parathyroid hormone, LymVas = lymphovascular invasion, M = mucosa; MCV = mean corpuscular volume, MEN-I = type I multiple endocrine neoplasia, MP = muscularis propria, MRI = magnetic resonance imaging, NET = neuroendocrine tumor, PET = positron emission tomography, SSA = sandostatin analogues, SM = submucosa; TH = thyroid hormone, ZES = Zollinger–Ellison syndrome.
Author contributions
Conceptualization: Chen-Shuan Chung, Cho-Lun Tsai, Yin-Yi Chu, Kuan-Chih Chen, Jung-Chun Lin, Chiung-Yu Chen, Hsiu-Po Wang.
Data curation: Chen-Shuan Chung, Cho-Lun Tsai, Yin-Yi Chu, Kuan-Chih Chen, Jung-Chun Lin, Bao-Chung Chen, Wei-Chih Sun, Hsu-Heng Yen, Chiung-Yu Chen, I-Chen Wu, Chao-Hung Kuo, Hisang-Yao Shih, Jack P Wang, Wen-Hao Hu, Chang-Shyue Yang, Ming-Lun Han, Tsu-Yao Cheng, Chao-Ming Tseng, Ming-Chang Tsai, Ming-Luen Hu, Hsiu-Po Wang.
Formal analysis: Chen-Shuan Chung, Yin-Yi Chu, Kuan-Chih Chen, Hsiu-Po Wang.
Methodology: Chen-Shuan Chung.
Resources: Cho-Lun Tsai, Yin-Yi Chu, Kuan-Chih Chen, Jung-Chun Lin, Bao-Chung Chen, Wei-Chih Sun, Hsu-Heng Yen, Chiung-Yu Chen, I-Chen Wu, Chao-Hung Kuo, Hisang-Yao Shih, Ming-Jong Bair, Jack P Wang, Wen-Hao Hu, Chang-Shyue Yang, Ming-Lun Han, Tsu-Yao Cheng, Chao-Ming Tseng, Ming-Chang Tsai, Ming-Luen Hu, Hsiu-Po Wang.
Supervision: Hsiu-Po Wang.
Validation: Chen-Shuan Chung, Cho-Lun Tsai, Yin-Yi Chu, Kuan-Chih Chen, Chiung-Yu Chen, I-Chen Wu, Chao-Hung Kuo, Hisang-Yao Shih, Ming-Jong Bair, Jack P Wang, Wen-Hao Hu, Chang-Shyue Yang, Ming-Lun Han, Tsu-Yao Cheng, Ming-Chang Tsai, Ming-Luen Hu, Hsiu-Po Wang.
Writing – original draft: Chen-Shuan Chung, Hsiu-Po Wang.
Writing – review & editing: Chen-Shuan Chung.
Chen-Shuan Chung orcid: 0000-0001-9224-4958
Supplementary Material
Footnotes
Abbreviations: ECL = enterochromaffin-like, EMR = endoscopic mucosal resection, ESD = endoscopic submucosal dissection, GEP-NET = gastroenteropancreatic neuroendocrine tumor, GI = gastrointestinal, GNET = gastric neuroendocrine tumor, LN = lymph node, MEN = multiple endocrine neoplasia, NCCN = National Comprehensive Cancer Network, NET = neuroendocrine tumor, SD = standard deviation, WHO = World Health Organization, ZES = Zollinger–Ellison syndrome.
C-SC, Y-YC, C-YC, and H-PW conceived and designed the study.
C-SC, Y-YC, K-CC, J-CL, W-CS, C-LT, H-HY, C-YC, I-CW, C-HK, H-YS, M-JB, J-P, W-HH, C-SY, M-LH, T-YC, C-MT, M-ST, M-LH, and H-PW analyzed and interpreted the data.
C-SC drafted the article.
C-SC and H-PW critically revised the article for important intellectual content.
C-SC, Y-YC, K-CC, J-CL, W-CS, C-LT, H-HY, C-YC, I-CW, C-HK, H-YS, M-JB, J-PW, W-HH, C-SY, M-LH, T-YC, C-MT, M-ST, M-LH, and H-PW did final approval of the article.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Oberndorfer S. Karzinoide tumoren des dunndarms. Frankf Z Pathol 1907;1:426–32. [Google Scholar]
- [2].Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol 2010;105:2563–9. [DOI] [PubMed] [Google Scholar]
- [3].Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- [4].Tsai HJ, Wu CC, Tsai CR, et al. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLoS One 2013;8:e62487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sonnenberg A, Genta RM. Prevalence of benign gastric polyps in a large pathology database. Dig Liver Dis 2015;47:164–9. [DOI] [PubMed] [Google Scholar]
- [6].Vatansever S, Akpınar Z, Alper E, et al. Gastric polyps and polypoid lesions: Retrospective analysis of 36650 endoscopic procedures in 29940 patients. Turk J Gastroenterol 2015;26:117–22. [DOI] [PubMed] [Google Scholar]
- [7].Li TT, Qiu F, Qian ZR, et al. Classification, clinicopathologic features and treatment of gastric neuroendocrine tumors. World J Gastroenterol 2014;20:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rindi G, Luinetti O, Cornaggia M, et al. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 1993;104:994–1006. [DOI] [PubMed] [Google Scholar]
- [9].Delle Fave G, T’Toole D, Sundin A, et al. ENET consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology 2016;103:119–24. [DOI] [PubMed] [Google Scholar]
- [10].Sato Y. Clinical features and management of type I gastric carcinoids. Clin J Gastroenterol 2014;7:381–6. [DOI] [PubMed] [Google Scholar]
- [11].Crosby DA, Donohoe CL, Fitzgerald L, et al. Gastric neuroendocrine tumors. Dig Surg 2012;29:331–48. [DOI] [PubMed] [Google Scholar]
- [12].Borch K, Ahrén B, Ahlman H, et al. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg 2005;242:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Solcia E, Kloppel G, Sobin LH. Histological Typing of Endocrine Tumours. Chapter: Definitions and Explanatory Notes. 2000;Berlin Heidelberg: Springer-Verlag, 15–74. [Google Scholar]
- [14].Sato Y, Imamura H, Kaizaki Y, et al. Management and clinical outcomes of type I gastric carcinoid patients: retrospective, multicenter study in Japan. Dig Endosc 2014;26:377–84. [DOI] [PubMed] [Google Scholar]
- [15].Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem. Am J Gastroenterol 2004;99:23–32. [DOI] [PubMed] [Google Scholar]
- [16].O’Toole D, Delle Fave G, Jensen RT. Gastric and duodenal neuroendocrine tumors. Best Pract Res Clin Gastroenterol 2012;26:719–35. [DOI] [PubMed] [Google Scholar]
- [17].Pape UF, Maasberg S, Jann H, et al. Management of follow-up of neuroendocrine neoplasias. Best Pract Res Clin Endocrinol Metab 2016;30:129–40. [DOI] [PubMed] [Google Scholar]
- [18].Delle Fave G, Kwekkeboom DJ, Van Cutsem E, et al. ENETS consensus guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology 2012;95:74–87. [DOI] [PubMed] [Google Scholar]
- [19].Kulke MH, Shah MH, Benson ABr, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw 2015;13:78–108. [DOI] [PubMed] [Google Scholar]
- [20].Merola E, Sbrozzi-Vanni A, Panzuto F, et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology 2012;95:207–13. [DOI] [PubMed] [Google Scholar]
- [21].Sarker S, Gutierrez JP, Council L, et al. Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy 2014;46:758–61. [DOI] [PubMed] [Google Scholar]
- [22].Uygun A, Kadayifci A, Polat Z, et al. Long-term results of endoscopic resection for type I gastric neuroendocrine tumors. J Surg Oncol 2014;109:71–4. [DOI] [PubMed] [Google Scholar]
- [23].Grozinsky-Glasberg S, Thomas D, Strosberg JR, et al. Metastatic type 1 gastric carcinoid: a real threat or just a myth. World J Gastroenterol 2013;19:8687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jung JH, Choi KD, Koh YW, et al. Risk factors of lymph node metastasis in patients with gastric neuroendocrine tumor with normal serum gastrin level. Hepatogastroenterology 2015;62:207–13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


