Abstract
Apatinib (Aitan®, brand name in China) is a new anti-antiangiogenic agent that has recently been approved for the treatment of advanced gastric cancer (GC) in China. Nevertheless, its therapeutic efficacy against other types of advanced solid tumors remains unclear. This meta-analysis examines the short-term efficacy and safety of apatinib or combination therapy for GC, hepatocellular carcinoma (HCC) and non-small-cell lung cancer (NSCLC); and provides a discussion of its anti-angiogenesis therapy applications. Seven clinical studies met the inclusion criteria. The treatment of cancers using apatinib was more successful compared to therapy without apatinib. Both objective response rates (ORRs) and disease control rates (DCRs) were significantly improved in the apatinib group compared to those in the control group (RR=2.18, 95% CI 1.30–3.65; RR=2.09, 95% CI 1.21–3.60). The DCR of 850 mg qd and 750 mg qd were higher than those in the control group (P<0.05). Based on the short-term acute adverse reactions of apatinib, significant differences between groups were found for hypertension, urine protein, hand foot syndrome, and gastrointestinal reactions (diarrhea), while no significant differences were found for myelosuppression, nausea and vomiting. Moreover, the results showed that apatinib prolonged patient survival (HR=0.38, 95% CI: 0.28–0.52), and the effect was more pronounced in patients treated with 750 mg qd or 850 mg qd of apatinib than in those treated with a dose of ≤500 mg qd. Additionally, compared to its second-line application, the third-line application was shown to further reduce the risk ratio in patients. Furthermore, overall survival was longer in patients treated with apatinib. Apatinib was shown to have certain short-term effects and survival benefits on GC, HCC, and NSCLC with controllable adverse effects.
Keywords: apatinib, vascular endothelial growth factor, malignant tumor, angiogenesis, meta-analysis
Introduction
Malignant tumors in middle and late stages are generally associated with poor outcomes. Existing studies have suggested that angiogenesis is a key requirement for malignant tumor growth and metastasis.1 Tumor angiogenesis is mainly achieved through the VEGF-VEGFR signaling pathway. The combination of vascular endothelial growth factor (VEGF) with vascular endothelial generated factor receptor 2 (VEGFR-2), induces automatic phosphorylation of the VEGFR-2 carboxyl terminal and phosphorylated kinase insert area, thus promoting a downstream signal cascade reaction, which in turn causes changes in vasodilation, distortion and permeability, and promotes tumor angiogenesis.2 Anti-angiogenesis targeted drugs can normalize tumor angiogenesis before the vessel fading by improving the blood density, expansion and seepage, and enhance the penetration of chemotherapeutic agents and oxygen supply in a short time to increase the sensitivity to chemoradiation.3 In recent years, major advances have been made in immunotherapy; the combination of antiangiogenic drugs and checkpoint blockers have become an attractive strategy in anti-tumor therapy.4 Normalization of the tumor vasculature can also reduce the immunosuppression exerted by Tregs and regulatory B cells. Moreover, this normalization can promote anti-tumor immunity by enhancing the uptake of antigen presentation in dendritic cells and activation of M1-associated macrophages and CD8+ cytotoxic T cells.5 Currently, anti-tumor angiogenesis drugs targeting the VEGF-VEGFR pathway achieve satisfactory clinical efficacy. For example, bevacizumab is a monoclonal antibody (mAb) that binds to VEGF and it has been approved for first- or second-line treatment in metastatic colorectal cancer and ramucirumab, as a novel human IgG1 mAb, selectively inhibits VEGFR2 and has been clinically used as second-line treatment for advanced or metastatic gastric and gastroesophageal cancer. In addition, sunitinib, sorafenib, etc. are also considered to be part of anti-vascular treatment in clinical practice.6
Apatinib (Aitan®, Hengrui Medicine, Lianyungang City, China) is a new type of small molecule tyrosine kinase inhibitor that mainly acts on VEGFR-2.2 Its clinical efficacy in advanced gastric cancer has been approved by the China Food and Drug Administration (CFDA) for patients with metastatic gastric adenocarcinoma or gastroesophageal conjunctive adenocarcinoma after the failure of second-line therapy.7 Several clinical studies have shown that short-term curative effects and survival benefits for patients with various types of middle−late solid tumor are obviously improved.8 Nevertheless, most of the relevant studies are retrospectively non-randomized controlled trials with small sample sizes and uncontrolled statistical analysis, and so far, there has been no assessment of the clinical efficacy of apatinib in advanced solid tumors based on a large sample of RCTs. This paper aims to analyze the efficacy and safety of the therapy with or without apatinib among adult patients with certain types of solid cancers.
Materials and methods
Literature search
We searched PubMed, Cochrane Library, Web of Science, EMBASE, and the CNKI database for articles published from the initiation of each database up to January 1, 2018. All articles were written in English. The key word used in the searches was “Apatinib.”
A total of 844 papers were collected. Finally, seven studies with 800 patients, were included after screening. Disagreements were discussed with a third arbiter until a consensus was reached.
Criteria for inclusion and exclusion
The meta-analysis inclusion criteria were as follows:
1) randomized controlled trial; 2) patients with a malignant solid tumor diagnosed and confirmed by cytology and pathology, TNM > III or failed first-line therapy, and with an expected survival time >1 month; 3) the main intervention measures of the experimental group and the control group were consistent with the use of apatinib; 4) reported the outcome indexes of interest, including short-time efficacy which was assessed by Response Evaluation Criteria In Solid Tumors (RECIST), survival analysis and adverse reactions; 5) high quality studies, newer publications and those with clearly reported data were selected preferentially among similar studies.
The exclusion criteria were as follows: 1) non-randomized controlled trial; 2) repeated publication of data; 3) number of cases <20; 4) the data was reported too vaguely to be extracted; 5) the outcome index was not reasonable or could not be merged.
Data extraction and quality assessment
The titles and abstracts of potentially relevant studies were independently screened by two reviewers according to the predefined selection criteria. Cochrane bias risk assessment criteria were used to evaluate the methodological quality of the RCTs,9 which were categorized into a low, unclear, or high risk of bias. This categorization was done according to the risk of bias for each important outcome within the included trials, including adequacy of the generation of allocation sequence, allocation concealment, blinding, and the presence of incomplete outcome data, selective outcome, or other sources of bias. Any disagreement was resolved through discussion and consensus with a third author. The extracted data were summarized as follows: authors, publication time, number of cases, basic information about the patients, intervention measures and outcome indicators.
Statistical analysis
Stata12.0 statistical software was used for meta-analysis and publication bias testing. The dichotomous data of the disease control rate (DCR), objective response rate (ORR) and adverse reactions were statistically based on Risk Ratio (RR), while survival data, including PFS, OS were based on Hazard Ratio (HR). The 95% confidence interval (CI) was calculated as a measure of estimate uncertainty. Heterogeneity was evaluated according to the sampling frame and statistical tests, which included chi-squared and I2 statistics; heterogeneity was considered low, moderate, or high for I2 values under 25%, between 25% and 50% and over 50%, respectively.10 The heterogeneity was judged according to clinical thinking. Finally, we conducted a simple assessment by sensitivity analysis and evaluation of publication bias, and the credibility of our research was confirmed.
Results
Search results and quality evaluation
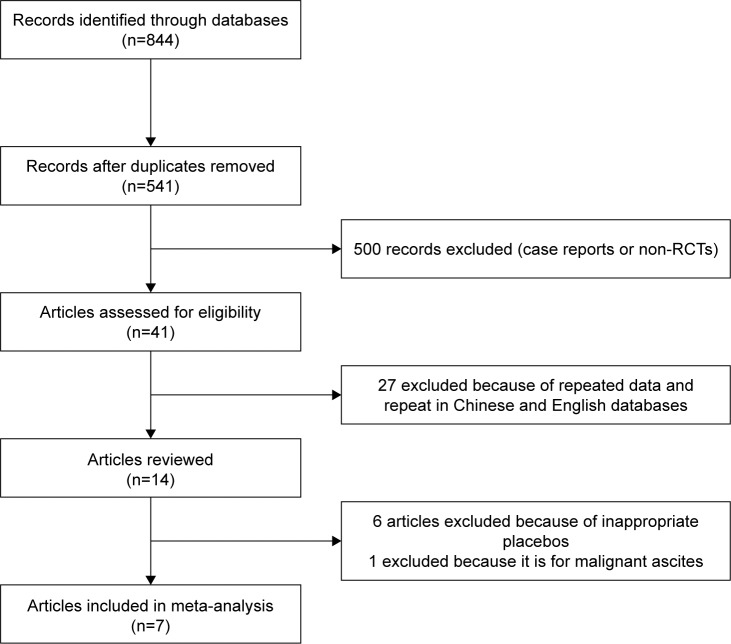
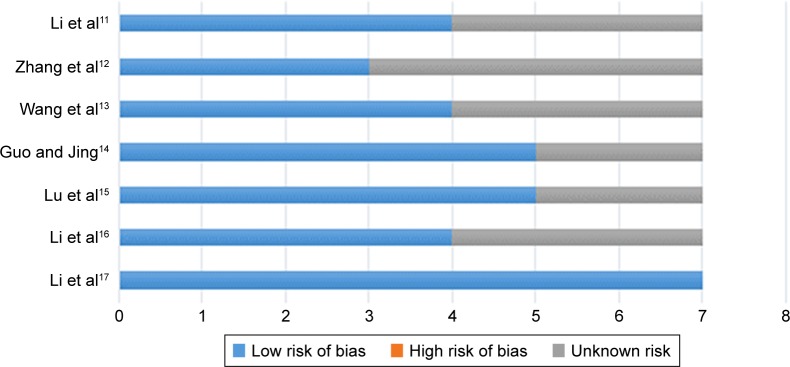
A total of 844 relevant papers were retrieved from five databases (PubMed, Cochrane Library, Web of Science, Embase and CNKI). The titles and abstracts were screened by two authors (JS and HL) respectively. Consequently, studies including case reports, non-randomized controlled trials, repetitive articles, and those containing the same set of published data were eliminated. Finally, seven trials were found to match the inclusion criteria and were included in the analysis.11–17 A flow chart of the search process is shown in Figure 1. Cochrane bias risk assessment criteria were then used to evaluate the methodological quality of the RCTs; any disagreement was resolved through discussion and consensus with a third author (JX). The quality of each trial is shown in Figure 2.
Figure 1.
Study flow diagram.
Figure 2.
The quality of each trial.
General characteristics of the included studies
All the included studies were RCTs which contained similar general characteristics of patients diagnosed with one of the three tumor types, commonly found in China: gastric cancer, hepatocellular carcinoma and non-small-cell lung cancer. Consequently, an additional article18 including patients with malignant ascites was excluded since the heterogeneity was greater when considering non-solid tumors. Meta-analysis was performed based on random-effect models considering heterogeneity from tumor type, drug dose, application line, and so forth.
The patients were almost all ethnic Chinese, and there was a similar number of male and female patients. For the intervention, the treatment with or without apatinib was the main difference, while apatinib dose, treatment line and tumor type were also analyzed. The primary outcomes referred to short efficacy and survival time of the patient. Basic information about the articles is shown in Table 1.
Table 1.
Basic information regarding the studies
| Study | Year | Tumor type | N | M/F ratio | Region | Participants
|
Line of apatinib | Outcome index | |
|---|---|---|---|---|---|---|---|---|---|
| Control | Treatment | ||||||||
| Li et al11 | 2013 | Gastric cancer | 144 | 109:33 | China | Placebo | Apatinib (850 mg qd or 425 mg bid) | Third line | ORR, DCR, PFS and OS, adverse reaction |
| Li et al16 | 2017 | Hepatocellular carcinoma | 40 | – | China | TACE | Oral apatinib (unknown dose) | Unknown | CR, PR, SD, PD, survival rate of half year/1 year, total survival rate, VEGF and MMP-9 level, adverse reaction |
| Lu et al15 | 2017 | Hepatocellular carcinoma | 44 | 33:9 | China | TACE | Oral apatinib (500 mg qd) + TACE | Unknown | AFP level, ORR, PFS, adverse reaction |
| Guo and Jing14 | 2017 | Nonsquamous non-small-cell lung cancer | 39 | 20:19 | China | Received chemotherapy | Oral apatinib (500 mg qd) + docetaxel | Second line | PFS, DCR, ORR, adverse reaction |
| Wang et al13 | 2017 | Nonsquamous non-small-cell lung cancer | 128 | 87:41 | China | Received chemotherapy | Apatinib + chemotherapy (unknown dose) | Second line | PFS, OS, CR, PR, SD, PD, adverse reaction |
| Zhang et al12 | 2012 | Nonsquamous non-small-cell lung cancer | 135 | – | China | Placebo | Apatinib (750 mg qd) | Third line | CR, PR, SD, PD, PFS, adverse reaction |
| Li et al17 | 2016 | Gastric cancer | 273 | 201:66 | China | Placebo | Apatinib (850 mg qd) | Third line | CR, PR, SD, PD, OS, PFS, adverse reaction |
Abbreviations: ORR, objective response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; CR, complete remission; PR, partial remission; SD, stable disease; PD, progression disease; AFP, alpha fetoprotein.
Short-term efficacy: ORR and DCR
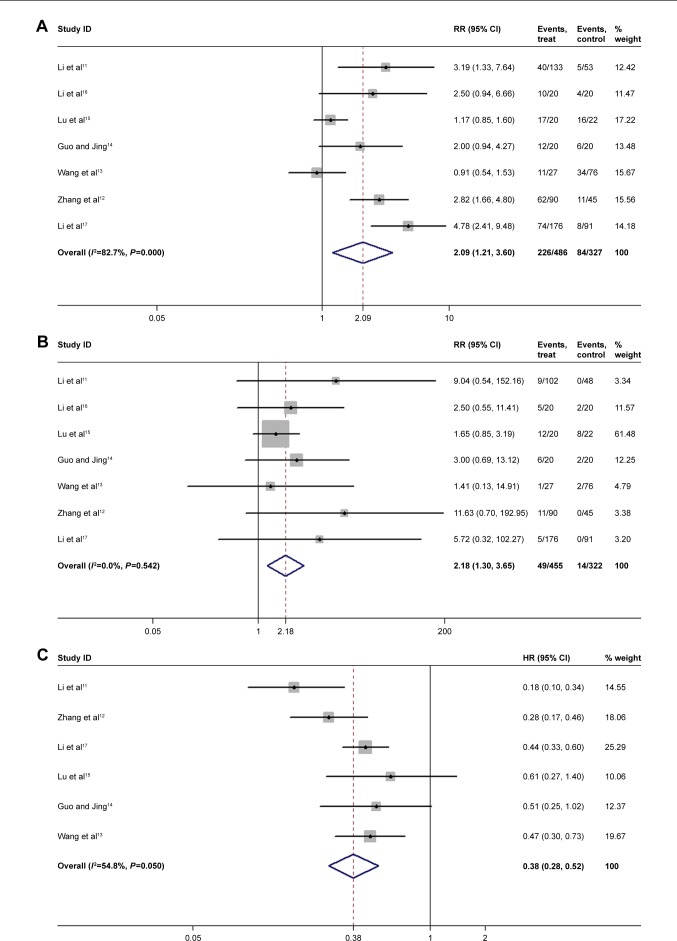
Seven studies that included 800 patients were used to evaluate the short-time efficacy. They contained ORR and DCR of apatinib on advanced solid cancers, including gastric cancer, hepatocellular carcinoma and non-small-cell lung cancer. Briefly, a significantly higher ORR and DCR were observed in the apatinib groups than in control groups (RR=2.18, 95% CI: 1.30–3.65; and RR=2.09, 95% CI: 1.21–3.60, respectively) (shown in Figure 3A and B). Furthermore, although no significant heterogeneity between studies was found in ORR (I2=0%, P=0.542), we performed subgroup analysis on the cancer types in light of their clinical differences. Surprisingly, the subgroup analysis suggested that there was no statistically significant difference in ORR between the apatinib and the control group (GC: RR=7.22, 95% CI: 0.96–54.32; HCC: RR=1.76, 95% CI: 0.96–3.22; NSCLC: RR=3.14, 95% CI: 1.00–9.86). In addition, according to an analysis based on apatinib’s different application stages, a difference in ORR between its third-line application (RR=8.49, 95% CI: 8.49–1.65) and second-line application (RR=2.43, 95% CI: 2.43–0.69) was observed. Compared with the control group, the third-line application of apatinib increased ORR (P<0.05), while the second-line application appeared to have no significant benefit. The same results were also seen in DCR (third line: RR=3.39, 95% CI: 2.32–4.95; second line: RR=1.28, 95% CI: 0.60–2.76). Moreover, the stratified analysis of the dose of apatinib indicated that the doses of 850 mg qd and 750 mg qd had a higher DCR than did the control group (P<0.05); but the dose of 500 mg qd seemed to have no effect (95% CI: 0.79–2.46) (shown in Table 2).
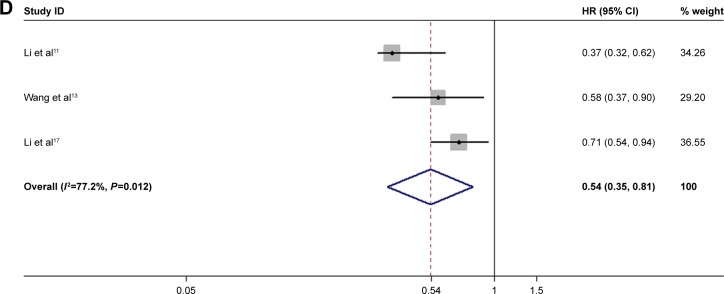
Figure 3.
Meta-analysis of the short-term efficacy and survival benefit of apatinib.
Notes: (A) DCR; (B) ORR; (C) PFS; and (D) OS. Weights are from random-effects analysis.
Abbreviations: DCR, disease control rate; ORR, objective response rate; PFS, progression-free survival; OS, overall survival.
Table 2.
Subgroup analysis of DCR
| Subgroup analysis | Total no of studies | Treatment group
|
Control group
|
RR (95% CI) of DCR | ||
|---|---|---|---|---|---|---|
| Total no of patients | Subjects (SD + PR + CR) | Total no of patients | Subjects (SD + PR + CR) | |||
| Tumor type | ||||||
| GC | 2 | 309 | 114 | 144 | 13 | 4.10 (2.39, 7.02) |
| HCC | 2 | 40 | 27 | 42 | 20 | 1.54 (0.66, 3.60) |
| NSCLC | 3 | 137 | 85 | 141 | 51 | 1.71 (0.81, 3.60) |
| Line | ||||||
| Second line | 2 | 47 | 23 | 96 | 40 | 1.28 (0.60, 2.76) |
| Third line | 3 | 399 | 176 | 189 | 24 | 3.39 (2.42, 4.95) |
| Apatinib dose | ||||||
| 850 mg | 2 | 309 | 114 | 144 | 13 | 4.10 (2.39, 7.02) |
| 750 mg | 1 | 90 | 62 | 45 | 11 | 2.82 (1.66, 4.80) |
| 500 mg | 2 | 40 | 29 | 42 | 22 | 1.40 (0.79, 2.46) |
Abbreviations: CR, complete remission; PR, partial remission; SD, stable disease; DCR, disease control rate; GC, gastric cancer; HCC, hepatocellular carcinoma; NSCLC, non-small-cell lung cancer.
PFS
Overall, apatinib prolonged the patients’ PFS (HR=0.38, 95% CI: 0.28–0.52) (shown in Figure 3C). Furthermore, we performed subgroup analysis of the tumor type based on the application stage and the dose of apatinib used. Our data showed that apatinib extended the patients’ mean PFS for gastric cancer (HR=0.29, 95% CI: 0.12–0.71) and NSCLC (HR=0.40, 95% CI: 0.27–0.58); nevertheless, there was not enough data to support these same conclusions in hepatocellular carcinoma. In addition, the third-line application of apatinib further reduced the risk ratio in patients (HR=0.30, 95% CI: 0.18–0.50 in third line; HR=0.48, 95% CI: 0.33–0.70 in second line). The dose of 750 mg qd and 850 mg qd of apatinib have been shown to be more efficient in improving PFS than was the dose of 500 mg qd or lower (shown in Table 3).
Table 3.
Subgroup analysis of PFS
| Subgroup analysis | Total no of studies | Total no of patients | HR of apatinib | 95% CI |
|---|---|---|---|---|
| Tumor type | ||||
| GC | 2 | 414 | 0.29 | 0.12, 0.71 |
| HCC | 2 | 44 | 0.61 | 0.27, 1.39 |
| NSCLC | 3 | 302 | 0.4 | 0.27, 0.58 |
| Line | ||||
| Second line | 2 | 127 | 0.48 | 0.33, 0.70 |
| Third line | 3 | 549 | 0.3 | 0.18, 0.50 |
| Apatinib dose | ||||
| 850 mg | 2 | 414 | 0.29 | 0.12, 0.71 |
| 750 mg | 1 | 135 | 0.28 | 0.17, 0.45 |
| 500 mg | 2 | 83 | 0.55 | 0.32, 0.94 |
Note: See Figure 3D.
Abbreviations: GC, gastric cancer; HCC, hepatocellular carcinoma; NSCLC, non-small-cell lung cancer.
OS
Apatinib prolonged the OS of patients. Three of the included articles indicated that the apatinib group had a 0.5 times higher OS than did that of the control group (HR=0.54, 95% CI: 0.35–0.81) (shown in Figure 3D).
Adverse reactions
In this paper, we analyzed short-term acute adverse reactions, such as myelosuppression, nausea and vomiting, hypertension, proteinuria, hand-foot syndrome, and gastrointestinal reactions. The results showed that the apatinib group had a significantly higher incidence of hypertension (RR=8.38, P<0.05), urine protein (RR=2.66, P<0.05), hand foot syndrome (RR=11.50, P<0.05), and gastrointestinal reaction (diarrhea) (RR=2.63, P=0.02); while no difference in bone marrow suppression, nausea and vomiting were found compared to the control group (Table 4). As defined in the original literature, most adverse reactions are classified below level 2 (according to Common Terminology Criteria for Adverse Events).
Table 4.
Adverse reactions of the apatinib group vs the control group
| Adverse reaction | Study no | RR | Min 95% CI | Max 95% CI | P-value |
|---|---|---|---|---|---|
| Myelosuppression | 5 | 1.45 | 0.52 | 4.06 | 0.48 |
| Nausea and vomiting | 3 | 1.00 | 0.63 | 1.58 | 0.99 |
| Hypertension | 5 | 8.38 | 4.89 | 14.37 | <0.005 |
| Proteinuria | 5 | 2.66 | 1.72 | 4.11 | <0.005 |
| Hand-foot syndrome | 5 | 11.50 | 5.68 | 23.68 | <0.005 |
| Diarrhea | 4 | 2.63 | 1.20 | 5.79 | 0.016 |
Publication bias assessment and sensitivity analyses
Because of the limited number of clinical studies included (n<10), we did not perform a statistical evaluation of publication bias. For sensitivity analyses, we tried to perform an analysis of each outcome indicator after excluding each article and doing the statistical analysis again by including an article which was excluded before. It was found that the excluded results did not differ from the previous ones (Table 5), suggesting that the sensitivity was low; and the results were robust and credible.
Table 5.
The sensitivity analyses of the study
| Sensitivity analyses | No of studies | RR (95% CI)
|
HR (95% CI)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies (no of patients) | DCR | Studies (no of patients) | ORR | Studies (no of patients) | PFS | Studies (no of patients) | OS | ||
| Total studies | 7 | 7 (n=800) | 2.09 (1.21, 3.60) | 7 (n=800) | 2.18 (1.30, 3.65) | 6 (n=760) | 0.38 (0.28, 0.52) | 3 (n=542) | 0.54 (0.35, 0.81) |
| Liu et al18 added | 8 | 8 (n=829) | – | 8 (n=829) | 2.16 (1.39, 3.16) | 6 (n=760) | 0.38 (0.28, 0.52) | 3 (n=542) | 0.54 (0.35, 0.82) |
| Li et al11 excluded | 6 | 6 (n=656) | 1.97 (1.10, 3.94) | 6 (n=656) | 2.07 (1.23, 3.50) | 5 (n=616) | 0.43 (0.35, 0.53) | 2 (n=398) | 0.67 (0.53, 0.85) |
| Li et al16 excluded | 6 | 6 (n=760) | 2.05 (1.12, 3.74) | 6 (n=760) | 2.21 (1.22, 3.98) | 6 (n=760) | 0.38 (0.28, 0.52) | 3 (n=542) | 0.54 (0.35, 0.81) |
| Lu et al15 excluded | 6 | 6 (n=756) | 2.35 (1.35, 4.09) | 6 (n=756) | 3.38 (1.47, 7.77) | 5 (n=726) | 0.36 (0.25, 0.51) | 3 (n=542) | 0.54 (0.35, 0.81) |
| Guo and Jing14 excluded | 6 | 6 (n=761) | 2.12 (1.31, 3.97) | 6 (n=761) | 2.08 (1.20, 3.61) | 5 (n=727) | 0.36 (0.25, 0.52) | 3 (n=542) | 0.54 (0.35, 0.81) |
| Wang et al13 excluded | 6 | 6 (n=672) | 2.45 (1.31, 4.58) | 6 (n=672) | 2.22 (1.31, 3.77) | 5 (n=632) | 0.36 (0.24, 0.53) | 2 (n=414) | 0.52 (0.27, 0.98) |
| Zhang et al12 excluded | 6 | 6 (n=665) | 1.99 (1.07, 3.72) | 6 (n=665) | 2.05 (1.21, 3.47) | 5 (n=625) | 0.41 (0.29, 0.58) | 3 (n=542) | 0.54 (0.35, 0.82) |
| Li et al17 excluded | 6 | 6 (n=530) | 1.79 (1.10, 2.90) | 6 (n=530) | 2.11 (1.25, 3.56) | 5 (n=490) | 0.36 (0.24, 0.55) | 2 (n=272) | 0.49 (0.25, 0.70) |
Abbreviations: DCR, disease control rate; ORR, objective response rate; PFS, progression free survival; OS, overall survival.
Discussion
In this article, we compared the efficacy and safety of apatinib in patients with various types of advanced malignant solid tumors. First, compared with the control group, the apatinib group showed significant improvements in ORR and DCR. Moreover, the third-line application seemed to have better benefits compared to second-line treatment, which suggests that apatinib could be used as a new treatment option for patients with advanced GC, HPC, and NSCLC who failed to respond to second-line treatment. With reference to the heterogeneity among the studies; the cancer types, dosage and application stages of apatinib were initially analyzed. The stratification of the clinical data related to each kind of tumor showed high heterogeneity (I2>50%, P<0.1). Consequently, we conducted a regression analysis of the apatinib dose used in the experimental groups; the specific dosage of apatinib, ie, 850 mg qd, 750 mg qd and 500 mg qd were investigated in five articles (it should be noted that in the 500 mg qd group, the apatinib dose was appropriately reduced when the adverse reactions were not tolerable). Briefly, our data showed that the heterogeneity was significantly decreased in each group (850 mg group: I2=0.00, P=0.47; 500 mg group: I2=53%, P=0.14; only one group of clinical data was included in the 700 mg group). In addition, logRR and the increased dosage showed an inversion proportion, and after the dosage was included as a covariate, T-squared was reduced from 0.5625 to 0.0023, indicating that the dosage was an important cause of heterogeneity, accounting for 99.08%. Through the analysis of the included literature, it was shown that the dose of 850 mg was the most beneficial for the short-term efficacy.
According to this literature review, we believe that apatinib shows a satisfactory short-term effect because of the time window of vascular normalization caused by the prophase application of apatinib. Tumor angiogenesis is different from normal blood vessels in structure and function, and it creates a tumor microenvironment characterized by high interstitial fluid pressure (IFP), hypoxia and acidosis in tissues, which in turn affects the curative effect and prognosis of the tumor.19 Anti-angiogenesis targeted drugs can normalize angiogenesis before the tumor vessels regressing, by improving the blood density, expansion and seepage, which enhance the penetration of chemotherapeutic agents and oxygen supply in a short time, thus increasing tumor sensitivity to chemoradiation.3
In terms of safety, this analysis was mainly focused on investigating the main adverse reactions of apatinib in clinical reports, such as bone marrow suppression (mainly leukopenia), hypertension, proteinuria, extremities syndromes, digestive tract reactions, etc. Like most other targeted drugs, the patients in the apatinib groups showed no significant difference in bone marrow suppression, nausea and vomiting compared with the control group but did experience induced hypertension, proteinuria and hand-foot syndrome. The specific mechanisms causing adverse reactions are unknown. Some studies have shown that VEGFR receptor inhibitors reduce the synthesis of NO and prostacyclin by inhibiting the VEGFR of endothelial cells,20 leading to elevated blood pressure. Furthermore, the occurrence of proteinuria may be associated with inhibition of VEGF of the foot and the vascular cells surrounding tubular cells.21 A retrospective cohort study showed that most of the adverse reactions occurred in the first cycle of the apatinib treatment. On the other hand, the risk of death was reduced by 10%, if the adverse events occurred one week earlier each additional adverse event reduced the risk of death by 23%.22 The original studies included in the meta-analysis, reported that most adverse reactions were grade 1 or grade 2 and were controllable. These data further suggest that it is possible to predict the curative effect by a series of mathematical conversions related to the adverse reactions that occur in the short term, which is beneficial for the exploration of the molecular markers of anti-vascular drugs. In addition, the author observed that there are some other adverse reactions, such as hemorrhagic cystitis and dyspnea, which can be improved by reduced dose administration and symptomatic treatment.
Preliminary analysis showed that the PFS in patients with advanced tumors was extended by apatinib (HR=0.38, 95% CI: 0.28, 0.52). Statistically, no significant differences in PFS were found among five of the studies included in this meta-analysis. In consideration of the clinical-related heterogeneity of each cancer type and the dosage and application stages of apatinib, we performed a subgroup analysis and once again, found no significant difference between studies. A total of three eligible articles were used to evaluate the patient’s OS. The meta-analysis suggested that the apatinib treatment can also prolong the patient’s OS, but the heterogeneity between studies could not be explained due to a lack of data.
Overall, multiple clinical reports have shown that apatinib has a certain effect on advanced liver cancer,23 lung cancer,24 ovarian cancer,25 and other types of malignancy. Nonetheless, there is no clinical research involving a large dataset that reveals its efficacy in malignant solid tumors. In this study, we performed a statistical analysis of the short-term effects, survival benefit and acute adverse reactions of apatinib in seven clinical studies and 800 patients. Because of the great achievements made in the field of immunotherapy, the combination of antiangiogenic drugs and checkpoint blockers has become an attractive strategy in anti-tumor therapy. Apatinib is a new type of small molecule tyrosine kinase inhibitor of VEGFR with a mechanism similar to that of ramucirumab, which has been approved by the Food and Drug Administration as a second-line treatment of advanced or metastatic gastric or gastroesophageal cancer. Apatinib can be combined with immunotherapy thus providing more treatment choices for patients.
The major limitations of this study are as follows: 1) here are not enough data for an in-depth analysis of PFS and OS; more clinical data are necessary to support the survival benefit of apatinib; 2) the current clinical study is limited to the Asian population, so whether the treatment effect of apatinib is different in other populations needs to be further investigated. ANGEL26 is an RCT study that is currently evaluating the efficacy and safety of apatinib in the treatment of advanced gastric cancer in North America, Europe and the subPacific, and its results are worth looking forward to; 3) among all the studies including combined apatinib with chemotherapy, we could not perform a subgroup analysis by chemotherapy drugs because of the limited number of studies, and this does not even include the other types of anti-tumor drugs that were used. The existing experimental results show that in the environment of tumor malnutrition induced by apatinib, the combination with Elemene Injections can induce protective autophagy and prevent human hepatoma cancer cells from undergoing apoptosis27 which suggests they should not be used concurrently.
Conclusion
In conclusion, the anti-tumor angiogenesis targeted drug apatinib shows a significant therapeutic effect in advanced solid tumors (gastric carcinoma, hepatocellular carcinoma and non-small-cell lung cancer), with controllable adverse effects. However, the present research has shown that even in the case of apatinib’s most common application for stomach cancer, the following limitations still exist in the literature: the details of early chemotherapy regimens are unclear; the analysis of predictive factors, and the ability to perform subgroup analysis. Therefore, more clinical studies on apatinib are required.
Acknowledgments
This study was supported by the Medical Scientific Research Project in Chongqing, People’s Republic of China (grant no 20141003); the Natural Science Foundation Project of Chongqing Science and Technology Commission (CSTC), People’s Republic of China (grant no cstc2013jcyjA10104); Chongqing Clinical Oncological Research Center, People’s Republic of China (grant no cstc2015yfpt_gcjsyjzx120010); the Natural Science Foundation Project of Chongqing Science and Technology Commission (CSTC), People’s Republic of China (grant no cstc2017jcyjA0630); and the Natural Science Foundation Project of Chongqing Science and Technology Commission (CSTC), China (grant no cstc2018jcyjAX0012).
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Krzywinska E, Kantari-Mimoun C, Kerdiles Y, et al. Loss of HIF-1α in natural killer cells inhibits tumour growth by stimulating non-productive angiogenesis. Nat Commun. 2017;8(1):1597. doi: 10.1038/s41467-017-01599-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. doi: 10.1111/j.1349-7006.2011.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin MI, Sessa WC. Antiangiogenic therapy: creating a unique “window” of opportunity. Cancer Cell. 2004;6(6):529–531. doi: 10.1016/j.ccr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204. doi: 10.1007/s10456-017-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med. 2014;2(12):123. doi: 10.3978/j.issn.2305-5839.2014.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang JF, Chen XY, Zhong DF. Metabolic research of domestically developed small molecule tyrosine kinase inhibitors. Yao Xue Xue Bao. 2016;51(2):248–256. [PubMed] [Google Scholar]
- 8.Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075–6081. doi: 10.2147/DDDT.S97235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration Version 5.10. 2011. [Accessed September 5, 2018]. Available from: www.cochrane-handbook.org.
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Shi M, Huang C, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J Clin Oncol. 2012;30(15 Suppl):7548. Abstract. [Google Scholar]
- 13.Wang X, Zhang W, Du W, et al. Efficacy and survival analysis of apatinib in patients with advanced nonsquamous non-small cell lung cancer after failure of first-line treatment. Zhongguo Fei Ai Za Zhi. 2017;20(11):761–768. doi: 10.3779/j.issn.1009-3419.2017.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Jing X. Efficacy and safety of docetaxel plus apatinib as a second-line treatment for advanced nonsquamous non-small cell lung cancer. Chinese J Clin Oncol. 2017;44(11):544–546. [Google Scholar]
- 15.Lu W, Jin XL, Yang C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther. 2017;18(6):433–438. doi: 10.1080/15384047.2017.1323589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Man W, Guo H, Yang P. Clinical research of apatinib with transcatheter arterial chemoembolization in treatment of advanced hepatocellular carcinoma. Anti-Tumor Pharmacy. 2017;7:74–78. [Google Scholar]
- 17.Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Chen S, Wang P, et al. Preliminary study of the treatment effect of apatinib mesylate upon malignant ascites and its safety. Transl Cancer Res. 2017;6(5):894–900. [Google Scholar]
- 19.Matsumoto S, Batra S, Saito K, et al. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 2011;71(20):6350–6359. doi: 10.1158/0008-5472.CAN-11-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang JR, Markham NE, Lin YJ, et al. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287(2):L344–L351. doi: 10.1152/ajplung.00291.2003. [DOI] [PubMed] [Google Scholar]
- 21.Launay-Vacher V, Deray G. Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs. 2009;20(1):81–82. doi: 10.1097/CAD.0b013e3283161012. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Qin S, Wang Z, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol. 2017;10(1):153. doi: 10.1186/s13045-017-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kou P, Zhang Y, Shao W, et al. Significant efficacy and well safety of apatinib in an advanced liver cancer patient: a case report and literature review. Oncotarget. 2017;8(12):20510–20515. doi: 10.18632/oncotarget.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng Y, Cui H, Liu Z, et al. Apatinib to combat EGFR-TKI resistance in an advanced non-small cell lung cancer patient with unknown EGFR status: a case report. Onco Targets Ther. 2017;10:2289–2295. doi: 10.2147/OTT.S130990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Wang Y, Lu W, et al. Apatinib treatment combined with chemotherapy for advanced epithelial ovarian cancer: a case report. Onco Targets Ther. 2017;10:1521–1525. doi: 10.2147/OTT.S126471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang YK, Boku N, Kang WK. A prospective, randomized, double-blinded, placebo-controlled, phase III study to evaluate the efficacy and safety of apatinib plus best supportive care (BSC) compared to placebo plus BSC in patients with advanced or metastatic gastric cancer: the ANGEL study. J Clin Oncol. 2017;35(15_suppl):TPS4138. Abstract. [Google Scholar]
- 27.Lin Y, Wang K, Hu C, et al. Elemene injection induced autophagy protects human hepatoma cancer cells from starvation and undergoing apoptosis. Evid Based Complement Alternat Med. 2014;2014:637528. doi: 10.1155/2014/637528. [DOI] [PMC free article] [PubMed] [Google Scholar]