Abstract
Purpose
Breast cancer (BC) is the leading cancer affecting Chinese women; however, the immune microenvironment between molecular subtypes is less reported. This study aimed to investigate the distribution of tumor-infiltrating lymphocyte (TIL) subpopulations, especially exhausted CD4+ and CD8+ TILs in Chinese BC patients.
Patients and methods
A total of 133 patients with breast invasive ductal carcinoma were recruited consecutively from January 1, 2012 to December 31, 2013, and TILs were detected in H&E-stained sections. Expression profiling of PD-1, CD4, and CD8 was determined by immunohistochemistry on 4 µm formalin-fixed paraffin-embedded tissue sections. The distribution of TILs was analyzed based on hormone receptor status and molecular subtypes.
Results
PD-1+, CD4+, and CD8+ TILs distributed differently based on molecular subtypes. Compared to Luminal A, triple-negative breast cancer (TNBC) patients had more PD-1+ TILs (39/high-power field [HPF] vs 11/HPF), PD-1+ helper T (CD4+) cells (28/HPF vs 10/HPF), and PD-1+ cytotoxic (CD8+) T-cells (3/HPF vs 2/HPF).
Conclusion
TILs are distributed differently based on molecular subtypes. TNBC patients exhibit more PD-1+ exhausted TILs, representing an inhibitory immune microenvironment. PD-1/PD-L1 pathway is a potential therapeutic target of TNBC.
Keywords: breast cancer, tumor-infiltrating lymphocyte, PD-1, molecular subtype
Introduction
Breast cancer (BC) is the most common malignancy in women, and 269,000 new cases were diagnosed in People’s Republic of China in 2015, accounting for 15% of all female cancers.1 In People’s Republic of China, the 5-year survival rate was 73%,2 compared to 90% in USA.3 This survival difference was reflective of a gap in therapeutic advancement and different pathological characteristics (such as low ER+ but high HER2+ status in Chinese cases).4,5 Comprehensive clinical management such as breast conservation surgery, endocrine therapy, targeted therapy, or immunotherapy was less applied in People’s Republic of China.6–8 Therefore, further exploration of emerging therapeutic technique is very meaningful to Chinese BC patients.
PD-1 is an immune checkpoint and inhibits biological functions of effector T-cells. PD-L1 is expressed on the surface of cancer cells, binds to PD-1, and then suppresses lymphocyte functions. Through presenting PD-L1 and binding to PD-1, cancer cells inhibit antitumor response of host immune system in tumor microenvironment. Recovery of antitumor response with checkpoint inhibitor against PD-1 has produced remarkable efficacy for non-small-cell lung carcinoma and melanoma.9,10 Patients with positive PD-L1 are eligible for checkpoint inhibitor application. In general, 12% of BC cases expressed PD-L1 in tumor tissue, and 32% of triple-negative breast cancer (TNBC) cases are PD-L1+.11
A high proportion of tumor-infiltrating lymphocytes (TILs) predicts a favorable prognosis of BC patients.12 High counts of intratumoral CD4+ and CD8+ lymphocytes conferred better survival in BC patients.13 Lymphocytes presenting positive PD-1 (PD-1+) indicated functional exhaustion.14 Intratumorally, PD-1+ TILs correlated with worse survival of BC.15 However, specific distribution of TIL subpopulations in Chinese BC patients remains unknown. This study aimed to investigate the distribution of exhausted TILs phenotypes, especially exhausted CD4+ and CD8+ TILs, and their relationship with pathological characteristics in Chinese BC patients.
Patients and methods
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethical committee of Beijing Shijitan Hospital, Capital Medical University, and following the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The ethical committee of Beijing Shijitan Hospital, Capital Medical University approved this study. This study had a retrospective design, and most of the patients were lost to follow-up, and so formal written informed consent consent was not required. Patients’ privacy and confidentiality were well protected and any identifiable data was recoded. Patients’ identity is unavailable to readers.
Patients
This cross-sectional study included 133 consecutively recruited invasive ductal BC patients. Patients had a diagnosis of operable BC and received surgical treatment at Department of Breast Surgery, Beijing Shijitan Hospital, Capital Medical University consecutively from January 1, 2012 to December 31, 2013. All of the cases were pathologically diagnosed with primary invasive BC.
Tissue collection
Archival formalin-fixed, paraffin-embedded (FFPE) BC samples were collected from all patients. Histopathologic feature was determined using serial 4 µm thickness sections derived from each specimen.
Immunohistochemistry (IHC)
Expression profiling of PD-1, Ki-67, CD4, and CD8 was assessed by IHC on 4 µm FFPE sections. Monoclonal antibodies against PD-1 (mouse anti-human, # UMAB199), Ki-67 (mouse anti-human, # MIB1), CD4 (rabbit anti-human, # EP204), and CD8 (# SP16) were purchased from Beijing Zhong Shan Golden Bridge Biotechnology Co. Ltd (Beijing, People’s Republic of China). Sections were baked at 60°C in a dehydration oven for 60 minutes, dewaxed, and rehydrated using xylene and graded alcohol washes. Antigen retrieval and deparaffinization were carried out using the EnVision™ FLEX Target Retrieval Solutions (Dako, Glostrup, Denmark). Sections were cooled to room temperature in TBST wash buffer for 5 minutes and then incubated with the primary antibodies. PD-1 and Ki-67 detection was visualized with DAB, whereas for CD4 and CD8 it was with AP-red. Slides were counterstained with hematoxylin.
TILs were detected in IHC sections. TILs were counted in randomly selected five different high-power fields (HPFs) to obtain an average number. PD-1 was expressed on the cytoplasm of lymphocytes colored brown, and CD4 and CD8 were expressed on the cytomembrane of lymphocytes colored red. Double staining of CD4/PD-1 and CD8/PD-1 showed red cytomembrane and brown cytoplasm of lymphocytes. We counted the PD-1-, CD4-, or CD8-positive cells in 100 lymphocytes, and then calculated the expression rate. The proportion of PD-1/CD4 coexpression or PD-1/CD8 coexpression was calculated in 100 CD4+ or CD8+ lymphocytes.
Ki-67 was expressed on nucleus of BC cells colored brown. Ki-67 index was estimated based on 100 tumor cells.
ER and PR classification
The status of ER and PR were determined by IHC, using the EnVision method with FLEX Ready-to-Use and Autostainer Link Solution (Dako). Monoclonal antibodies against ER (rabbit anti-human, # IR084 Clone EP1) and PR (rabbit anti-human, # IR068 Clone PgR 636) were purchased from Gene Tech Co. Ltd. (Shanghai, People’s Republic of China). ER and PR were expressed in the nucleus colored brown. Positive ER or PR expression was defined as ≥1% tumor cells with staining.
HER2 classification
Expression of HER2 was assessed by IHC using Ventana HER2 method (Roche BenchMark XT, Roche Diagnostics, Rotkreuz, Switzerland). HER2 rabbit anti-human monoclonal antibody was purchased from Roche Diagnostics GmbH (clone # 4B5). HER2 was expressed on the cytomembrane. HER2 amplification was defined as a score of 3+, equivocal HER2 expression was defined as a score of 2+, and negative HER2 expression was defined as a score of 0/1+. For equivocal stating, fluorescence in situ hybridization was performed, and HER2 amplification was defined as a ratio of HER2/CEP17 >2.2.
Definition of molecular subtypes
According to the hormone receptor (HR) ER, PR, and HER2 status and Ki-67 index, the BC cases were divided into four subtypes: Luminal A, Luminal B, HER2 overexpression, and TNBC. Luminal A was defined as positive HR (positive ER and/or positive PR) and negative HER2. Luminal B was defined as positive HR and HER2 amplification, or positive HR, negative HER2, but Ki-67 index ≥14%. HER2 overexpression was defined as negative HR (negative ER and PR) and HER2 amplification. TNBC was defined as negative HR and HER-2.
Statistical analysis
SPSS version 19.0 (IBM Corporation, Armonk, NY, USA) was used to conduct data analyses. Phenotypes of TIL were described by median and interquartile range (IQR). The difference between ER, PR, and HER2 status was evaluated by Wilcoxon test. The difference of TILs phenotypes among molecular subtypes was analyzed by Kruskal–Wallis test. The difference between Luminal A and TNBC subtypes was estimated by Wilcoxon test. All tests were two-sided, with a significant level of P<0.05.
Results
The average age of the 133 patients was 57.8, and more than 85% patients were in histological grade I and II (Table 1). The expression rate of ER and PR was 73.8% and 65.1% (Table 1). The amplification rate of HER2 was 25.6% (Table 1). 54.2% patients had subtype Luminal A, 28.3% patients had Luminal B, 8.3% patients had HER2 overexpression, and 9.2% patients had TNBC (Table 1).
Table 1.
Patient characteristics in the study
| Items | |
|---|---|
| Age, mean±SD | 57.8±13.6 |
| Histological grade, n (%) | |
| I | 14 (11.3) |
| II | 82 (66.1) |
| III | 28 (22.6) |
| ER expression, n (%) | |
| Negative | 33 (26.2) |
| Positive | 93 (73.8) |
| PR expression, n (%) | |
| Negative | 44 (34.9) |
| Positive | 82 (65.1) |
| HER2 amplification, n (%) | |
| Negative | 64 (74.4) |
| Positive | 22 (25.6) |
| Molecular subtype, n (%) | |
| Luminal A | 65 (54.2) |
| Luminal B | 34 (28.3) |
| HER2 overexpression | 10 (8.3) |
| TNBC | 11 (9.2) |
Abbreviation: TNBC, triple-negative breast cancer.
Patients with negative ER had more TILs than those with positive ER (P<0.05, Table 1). The median number of TILs was 100/HPF in negative ER patients, whereas it was 70/HPF in positive ER patients (P<0.05, Table 2). Count of CD4+ TILs was higher among patients with negative ER (56/HPF) than those with positive ER (44/HPF) (P<0.05, Table 2). The median number of PD-1+ TILs in patients with negative ER was 33/HPF, significantly higher than those with positive ER expression (16/HPF) (P<0.05, Table 2). Additionally, CD4+/PD-1+ TILs and CD8+/PD-1+ TILs were both higher in patients with negative ER than those with positive ER (28/HPF vs 12/HPF, and 3/HPF vs 2/HPF, respectively, P<0.05, Table 2). Phenotypes of TILs showed no associations with PR or HER2 status (Table 2).
Table 2.
Relationship between phenotypes of TILs and receptor status
| Phenotypes of TILs | ER, n (%)
|
PR, n (%)
|
HER2, n (%)
|
|||
|---|---|---|---|---|---|---|
| Negative (n=33) |
Positive (n=93) |
Negative (n=44) |
Positive (n=82) |
Negative (n=64) |
Positive (n=22) |
|
|
| ||||||
| TILs | ||||||
| Median (IQR) | 100 (100) | 70 (65) | 80 (78) | 80 (80) | 75 (78) | 85 (65) |
| P-value | 0.027 | 0.498 | 0.38 | |||
| CD4+ TILs | ||||||
| Median (IQR) | 56 (78) | 44 (52) | 51 (61) | 45 (57) | 45 (73) | 52 (40) |
| P-value | 0.030 | 0.556 | 0.403 | |||
| CD8+ TILs | ||||||
| Median (IQR) | 21 (31) | 16 (24) | 15 (25) | 19 (25) | 16 (30) | 22 (17) |
| P-value | 0.083 | 0.618 | 0.473 | |||
| PD-1+ TILs | ||||||
| Median (IQR) | 33 (48) | 16 (26) | 24 (44) | 16 (28) | 16 (36) | 25 (37) |
| P-value | 0.003 | 0.253 | 0.076 | |||
| CD4+/PD-1+ TILs | ||||||
| Median (IQR) | 28 (45) | 12 (27) | 22 (41) | 13 (28) | 11 (34) | 24 (27) |
| P-value | 0.004 | 0.204 | 0.057 | |||
| CD8+/PD-1+ TILs | ||||||
| Median (IQR) | 3 (8) | 2 (5) | 3 (4) | 2 (7) | 2 (7) | 3 (6) |
| P-value | 0.009 | 0.678 | 0.198 | |||
Abbreviations: IQR, interquartile range; TIL, tumor-infiltrating lymphocyte.
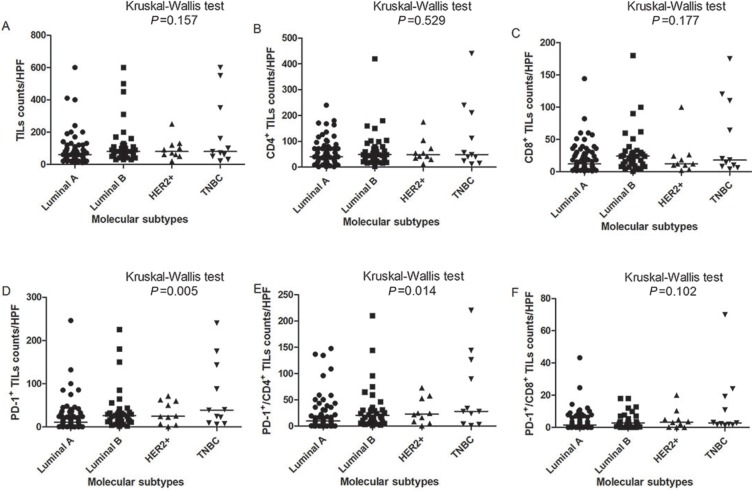
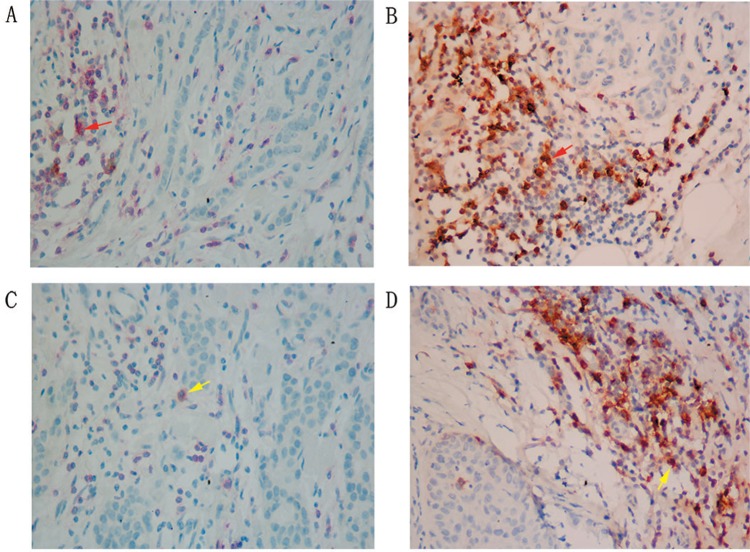
PD-1+ TILs and CD4+/PD-1+ TILs were distributed differently among different molecular subtypes (Figure 1). The median count of PD-1+ TILs in TNBC was significantly higher than that in Luminal A subtype (39/HPF vs 11/HPF, Table 3). CD4+/PD-1+ TILs in TNBC were much higher than that in Luminal A (28/HPF vs 10/HPF, Table 3). CD8+/PD-1+ TILs were also more common in TNBC than Luminal A (3/HPF vs 2/HPF, P<0.05, Table 3). From pathological diagnosis, TNBCs had higher amount of CD4/ PD-1 coexpression and CD8/PD-1 coexpression on TILs than Luminal A (Figure 2).
Figure 1.
Distribution of TIL phenotypes between molecular subtypes.
Note: (A) TILs, (B) CD4+ TILs, (C) CD8+ TILs, (D) PD-1+ TILs, (E) PD-1+/CD4+ TILs, (F) PD-1+/CD8+ TILs.
Abbreviations: TIL, tumor-infiltrating lymphocyte; HPF, high-power field.
Table 3.
TIL phenotype differences between Luminal A and TNBC
| Luminal A n (%) |
TNBC n (%) |
P-value | |
|---|---|---|---|
|
| |||
| TILs | |||
| Median (IQR) | 60 (60) | 80 (300) | 0.195 |
| CD4+ TILs | |||
| Median (IQR) | 40 (52) | 48 (186) | 0.262 |
| CD8+ TILs | |||
| Median (IQR) | 12 (24) | 18 (102) | 0.184 |
| PD-1+ TILs | |||
| Median (IQR) | 11 (24) | 39 (134) | 0.009 |
| CD4+/PD-1+ TILs | |||
| Median (IQR) | 10 (25) | 28 (122) | 0.027 |
| CD8+/PD-1+ TILs | |||
| Median (IQR) | 2 (6) | 3 (17) | 0.022 |
Abbreviations: IQR, interquartile range; TIL, tumor-infiltrating lymphocyte; TNBC, triple-negative breast cancer.
Figure 2.
Coexpression of CD4/PD-1 and CD8/PD-1 on TILs in Luminal A and TNBC (×400 magnification).
Notes: (A) Coexpression of PD1 and CD4 in Luminal A. (B) Coexpression of PD1 and CD4 in TNBC. (C) Coexpression of PD1 and CD8 in Luminal A (IHC, ×400). (D) Coexpression of PD1 and CD8 in TNBC (IHC, 400×). Red arrows indicate CD4+/PD-1+ lymphocytes, yellow arrows indicate CD8+/PD-1+ lymphocytes. The cells were scored at ×400 magnification.
Abbreviations: IHC, immunohistochemistry; TIL, tumor-infiltrating lymphocyte; TNBC, triple-negative breast cancer.
Discussion
TIL phenotypes were significantly associated with pathological characteristics of BC. The distribution of TIL phenotypes was different between molecular subtypes of BC. TNBC patients tended to have a higher count of exhausted CD4+ and CD8+ TILs.
TILs are a kind of mononuclear immune cells. TIL infiltration into tumor tissue occurs in several tumor types, including BC. TILs are distributed in both stromal and intratumoral tissue. TILs BC were described as a lymphocyte population consisted of varying proportions of cytotoxic (CD8+) T-cells, helper (CD4+) T-cells, CD19+ B cells, and NK cells in BC, whereas, T-cells were the main phenotype.16 High proportions of effector TILs have consistently been associated with good prognosis, lower recurrence, and better clinical efficacy of patients with early-stage TNBC and HER2-amplified BC.17–19 TILs also has a relationship with response to anthracycline-containing adjuvant chemotherapy, and a high percentage of TILs was associated with better clinical efficacy and prolonged survival among HER2-positive BC cases.18
TILs had significant effects on survival of BC patients, depending on different phenotypes. A high level of CD8+ TILs predicted prolonged overall survival of BC patients, where a high CD8+/FOXP3+ ratio might reduce the risk of death by 29%.20 High counts CD4+ TILs correlated with improved outcome of ductal BC.13 CD4 (helper) and CD8 (cytotoxic) T-cells perform host antitumor immune response. The presence of PD-1 on the helper and cytotoxic T-cells represents exhausted function and dampens the host’s anti-tumor effect. PD-1 plays a role in establishing peripheral tolerance and inhibiting the proliferation and function of T-cells. The exhausted T-cells had an upregulated expression of PD-1.14 During acute infection, the expression of PD-1 on effector T-cells was transient and not presented on functionally competent memory T-cells,21 and a high level of PD-1+ TILs has been associated with poor survival of BC patients.15,22 The PD-1/PD-L1 pathway provides a potential mechanism of implementing immunotherapy in BC. In this study, TNBCs had higher counts of exhausted effector cells than Luminal A subtype, indicating the immune-suppressive microenvironment among TNBC patients.
TNBCs had a special immune microenvironment. TNBCs had the highest amounts (70%) of FOXP3+ regulatory T-cells (Tregs) compared to other types of BC.23 The function of Tregs is to regulate and suppress immune response and prevent autoimmune diseases. Traditionally, it has been believed that Tregs can suppress other effector cells and prevent effective immune response in the tumor microenvironment.24 PD-1/PD-L1 was more likely to be positive in TNBC patients. Compared with non-TNBC patients, PD-1 (70% vs 25%) or PD-L1 (59% vs 33%) expression was much higher among TNBC patients.25 The immune microenvironment in TNBC was in an inhibitory status. Similarly, the exhausted T-cells were much higher in TNBC than Luminal A. Compared to Luminal A patients, TNBC patients had much more PD-1+ TILs (27.3% vs 4.7%)15 and PD-L1 expression (30.7% vs 12.7%).26 PD-1 expression was correlated with ER status: expression rate of PD-1 was 12% for positive ER patients, compared with 25% for negative ER patients.15 PD-1 expression had no association with HER2 status.15 Ras/MAPK pathway activation caused immune suppression in TNBC, and the inhibition of this pathway improved immune response.27 Although TNBC was more aggressive than other subtypes, the suppressive immune microenvironment provided an opportunity for immunotherapy. Further studies were warranted to investigate the feasibility of immunotherapy for TNBC treatment.
A small sample size was the first limitation of our study. Second, we did not analyze intratumoral and stromal TILs separately. Third, IHC was the sole detection method, and flow cytometry was not performed to detect TILs phenotypes. Double staining CD4, CD8, and PD-1 was not in an easy distinguished way that fast red and brown might not be separated easily. The number of CD4+/PD-1+ and CD8+/ PD-1+ TILs was too small. FOXP3+/CD4+ and Th1+/CD4+ T-cells were not double stained.
Conclusion
BC molecular subtypes had different patterns of distribution with regard to TILs phenotypes. TNBCs had higher counts of PD-1+ effector T-cells compared to Luminal A. The immune microenvironment was in an inhibitory status in Chinese TNBC patients. PD-1/PD-L1 pathway was a potential therapeutic strategy for TNBC.
Acknowledgments
This study was supported by Beijing Shijitan Hospital, Capital Medical University (QKS grant number 2017-QB03), Beijing Key Laboratory of Cancer Therapeutic Vaccine (FS grant number 2017-KF01), Beijing Municipal Commission of Health and Family Planning (QKS grant number 2015-3-057), Ministry of Railway (QKS grant number J2017Z604), and Beijing Municipal Administration of Hospitals (QKS grant number PX2018029). The supporting organizations had no role in study design, data collection, analysis, and interpretation.
Footnotes
Author contributions
QKS, FS, and HC designed the study. FS, YJZ, and QZ collected the data. QKS and GJW analyzed the data. QKS, FS, HC, YJZ, QZ, and GJW were involved in manuscript writing, modification of the manuscript, and approval for submission. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer. 2015;136(8):1921–1930. doi: 10.1002/ijc.29227. [DOI] [PubMed] [Google Scholar]
- 3.Melnikova V, Vibat CRT, Ning Y, et al. Quantitative urinary KRAS for treatment decisions in patients with metastatic colorectal cancer (mCRC) Journal of Clinical Oncology. 2016;34(15 Suppl):e15011. [Google Scholar]
- 4.Zheng S, Bai JQ, Li J, et al. The pathologic characteristics of breast cancer in China and its shift during 1999–2008: a national-wide multicenter cross-sectional image over 10 years. Int J Cancer. 2012;131(11):2622–2631. doi: 10.1002/ijc.27513. [DOI] [PubMed] [Google Scholar]
- 5.Song Q, Huang R, Li J, et al. The diverse distribution of risk factors between breast cancer subtypes of ER, PR and HER2: a 10-year retrospective multi-center study in China. PLoS One. 2013;8(8):e72175. doi: 10.1371/journal.pone.0072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SL, Li YX, Zhang BN, et al. Epidemiologic study of radiotherapy use in China in patients with breast cancer between 1999 and 2008. Clin Breast Cancer. 2013;13(1):47–52. doi: 10.1016/j.clbc.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Song Q, Zhang B, et al. A 10-year (1999 ~ 2008) retrospective multi-center study of breast cancer surgical management in various geographic areas of China. Breast. 2013;22(5):676–681. doi: 10.1016/j.breast.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, He J, Li J, et al. A nation-wide multicenter 10-year (1999–2008) retrospective clinical study of endocrine therapy for Chinese females with breast cancer. PLoS One. 2014;9(7):e100159. doi: 10.1371/journal.pone.0100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reck M, Rodríguez-Abreu D, KEYNOTE-024 Investigators Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 10.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 11.Dill EA, Gru AA, Atkins KA, et al. PD-L1 Expression and Intratumoral Heterogeneity Across Breast Cancer Subtypes and Stages: An Assessment of 245 Primary and 40 Metastatic Tumors. Am J Surg Pathol. 2017;41(3):334–342. doi: 10.1097/PAS.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 12.Salgado R, Denkert C, Campbell C, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1(4):448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathore AS, Kumar S, Konwar R, Makker A, Negi MP, Goel MM. CD3+, CD4+ & CD8+ tumour infiltrating lymphocytes (TILs) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian J Med Res. 2014;140(3):361–369. [PMC free article] [PubMed] [Google Scholar]
- 14.Flies DB, Sandler BJ, Sznol M, Chen L. Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J Biol Med. 2011;84(4):409–421. [PMC free article] [PubMed] [Google Scholar]
- 15.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12(5):1463–1466. [PubMed] [Google Scholar]
- 17.Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148(3):467–476. doi: 10.1007/s10549-014-3185-2. [DOI] [PubMed] [Google Scholar]
- 18.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 19.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 20.Liu F, Lang R, Zhao J, et al. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130(2):645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 21.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129(4):474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun S, Fei X, Mao Y, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014;63(4):395–406. doi: 10.1007/s00262-014-1519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 25.Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965–2970. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 26.Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146(1):15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin Cancer Res. 2016;22(6):1499–1509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]