Abstract
Purpose
To examine the trends of analgesic prescribing at public tertiary hospital outpatient settings and explore the patterns of their utilization in nonsteroidal anti-inflammatory drugs (NSAIDs), tramadol, and opioid patients.
Patients and methods
This cross-sectional study was conducted from 2010 to 2016 using the prescription databases of two tertiary hospitals in Malaysia. Prescriptions for nine NSAIDs (ketoprofen, diclofenac, celecoxib, etoricoxib, ibuprofen, indomethacin, meloxicam, mefenamic acid, and naproxen), tramadol, and five other opioids (morphine, fentanyl, oxycodone, dihydrocodeine, and buprenorphine) were included in this study. Annual number of patients and prescriptions were measured in repeat cross-sectional estimates. Descriptive statistics and linear trend analysis were performed using Stata version 13.
Results
A total of 192,747 analgesic prescriptions of the nine NSAIDs, tramadol, and five other opioids were given for 97,227 patients (51.8% NSAIDs patients, 46.6% tramadol patients, and 1.7% opioid patients) from 2010 to 2016. Tramadol (37.9%, n=72,999) was the most frequently prescribed analgesic, followed by ketoprofen (17.5%, n=33,793), diclofenac (16.2%, n=31,180), celecoxib (12.2%, n=23,487), and other NSAIDs (<4.5%). All the analgesics were increased over time except meloxicam, indomethacin, and mefenamic acid. Opioids, primarily morphine (2.2%, n=4,021) and oxycodone (0.5%, n=1,049), were prescribed the least, but the rate of increase was the highest.
Conclusion
Tramadol was the most frequently prescribed analgesic in hospital outpatient settings in Malaysia. Opioids were prescribed the least, but noted the highest increase in utilization.
Keywords: trends of prescribing, patterns, analgesics, tramadol, NSAIDs, opioids, Malaysia
Introduction
Analgesics are commonly prescribed for pain relief in all types of patient care settings.1 In the last three decades, analgesic utilization has increased steadily in both developed and developing countries.2 According to the World Health Organization (WHO) analgesic ladder, paracetamol or nonsteroidal anti-inflammatory drugs (NSAIDs) are to be used prior to weak opioids (eg, codeine and tramadol). If weak opioids do not provide adequate pain relief, strong opioids such as morphine, oxycodone, and fentanyl are indicated.3
Although the use of analgesics provides a number of benefits, they are also associated with unwanted consequences. For example, NSAIDs are associated with serious gastrointestinal (GI) bleeding4 and renal injury.5 It was also reported that one fourth of peptic ulcer cases were caused by NSAIDs.6 NSAIDs may also increase the risk of cardiovascular events such as myocardial infarction, stroke, and heart failure.7,8 Many of these events are avoidable, and it was estimated that unnecessary prescriptions for NSAIDs were written in 42% of physician visits.9
Opioid analgesics have also been associated with adverse events such as addiction, abuse, and misuse, particularly when they are used for chronic noncancer pain (CNCP). In the past two decades, the prescribing of opioid medications to treat CNCP has increased dramatically, resulting in a parallel increase in opioid abuse/dependence and accidental overdose.10
The utilization of analgesics is not without risk and has now become one of a major public health issues. Inappropriate or irrational use of analgesics may have contributed to increased morbidity and mortality rates and deterioration of quality of life. This will lead to increased demand on health care resources and increased health care costs. Thus, it is imperative to have safe and appropriate use of analgesics.
Thus far, no large-scale study has been conducted to evaluate the trends and patterns of analgesic utilization in Malaysia. The available drug utilization studies involving face-to-face interviews have provided useful data, but these studies were conducted in a short period and hence do not provide any information on trends and patterns of analgesic usage. Accordingly, the current study aims to 1) address the trends of analgesic prescribing in Malaysia for a period of 7 years using large databases and 2) examine the patterns of their utilization of NSAIDs, tramadol, and opioid patients.
Methods
Study design and data source
This cross-sectional study was conducted from 2010 to 2016 using the prescription databases of two public tertiary hospitals in Malaysia. This study has been granted ethical approval from the Medical Research Ethical Committee, Ministry of Health Malaysia. The data on patient information were de-identified and only aggregate results were reported. There was no direct involvement of patients in this study, so the committee waived the need for informed consent.
All prescriptions for nine NSAIDs (ketoprofen, diclofenac, celecoxib, etoricoxib, ibuprofen, indomethacin, meloxicam, mefenamic acid, and naproxen) and six opioids (tramadol, morphine, fentanyl, oxycodone, dihydrocodeine, and buprenorphine) were included in this study. Information extracted from the prescriptions includes item name and strength, prescription date, frequency, quantity, issuing department, and patient age and gender. These prescription data were collected at the point when a prescription was written. Patients of all ages were included in the study if they were using at least one prescription for analgesic during the study period. The calculation of patients’ age was based on the date of the first encounter prescription in the database. The patients’ diagnoses were not available because the data for this study were extracted from the prescription database.
Patients were stratified into NSAID, tramadol, and opioid groups (morphine, fentanyl, oxycodone, dihydrocodeine, and buprenorphine) based on the type of analgesic used. The number of patients was calculated more than once if the patients were using more than one analgesic group. Patients receiving tramadol were analyzed separately from other opioid patients because tramadol is a unique centrally acting analgesic with opioid agonist properties and a weak inhibitor of norepinephrine and serotonin reuptake compared with the other opioids included in this study. Tramadol is also widely used and does not require strict procedures, which is the case for other opioids such as dihydrocodeine, oxycodone, and morphine (controlled drugs) in Malaysia. Paracetamol was also not included because the usage was very common and it was also prescribed not only for analgesia but also for fever. Patients in this study refer to those who received an analgesic prescription during the study period and this does not necessarily mean that they filled the prescription or even consumed it.
Data analysis
The annual number of patients, prescriptions, and prescriptions per patient was measured in repeat cross-sectional estimates. This means that the number of patients was calculated more than once if the patients appeared in following calendar years after the patient was first identified in the database. The issuing departments for the analgesic were also noted. The mean, SD, and range were calculated for patient demographics. Descriptive statistics and linear trend analysis were performed using Stata v13 (Stata Corp LP, College Station, TX, USA; 2011).
Results
Number of analgesic users
A total of 192,747 analgesic prescriptions of the nine NSAIDs, tramadol, and five other opioids were prescribed for 97,227 patients (48.6% female) from 2010 to 2016 (Table 1). The mean age of total patients prescribed with analgesics was 46.3±18.3 years (mode: 56; range: 1-106 years). Of the six age ranks, patients aged 18-40 years recorded the highest proportion (40.6%), followed by patients aged 61-80 (21.9%), 51-60 (17.1%), and 41-50 years (14.9%). During the study period, the number of all patients prescribed with analgesics each year slightly increased over time (from 11,694 to 11,983 or 2.5% increase).
Table 1.
Patient demographics
| Descriptions | NSAIDs | Opioids | Tramadol | Total |
|---|---|---|---|---|
| Patients (n) | 50,351 | 1,618 | 45,258 | 97,227 |
| % | 51.79 | 1.66 | 46.55 | 100 |
| Age | ||||
| Mean | 42.55 | 62.43 | 49.83 | 46.27 |
| Median | 40 | 64 | 51 | 45 |
| Mode | 28 | 64 | 56 | 56 |
| Range | 1–106 | 3–103 | 1–105 | 1–106 |
| SD | 17.61 | 15.81 | 18.03 | 18.26 |
| Age group, years | ||||
| ≤17 (n) | 2,008 | 10 | 469 | 2,487 |
| % | 3.99 | 0.62 | 1.04 | 2.56 |
| 18–40 (n) | 23,928 | 155 | 15,385 | 39,468 |
| % | 47.52 | 9.58 | 33.99 | 40.59 |
| 41–50 (n) | 7,586 | 183 | 6,715 | 14,484 |
| % | 15.07 | 11.31 | 14.84 | 14.9 |
| 51–60 (n) | 7,573 | 321 | 8,774 | 16,668 |
| % | 15.04 | 19.84 | 19.39 | 17.14 |
| 61–80 (n) | 8,397 | 765 | 12,107 | 21,269 |
| % | 16.68 | 47.28 | 26.75 | 21.88 |
| >80 (n) | 859 | 184 | 1,808 | 2,851 |
| % | 1.71 | 11.37 | 3.99 | 2.93 |
| Gender | ||||
| Female (n) | 25,533 | 863 | 20,825 | 47,221 |
| % | 50.7 | 53.3 | 46 | 48.6 |
| Male (n) | 24,818 | 755 | 24,433 | 50,006 |
| % | 49.3 | 46.7 | 53.9 | 51.43 |
Abbreviation: NSAIDs, nonsteroidal anti-inflammatory drugs.
Of the 97,227 patients prescribed with analgesics, 50,351 (51.8%) were NSAID patients, 45,258 (46.6%) were tramadol patients, and 1,618 (1.7%) were opioid patients. A slightly higher proportion of female patients were those in the opioid group (53.3%) than those in the NSAIDs (50.7%) and tramadol (46%) groups. The mean age of NSAID patients (42.6±17.6; mode 28; range: 1-106 years) and tramadol patients (49.8±18.0; mode 56; range 1-105 years) was lower than that of the opioid patients (62.4±15.8, mode 64; range: 3-105 years). A higher proportion of younger patients aged 18-40 years were recorded for the NSAIDs (47.5%) and tramadol (33.9%) groups, compared with other age groups. For opioids patients, most of them (47.3%) were older patients aged 61-80 years (Table 1).
During the 7-year study period, the annual number of opioid patients increased (from 116 to 435 patients, 275.0% increase) each year higher than that of tramadol (from 5,856 to 10,220 patients, 74.5% increase) and NSAIDs (from 6,368 to 10,534 patients, 65.4% increase) patients.
Number of prescriptions
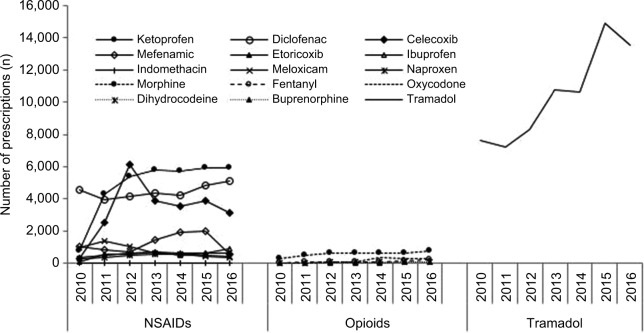
Of all analgesic prescriptions (n=192,747), tramadol was the most frequently prescribed analgesic (37.8%, n=72,999 prescriptions) followed by ketoprofen (17.5%, n=33,793), diclofenac (16.2%, n=31,180), and celecoxib (12.2%, n=23,487) during the 7-year study period (Figure 1). The prescriptions for other NSAIDs were <4.5% (mefenamic acid [4.4%, n=8,477], meloxicam [2.8%, n=5,346], ibuprofen [2.2%, n=4,140], etoricoxib [1.9%, n=3,692], naproxen [1.6%, n=3,115], and indomethacin [0.1%, n=206]). Opioids were prescribed the least, with the majority being morphine (2.1%, n=4,021) and oxycodone (0.5%, n=1,049).
Figure 1.
Number of prescriptions for NSAIDs, opioids, and tramadol from 2010 to 2016.
Abbreviation: NSAIDs, nonsteroidal anti-inflammatory drugs.
The annual number of NSAID prescriptions increased over time, with the largest increase noted for celecoxib (from 290 to 3,168, 992% increase, P>0.05), followed by ketoprofen (from 769 to 5,920, 669.8% increase, P<0.05), etoricoxib (from 81 to 591, 629% increase, P>0.05), ibuprofen (from 368 to 879, 138.8% increase, P<0.05), naproxen (from 269 to 415, 54.3% increase, P>0.05), and diclofenac (from 4,552 to 5,099, 12.0% increase, P>0.05). In contrast, the prescription of mefenamic acid (from 1,043 to 583, 44% decrease, P>0.05), indomethacin (from 27 to 12, 55% decrease, P>0.05), and meloxicam (from 965 to 729, 60% decrease, P<0.05) moderately decreased during the same study period. For tramadol, the annual number of prescriptions moderately increased from 7,643 to 13,533 (77.0% increase, P<0.005).
Although the utilization of opioids was lower than that of tramadol and NSAIDs, the annual number of prescriptions for opioids recorded the largest increase compared with the prescription of other analgesics. The prescriptions of buprenorphine increased the most (from 3 to 89 prescriptions, 2,866.6% increase, P<0.005), followed by the prescription of dihydrocodeine (from 7 to 88, 1,157.1% increase, P<0.05), oxycodone (from 28 to 275, 882.1% increase, P<0.005), fentanyl (from 24 to 197, 720.8% increase, P>0.05), and morphine (from 297 to 729, 145.4% increase, P<0.05). The overall increase in opioid utilization was high, but clinically it was affecting a smaller number of patients compared with other analgesics.
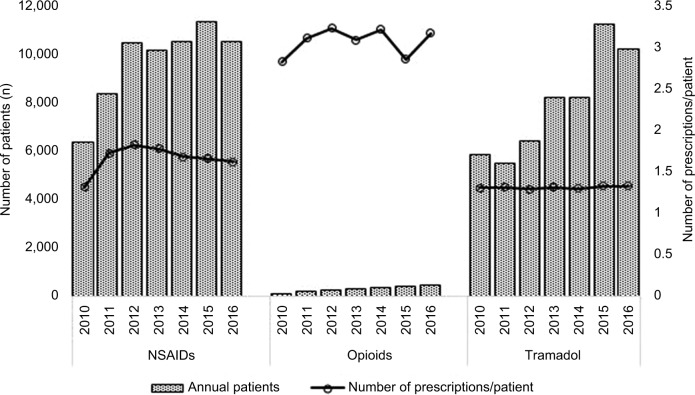
During the study period, the mean number of prescriptions per patient per year was higher in the opioid group (3.06 prescriptions/patient) than in the NSAID (1.65) and tramadol groups (1.30) (Figure 2). However, the annual number of prescriptions per patient increased more in the NSAID group (from 1.31 in 2010 to 1.61 in 2016; 23.2% increase) than in the opioid (from 2.82 to 3.16, 12.0% increase) and tramadol (from 1.30 to 1.32; 1.45% increase) groups.
Figure 2.
Annual number of patients and number of prescriptions per patient for NSAIDs, opioids, and tramadol from 2010 to 2016.
Abbreviation: NSAIDs, nonsteroidal anti-inflammatory drugs.
Issuing department
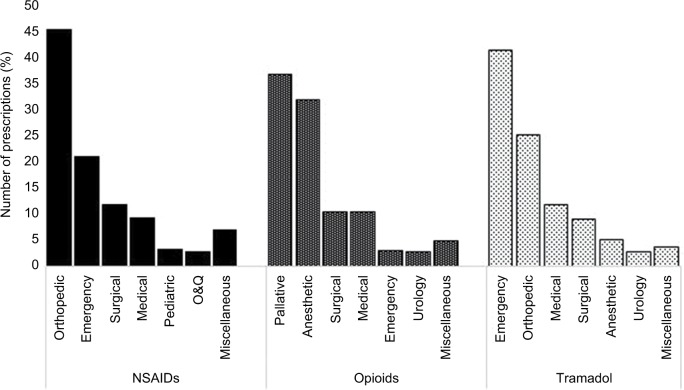
NSAIDs were most commonly used by the orthopedic (45.4%) and emergency (21%) departments, while opioids were primarily used by the palliative care (36.8%) and anesthetic (31.98%) departments. For tramadol, the emergency (41.3%) and orthopedic (25.2%) were the predominant issuing departments (Figure 3).
Figure 3.
Prescribing of NSAIDs, opioids, and tramadol by issuing department.
Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs; O&G, obstetrics and gynecology.
Discussion
This study found that tramadol was the most frequently prescribed analgesic at public hospital outpatient settings in Malaysia from 2010 to 2016. Ketoprofen, diclofenac, and celecoxib were the most commonly prescribed NSAIDs, while morphine and oxycodone were the most common opioids used. The utilization of all analgesics increased over time, except mefenamic acid, meloxicam, and indomethacin, which decreased. Although accounting for the least number of prescriptions, opioids showed the largest rate of increase during the study period.
Tramadol utilization was found to outnumber that of individual NSAIDs, reflecting the preference to use tramadol over NSAIDs for pain management in Malaysian public hospitals. Studies from other countries also showed a similar pattern, for example, in Germany, tramadol was the most prescribed analgesic,11 while in the USA, tramadol was the second most commonly prescribed narcotic pain reliever, outranking oxycodone in 2012.12 In Australia, tramadol was the second most frequently used drug after codeine.13
One reason for the popularity of tramadol as an analgesic in Malaysia is that it is not categorized as a controlled drug, unlike all the other opioids. Other countries such as the UK, Australia, and Sweden, and some states of America have rescheduled their classification of tramadol to a controlled substance in response to an increased number of deaths associated with its diversion and misuse.14–16 The fatalities were commonly observed when tramadol was used concurrently with other central nervous depressant drugs or alcohol. In Iran, tramadol poisoning is one of the most common causes of poisoning, concurrent with the increase in tramadol abuse and overdose.17,18 Further studies are warranted to evaluate whether such tramadol-related deaths have also occurred in Malaysia.
The high use of tramadol observed in the current study may also be attributed to the recommendations in Malaysian clinical practice guidelines (CPG) on management of osteoarthritis to use tramadol or NSAIDs as second-line drugs if pain is not relieved by paracetamol and topical NSAIDs (step 1).19 Guidelines in other countries, such as by European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and The American College of Rheumatology (ACR), have slightly different recommendations by indicating tramadol for a short-term use if the pain is not controlled following step 1 (topical NSAIDs) and step 2 (oral NSAIDs) treatments20 or if coxibs, nonopioid analgesics, and nonselective NSAIDs are ineffective, poorly tolerated, or contraindicated.21
Although tramadol was reported to be less likely to be abused and misused compared with other opioids, the analgesic has also been associated with adverse events such as seizures, respiratory depression, and a potentially fatal drug reaction known as serotonin syndrome.22–25 The increasing use of tramadol was reported to be parallel with the increasing emergency department visits involving tramadol adverse reactions, misuse and abuse, and suicidal attempts.26 In individuals with a history of substance abuse, tramadol is also associated with high risk of addiction when it is consumed repetitively and/or for a prolonged period.27–29
As indicated in the current study, ketoprofen, diclofenac, and celecoxib were among the most frequently prescribed NSAIDs. The availability of ketoprofen in a patch form could be the likely reason for it to be highly used. Diclofenac is an old NSAID, which has always been the preferred analgesic. From a study that used a country’s sales data to compare the individual sale of NSAIDs among low-, middle- and high-income countries, diclofenac was found to be the most popular NSAID in all countries despite being associated with high risk of cardiovascular (CV) events. This study did not include ketoprofen in its analysis.1
The above-mentioned study also showed that the sales of celecoxib were almost similar to etoricoxib in Malaysia. This finding was slightly different from the current study, in which the utilization of etoricoxib was small (<4.5% of total analgesic usage) compared with celecoxib. This may be best explained by the sales data used in the previous study that was referred to the analgesic utilization by various health settings.
Although NSAIDs are still considered relatively safe drugs, there is wide disparity in the adverse event risk of GI and CV events among different oral NSAIDs, which have contributed to the limited use of NSAIDs. Patients with high GI risk should be coprescribed cyclooxygenase-2 (COX-2) selective NSAIDs with a proton pump inhibitor, and nonselective NSAIDs should be avoided.30 In high CV risk patients, both selective and non-selective NSAIDs should be avoided. However, naproxen can be considered because it is associated with lower risk of CV events.30 Taken together, the risks can be minimized by appropriate selection of analgesics according to patient’s suitability, which would help to maintain the clinical benefit of treatment.20
The current study also demonstrated that opioids (other than tramadol) were prescribed the least compared with NSAIDs and tramadol. However, the utilization of opioids noted the largest increase. It is not surprising for opioids to have a low usage in this study because opioids are categorized as a controlled drug that requires strict procedures of prescribing, which are not required for NSAIDs and tramadol.31
As shown in the study, the largest increase in opioid utilization compared with other analgesics may need further attention in view of the current issues related to opioid utilization, which include addiction, abuse, misuse, and overdose deaths, particularly in patients with noncancer pain. Early measures taken to manage these issues could prevent those unwanted consequences. It is also possible that the large increase in opioids is due to the small number of patients prescribed opioids in the first place, as well as the educational efforts to increase awareness among doctors about the role of morphine and other strong opioids in the treatment of cancer pain. A previous study reported that ~60% of the opioid users at a Malaysian public hospital were patients with cancer pain, and morphine was mainly used for cancer pain, while oxycodone was primarily used for noncancer pain.32 However, we have to be vigilant that this increase does not occur in those patients with noncancer pain as this has been shown in other countries to lead to addiction, abuse, misuse, and overdose deaths.33
The present study also found that the mean annual number of prescriptions issued per patient per year was higher in the opioid group (3.06 prescriptions/patient) than in the tramadol (1.30) and NSAID (1.65) groups. This may likely reflect the chronic use of opioids among opioid users who required repeat opioid prescriptions; the case may not provide any benefit in treating pain or improving function in patients with CNCP.10 However, the present study was unable to evaluate the indication of opioid use because of limited information available on patient’s diagnosis.
It was also observed in the present study that the orthopedic and emergency departments were the most commonly issuing departments for NSAIDs and tramadol, whereas opioids were primarily prescribed by palliative care and anesthetic departments. It is expected that those departments highly use the above-mentioned analgesics because they were frequently dealing with patients suffering from pain. However, the high usage of tramadol by the emergency department requires further research to ascertain the prescribing indication and whether this was the first time patients initiated with opioid analgesic. For these naïve opioid users, they are recommended to be started with a low dose and a short duration of opioid therapy to prevent the progression to long-term use, which is associated with much controversy due to the lack of evidence supporting the long-term effectiveness of opioids.
The strength of the present study includes the long follow-up period and inclusion of the most common analgesics available at study settings. This study used real-world dataset that is likely to reflect the real practice. However, the findings should be interpreted in light of the fact that they were based on outpatient tertiary settings dataset that might not reflect the prescribing of analgesics in other settings in Malaysia. This study referred to prescribing data and there was no information whether the medications were dispensed and were actually consumed. The lack of information on diagnoses limited further analysis. Some departments at the study settings were not using electronic prescriptions, and thus the data were not captured in the current study. Such a constraint was likely to result in an underestimation of analgesics use. However, this limitation should not affect the results because the large number of patients included in the study is more likely to represent the patient population.
Conclusions
Based on individual analgesic consumption, the finding from this study demonstrates that tramadol has been the most frequently prescribed analgesic at outpatient hospital settings in Malaysia. The prescriptions for NSAIDs were primarily for ketoprofen, diclofenac, and celecoxib, whereas the opioids predominantly prescribed were morphine and oxycodone. The overall utilization of analgesics in Malaysia has increased steadily, with opioid utilization increasing at a greater rate than tramadol and NSAIDs. On the whole, the decision to use analgesics must consider the benefits and risks of the therapy. If the selected analgesic is inappropriate, then the benefits and risks of the alternatives also need to be considered.
Footnotes
Authors contributions
CSZ initiated and developed the research questions and study design, conducted data management and analysis, and led the drafting the manuscript. All of the authors contributed to the data acquisition, interpretation of the data, critically revised the manuscript, approved the final version submitted for publication, and agree to be accountable for all aspects of the work.
Disclosure
CSZ was supported by a research grant from The Ministry of Education Malaysia (Fundamental Research Grant Scheme, FRGS 15-195-0436). The authors report no other conflicts of interest in this work.
References
- 1.Mcgettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med. 2013;10(2):e1001388. doi: 10.1371/journal.pmed.1001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob L, Kostev K. Prevalence of pain medication prescriptions in France, Germany, and the UK - a cross-sectional study including 4,270,142 patients. Postgrad Med. 2018;130(1):32–36. doi: 10.1080/00325481.2018.1391658. [DOI] [PubMed] [Google Scholar]
- 3.Carlson CL. Effectiveness of the World Health Organization cancer pain relief guidelines: an integrative review. J Pain Res. 2016;9:515–534. doi: 10.2147/JPR.S97759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S3. doi: 10.1186/ar4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26(4):285–291. doi: 10.1016/j.ejim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Kurata JH, Nogawa AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol. 1997;24(1):2–17. doi: 10.1097/00004836-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Ungprasert P, Srivali N, Thongprayoon C. Nonsteroidal Anti-inflammatory Drugs and Risk of Incident Heart Failure: A Systematic Review and Meta-analysis of Observational Studies. Clin Cardiol. 2016;39(2):111–118. doi: 10.1002/clc.22502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross SJ, Elgendy IY, Bavry AA. Cardiovascular safety and bleeding risk associated with nonsteroidal anti-inflammatory medications in patients with cardiovascular disease. Curr Cardiol Rep. 2017;19(1):8. doi: 10.1007/s11886-017-0814-5. [DOI] [PubMed] [Google Scholar]
- 9.Durrance SA. Older adults and NSAIDs: avoiding adverse reactions. Geriatr Nurs. 2003;24(6):348–352. doi: 10.1016/j.gerinurse.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain–United States. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tholen K, Hoffmann F. High use of tramadol in Germany: an analysis of statutory health insurance data. Pharmacoepidemiol Drug Saf. 2012;21(9):1013–1021. doi: 10.1002/pds.3266. [DOI] [PubMed] [Google Scholar]
- 12.Health IMS Top 25 medicines by dispensed prescriptions (U. S.) 2012. Dec, [Accessed December 31, 2017]. Available from: http://www.imshealth.com/deployedfiles/imshealth/Global/Content.
- 13.Hollingworth SA, Gray PD, Hall WD, Najman JM. Opioid analgesic prescribing in Australia: a focus on gender and age. Pharmacoepidemiol Drug Saf. 2015;24(6):628–636. doi: 10.1002/pds.3767. [DOI] [PubMed] [Google Scholar]
- 14.Spiller HA, Scaglione JM, Aleguas A, et al. Effect of scheduling tramadol as a controlled substance on poison center exposures to tramadol. Ann Pharmacother. 2010;44(6):1016–1021. doi: 10.1345/aph.1P064. [DOI] [PubMed] [Google Scholar]
- 15.Randall C, Crane J. Tramadol deaths in Northern Ireland: a review of cases from 1996 to 2012. J Forensic Leg Med. 2014;23:32–36. doi: 10.1016/j.jflm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Hawkes N. Deaths from tramadol and legal highs reach new highs in England and Wales. BMJ. 2013;347:f5336. doi: 10.1136/bmj.f5336. [DOI] [PubMed] [Google Scholar]
- 17.Alinejad S, Zamani N, Abdollahi M, Mehrpour O. A narrative review of acute adult poisoning in Iran. Iran J Med Sci. 2017;42(4):327–346. [PMC free article] [PubMed] [Google Scholar]
- 18.Shadnia S, Esmaily H, Sasanian G, Pajoumand A. Hassanian-Moghaddam, H Abdollahi M. Pattern of acute poisoning in Tehran-Iran in 2003. Hum Exp Toxicol. 2007;26:753–756. doi: 10.1177/0960327107083017. [DOI] [PubMed] [Google Scholar]
- 19.Academy of Medicine Malaysia . Clinical Practice Guidelines. Management of Osteoarthritis. 2nd ed. Academy of Medicine Malaysia; 2015. [Accessed August 25, 2018]. Available from: http://www.acadmed.org.my/index.cfm?&menuid=67. [Google Scholar]
- 20.Bruyère O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 Suppl):S3–S11. doi: 10.1016/j.semarthrit.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 22.Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in tramadol: pharmacology, metabolism, and misuse. Anesth Analg. 2017;124(1):44–51. doi: 10.1213/ANE.0000000000001683. [DOI] [PubMed] [Google Scholar]
- 23.Nelson EM, Philbrick AM. Avoiding serotonin syndrome: the nature of the interaction between tramadol and selective serotonin reuptake inhibitors. Ann Pharmacother. 2012;46(12):1712–1716. doi: 10.1345/aph.1Q748. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita J, Litzinger MH. Serotonin syndrome associated with tramadol. Clin Psychiatry. 2009;11(5):273. doi: 10.4088/PCC.08l00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tashakori A, Afshari R. Tramadol overdose as a cause of serotonin syndrome: a case series. Clin Toxicol. 2010;48(4):337–341. doi: 10.3109/15563651003709427. [DOI] [PubMed] [Google Scholar]
- 26.Bush DM. The DAWN Report: Emergency Department Visits for Drug Misuse or Abuse Involving the Pain Medication Tramadol. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health; 2015. p. 54. [PubMed] [Google Scholar]
- 27.Tjäderborn M, Jönsson AK, Ahlner J, Hägg S. Tramadol dependence: a survey of spontaneously reported cases in Sweden. Pharmacoepidemiol Drug Saf. 2009;18(12):1192–1198. doi: 10.1002/pds.1838. [DOI] [PubMed] [Google Scholar]
- 28.Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69(3):233–241. doi: 10.1016/s0376-8716(02)00321-6. [DOI] [PubMed] [Google Scholar]
- 29.Cicero TJ, Inciardi JA, Adams EH, et al. Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol Drug Saf. 2005;14(12):851–859. doi: 10.1002/pds.1113. [DOI] [PubMed] [Google Scholar]
- 30.Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ministry of Health Medicines Formulary No 2/2017. Aug, 2017. [Accessed April 2, 2018]. Available from: https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/formulari%20ubat%20kkm%202-2017%20ogos%20eng.pdf.
- 32.Zin CS, Rahman NA, Ismail CR, Choy LW. Dose and duration of opioid use in patients with cancer and noncancer pain at an outpatient hospital setting in Malaysia. Pain Pract. 2017;17(6):774–781. doi: 10.1111/papr.12525. [DOI] [PubMed] [Google Scholar]
- 33.Häuser W, Schug S, Furlan AD. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: a perspective from different continents. Pain Rep. 2017;2(3):e599. doi: 10.1097/PR9.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]