Abstract
Zika virus has challenged the assumed knowledge regarding the pathobiology of flaviviruses. Despite causing sporadic and mild disease in the 50 years since its discovery, Zika virus has now caused multiple outbreaks in dozens of countries worldwide. Moreover, the disease severity in recent outbreaks, with neurological disease in adult and devastating congenital malformations in fetuses, was not previously seen. One hypothesis is that the virus has acquired mutations that have increased its virulence. Indeed, mutations in other arboviruses like West Nile, chikungunya and Venezuelan equine encephalitis viruses have enhanced outbreaks. This possibility, as well as alternative hypotheses, are explored here.
Keywords: Zika, Zika virus, evolution, adaptive mutations, outbreak
A Serendipitous Finding
Zika virus (ZIKV) was a relatively obscure virus at the turn of the last century. To many, it was a mundane virus within the genus Flavivirus that caused only a handful of self-limited and seemingly benign infections in Africa (reviewed in (1)). Even its original isolation was more of an accident. During a yellow fever epidemiological campaign sponsored by the Rockefeller Institution in the Ziika Forest of Uganda, ZIKV was isolated from a sentinel monkey who became febrile during the observation phase (2). This serendipitous finding yielded the prototype ZIKV strain, MR766. Subsequent serological studies placed ZIKV in the family Flaviviridae, genus Flavivirus alongside other well-known arboviruses including West Nile, yellow fever and dengue viruses. Flaviviruses contain single-stranded, positive-sense RNA genomes which contain all the genes required for replication in both mammalian and insect cells. The ends of the genome have structures at the 5’ and 3’ ends called untranslated regions (UTRs) which are essential for viral replication. The genes encoding the structural proteins, capsid (C), pre/membrane (prM) and envelope (E) are located within the first third of the genome at the 5’ end. The latter two-thirds encode the structural proteins from seven nonstructural proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5.
Recent studies have definitely identified species in the Aedes (Ae.) genus (species aegypti and albopictus during epidemics) to be the main vectors in nature (reviewed in (3)) despite a few studies showing other species may also play a role (4–6). Arboviruses need to be able to replicate in different alternating hosts (non-human primates/humans - Aedes spp.), which subject the virus to unique selective pressures. Furthermore, ZIKV is maintained in an enzootic cycle, which may spill over and initiate epidemics in the human transmission cycle (FIGURE 1). These arboviral characteristics greatly influence the virus capacity to cause outbreaks, which will be expanded upon below.
Figure 1. Transmission Cycle of Zika Virus.
The sylvatic cycles and urban cycles co-exist and mix within the zone of emergence. Red text shows areas where mutations might effect disease and transmission cycles.
Marching East to Go West- Overview of Recent Outbreaks
Since its isolation in 1947, only a few outbreaks of ZIKV disease have been described (reviewed in (1)). Although there were sporadic foci of ZIKV transmission in Africa and Asia evidenced by serology of primates and humans as well as viral isolations from mosquitoes, the first major outbreak since the 1940s occurred in Yap Island, Micronesia, in 2007 (reviewed in (3)). Then in 2013, thousands of patients in French Polynesia became infected and the neurological Guillain-Barre syndrome was noted for the first time to be associated with ZIKV infection (7). The virus then spread to other areas in Southeast Asia and in 2014 was introduced in Brazil. This explosive outbreak established the causal link between birth defects (defined as congenital Zika syndrome [CZS]) and ZIKV infection in utero (8, 9). This outbreak spread to neighboring countries in South and Central America and Mexico. The United States also had two documented foci of autochthonous ZIKV transmission in Florida (10) and Texas (11), although the latter not in the magnitude observed in Florida.
Nucleotide changes in the viral RNA that have accumulated over time and geographical space have resulted in the presence of two main lineages of ZIKV. Phylogenetic trees generated from multiple partial or full-length ZIKV genomes clearly show that the nearest relative is Spondee virus and the presence of an African lineage, comprised of the prototype MR766 and other African isolates from Senegal and Uganda, and an Asian lineage that contains viruses from Yap Island, Cambodia, Philippines (12, 13). Some researchers are considering a third clade that splits the Asian lineage in two: the Asian strain comprised of isolates from Asian countries and the American lineage that arose after the introduction and rapid expansion of ZIKV in the Western Hemisphere (12). Regardless of lineage, there is only one serotype of ZIKV since antibodies produced against one strain can cross-neutralize all others (14). The Asian and American lineage viruses have resulted in the epidemics associated with severe neurological and developmental diseases and potential reasons why are discussed below. The reasons why African lineage strains have not been associated with notable outbreaks are likely multifactorial and could be due to the virus itself, preexisting flavivirus immunity in the population, differences in the native mosquito species or due to the paucity of epidemiological data often associated with resource-poor countries.
Could ZIKV’s Unique Attributes Contribute to the Epidemic?
Members of the genus Flavivirus cause a variety of diseases, including hemorrhagic (like dengue and yellow fever viruses) and encephalitic (like West Nile, Saint Loius and Japanenece encephalitis viruses). Most flavivirus infections result in subclinical or mild disease, leading reserachers to develop the “iceberg” model, showing a smaller percentage of the infections resulting in severe disease, or the patients that seek medical treatment are the “tip of the iceberg” in an outbreak. That percentage of severe disease will vary based upon the virus (and sometimes the strain). This often leads to under-reporting of the true disease burden during an outbreak since many people will never show signs of infection but could still spread disease (15). Zika virus is a typical flavivirus in that the majority of infections are asymptomatic or mild (summarized in (3)).
ZIKV has also been found in tissues that have not typically been associated with flaviviral infections such as the eyes, reproductive organs and bodily secretions. ZIKV genetic material has been identified in saliva (16) and urine (17). Ocular infections might be a result of neurological infections, particularly in the young (18) and interestingly, tears from experimentally infected mice may contain infectious ZIKV (19),. Long-lasting viremia, albeit transient, has also been observed in pregnant women (20) with a current unconfirmed hypothesis pointing to the infected fetus as the virus source. Virus from these “non-traditional” sources like saliva and tears may be infectious, as was hypothesized from the non-vectored transmission between a ZIKV-infected patient and his caregiver (21), but the contribution to overall transmission in nature is likely low. Indeed, these transmission patterns cannot be studied in ZIKV endemic areas as most infections are mosquito-vectored.
Sexual transmission has also been documented and is the most prevalent form of non-vectored transmission in nature. It is difficult to assess the contribution of sexual transmission in endemic areas; case reports are relatively few, originate from non-endemic countries and do not address the epidemiological impact of this type of transmission mode (reviewed in (22)). A recent mathematical model which used data from the 2015 Brazil outbreak suggested sexual transmission in a non-outbreak scenario is likely but low (23). Furthermore, virus or the virus’ genetic material can be present in semen for months (22, 24), even in asymptomatic men (25), and sexually transmitted virus can infect a developing fetus (26). At this time, the correlation between disease and transmission route (mosquito vs sexual) is unknown. Sexual transmission models using Asian and American lineage ZIKV, including characterization of infected male reproductive tracts, have also been developed in mice (26–28). To the best of our knowledge, no genetic studies have been performed on viruses isolated in these modes of transmission. This represents a gap in knowledge and a key opportunity for understanding the unique properties of a sexually transmitted flavivirus. Notably, the closely related Spondweni virus has recently been shown to be transmitted by the sexual route, albeit inefficiently, compared to ZIKV (29).
To date, severe Zika disease and sexual transmission have primarily been described for Asian (primarily American) lineage viruses (30). The lack of association with African strains could be due to several factors. First, contemporary African lineage viruses have not been as freely available for experimentation as Asian lineage strains, despite being consistently isolated during the last few decades (31), so experimental comparisons rely on older African strains typically from the 1950s and 1960s. This limited dataset forces researchers to focus on only a few strains, which might not fully represent the viruses circulating currently. Second, “explosive” epidemics have not been recorded in Africa. Seroprevelance data and documented human infections from Africa shows circulating ZIKV (32, 33). However, given the notorious cross-reactivity between flaviviruses, determining recent cases or seroprevelance in hyperendemic places like Africa make identifying and tracking recent ZIKV extremely difficult (reviewed in (3)). Indeed, historical case reports must be interpreted cautiously as some of these infections based only on serology may be from Spondweni infections (34). Therefore, ascribing severe Zika disease to African lineage viruses has not been done, even if it is true. Third, misdiagnosis is also common in areas endemic for viruses that cause similar diseases like dengue or Spondweni fever, so unless a laboratory confirmation is performed, the true burden of ZIKV might be under-reported. It is also worth noting that a small outbreak in the Cape Verde Islands has been associated with microcephaly (35) but the virus may have come from the Americas (3).
Using Phylogenetics to Identify Mutations of Interest
The most straightforward method to determine whether the recent outbreak in the Americas was caused by a mutated ZIKV is to compare currently circulating strains to those in archived collections and/or sequences in GenBank. Like other RNA viruses, ZIKV undergoes error-prone replication due to the lack of error-checking and mismatch-repair mechanisms within the virally encoded RNA-dependent-RNA-polymerase. While the vast majority of mutations to the viral genome are negative and rapidly removed by purifying selection, some are selectively neutral and may be maintained over time because they do not alter virus fitness. Some mutations, however, have the ability to increase virus fitness in mosquitoes and/or vertebrates, altering the course of epidemics. Several examples of this are well established in the literature and span several viral taxa. Excellent examples of epidemic enhancing mutations include WNV adaptation to efficient transmission by North American Culex mosquitoes, CHIKV adaptation to Ae. albopictus, and Venezuelan equine encephalitis virus (VEEV) adaptations that confer high titers in horses (36–38)(39).
The error-prone nature of RNA genome viruses creates a problem for analyzing the contributions of mutations in an outbreak due to their frequency because of the large number of mutations that may be of potential interest. For this reason, additional information is usually needed to reduce the number of interesting candidates for analysis. Mutations that occur at the nodes of phylogenies are an attractive starting point since they represent the changes that occur in all viruses in that branch (FIGURE 2). These mutations might represent those which have fitness advantages and might be useful for further investigation. However, these mutations might also have a neutral fitness and represent a founder’s effect if relatively few isolates generated this new branch (as a single introduction in a new area resulting in an outbreak). An additional hurdle to analyzing the role of individual mutations is epistasis, or the contribution of more than one mutation in concert to result in a phenotype. If mutations in this situation are examined alone, the previously observed phenotype might not be observed and lead to a false conclusion. This approach has been taken by several groups to begin to ascertain the potential mutations of interest, some of which are discussed below (40).
Figure 2. An Example of a Rooted Tree with Meaningless Branch Lengths.
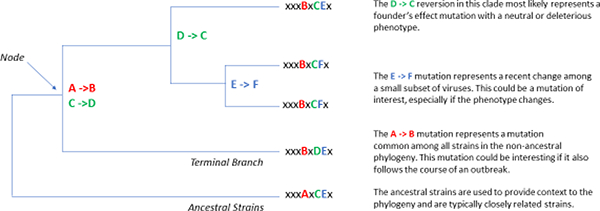
Here, mutations accumulate which are denoted at the nodes of new branching patterns.
The phenotype of interesting mutations can be analyzed using reverse-genetics. Full-length infectious clones containing the cDNA sequence of RNA viruses allows for mutations to be engineered using standard molecular cloning techniques. Viruses containing the desired mutations can be generated and compared directly to the original wild-type virus. Infectious clones for ZIKV are available for a variety of strains and the phenotype of rescued viruses is similar to the isolate (41–44). One important distinction between natural isolates and clone-derived virus is the number of viral intragenic variation (commonly but incorrectly referred to by many virologists as ‘quasispecies’) present. Natural isolates contain a much more diverse set of SNPs which are not present in the well-defined sequence of the cDNA used to create infectious clone-derived virus (45), and the level of genetic diversity might also influence disease. However, when comparing the roles of individual mutations, this lack of intra-host variation is usually not a problem.
Phenotypic Differences Between African, Asian (and American) Lineages of ZIKV
Almost as soon as researchers began to characterize ZIKV in vitro and in vivo, notable differences between the African and Asian (and American) lineages appeared. The hypothesis at the time that Asian lineage viruses associated with microcephaly would be more virulent or more transmissible by mosquitoes was proven incorrect by many studies. In tissue culture, MR766 and other African lineage strains like Uganda 976 and DakAR41524 grow to higher titers and result in more apoptosis in cell culture (Vero, HEK-293, human neuroprogenetor [hNPC], neuroblastoma, glioblastoma, monocyte-derived dendritic and trophoblast cells) (46–51). African lineage viruses are also more pathogenic in mice. As originally shown in the 1950s (52) and confirmed more recently (53), immunocompetent mice are resistant to Zika disease. Mice with innate immune deficiencies, particularly in the type-I interferon signaling pathways, have been useful yet imperfect models. In general, African lineage ZIKV resulted in higher mortality, increased morbidity and higher viral loads in key tissues like brain and testes when compared to Asian lineage infected mice (47, 54) (reviewed in (55)).
Increased vector competence could also contribute to the severity of an outbreak; if Asian lineage virus was more transmissible by North American mosquito species, this might increase the number of infections and also increase the change of seeing devastating but rare clinical outcomes. However, mosquito populations are also more susceptible for African lineage ZIKV (56). Low felial (F) generation Ae. aegypti from several locations in North America also vector ZIKV DakAR41525 strain more efficiently than other Asian lineages viruses tested (57, 58). A more nuanced study comparing two Asian lineage viruses (French Polynesia 2013 [Asian] and Brazil 2015 [BE H 815744] showed mosquitoes from Singapore were more susceptible with the Brazilian isolate (59).
Taken together, researchers suggest that Asian (and American) lineage viruses may cause less severe disease than African strains, at least in the systems used, but still damage the host and lead to some of the severe neurological diseases, especially in the young. Given these counterintuitive results and the way the virus spread globally, perhaps the comparison between Asian lineage viruses before the outbreaks in the 2000s and after might be more appropriate. Indeed, if the African lineage viruses are this virulent in cell culture and animal models, why does Zika appear to be a mild disease in areas where the African strain is still present? Futher investigation is needed, but heterologous protection from other cross-reactive flaviviruses may have a protective effect in endemic areas. Furthermore, perhaps the “less virulent” Asian strains are able to cause damage during development but still allow for fetal development whereas the African strains could have more devastating effects resulting in fetal death (49).
It is important to note that the prototype MR766 strain should not be used for many genotypic and phenotypic studies. This strain, while attractive as it’s found in many collections for collaboration or purchase, has been passaged through white mice over 150 times, resulting in a murine-adapted virus which may poorly reflect the original isolate made in 1947 (52).
The Contribution of Mutations in the Americas Epidemic
Despite the aforementioned challenges that exist, mutations of interest can still be investigated. Most of the published manuscripts to date focus on the structural proteins or immunogenic nonstructural proteins as evidenced below. These targets are intuitive since changes to these proteins in other arboviral have led to phenotypic changes that influence the course of viral infection and/or outbreak.
PrM S139N results in increased neurovirulence in murine neonates
Flavivirus prM protein is a structural scaffold maintained during the immature form of the virion. During particle maturation, the prM protein is cleaved into its mature form, M (membrane), and allows for the E (envelope) protein to fold into a head-to-tail dimer to form the mature particle.
Yuan and colleagues compared three ZIKV isolates from the 2015–2016 epidemic and compared them to a strain isolated from a child in Cambodia in 2010 (ZIKV FSS13025) (60). The more recent strains showed increased neurovirulence in neonatal mice accompanied by increased replication, apoptosis and reduction of neurons compared to the ZIKV FSS13025 strain. Phylogenetic analyses identified seven mutations of interest, which were then incorporated back into the FSS13025 infectious clone and mutant viruses were rescued. Only a serine to asparagine mutation S139N within the prM protein rescued the neuro virulent phenotype. ZIKV S139N caused more severe microcephaly in the mouse fetus after directly injected into lateral ventricle of embryonic littermate brains. It also led to higher mortality rates in neonatal mice after intracranial infection. Further evolutionary analysis indicates that the S139N substitution, which likely originated around May 2013, has been stably maintained since the 2013 outbreak in French Polynesia and was not found on more ancestral Asian lineage ZIKV strains (40). Two caveats to this study are the limited viral strains and the animal model used. All were contemporary from Asian lineage isolates and the phenotype of the African strains were not compared. Also, only neurovirulence and not neuroinvasion was examined. The contribution of this phenotype needs to be analyzed in the context of a peripheral infection to fully appreciate it’s phenotype in relation to other strains. As previously noted, African strains show considerable neurovirulence and the addition of that comparison would have been useful. Nonetheless, additional studies to determine the phenotype of this mutation on mosquito transmissibility has not yet been tested.
NS1 mutations increase mosquito transmission and (maybe) microcephaly
Flavivirus nonstructural protein 1 (NS1) is a multi-functional glycoprotein. The viral protein is made in the ER lumen in the infected cell and exists in many forms: monomers, dimers and hexamers. A truncated form of the protein is also secreted from cells and found in the bloodstream. NS1 is critical for virus replication and host immune evasion.
Liu and colleagues compared the phenotype of a virus isolated from a traveler returning from Venezuela in 2016 (GZ01) to the aforementioned Cambodia 2010 (ZIKV FSS13025) strain in their ability to infect mosquitoes feeding from viremic mice (61). Aedes aegypti were far more susceptible after feeding from GZ01-infected mice than FSS13025-infected mice despite similar viremia levels. The NS1 levels, however, were far greater in the blood of GZ01-infected mice. Indeed, blocking NS1 with an antibody or spiking exogenous NS1 into mosquito bloodmeals resulted in a reduction or increase in infected mosquitoes, respectively. Of the two mutations found between the two isolates in the prM-NS1 gene segment, only the A188V mutation conferred the increased NS1 secretion and enhanced mosquito infectivity phenotype. A phylogenetic study places the initial occurrence of this mutation in Southeast Asia between 2003 and 2007 (62). It is worth noting that African lineage strains also have the valine residue at position 188 (61), which might in part account for their high infectivity of Ae. aegypti mosquitoes.
The NS1 A188V might have multiple functions. Xia and colleagues reported the same A188V mutation confers NS1 to inhibit interferon-β induction (63). The A188V mutation enables NS1 binding to TBK1 and reduces TBK1 phosphorylation. Engineering the mutation into a pre-epidemic ZIKV strain confers the ability to inhibit interferon-β induction while reversing the mutation in an epidemic ZIKV strain showed reduction in inhibiting interferon-β induction.
In addition, a threonine-to-alanine substitution at residue 233 of NS1 was identified from the brain of infected fetus with neonatal microcephaly (64). The T233A mutation was shown to disrupt central hydrogen bonding network at NS1 dimer surface and to destabilize the NS1 dimeric assembly in vitro (65). However, the biological relevance of this mutation in causing neurological diseases remains to be experimentally evaluated.
Concluding Remarks: Did Zika Virus Mutate to Cause Severe Disease in the Americas?
A large body of work has been performed by scientists and clinicians since the first few cases of Zika disease were described in the Americas in 2015. Mutations that may have contributed to these epidemics have only begun to be described and undoubtedly many additional manuscripts will be reported in the years to come. However, it is also likely that ZIKV did not accumulate mutations that lead to increased virulence in patients or increased transmissibility in mosquitoes. The alternative hypothesis is that all strains of ZIKV, regardless of their origin, are capable of causing CZS, Guillan-Barré syndrome, sexual transmission and other severe rare outcomes. However, since these events are rare, the number of vertebrate hosts (patients) smaller outbreaks in Yap Island with only 5,000 cases may have been insufficient to see these diseases (66). Guillan-Barré syndrome was first observed in the French Polynesian epidemic, which had approximately 30,000 cases (7, 67). Although the actual number of infections is unknown, for the American epidemic, greater than 700,000 suspected cases (with over 175,000 of these laboratory-confirmed) were identified which might reflect the alarming occurrence of these devastating events (68).
The reason(s) underlying the rapid worldwide spread of ZIKV may never be known. This review has made one critical assumption while tying to answer it’s central question, and that is all events related to the severity of the outbreak are virus-derived and the host has no contribution on the outcome of infection. Largely, the intrinsic properties aside from previous flavivirus immunity of the patient were not considered, mostly for clarity. This half of the equation is critical to understanding severe outbreaks, but studies to ascertain the contribution of human genetics are extremely difficult to perform and complex to analyse. Discordant and dizygotic twins (non-identical twins of different sizes) from ZIKV-infected mothers has shown that the genetic makeup of the indivudal greatly influences the severity of ZIKV infection, even when infected with the same strain (69). These observation studies are good first step and similar studies are likely to be published. However, when considering only the virus, it is likely it was not one single event but the synergistic effect of several small changes under favorable epidemic conditions. This does not preclude the role of individual mutations, which may have a small chance in fitness in a given environment and cannot be experimentally determined. However, at the time of this review, there is no smoking gun to point to why millions of people worldwide were so affected by these debilitation ZIKV outbreaks.
Highlights.
Zika virus (ZIKV) was originally identified in Africa in the 1940s. Human infection was confirmed in the 1950s.
ZIKV caused several severe world-wide outbreaks in the last decade, with millions of people at risk of infection. Hundreds of thousands are suspected to have been infected with disease signs and symptoms ranging from subclinical to fever in adults. The highest burden of disease is in the unborn.
Severe outbreaks have been associated with the Asian and American lineage viruses. The other African lineage viruses are virulent in immunocompromised mouse models, and it is unclear if there is a genetic component to the outbreaks associated only with Asian or American ZIKV viruses.
Several mutations are observed in Asian/American lineage ZIKV and not in African lineage ZIKV. Published mutations in prM (S139N) and NSI (A188V and T233A) have shown to increase virulence in mouse models.
Outstanding Questions.
How did ZIKV spread from Africa, and then from Asia? Was this from viremic travelers or infected mosquitoes into new areas?
Is there a difference between the viruses circulating in Africa and the viruses circulating in Asia and the Americas?
What is the contribution of sexual transmission on maintaining ZIKV in nature? How often does this occur in the human population? Does this infection route alter the course of disease?
Are there mutations that have contributed to the severity of the American outbreak? If so, what part of the transmission cycle is affected, the mosquito or the vertebrate? When did these changes occur?
How likely is it that ZIKV will mutate again and spread to new areas not currently affected?
Acknowledgements
We kindly thank Christopher Roundy for his proofreading help. This work was supported in part by the U.S. National Institutes of Health grant U01 AI115577 for N.V., AI067380 and AI125996 for G.D.E and the Joint Vaccine Accquisition Program W911QY-15–1-0014 for S.R.. P.-Y.S. and C. S. was supported by CDC grant for the Western Gulf Center of Excellence for Vector-Borne Diseases, Kleberg Foundation Award, UTMB CTSA UL1TR-001439, and NIH grant AI127744.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, et al. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20. [DOI] [PubMed] [Google Scholar]
- 3.Aliota MT, Bassit L, Bradrick SS, Cox B, Garcia-Blanco MA, Gavegnano C, et al. Zika in the Americas, year 2: What have we learned? What gaps remain? A report from the Global Virus Network. Antiviral Res. 2017;144:223–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epelboin Y, Talaga S, Epelboin L, Dusfour I. Zika virus: An updated review of competent or naturally infected mosquitoes. PLoS Negl Trop Dis. 2017;11(11):e0005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One. 2014;9(10):e109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo XX, Li CX, Deng YQ, Xing D, Liu QM, Wu Q, et al. Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg Microbes Infect. 2016;5(9):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Araujo TVB, Ximenes RAA, Miranda-Filho DB, Souza WV, Montarroyos UR, de Melo APL, et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis. 2018;18(3):328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Likos A, Griffin I, Bingham AM, Stanek D, Fischer M, White S, et al. Local Mosquito-Borne Transmission of Zika Virus - Miami-Dade and Broward Counties, Florida, June-August 2016. MMWR Morb Mortal Wkly Rep. 2016;65(38):1032–8. [DOI] [PubMed] [Google Scholar]
- 11.Hall NB, Broussard K, Evert N, Canfield M. Notes from the Field: Zika Virus-Associated Neonatal Birth Defects Surveillance - Texas, January 2016-July 2017. MMWR Morb Mortal Wkly Rep. 2017;66(31):835–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubler DJ, Vasilakis N, Musso D. History and Emergence of Zika Virus. J Infect Dis. 2017;216(suppl_10):S860–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6(2):e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith ARY, Goo L, et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep. 2016;16(6):1485- 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghadas SM, Shoukat A, Espindola AL, Pereira RS, Abdirizak F, Laskowski M, et al. Asymptomatic Transmission and the Dynamics of Zika Infection. Sci Rep. 2017;7(1):5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–5. [DOI] [PubMed] [Google Scholar]
- 17.Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21(1):84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aleman TS, Ventura CV, Cavalcanti MM, Serrano LW, Traband A, Nti AA, et al. Quantitative Assessment of Microstructural Changes of the Retina in Infants With Congenital Zika Syndrome. JAMA Ophthalmol. 2017;135(10):1069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016;16(12):3208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suy A, Sulleiro E, Rodo C, Vazquez E, Bocanegra C, Molina I, et al. Prolonged Zika Virus Viremia during Pregnancy. N Engl J Med. 2016;375(26):2611–3. [DOI] [PubMed] [Google Scholar]
- 21.Krow-Lucal ER, Novosad SA, Dunn AC, Brent CR, Savage HM, Faraji A, et al. Zika Virus Infection in Patient with No Known Risk Factors, Utah, USA, 2016. Emerg Infect Dis. 2017;23(8):1260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect. 2017;23(5):296–305. [DOI] [PubMed] [Google Scholar]
- 23.Maxian O, Neufeld A, Talis EJ, Childs LM, Blackwood JC. Zika virus dynamics: When does sexual transmission matter? Epidemics. 2017;21:48–55. [DOI] [PubMed] [Google Scholar]
- 24.Arsuaga M, Bujalance SG, Diaz-Menendez M, Vazquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis. 2016;16(10):1107. [DOI] [PubMed] [Google Scholar]
- 25.Musso D, Richard V, Teissier A, Stone M, Lanteri MC, Latoni G, et al. Detection of Zika virus RNA in semen of asymptomatic blood donors. Clin Microbiol Infect. 2017;23(12):1001 e1- e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166(5):1247–56 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duggal NK, Ritter JM, Pestorius SE, Zaki SR, Davis BS, Chang GJ, et al. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep. 2017;18(7):1751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng ZY, Gao N, Wang ZY, Cui XY, Zhou DS, Fan DY, et al. Sertoli Cells Are Susceptible to ZIKV Infection in Mouse Testis. Front Cell Infect Microbiol. 2017;7:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald EM, Duggal NK, Brault AC. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Negl Trop Dis. 2017;11(10):e0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374(22):2195–8. [DOI] [PubMed] [Google Scholar]
- 31.Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014;8(1):e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond). 1979;83(2):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haddow AD, Woodall JP. Distinguishing between Zika and Spondweni viruses. Bull World Health Organ. 2016;94(10):711-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lourenco J, de Lourdes Monteiro M, Valdez T, Monteiro Rodrigues J, Pybus O, Rodrigues Faria N. Epidemiology of the Zika Virus Outbreak in the Cabo Verde Islands, West Africa. PLoS Curr. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebel GD, Carricaburu J, Young D, Bernard KA, Kramer LD. Genetic and phenotypic variation of West Nile virus in New York, 2000–2003. Am J Trop Med Hyg. 2004;71(4):493–500. [PubMed] [Google Scholar]
- 37.Brault AC, Powers AM, Holmes EC, Woelk CH, Weaver SC. Positively charged amino acid substitutions in the e2 envelope glycoprotein are associated with the emergence of Venezuelan equine encephalitis virus. J Virol. 2002;76(4):1718–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirato K, Miyoshi H, Goto A, Ako Y, Ueki T, Kariwa H, et al. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol. 2004;85(Pt 12):3637–45. [DOI] [PubMed] [Google Scholar]
- 40.Pettersson JH, Eldholm V, Seligman SJ, Lundkvist A, Falconar AK, Gaunt MW, et al. How Did Zika Virus Emerge in the Pacific Islands and Latin America? MBio 2016;7(5) e01239–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shan C, Xie X, Muruato AE, Rossi SL, Roundy CM, Azar SR, et al. An Infectious cDNA Clone of Zika Virus to Study Viral Virulence, Mosquito Transmission, and Antiviral Inhibitors. Cell Host Microbe. 2016;19(6):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsetsarkin KA, Kenney H, Chen R, Liu G, Manukyan H, Whitehead SS, et al. A Full-Length Infectious cDNA Clone of Zika Virus from the 2015 Epidemic in Brazil as a Genetic Platform for Studies of Virus-Host Interactions and Vaccine Development. MBio. 2016;7(4) e01114–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weger-Lucarelli J, Duggal NK, Bullard-Feibelman K, Veselinovic M, Romo H, Nguyen C, et al. Development and Characterization of Recombinant Virus Generated from a New World Zika Virus Infectious Clone. J Virol. 2017;91(1):e01765–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Widman DG, Young E, Yount BL, Plante KS, Gallichotte EN, Carbaugh DL, et al. A Reverse Genetics Platform That Spans the Zika Virus Family Tree. MBio. 2017;8(2): 10.1155/2015/409596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Boheemen S, Tas A, Anvar SY, van Grootveld R, Albulescu IC, Bauer MP, et al. Quasispecies composition and evolution of a typical Zika virus clinical isolate from Suriname. Sci Rep. 2017;7(1):2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonin Y, Loustalot F, Desmetz C, Foulongne V, Constant O, Fournier-Wirth C, et al. Zika Virus Strains Potentially Display Different Infectious Profiles in Human Neural Cells. EBioMedicine. 2016;12:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao Q, Herrlinger S, Zhu YN, Yang M, Goodfellow F, Stice SL, et al. The African Zika virus MR-766 is more virulent and causes more severe brain damage than current Asian lineage and dengue virus. Development. 2017;144(22):4114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowen JR, Quicke KM, Maddur MS, O’Neal JT, McDonald CE, Fedorova NB, et al. Zika Virus Antagonizes Type I Interferon Responses during Infection of Human Dendritic Cells. PLoS Pathog. 2017;13(2):e1006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A. 2017;114(9):E1587–E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith DR, Sprague TR, Hollidge BS, Valdez SM, Padilla SL, Bellanca SA, et al. African and Asian Zika Virus Isolates Display Phenotypic Differences Both In Vitro and In Vivo. Am J Trop Med Hyg. 2018. 98(2):432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anfasa F, Siegers JY, van der Kroeg M, Mumtaz N, Stalin Raj V, de Vrij FMS, et al. Phenotypic Differences between Asian and African Lineage Zika Viruses in Human Neural Progenitor Cells. mSphere. 2017;2(4) e00292–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–34. [DOI] [PubMed] [Google Scholar]
- 53.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, et al. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016;94(6):1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dowall SD, Graham VA, Rayner E, Hunter L, Atkinson B, Pearson G, et al. Lineage-dependent differences in the disease progression of Zika virus infection in type-I interferon receptor knockout (A129) mice. PLoS Negl Trop Dis. 2017;11(7):e0005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simonin Y, van Riel D, Van de Perre P, Rockx B, Salinas S. Differential virulence between Asian and African lineages of Zika virus. PLoS Negl Trop Dis. 2017;11(9):e0005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weger-Lucarelli J, Ruckert C, Chotiwan N, Nguyen C, Garcia Luna SM, Fauver JR, et al. Vector Competence of American Mosquitoes for Three Strains of Zika Virus. PLoS Negl Trop Dis. 2016;10(10):e0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azar SR, Roundy CM, Rossi SL, Huang JH, Leal G, Yun R, et al. Differential Vector Competency of Aedes albopictus Populations from the Americas for Zika Virus. Am J Trop Med Hyg. 2017;97(2):330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roundy CM, Azar SR, Rossi SL, Huang JH, Leal G, Yun R, et al. Variation in Aedes aegypti Mosquito Competence for Zika Virus Transmission. Emerg Infect Dis. 2017;23(4):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pompon J, Morales-Vargas R, Manuel M, Huat Tan C, Vial T, Hao Tan J, et al. A Zika virus from America is more efficiently transmitted than an Asian virus by Aedes aegypti mosquitoes from Asia. Sci Rep. 2017;7(1):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science. 2017;358(6365):933–6. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Liu J, Du S, Shan C, Nie K, Zhang R, et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature. 2017;545(7655):482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delatorre E, Mir D, Bello G. Tracing the origin of the NS1 A188V substitution responsible for recent enhancement of Zika virus Asian genotype infectivity. Mem Inst Oswaldo Cruz. 2017;112(11):793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia H, Luo H, Shan C, Muruato AE, Nunes BTD, Medeiros DBA, et al. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat Commun. 2018;9(1):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374(10):951–8. [DOI] [PubMed] [Google Scholar]
- 65.Wang D, Chen C, Liu S, Zhou H, Yang K, Zhao Q, et al. A Mutation Identified in Neonatal Microcephaly Destabilizes Zika Virus NS1 Assembly in Vitro. Sci Rep. 2017;7:42580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–43. [DOI] [PubMed] [Google Scholar]
- 67.Musso D, Bossin H, Mallet HP, Besnard M, Broult J, Baudouin L, et al. Zika virus in French Polynesia 2013–14: anatomy of a completed outbreak. Lancet Infect Dis. 2017;18(5):e172–e82. [DOI] [PubMed] [Google Scholar]
- 68.Ikejezie J, Shapiro CN, Kim J, Chiu M, Almiron M, Ugarte C, et al. Zika Virus Transmission Region of the Americas, May 15, 2015-December 15, 2016. Am J Transplant. 2017;17(6):1681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caires-Junior LC, Goulart E, Melo US, Araujo BHS, Alvizi L, Soares-Schanoski A, et al. Discordant congenital Zika syndrome twins show differential in vitro viral susceptibility of neural progenitor cells. Nat Commun. 2018;9(1):475. [DOI] [PMC free article] [PubMed] [Google Scholar]


