Abstract
Background and Aims: Recurrent hepatitis C (HCV) disease in liver transplant (LT) recipients is associated with significant morbidity and mortality. With the availability of noninterferon-based therapy, eliminating HCV may be achievable in LT recipients.
Methods: We studied all consecutive recipients who underwent LT at the University of California Los Angeles between January 2005 and June 2017. We collected data on date of transplant and last follow-up, as well as laboratory values. We also recorded type and timing of antiviral therapy relative to LT. Analyses were performed to assess the proportion of LT recipients who are viremic after transplant.
Results: Six hundred thirty-four patients underwent LT with a diagnosis of HCV. There was a statistically significant trend for patients to be cured before (p < 0.001) and after liver transplantation (p < 0.001) for the study period of 2014 to 2016 relative to 2005 and 2013, respectively. Of the 634 recipients eligible for therapy, 8% and 74% were treated within 12 months of transplant for the study periods 2005 to 2013 and 2014 to 2016, respectively. There was a significant decrease between the two study periods in the proportion of patients undergoing re-LT 1 year after the original LT: 5.5% (n = 28/510) and 1.5% (n = 2/124) respectively for study periods 2005 to 2013 and 2014 to 2016 respectively (p = 0.011).
Conclusions: The proportion of LT recipients who are viremic has decreased over time. Eliminating HCV in LT recipients is feasible after the introduction of direct-acting agents. Curing HCV should translate to improved clinical outcomes in LT recipients who were transplanted for HCV infection with longer follow-up. Preliminary results suggest the decreased need for transplant in the direct-acting agents era.
Keywords: Hepatitis C, Direct-acting agents, Liver transplant, Elimination
Introduction
Chronic hepatitis C (HCV) remains one of the most common indications for liver transplantation (LT) in the USA.1,2 Recurrent infection is universal, and can lead to both patient and graft loss.3,4 Treatment of HCV has substantially evolved over the years.5–7 The sustained viral response (SVR) or ‘cure’ rates with pegylated-interferon and ribavirin were approximately 23%.6,7 The first generation of direct-acting agents (DAAs), with the backbone of interferon, was associated with increased cure rates but with substantial adverse effects, permitting only select LT recipients, generally those with progressive liver disease, to be eligible for treatment.5 Thus, when all-oral DAAs became available, LT recipients were recommended to have priority treatment allocation.6 The use of all-oral DAAs has been found to be safe, effective and tolerable after LT.7,8
With increasing access and identification of patients infected with HCV, it is believed that viral infection can become a rare disease in the general population within the next couple of decades.9 There are already several examples of elimination strategies of HCV from various cohorts.10–13 Liver transplant recipients infected with HCV would be an ideal cohort to attempt elimination or eradication because recurrent infection clearly impacts patient and graft survival, as well as health care utilization.3,4,14 Although patients can be treated with DAAs both prior to and after LT, the optimal timing of antiviral therapy is unclear.15,16 Progressive liver disease in liver transplant candidates may lower the likelihood of SVR and increase the adverse effects of DAAs.16–18 Nevertheless, the impact of treating LT candidates with DAAs has begun to be realized across the USA, as evidenced by changes in the number of HCV patients registered on the LT waitlist.19–21
Because of the medical impact of recurrent HCV infection and the safety and efficacy of DAAs in LT recipients, we sought to describe the impact of DAAs on the elimination of HCV in LT recipients. Our hypothesis was that HCV can be eliminated in LT recipients with the use of DAAs.
Methods
We performed a retrospective chart review of all adult (age >18 years) recipients who underwent a liver transplant for HCV at the University of California Los Angeles Medical Center (UCLA) between January 2005 and June 2017. Inclusion criteria included adult recipients (age 18 years or older) with HCV infection confirmed by a detectable HCV RNA at any time or a clinical history of having HCV infection without documentation of antiviral therapy. We assumed no spontaneous clearance. Exclusion criteria was met for recipients who expired within 30 days of the LT. The study was approved by the UCLA Institutional Review Board. Because of the nature of the current study, obtained informed consent was not required.
Data were obtained by medical chart review and the UCLA Liver Transplant database. Demographic data (age, sex), date of liver transplant and HCV therapeutic regimen were collected. Recipients received the standard immunosuppressant regimen including prednisone, tacrolimus and mycophenolate acid after transplant, and were eventually weaned off to only tacrolimus if there was no renal insufficiency or if recipients had complete renal failure requiring hemodialysis. Mycophenolate was utilized with tacrolimus in recipients with renal insufficiency but who were not on hemodialysis. SVR was defined as the absence of HCV RNA at least 12 weeks after completing therapy. Date of last follow-up, death or graft failure was also recorded. Dates when different antiviral regimens were used are shown in Table 1.5,22–25 All-oral DAA regimens were used starting in 2014. The regimens utilized were: sofosbuvir+ribavirin; sofosbuvir+simeprevir; sofosbuvir+ledipasvir; sofosbuvir+daclatasvir; sofosbuvir+elbasvir+grazoprevir+ribavirin.
Table 1. Approximate dates of antiviral therapy used at the University of California Los Angeles.
| Drug regimen | Years utilized |
| Pegylated-interferon and ribavirin | 2005 to 2010 |
| Pegylated-interferon, ribavirin, and boceprevir | 2011 to 2013 |
| All-oral direct-acting agents* | 2014 |
sofosbuvir+ribavirin; sofosbuvir+simeprevir; sofosbuvir+ledipasvir; sofosbuvir+daclatasvir; sofosbuvir+elbasvir+grazoprevir+ribavirin.
Differences in SVR were summarized using two different models for the time periods of 2005 to 2013 and 2014 to 2016, namely the spline model and Cox proportional hazards model. Median with interquartile range (IQR) was used to describe data distribution. Each model had an indicator for whether patients were transplanted prior to or after January 1, 2014. This time was chosen because this is when DAAs without interferon were made available at our institution. The spline model was a simple linear spline with an interaction between time from start of study and the indicator for availability of DAAs without interferon. The Cox proportional hazards model censored patients at their last clinical follow-up or at the date of SVR. Time to sustained viral suppression was measured from last transplant if the patient had multiple LTs. A p-value below 0.05 was considered statistically significant and the statistical analysis was performed in the R Statistical Computing Environment (R Core Team; Vienna, Austria).
Results
We identified 634 consecutive liver transplant recipients who were transplanted for HCV at our institution during the study period. The demographics are shown in Table 2. Most recipients were men (70%), and the median age (IQR) was 58 (53 – 63) years at the time of liver transplant. The median (IQR) follow-up was 2.69 (0.89 – 5.83) years. Thirty recipients required re-LT during the first year of LT. There was a significant difference in re-LT rate between the two periods at 1 year. The re-LT rate at 1-year for patients originally transplanted pre-2014 was 5.5% (n = 28/510), compared to 1.5% (n = 2/124; p = 0.011) for recipients transplanted after 2014.
Table 2. Patient demographics.
| Cohort | |||
| Overall | 2005–2013 | 2014–2016 | |
| Median (IQR) age in years at start of antiviral treatment | 58 (53–63) | 57 (53–62) | 60 (55–64) |
| Sex, M/F | 441/193 | 353/154 | 88/39 |
| Time (IQR) in years since liver transplantation | 7.7 (4.4–10.1) | 8.8 (6.4–10.6) | 2.2 (1.3–3.0) |
| Median (IQR) follow-up in years since SVR | 2.8 (0.9–5.8) | 3.9 (1–6.5) | 1.3 (0.8–2.1) |
Abbreviations: F, female; IQR, interquartile range; M, male; SVR, sustained viral response.
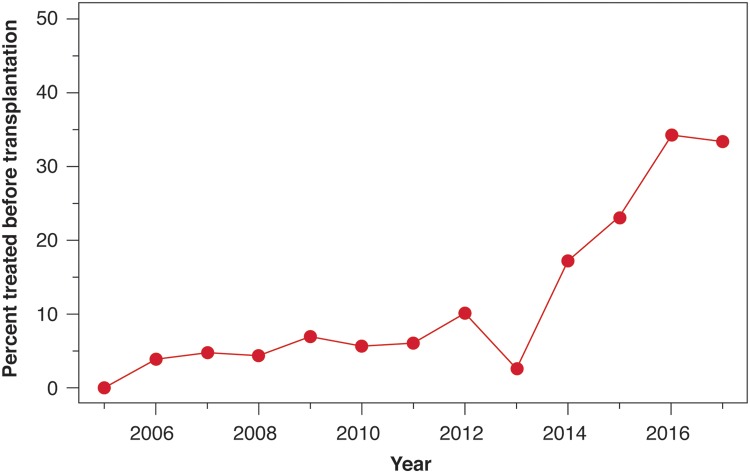
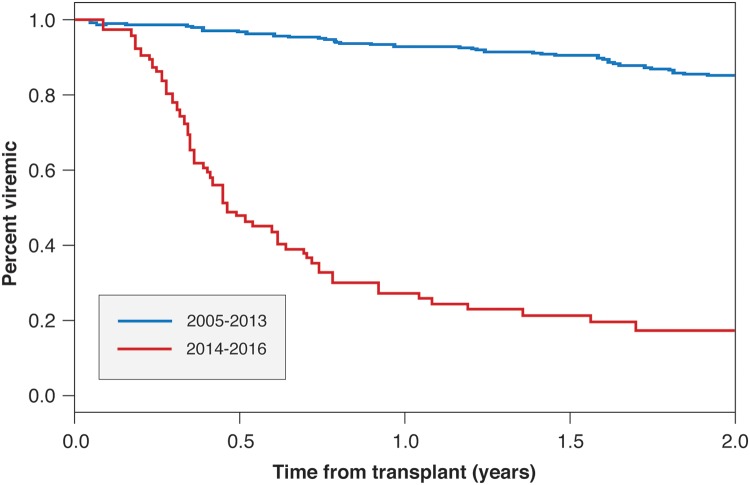
The use of DAAs without interferon began in our institution in 2013. Less than 10% (57/634) of the recipients were cured of HCV prior to the LT. There was a statistically significant higher rate of pretransplant HCV treatment in recipients undergoing LT after 2013 (p < 0.001) (Fig. 1). The median time from transplant to treatment was 6.21 (confidence interval [CI]: 5.42 to 6.86) years for the time period 2005 to 2013, compared to 0.466 years (CI: 0.40 to 0.69) for LT recipients in the later era of 2014–2016 (hazard ratio: 14.2, 95%CI: 9.87 to 20.45) (Fig. 2). Prior to the year 2014, 5% (25/506) of LT recipients had been treated before LT. After 2014, 25% (32/128) of LT recipients had been treated prior to surgery.
Fig. 1. Percentage of recipients treated before liver transplantation by year.
Fig. 2. Time to sustained viral response after liver transplant in eligible recipients.
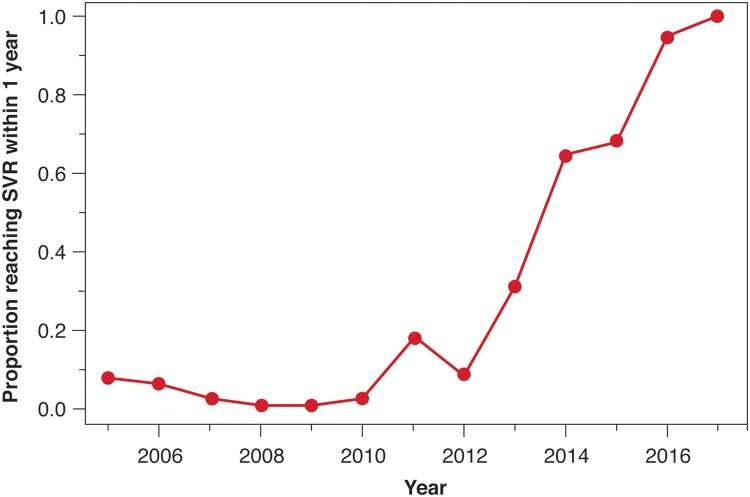
Over the study period, 42% (236/558) of LT recipients who were viremic and did not expire within 30 days of transplant were cured after transplant. There was a statistically significant trend to treat HCV earlier after LT during the period of 2014 and 2016 compared to 2005 and 2013 (Fig. 3) (p < 0.001). Between 2005 and 2013, 6% (29/506) of LT recipients were treated within the first year of transplant. Between 2014 and 2016, 33% (42/128) of LT recipients were treated within the first year of transplant. Within 2 years of LT, the cumulative proportion of LT recipients cured of HCV increased from 18% (90/506) in the earlier era (2005–2013) to 78% (100/128) in the later era (2014–2016) (p < 0.001).
Fig. 3. Proportion by year of recipients achieving a sustained viral response within 1 year of liver transplant.
Discussion
The results of our study demonstrate the proportion of recipients transplanted for HCV who are viremic after surgery has significantly decreased with the introduction of DAAs. Whereas most of our recipients were treated after liver transplant, a substantial percentage of recipients appear to have been treated even before LT. Such a strategy has been recently demonstrated to be cost effective.26 However, differences in the need for re-LT has not been seen, likely as a result of limited follow up in the latter cohort.
The timing of antiviral therapy is somewhat controversial. Most providers agree that patients with cirrhosis Child–Pugh class A should be offered therapy, but the role of DAAs in patients with Child–Pugh class B and C is less clear. In patients with Child-Pugh class C, the benefits of antiviral therapy are not as clearly defined as those patients with Child-Pugh class B. Indeed, there are reports of the model for end-stage liver disease threshold, beyond which may be associated with adverse effects from therapy.16 Treatment may be limited in patients with renal insufficiency since regimens approved for patients with decompensated liver disease are sofosbuvir-based regimens. Most therapeutic regimens include ribavirin, which has tolerability and safety issues in patients with advanced liver disease. However, those studies that highlighted potential adverse effects utilized protease inhibitors in their treatment regimen. The metabolism of a protease inhibitor occurs in the liver, so levels increase with worsening liver function.16
Achieving SVR in patients waiting for LT is associated with improved model for end-stage liver disease score/Child-Pugh class and the prevention of reinfection in the new liver graft. The treatment of liver transplant candidates has been shown to be cost effective.26 Although SVR is generally lower in patients with decompensated liver disease, two DAA drug combinations have been recently approved by the USA’s Federal Drug Administration for patients who failed DAA treatment.27,28 Until DAAs became readily available, interferon-based therapy was limited to not necessarily all viremic patients but only those patients with established recurrent HCV disease.
Liver transplant recipients are an ideal population to achieve HCV elimination. Treatment is biologically and technically feasible, cost and benefits favor therapy, and there are strong societal and political considerations.29 For instance, diagnosis of both viral infection and recurrent disease is readily made and noncontroversial. Antiviral therapy is not only highly effective, but safe and tolerable. Although several DAA regimens list the use of cyclosporine as a contraindication, most liver transplant recipients receive tacrolimus as their backbone immunosuppression. The treatment of HCV has been considered cost effective across all cohorts studied.30–32 There is also a high level of political consensus and support for eliminating HCV in liver transplant recipients. Reinfection is universal and recurrent disease clearly impairs patient and graft survival.3,4 Moreover, the role of re-LT for HCV is controversial, with practices varying throughout the country.
Our study has several important limitations. First, the study represents a single institution’s experience, and the results may not be generalizable to other settings. Nevertheless, our cohort included over 500 recipients whose care spanned over a decade. Another limitation is the brief follow-up in the era of DAAs. Although we were able to demonstrate that HCV is being eliminated from the LT recipient population, the recent utilization of DAAs prevents observing differences in re-LT rates for recurrent HCV. We suspect that the utilization of DAAs both pre- and posttransplant will decrease the need for re-LT with longer follow-up. Curing HCV has already been shown to lead to decreased health care utilization in LT recipients.14 We were also not able to define the SVR rates before LT. As demonstrated by our results, there is an increasing number of LT candidates being treated unbeknownst to our center. Thus, we are unable to comment on the SVR, since the total number of candidates treated are unknown to us. The SVR in liver transplant recipients has been previously reported by us.5,22–25 The range of SVR described using pegylated-interferon+ribavirin, pegylated-interferon+ribavirin+boceprevir, and all-oral DAAs has been 10% to 30%, 40% to 60% and 90% to 95%, respectively.7
Hepatitis B (HBV) was once considered a relative contraindication for LT because of decreased patient and graft survival compared to other indications for LT.33 Advancement in the treatment to prevent HBV reinfection in LT recipients has resulted in HBV being associated with among the best survival rates among all indications for LT.34,35 In the recent past, HCV was also associated with one of the worst survival rates for any of the major indications for LT.36 However, HCV can be eliminated from the liver transplant cohort in this country with treatment commencing either before or after liver transplantation. The safety, efficacy and tolerability has resulted in a greater proportion of patients being treated before their LT. Elimination of HCV should eventually translate to improved patient and graft survival.
Abbreviations
- CI
confidence interval
- DAAs
direct-acting agents
- HBV
hepatitis B
- HCV
chronic hepatitis C
- IQR
interquartile range
- LT
liver transplantation
- SVR
sustained viral response
- UCLA
University of California Los Angeles Medical Center
References
- 1.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015;15(Suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–1099.e1. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 4.Firpi RJ, Clark V, Soldevila-Pico C, Morelli G, Cabrera R, Levy C, et al. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl. 2009;15:1063–1071. doi: 10.1002/lt.21784. [DOI] [PubMed] [Google Scholar]
- 5.Saab S, Manne V, Bau S, Reynolds JA, Allen R, Goldstein L, et al. Boceprevir in liver transplant recipients. Liver Int. 2015;35:192–197. doi: 10.1111/liv.12548. [DOI] [PubMed] [Google Scholar]
- 6.AASLD/IDSA HCV Guidance Panel Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 7.Suraweera D, Sundaram V, Saab S. Treatment of hepatitis C virus infection in liver transplant recipients. Gastroenterol Hepatol (N Y) 2016;12:23–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Brown RS, Jr, O’Leary JG, Reddy KR, Kuo A, Morelli GJ, Burton JR, Jr, et al. Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: Real-world experience from the hepatitis C therapeutic registry and research network. Liver Transpl. 2016;22:24–33. doi: 10.1002/lt.24366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161:170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soipe AI, Razavi H, Razavi-Shearer D, Galárraga O, Taylor LE, Marshall BD. Chronic hepatitis C virus (HCV) burden in Rhode Island: modelling treatment scale-up and elimination. Epidemiol Infect. 2016:1–11. doi: 10.1017/S0950268816001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mera J, Vellozzi C, Hariri S, Carabin H, Drevets DA, Miller A, et al. Identification and clinical management of persons with chronic hepatitis C virus infection - Cherokee Nation, 2012–2015. MMWR Morb Mortal Wkly Rep. 2016;65:461–466. doi: 10.15585/mmwr.mm6518a2. . [DOI] [PubMed] [Google Scholar]
- 12.Gvinjilia L, Nasrullah M, Sergeenko D, Tsertsvadze T, Kamkamidze G, Butsashvili M, et al. National progress toward hepatitis C elimination - Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep. 2016;65:1132–1135. doi: 10.15585/mmwr.mm6541a2. [DOI] [PubMed] [Google Scholar]
- 13.He T, Li K, Roberts MS, Spaulding AC, Ayer T, Grefenstette JJ, et al. Prevention of hepatitis C by screening and treatment in U.S. prisons. Ann Intern Med. 2016;164:84–92. doi: 10.7326/M15-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aby E, Jimenez MA, Grotts JF, Agopian V, French SW, Busuttil RW, et al. Diminishing use of liver biopsy among liver transplant recipients for hepatitis C. J Clin Transl Hepatol. 2017;5:197–202. doi: 10.14218/JCTH.2016.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suraweera D, Saab EG, Tong MJ, Saab S. Timing of hepatitis C antiviral therapy in liver transplant recipients with direct-acting agents. Exp Clin Transplant. 2016;14:243–251. [PubMed] [Google Scholar]
- 16.Suraweera D, Saab S. Hepatitis C treatment threshold in patients with decompensated liver disease. Hepatology. 2017;65:1789–1791. doi: 10.1002/hep.29175. [DOI] [PubMed] [Google Scholar]
- 17.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 19.Belli LS, Berenguer M, Cortesi PA, Strazzabosco M, Rockenschaub SR, Martini S, et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol. 2016;65:524–531. doi: 10.1016/j.jhep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Saab S, Greenberg A, Li E, Bau SN, Durazo F, El-Kabany M, et al. Sofosbuvir and simeprevir is effective for recurrent hepatitis C in liver transplant recipients. Liver Int. 2015;35:2442–2447. doi: 10.1111/liv.12856. [DOI] [PubMed] [Google Scholar]
- 23.Saab S, Jimenez M, Bau S, Goo T, Zhao D, Durazo F, et al. Treating fibrosing cholestatic hepatitis C with sofosbuvir and ribavirin: a matched analysis. Clin Transplant. 2015;29:813–819. doi: 10.1111/ctr.12584. [DOI] [PubMed] [Google Scholar]
- 24.Saab S, Rheem J, Jimenez M, Bau S, Choi G, Durazo F, et al. Curing hepatitis C in liver transplant recipients is associated with changes in immunosuppressant use. J Clin Transl Hepatol. 2016;4:32–38. doi: 10.14218/JCTH.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saab S, A Jimenez M, N Bau S, Choi G, Durazo FA, M El-Kabany M, et al. Use of sofosbuvir-based treatment of chronic hepatitis C in liver transplant recipients on hemodialysis. J Clin Gastroenterol. 2017;51:167–173. doi: 10.1097/MCG.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed A, Gonzalez SA, Cholankeril G, Perumpail RB, McGinnis J, Saab S, et al. Treatment of patients waitlisted for liver transplant with all-oral direct-acting antivirals is a cost-effective treatment strategy in the United States. Hepatology. 2017;66:46–56. doi: 10.1002/hep.29137. [DOI] [PubMed] [Google Scholar]
- 27.Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376:2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 28.Poordad F, Felizarta F, Asatryan A, Sulkowski MS, Reindollar RW, Landis CS, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology. 2017;66:389–397. doi: 10.1002/hep.29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowdle WR. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76:22–25. [PMC free article] [PubMed] [Google Scholar]
- 30.Saab S, Virabhak S, Parisé H, Johnson S, Wang A, Misurski D, et al. Cost-effectiveness of genotype 1 chronic hepatitis C virus treatments in patients coinfected with human immunodeficiency virus in the United States. Adv Ther. 2016;33:1316–1330. doi: 10.1007/s12325-016-0362-1. [DOI] [PubMed] [Google Scholar]
- 31.Saab S, Gonzalez YS, Huber C, Wang A, Juday T. Cost-effectiveness of ombitasvir/paritaprevir/ritonavir, dasabuvir+ribavirin for US post-liver transplant recurrent genotype 1 HCV. Liver Int. 2016;36:515–521. doi: 10.1111/liv.13033. [DOI] [PubMed] [Google Scholar]
- 32.Younossi ZM, Park H, Saab S, Ahmed A, Dieterich D, Gordon SC. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2015;41:544–563. doi: 10.1111/apt.13081. [DOI] [PubMed] [Google Scholar]
- 33.Kim WR, Terrault NA, Pedersen RA, Therneau TM, Edwards E, Hindman AA, et al. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009;137:1680–1686. doi: 10.1053/j.gastro.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saab S, Chen PY, Saab CE, Tong MJ. The management of hepatitis B in liver transplant recipients. Clin Liver Dis. 2016;20:721–736. doi: 10.1016/j.cld.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Young K, Liu B, Bhuket T, Younossi Z, Saab S, Ahmed A, et al. Long-term trends in chronic hepatitis B virus infection associated liver transplantation outcomes in the United States. J Viral Hepat. 2017;24:789–796. doi: 10.1111/jvh.12703. [DOI] [PubMed] [Google Scholar]
- 36.Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, et al. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg. 2013;258:409–421. doi: 10.1097/SLA.0b013e3182a15db4. [DOI] [PubMed] [Google Scholar]