Abstract
Although type 2 diabetes (T2D) results from metabolic defects in insulin secretion and insulin sensitivity, most of the genetic risk loci identified to date relates to insulin secretion. We reported that T2D loci influencing insulin sensitivity may be identified through interactions with insulin secretion loci, thereby leading to T2D. Here, we hypothesize that joint testing of variant main effects and interaction effects with an insulin secretion locus increases power to identify genetic interactions leading to T2D. We tested this hypothesis with an intronic MTNR1B SNP, rs10830963, which is associated with acute insulin response to glucose (AIRg), a dynamic measure of insulin secretion. rs10830963 was tested for interaction and joint (main + interaction) effects with genome-wide data in African Americans (2,452 cases and 3,772 controls) from five cohorts. Genome-wide genotype data (Affymetrix HumanGenome 6.0 array) was imputed to a 1000 Genomes Project reference panel. T2D risk was modeled using logistic regression with rs10830963 dosage, age, sex, and principal component as predictors. Joint effects were captured using the Kraft 2 degree-of-freedom test. Genome-wide significant (P<5×10−8) interaction with MTNR1B and joint effects were detected for CMIP intronic SNP rs17197883 (Pinteraction=1.43×10−8; Pjoint=4.70×10−8). CMIP variants have been nominally associated with T2D, fasting glucose, and adiponectin in individuals of East Asian ancestry, with high density lipoprotein (HDL), and with waist-to-hip ratio (WHR) adjusted for body mass index (BMI) in Europeans. These data support the hypothesis that additional genetic factors contributing to T2D risk, including insulin sensitivity loci, can be identified through interactions with insulin secretion loci.
Keywords: Gene-gene interactions, insulin resistance, insulin sensitivity
Introduction
Type 2 diabetes (T2D), a disease twice as prevalent in African Americans compared to European Americans, is characterized by elevated plasma glucose resulting from loss of glucose homeostasis through beta cell dysfunction impacting insulin secretion and impaired insulin sensitivity(“2014 Statistics Report | Data & Statistics | Diabetes | CDC,” n.d.). Genome-wide association studies (GWAS) have identified over 120 T2D loci with the biological basis for most of these loci assumed to be related with beta cell dysfunction(Prasad & Groop, 2015).
We hypothesized that T2D risk loci, particularly those loci affecting insulin sensitivity, could be identified by interaction analyses with insulin secretion loci(Keaton et al., 2016). This hypothesis was based on physiological observations that strongly suggest non-additive interaction between insulin secretion deficits and insulin resistance resulting in T2D(Kahn et al., 1993; Lillioja et al., 1993). To test this hypothesis, a single nucleotide polymorphism (SNP) associated with acute insulin response to glucose (AIRg) in samples from African American in the Insulin Resistance Atherosclerosis Family Study (IRASFS) were analyzed for genome-wide interactions contributing T2D risk in a pooled cohort of African Americans.
Interaction analysis based solely on significance of interaction terms presents power challenges for identifying interacting loci. In this report, we extend genome-wide interaction analysis by incorporating both main and interaction effects in a two degree-of-freedom joint test to search for variants with marginal effects as well as interacting with the intronic insulin secretion variant rs10830963 in MTNR1B, a genetic variant powerfully associated with AIRg (P=1.20×10−5 in a study of 492 African American individuals examining association with 247,870 variants from an exome microarray) and fasting glucose (P=9.29×10−15 in 20,209 African Americans), to increase T2D risk in African Americans(Keaton et al., 2016, Liu et al, 2016). This approach is more powerful than 1 degree-of-freedom tests when both marginal and interaction effects exist (Manning et al, 2011). Considering the higher prevalence rate of T2D, insulin resistance, and obesity with strong genetic predisposition, African Americans are optimal for the study of genetic interactions that contribute to T2D risk.
Research Design and Methods
Study Populations
A pooled cohort of African Americans was created from five cohorts. Participants included African American participants from the Atherosclerosis Risk in Communities study (ARIC; n=820 T2D cases, 371 controls), the Coronary Artery Risk Development in Young Adults study (CARDIA; n=94 T2D cases, 652 controls), the Jackson Heart Study (JHS; n=244 T2D cases, 1,089 controls), the Multi-Ethnic Study of Atherosclerosis (MESA; n=404 T2D cases, 773 controls), and the Wake Forest School of Medicine study (WFSM; n=890 T2D cases, 887controls) cohorts(Bild et al., 2002; Friedman et al., 1988; McDonough et al., 2011; Palmer et al., 2012; Taylor et al., 2005; The ARIC Investigators, 1989). Inclusion and exclusion criteria for T2D cases and controls have previously been described(Ng et al., 2013). Briefly, T2D was diagnosed according to the American Diabetes Association criteria with at least one of the following: fasting glucose ≥126 mg/dL, 2-h oral glucose tolerance test glucose ≥200 mg/dL, random glucose ≥200 mg/dL, use of oral hypoglycemic agents and/or insulin, or physician diagnosed diabetes. Subjects diagnosed before 25 years of age were excluded. Normal glucose tolerance was defined as fasting glucose <100 mg/dL and 2-h oral glucose tolerance test glucose <140 mg/dL (if available) without reported use of diabetes medications or clinically diagnosed diabetes. Controls <25 years of age were excluded.
Primary associations with AIRg in IRASFS African Americans
Glucose homeostasis traits were measured by the frequently sampled intravenous glucose tolerance test (FSIGT)(Henkin et al., 2003). Briefly, a 50% glucose solution (0.3g/kg) and regular human insulin (0.03units/kg) were injected intravenously at 0 and 20 minutes, respectively. Blood was collected at −5, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 minutes for measurement of plasma glucose and insulin. AIRg was calculated as the increase in insulin at 2–8 minutes above the basal (fasting) insulin level after the bolus glucose injection at 0–1 minute.
SNP genotyping, imputation, and quality control
Genotyping and quality control for the IRASFS samples were performed using the Illumina Infinium HumanExome BeadChip v1.0 as previously described(Hellwege et al., 2014). The exome chip contained 247,870 variants (92% protein coding). In addition, the chip included 64 SNPs associated with T2D from previous GWAS in Europeans, many of which have been implicated in insulin secretion (exome chip design: http://genome.sph.umich.edu/wiki/Exome_Chip_Design). Sample and autosomal SNP call rates were ≥99%, and SNPs with poor cluster separation (<0.35) were excluded.
For the ARIC, CARDIA, JHS, MESA, and WFSM cohorts, samples were genotyped on the Affymetrix Genome-Wide Human SNP Array 6.0. Genotyping and quality control were completed by the National Heart, Lung, and Blood Institute’s (NHLBI’s) Candidate Gene Association Resource (CARe) at the Broad Institute for all cohorts excluding WFSM(Lettre et al., 2011). Genotyping for the WFSM study was performed at the Center for Inherited Disease Research (CIDR). In a pooled analysis of all studies, pre-phasing was performed using SHAPEIT2 and imputation was performed using IMPUTEv2 to obtain missing genotypes and replace genotypes inconsistent with reference haplotypes(Howie, Donnelly, & Marchini, 2009; O’Connell et al., 2014). SNPs with call rate ≥95%, minor allele frequency (MAF) ≥1%, and Hardy-Weinberg Equilibrium P <1×10−4 that passed study-specific quality control were used for imputation(Hester et al., 2012; Lettre et al., 2011). The 1000 Genomes Project cosmopolitan reference panel (Phase I Integrated Release Version 3, March 2012) was used as reference(1000 Genomes Project Consortium et al., 2012). A total of 9,085,034 autosomal SNPs with MAF ≥5% and imputation quality (INFO) ≥0.5 were included in subsequent data analyses.
To account for the effect of population structure on genetic association in these African American samples, principal components analysis (PCA) was computed for all samples collectively using genotyped SNPs that passed quality control standards after exclusion of regions of high linkage disequilibrium (LD) and inversions. To adjust for population substructure, the first PC (PC1) was used as a covariate in all analyses.
Potential relatedness was assessed in the combined analysis of WFSM, ARIC, CARDIA, JHS, and MESA using identity-by-descent (IBD) performed in PLINK(Purcell et al., 2007). A total of 1065 duplicates (pi-hat >0.9) and first-degree relatives were removed according to sample call rate and phenotype to retain only unique unrelated subjects for analysis. Samples reflecting low call rate, gender mismatch, or population outliers were also excluded. After cleaning, a total of 2,452 T2D cases and 3,772 controls remained for analysis.
Analytic Methods
Primary inferences of association with insulin secretion were derived from African American participants (n=492 individuals from 42 families) in the Insulin Resistance Atherosclerosis Family Study (IRASFS), a metabolically well-characterized cohort(Henkin et al., 2003).
Logistic regression with T2D as the outcome was modeled including genetic main and gene × gene interaction effects as well as rs10830963 dosage, age, sex, and principal component covariates for all samples. One additional model was computed to additionally adjust for body mass index (BMI).
Y is the log odds of T2D, S is the dosage for the MTNR1B SNP rs10830963, G is the dosage of the imputed variant, and C is the vector of all remaining covariates. Both the rs10830963 and imputed variant dosages were additively coded with values between 0 and 2. The ProbABEL package from the GenABEL suite of programs (http://www.genabel.org/) was used to calculate the genetic main effect β2, the interaction effect β3, and the corresponding robust standard errors and covariance used to construct the 2×2 covariance matrix for the Kraft test(Kraft, Yen, Stram, Morrison, & Gauderman, 2007). Hypothesis testing included a Wald test statistic following a chi-squared distribution with 1 degree-of-freedom under the null H0:β3 = 0, as well as the Kraft test statistic following a chi-squared distribution with 2 degrees-of-freedom under the null H0:β2 = 0, β3 = 0. Test statistics and corresponding p-values were calculated in the statistical computing environment R(R Core Team, 2015).
Results
Study characteristics
A genome-wide interaction analysis with intronic insulin secretion SNP (rs10830963 in MTNR1B) was performed to detect interactions affecting T2D risk. The combined analysis included African American subjects from the ARIC, CARDIA, JHS, MESA, and WFSM studies. Characteristics of study participants by cohort are presented in Table 1. The percentage of male subjects was modestly increased (+2.6%) among control subjects compared to T2D cases across studies. The percentage of male subjects was highest (47.0%) among MESA T2D cases and lowest (19.1%) among CARDIA T2D cases. On average, T2D cases were 10.2 years older than controls across studies. The average older age for cases across all studies is driven by the average older age for cases in the WFSM study (15.1 years). WFSM includes cases with both T2D and end-stage renal disease (ESRD) with age at T2D diagnosis preceding age at ESRD diagnosis by an average of 16.4 years(McDonough et al., 2011). Healthy controls (i.e. no T2D or ESRD diagnosis) from WFSM were on average 9.5 years older than the age of T2D diagnosis of cases(McDonough et al., 2011). The ascertainment method of selecting cases with a long duration of T2D before ESRD diagnosis and selecting controls 10 years older than the age of T2D diagnosis in cases was employed to capture the largest possible sample to specifically examine the genetic determinants of T2D-ESRD and resulted in a larger age difference between cases and controls than observed in other cohorts included in this study. The average age of controls in this study was 51.1 years (Table 1) and controls <25 years of age were excluded. Thus, misclassification of controls with undiagnosed T2D cases is unlikely. MESA T2D cases had the highest mean age (67.6 years) while CARDIA controls had the lowest mean age (38.2 years), reflecting the differences in ascertainment ages between the cohorts. Average BMI was 1.7 kg/m2 higher in T2D cases compared to controls across studies. Highest mean BMI (34.5 kg/m2) was reported in JHS T2D cases and lowest mean BMI (27.6 kg/m2) was reported in ARIC controls. A detailed description of each study is provided in the Supplementary Methods.
Table 1.
Descriptive characteristics of African American cases with type 2 diabetes and controls.
| ARIC | CARDIA | JHS | MESA | WFSM | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | T2D cases | Controls | T2D cases | Controls | T2D cases | Controls | T2D cases | Controls | T2D cases | Controls | T2D cases | Controls |
| N | 820 | 371 | 94 | 652 | 244 | 1089 | 404 | 773 | 890 | 887 | 2452 | 3772 |
| Male (%) | 36.0 | 32.1 | 19.1 | 38.3 | 36.1 | 40.8 | 47.0 | 42.8 | 38.4 | 43.9 | 38.0 | 40.6 |
| Age (years)b | 61.4±5.9 | 59.4±6.4 | 40.5±3.8 | 38.2±4.4 | 55.6±10.7 | 49.1±10.7 | 67.6±9.3 | 65.3±10.3 | 62.2±10.1 | 47.1±11.7 | 61.3±10.1 | 51.1±13.3 |
| BMI (kg/m2)b | 32.1±6.7 | 27.6±5.8 | 33.8±8.1 | 29.6±6.8 | 34.5±7.3 | 31.1±7.3 | 31.7±6.2 | 28.7±5.8 | 29.6±7.1 | 30.1±7.1 | 31.5±7.0 | 29.8±6.8 |
Data are shown as count, percentage, or mean ± SD.
Age and BMI are shown for the last available visit for the prospective studies including ARIC, CARDIA, and MESA (Exam 4); and the baseline visit for JHS and WFSM.
MTNR1B interactions
The variant rs10830963 (MTNR1B) was selected to test for genome-wide gene-gene interactions based on the prior association with AIRg (P=1.20×10−5) and fasting glucose (P=9.39×10−15) in African Americans (Keaton et al., 2016, Liu et al,. 2016). In this study, the AIRg and fasting glucose-lowering allele of rs10830963, G, had a frequency of 7.06% and was not associated with T2D in single variant association analysis (odds ratio [OR]=1.09; P=0.76). Genome-wide interactions with rs10830963 were tested by logistic regression modeled with T2D as the outcome and including genetic main and gene-gene interaction effects as well as rs10830963 dosage, age, sex, and principal component covariates in all samples. A secondary model adjusting for all prior covariates plus BMI was computed. Both interaction and joint effects were analyzed. Interaction and joint tests, with and without adjustment for BMI, were interrogated for genomic inflation through estimation of lambda values. Lambda values ranged from 1.019 to 1.062. The quantile-quantile plots with corresponding lambda values for each hypothesis test are presented in Supplementary Figure 1 and show adequate control for inflation. The most significant results (PJOINT <5×10−6) of this analysis are presented in Table 2. The effect allele, other allele, and effect allele frequency for variants in Table 2 are presented in Supplementary Table 1.
Table 2.
Top signals (PJOINT<5×10−6) from the 2 degree-of-freedom test with the MTNR1B SNP rs10830963 interaction effect and the marginal SNP effect regressed on T2D risk in pooled analysis of ARIC, CARDIA, JHS, MESA, and WFSM African American cases with type 2 diabetes and controls.
| SNPa (Nearest Gene) | Chr | Positionb | SNP Function | MAFc | βMAIN (SEMAIN)d | βINTXN (SEINTXN)e | PINTXNf | PJOINTf | PINTXN_ADJ_BMIg | PJOINT_ADJ_BMIg |
|---|---|---|---|---|---|---|---|---|---|---|
| rs7903146 (TCF7L2) | 10 | 114758349 | intronic | 0.30 | 0.29 (0.05) | 0.14 (0.14) | 3.09E-01 | 5.47E-11 | 6.23E-01 | 1.44E-10 |
| rs34872471 (TCF7L2) | 10 | 114754071 | intronic | 0.35 | 0.25 (0.05) | 0.00 (0.13) | 9.94E-01 | 3.36E-08 | 6.35E-01 | 3.15E-08 |
| rs17197883 (CMIP) | 16 | 81523013 | intronic | 0.11 | −0.24 (0.08) | 1.12 (0.20) | 1.43E-08 | 4.70E-08 | 2.53E-07 | 8.91E-07 |
| rs35198068 (TCF7L2) | 10 | 114754784 | intronic | 0.36 | 0.25 (0.05) | 0.00 (0.13) | 9.77E-01 | 5.88E-08 | 5.99E-01 | 5.72E-08 |
| rs2836995 (WRB) | 21 | 40748801 | intergenic | 0.49 | 0.00 (0.04) | −0.58 (0.11) | 3.49E-07 | 2.97E-07 | 2.70E-06 | 8.43E-07 |
| rs11819613 (TCF7L2) | 10 | 114746717 | intronic | 0.11 | 0.36 (0.07) | −0.24 (0.20) | 2.30E-01 | 3.28E-07 | 1.00E-01 | 8.46E-07 |
| rs4818031 (WRB) | 21 | 40747523 | intergenic | 0.37 | −0.01 (0.05) | −0.60 (0.12) | 5.23E-07 | 3.31E-07 | 2.83E-06 | 5.24E-07 |
| rs4818032 (WRB) | 21 | 40747583 | intergenic | 0.36 | 0.00 (0.05) | −0.61 (0.12) | 3.03E-07 | 3.52E-07 | 1.34E-06 | 5.97E-07 |
| rs2836994 (WRB) | 21 | 40748006 | intergenic | 0.48 | −0.01 (0.04) | −0.58 (0.12) | 5.61E-07 | 3.72E-07 | 5.64E-06 | 1.09E-06 |
| rs4818033 (WRB) | 21 | 40747624 | intergenic | 0.48 | −0.01 (0.04) | −0.58 (0.12) | 5.61E-07 | 3.92E-07 | 5.60E-06 | 1.15E-06 |
| rs13230 (WRB) | 21 | 40769290 | untranslated-3' | 0.45 | −0.01 (0.04) | 0.60 (0.12) | 3.68E-07 | 5.25E-07 | 8.07E-07 | 1.39E-06 |
| rs56140527 (CMIP) | 16 | 81515361 | intronic | 0.12 | −0.23 (0.07) | 0.94 (0.18) | 2.64E-07 | 5.41E-07 | 1.57E-06 | 4.01E-06 |
| rs7069007 (TCF7L2) | 10 | 114756285 | intronic | 0.11 | 0.34 (0.07) | −0.16 (0.19) | 3.99E-01 | 7.81E-07 | 1.95E-01 | 1.84E-06 |
| rs56155262 (CMIP) | 16 | 81511653 | intronic | 0.12 | −0.25 (0.07) | 0.90 (0.18) | 6.73E-07 | 8.29E-07 | 3.31E-06 | 5.52E-06 |
| rs61329995 (TCF7L2) | 10 | 114748105 | intronic | 0.11 | 0.35 (0.07) | −0.21 (0.20) | 2.78E-01 | 8.61E-07 | 1.25E-01 | 2.18E-06 |
| rs79029870 (---) | 2 | 35998316 | intergenic | 0.11 | 0.34 (0.07) | 0.10 (0.20) | 6.28E-01 | 1.10E-06 | 5.43E-01 | 1.34E-06 |
| rs116123815 (C2orf65) | 2 | 74873329 | intronic | 0.05 | 0.13 (0.10) | −1.49 (0.29) | 2.05E-07 | 1.33E-06 | 2.22E-06 | 1.23E-05 |
| rs116472028 (C2orf65) | 2 | 74872566 | intronic | 0.05 | 0.13 (0.10) | −1.49 (0.29) | 2.05E-07 | 1.33E-06 | 2.22E-06 | 1.23E-05 |
| rs73364034 (WRB) | 21 | 40744651 | intergenic | 0.36 | 0.01 (0.05) | −0.59 (0.12) | 8.20E-07 | 1.37E-06 | 2.83E-06 | 1.89E-06 |
| rs8131718 (WRB) | 21 | 40766181 | intronic | 0.45 | −0.01 (0.04) | 0.59 (0.12) | 6.77E-07 | 1.41E-06 | 1.61E-06 | 3.97E-06 |
| rs73659516 (AK128880) | 8 | 2587387 | intergenic | 0.14 | −0.08 (0.07) | −0.90 (0.20) | 1.16E-05 | 1.48E-06 | 7.05E-06 | 7.63E-07 |
| rs10438632 (CMIP) | 16 | 81510920 | intronic | 0.12 | −0.24 (0.07) | 0.88 (0.18) | 1.20E-06 | 1.55E-06 | 5.38E-06 | 9.35E-06 |
| rs73659517 (AK128880) | 8 | 2587464 | intergenic | 0.16 | −0.07 (0.07) | −0.88 (0.20) | 8.99E-06 | 1.86E-06 | 7.20E-06 | 1.20E-06 |
| rs2924879 (CSMD1) | 8 | 2702216 | intergenic | 0.39 | 0.02 (0.05) | −0.59 (0.12) | 6.73E-07 | 1.87E-06 | 4.43E-06 | 1.33E-05 |
| rs6043626 (MACROD2) | 20 | 15916192 | intronic | 0.45 | 0.18 (0.04) | 0.17 (0.12) | 1.42E-01 | 1.93E-06 | 3.30E-01 | 9.55E-06 |
| rs4319449 (TCF7L2) | 10 | 114769406 | intronic | 0.09 | 0.38 (0.08) | −0.10 (0.22) | 6.31E-01 | 2.10E-06 | 3.48E-01 | 4.13E-06 |
| rs1369455 (YTHDF3) | 8 | 64082963 | intergenic | 0.07 | −0.37 (0.09) | −0.28 (0.22) | 2.07E-01 | 2.17E-06 | 2.40E-01 | 2.25E-05 |
| rs2043525 (YTHDF3) | 8 | 64107847 | intergenic | 0.07 | −0.37 (0.09) | −0.26 (0.22) | 2.40E-01 | 2.25E-06 | 2.72E-01 | 2.37E-05 |
| rs10866267 (LOC728175) | 4 | 185239161 | near-gene-5' | 0.36 | −0.12 (0.05) | −0.42 (0.13) | 9.05E-04 | 2.27E-06 | 3.86E-03 | 6.87E-06 |
| rs9842579 (NAALADL2) | 3 | 174700592 | intronic | 0.19 | 0.29 (0.06) | −0.15 (0.17) | 3.59E-01 | 2.33E-06 | 2.88E-01 | 5.99E-07 |
| rs812665 (LOC728175) | 4 | 185245310 | intergenic | 0.34 | −0.11 (0.05) | −0.41 (0.12) | 7.08E-04 | 2.39E-06 | 1.90E-03 | 6.24E-06 |
| rs2836997 (WRB) | 21 | 40751811 | near-gene-5' | 0.49 | −0.02 (0.04) | −0.55 (0.12) | 5.11E-06 | 2.56E-06 | 2.34E-05 | 4.12E-06 |
| rs1807685 (CRYBB2) | 22 | 25639858 | intergenic | 0.23 | 0.28 (0.06) | −0.40 (0.15) | 9.40E-03 | 2.81E-06 | 5.68E-03 | 1.21E-06 |
| rs115597883 (SLC19A3) | 2 | 228559189 | intronic | 0.11 | 0.35 (0.07) | −0.69 (0.20) | 7.17E-04 | 2.93E-06 | 2.93E-04 | 1.77E-06 |
| rs2291268 (MMP19) | 12 | 56236043 | intergenic | 0.32 | −0.22 (0.05) | −0.15 (0.13) | 2.53E-01 | 2.96E-06 | 3.37E-01 | 1.72E-05 |
| rs1060180 (WRB) | 21 | 40769017 | untranslated-3' | 0.46 | −0.01 (0.04) | 0.57 (0.12) | 1.38E-06 | 3.03E-06 | 3.95E-06 | 9.47E-06 |
| rs1360814 (---) | 13 | 80403917 | intergenic | 0.18 | 0.24 (0.06) | 0.15 (0.15) | 3.13E-01 | 3.19E-06 | 4.28E-01 | 2.85E-06 |
| rs17017402 (---) | 2 | 36003758 | intergenic | 0.11 | 0.31 (0.07) | 0.07 (0.19) | 7.02E-01 | 3.35E-06 | 5.58E-01 | 3.49E-06 |
| rs1681087 (DNAJC14) | 12 | 56180764 | intronic | 0.26 | 0.21 (0.05) | 0.17 (0.13) | 1.85E-01 | 3.47E-06 | 2.48E-01 | 1.55E-05 |
| rs114812021 (SLC19A3) | 2 | 228559188 | intronic | 0.11 | 0.35 (0.07) | −0.68 (0.20) | 8.43E-04 | 3.53E-06 | 3.44E-04 | 2.05E-06 |
| rs9845886 (NAALADL2) | 3 | 174704267 | intronic | 0.26 | 0.24 (0.05) | −0.17 (0.14) | 2.23E-01 | 3.58E-06 | 1.55E-01 | 3.96E-07 |
| rs58538128 (FCGR3A) | 1 | 161520858 | near-gene-5' | 0.14 | 0.35 (0.08) | 0.08 (0.20) | 7.04E-01 | 3.59E-06 | 7.93E-01 | 3.93E-05 |
| rs2837021 (LCA5L) | 21 | 40796623 | intronic | 0.36 | −0.02 (0.05) | −0.56 (0.12) | 5.26E-06 | 3.60E-06 | 3.02E-06 | 2.86E-06 |
| rs4739079 (YTHDF3) | 8 | 64200821 | intergenic | 0.09 | 0.35 (0.08) | 0.08 (0.19) | 6.62E-01 | 3.72E-06 | 8.58E-01 | 1.27E-05 |
| rs59246912 (NAALADL2) | 3 | 174704393 | intronic | 0.26 | 0.24 (0.05) | −0.16 (0.14) | 2.27E-01 | 3.82E-06 | 1.57E-01 | 4.14E-07 |
| rs9862955 (NAALADL2) | 3 | 174703978 | intronic | 0.26 | 0.24 (0.05) | −0.17 (0.14) | 2.26E-01 | 3.88E-06 | 1.57E-01 | 4.22E-07 |
| rs9862818 (NAALADL2) | 3 | 174703904 | intronic | 0.26 | 0.24 (0.05) | −0.17 (0.14) | 2.26E-01 | 3.88E-06 | 1.57E-01 | 4.20E-07 |
| rs16846841 (HECW2) | 2 | 197063250 | intergenic | 0.20 | 0.06 (0.06) | −0.81 (0.16) | 6.80E-07 | 3.89E-06 | 2.22E-06 | 1.09E-05 |
| rs9862801 (NAALADL2) | 3 | 174703876 | intronic | 0.26 | 0.24 (0.05) | −0.17 (0.14) | 2.26E-01 | 3.90E-06 | 1.57E-01 | 4.25E-07 |
| rs7228666 (SLMO1) | 18 | 12427118 | intronic | 0.23 | −0.17 (0.05) | 0.68 (0.14) | 1.77E-06 | 3.93E-06 | 4.21E-06 | 8.64E-06 |
| rs9821147 (NAALADL2) | 3 | 174703737 | intronic | 0.26 | 0.24 (0.05) | −0.17 (0.14) | 2.26E-01 | 3.94E-06 | 1.57E-01 | 4.31E-07 |
| rs13323013 (NAALADL2) | 3 | 174703232 | intronic | 0.26 | 0.24 (0.05) | −0.17 (0.14) | 2.26E-01 | 4.07E-06 | 1.57E-01 | 4.50E-07 |
| rs1335279 (---) | 13 | 80399950 | intergenic | 0.21 | 0.21 (0.05) | 0.23 (0.15) | 1.21E-01 | 4.10E-06 | 1.73E-01 | 1.04E-05 |
| rs56864167 (---) | 2 | 35969614 | intergenic | 0.11 | 0.31 (0.07) | 0.07 (0.19) | 7.12E-01 | 4.10E-06 | 5.87E-01 | 4.52E-06 |
| rs9820271 (NAALADL2) | 3 | 174703026 | intronic | 0.26 | 0.24 (0.05) | −0.17 (0.14) | 2.25E-01 | 4.13E-06 | 1.56E-01 | 4.59E-07 |
| rs10412272 (USP29) | 19 | 57597884 | intergenic | 0.05 | 0.45 (0.10) | 0.14 (0.27) | 5.87E-01 | 4.34E-06 | 7.59E-01 | 6.81E-06 |
| rs2223028 (LCA5L) | 21 | 40810373 | intronic | 0.34 | 0.01 (0.05) | −0.60 (0.13) | 2.26E-06 | 4.44E-06 | 1.01E-06 | 2.16E-06 |
| rs7016339 (YTHDF3) | 8 | 64121730 | intergenic | 0.07 | −0.36 (0.09) | −0.30 (0.23) | 1.91E-01 | 4.48E-06 | 2.25E-01 | 4.53E-05 |
| rs76240879 (NAALADL2) | 3 | 174704464 | intronic | 0.26 | 0.24 (0.05) | −0.17 (0.14) | 2.25E-01 | 4.50E-06 | 1.55E-01 | 4.49E-07 |
| rs9810343 (NAALADL2) | 3 | 174701501 | intronic | 0.26 | 0.24 (0.05) | −0.18 (0.14) | 1.94E-01 | 4.60E-06 | 1.33E-01 | 5.23E-07 |
| rs6735529 (LINC00299) | 2 | 8614384 | intergenic | 0.05 | 0.23 (0.10) | 0.78 (0.24) | 1.41E-03 | 4.67E-06 | 5.54E-03 | 2.97E-05 |
| rs16870669 (RIMS2) | 8 | 104773461 | intronic | 0.11 | 0.22 (0.07) | 0.47 (0.19) | 1.41E-02 | 4.98E-06 | 2.82E-02 | 1.30E-06 |
SNP interacting with rs10830963.
NCBI build 37.
Minor allele frequency.
Marginal genetic effect from interaction models adjusted for age, gender, and PC1.
Interaction effect from interaction models adjusted for age, gender, and PC1.
Interaction and joint p-values from interaction models adjusted for age, gender, and PC1.
Interaction and joint p-values from interaction models adjusted for age, gender, PC1, and BMI.
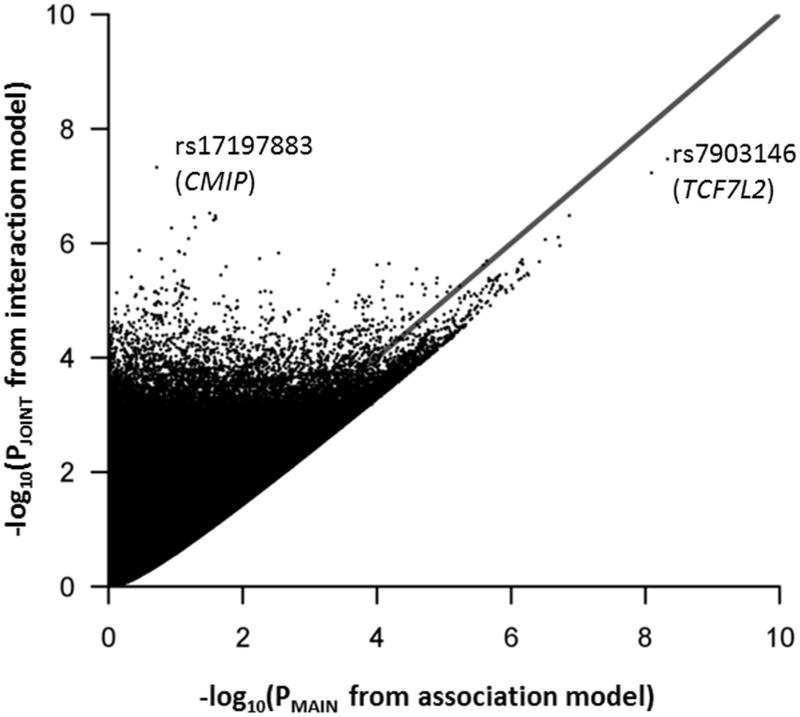
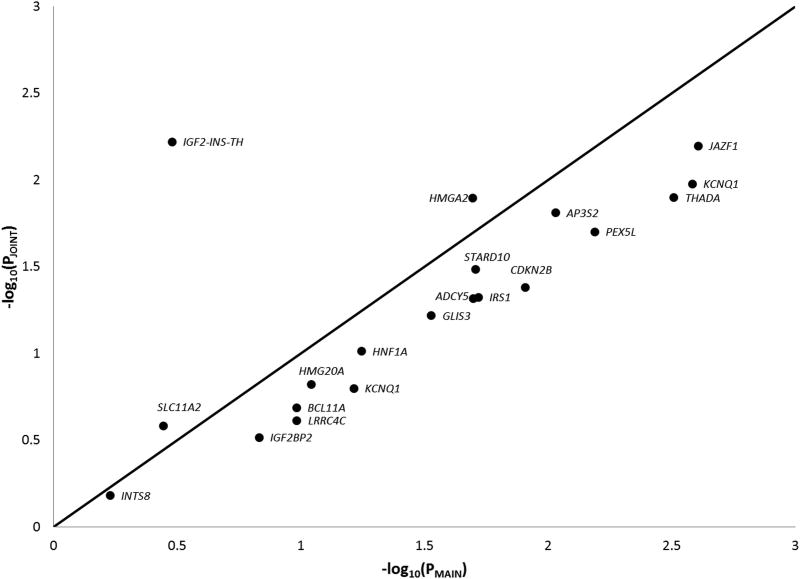
Figure 1 shows a comparison of main effect P-values from a single-SNP association model adjusted for age, sex, and principal component covariates (X-axis) and joint effect P-values from the interaction model (Y-axis). The line of identity in this figure represents equal power for the univariate association model SNP main effect hypothesis test and the interaction model joint hypothesis test. Similarly, Figure 2 shows the same comparison for 20 SNPs indexing established T2D loci which exhibit trans-ethnic transferability (locus-wide P<0.05, SNP P<1×10−3) in the Meta-analysis of T2D in African Americans (MEDIA) consortium(Ng et al., 2013).
Figure 1.
A comparison of P-values from association and interaction models. The X-axis represents negative logarithm transformed P-values from the 1 degree-of freedom hypothesis test of SNP main effects from the univariate association model not including the interaction term, the Y-axis represents negative logarithm transformed P-values from the 2 degree-of freedom joint hypothesis test of SNP main and interaction effects from the interaction model, and the line of identity represents equal power between the 2 tests. Each point represents p-values for a genomic SNP depending upon the model.
Figure 2.
A comparison of P-values from association and interaction models for established T2D loci exhibiting transferability in African Americans. In this figure, the X-axis represents negative logarithm transformed P-values from the 1 degree-of freedom hypothesis test of SNP main effects from the univariate association model not including the interaction term, the Y-axis represents negative logarithm transformed P-values from the 2 degree-of freedom joint hypothesis test of SNP main and interaction effects from the interaction model, and the line of identity represents equal power between the 2 tests. Each point represents p-values for a trans-ethnic transferrable SNP depending upon the model.
The most significant joint association was with TCF7L2 intronic SNP rs7903146 (PJOINT=5.47×10−11), with another TCF7L2 intronic SNP, rs34872471, attaining genome-wide significance (PJOINT=3.36×10−8). Neither rs7903146 (PINTXN=0.31) nor rs34872471 (PINTXN=0.99) exhibited a significant association with the interaction effect alone. A novel association was observed with the CMIP intronic SNP rs17197883 (PJOINT=4.70×10−8, PINTXN=1.43×10−8). In this study, the rs17197883 T2D effect allele, C, had a frequency of 10.90% and was not associated with T2D in single variant association analysis of pooled samples (OR=1.07; P=0.19). However, when samples were stratified by rs10830963 (MTNR1B) AIRg-lowering allele carriers versus non-carriers, we observed a suggestively significant opposite effect in both groups. rs17197883 exhibited a T2D risk effect in carriers (OR=2.29, P=7.64×10−6) and a protective effect in non-carriers (OR=0.78, P=8.00×10−4), suggesting an antagonistic pattern of interaction (Table 3). In general, observed associations were robust to adjustment for BMI (Table 2).
Table 3.
Association of CMIP variant rs17197882 with T2D stratified by MTNR1B genotype
| MTNR1B genotype | β (SE)a | OR (95%CI)b | P |
|---|---|---|---|
| CC | −0.25 (0.07) | 0.78 (0.67–0.90) | 8.00E-04 |
| CG or GG | 0.83 (0.18) | 2.29 (1.59–3.28) | 7.64E-06 |
Effect and standard error from a simple T2D association model adjusted for age, sex, and PC1.
Odds ratio and 95% confidence interval.
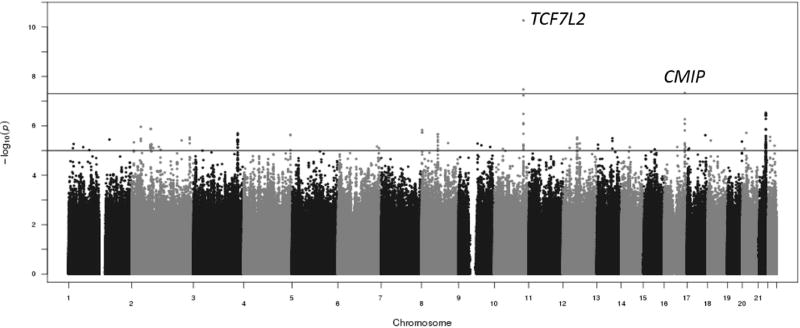
Additional SNPs in loci (e.g., WRB) exhibited a nominal association (PJOINT<5×10−6) in joint tests of marginal and interaction effects. The majority of these loci were well-supported with multiple SNPs showing evidence of nominally significant association as shown in Figure 3. The association of these SNPs is driven, for the most part, by the interaction effect. The most significantly associated SNP in prior interaction analysis with the MTNR1B SNP rs10830963 in HapMap imputed data, rs7277627 at the LCA5L locus (PINTXN=1.65×10−6), replicated in this study (PJOINT=5.46×10−6)(Keaton et al., 2016). However, the use of a denser imputation panel (i.e., 1000 Genomes Project) provided the identification the SNP at the LCA5L locus, rs2223028, that exhibited stronger evidence of association (PJOINT=4.44×10−6).
Figure 3.
Manhattan plot of results from the 2 degree-of-freedom test. Top line denotes genome-wide significance at P < 5×10−8. Second line denotes suggestive significance at P < 5×10−6
Discussion
A genome-wide analysis of interaction and joint effects with MTNR1B intronic insulin secretion SNP rs10830963 resulting in risk of T2D revealed significant associations at two loci, TCF7L2 and CMIP. The strongest joint association was with TCF7L2 intronic SNP rs7903146. Association with rs7903146 has been replicated in numerous African American studies suggesting that it is a key genetic determinant in development of T2D(Lewis et al., 2008; Long et al., 2012; Moore et al., 2008; Palmer et al., 2012; Saxena et al., 2012; Waters et al., 2010). Palmer et al. reported that rs7903146 is likely the causal variant contributing T2D susceptibility at the TCF7L2 locus using a resequencing approach(Palmer et al., 2011). However, rs7903146 was not associated with fasting glucose in African Americans (Liu et al., 2016). The lack of interaction effect in the current study suggests that the joint signal is driven by marginal (i.e., additive) genetic effects.
The strongest novel association was with CMIP intronic SNP rs17197883 (PJOINT=4.70×10−8, PINTXN=1.43×10−8). Variants at this locus have previously been associated with HDL cholesterol (rs56823429, P=2×10−8), adiponectin levels (rs12051272, P=6×10−48), and WHR adjusted for BMI (rs2925979, P=7×10−13) in populations of European (EUR) descent and adiponectin levels (rs2925979, P=2×10−10), fasting glucose (rs16955379, P=0.03), and T2D (rs16955379, P=3×10−7) in populations of East Asian (EAS) descent(Cho et al., 2012; Dastani et al., 2012; Global Lipids Genetics Consortium et al., 2013; Sakai et al., 2013; Shungin et al., 2015; Surakka et al., 2015; Wu et al., 2014). All of the previously described variants at the CMIP locus are in weak LD with rs17197883 (r2<0.2). There is no surrogate SNPs in high LD (r2 > 0.6 in 1000 Genomes AFR population) with rs17197883, In addition, rs17197883 did not reside in a regulatory region and did not regulate nearby gene expression by HaploReg analysis. Interestingly, the frequency of the rs10830963 AIRg-lowering allele (G) is much higher in these populations compared to African Americans (AfA) (EAS=42%, EUR=29%, AfA=7%). Thus, associations of SNPs at the CMIP locus with T2D and biomarkers related to insulin resistance in individuals of EUR and EAS descent may reflect an underlying interaction with rs10830963 that is partially unmasked in these populations. In IRASFS, neither previously reported SNPs in the CMIP region nor the interacting SNP rs17197883 showed evidence of association with measures of insulin sensitivity, adiponectin levels, WHR adjusted for BMI, nor fasting glucose(Gao et al., 2015; Hellwege et al., 2014, 2017). However, 2 previously reported SNPs at the CMIP locus, rs2925979 (P=0.02) and rs56823429 (P=0.009), and the interacting SNP rs17197883 (P=0.002) were nominally associated with HDL cholesterol in IRASFS Hispanics(Hellwege et al., 2017). The interacting CMIP variant, rs17197883, was not associated with T2D in the current study in a model adjusted for age, sex, and PC1 (P=0.19).
This study was conducted to explore the impact of three methodological additions to an analysis model focused solely on the interaction term. First, after observing homogeneity in genetic effects across cohorts in a prior study(Keaton et al., 2016), we pooled (as opposed to meta-analyzed) samples from five African American T2D studies. Sung et al. suggested that results from pooled analysis and meta-analysis for main, interaction, and joint effects are largely consistent(Sung et al., 2014). Notably, we detected an interaction P-value reaching genome-wide significance (rs17197883, PINTXN=1.43×10−8) in a pooled analysis, which is an order of magnitude smaller compared to the most significant P-value (rs16924460, PINTXN=1.70×10−7) in our previous meta-analyzed study(Keaton et al., 2016). However, it is difficult to assess the impact of pooling samples due to additional methodological differences. For example, additional quality control identified some samples that were duplicated or had other quality control issues.
Second, we used genotype data imputed to a 1000 Genomes Project phase 1 reference panel(The 1000 Genomes Project Consortium, 2015). Compared to the HapMap reference panel previously used for imputation, the 1000 Genomes Project panel provides greater coverage of common variation and facilitates overall improvement of imputation quality(Wood et al., 2013). This expanded genotype data allowed for analysis of 9,085,034 autosomal SNPs with MAF >5% in our study cohorts, compared to 2,907,086 autosomal SNPs with a MAF >1% in the prior analysis(Keaton et al., 2016). The MAF threshold was increased in the current study to overcome the inherent power loss and potential for false positives for interaction analyses incorporating a low frequency exposure(Zhang, Lewinger, Conti, Morrison, & Gauderman, 2016).
Finally, in addition to hypothesis testing of the interaction term in our models, this analysis incorporates the Kraft 2 degree-of-freedom test to jointly analyze marginal and interaction effects(Kraft et al., 2007). This joint test allows for the detection of variants with both additive and non-additive effects contributing to T2D risk. The skew toward more significant joint effect P-values in Figure 1 suggests that genome-wide interaction analysis with a well-defined insulin secretion variant (e.g., MTNR1B SNP rs10830963) incorporating a 2 degrees-of-freedom hypothesis test is a powerful approach for detection of novel T2D risk loci. In this figure, 3,435,184 out of 9,085,034 (37.8%) SNPs had a more significant joint effect P-value compared to the main effect P-value from an association model excluding the interaction term. The SNPs in TCF7L2 fall below the line of identity on the main effect axis, while the CMIP SNP, plus many others fall above the line of identity, thus comparing power between the two tests. These results suggest that established T2D loci, originally identified by strong SNP main effects in a typical univariate association analysis and largely involved in insulin secretion biology themselves, are not likely candidates for interaction with insulin secretion loci. Alternatively, these established T2D loci, primarily discovered in European populations, do not exhibit strong SNP main effects in African American populations and may have a limited interaction effect to impact association with T2D.
To analyze performance of the joint test for known T2D loci, we compared main and joint effect P-values for the most significant African American SNP at 20 established T2D loci that exhibited trans-ethnic transferability in MEDIA(Ng et al., 2013). Figure 2 shows that SNPs from 3 loci, IGF2-INS-TH, SLC11A2, and HMGA2, had a more significant joint effect P-value compared to the main effect P-value. This result combined with the observation from Figure 1 suggests that the joint test is a powerful approach for detection of novel T2D loci, but a typical association model may be more powerful for established loci.
This study has limitations. To validate these findings, genome-wide interactions with rs10830963 associated with T2D must be replicated in additional studies, including studies of other ethnicities. Additionally, we did not account for gene-environment interactions which may account for T2D risk. Interactions with or stratified analysis of sex, age, BMI, and age at diagnosis may be appropriate in follow-up studies. It is also important to note that statistical interaction does not imply biological interaction, as the statistical interaction may be mediated through multiple biological factors. However, statistical interaction may revealed novel loci which may not have strong marginal effect.
In summary, the present findings demonstrate that analysis of physiologically defined genome-wide interactions with variants strongly associated with insulin secretion is a potentially powerful approach for discovery of novel T2D loci and for expanding the knowledgebase of disease etiology. A similar approach examining interactions with variants associated with key biomarkers may be of wider relevance in other complex human diseases. Results highlight the need for further study of genetic variation underlying T2D risk in African Americans as a means to improve our overall understanding of this disease.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the contributions of the involved research institutions, study investigators, field staff, and study participants of ARIC, CARDIA, JHS, MESA, and WFSM.
Funding
Genotyping services for the WFSM study were provided by CIDR. CIDR is fully funded through a federal contract from the National Institutes of Health (NIH) to The Johns Hopkins University (Contract HHSC268200782096C). The work at Wake Forest was supported by NIH grants K99-DK-081350 (N.D.P.), R01-DK-066358 (D.W.B., M.C.Y.N.), R01-DK-053591 (D.W.B.), R01-DK-087914 (M.C.Y.N), U01-DK-105556 (M.C.Y.N.), R01-HL-56266 (B.I.F.), and R01-DK-070941 (B.I.F.), and in part by the General Clinical Research Center of the WFSM Grant M01-RR-07122. This work was also supported by the NHLBI.
The following four parent studies have contributed parent study data, ancillary study data, and DNA samples through the Massachusetts Institute of Technology-Broad Institute (N01-HC-65226) to create this genotype/phenotype database for wide dissemination to the biomedical research community:
ARIC, CARDIA, JHS, and MESA.
The Atherosclerosis Risk in Communities (ARIC) Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The authors thank the staff and participants of the ARIC study for their important contributions.
The Coronary Artery Risk Development in Young Adults (CARDIA) Study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging. Genotyping was funded as part of the NHLBI Candidate-gene Association Resource (N01-HC-65226) and the NHGRI Gene Environment Association Studies (GENEVA) (U01-HG004729, U01-HG04424, and U01-HG004446). This manuscript has been reviewed and approved by CARDIA for scientific content.
The Jackson Heart Study (JHS) is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. We thank the Jackson Heart Study participants and staff for their contributions to this work.
Multi-Ethnic Study of Atherosclerosis (MESA), and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-001079, UL1-TR-000040, and DK063491. The MESA CARe data used for the analyses described in this manuscript were obtained through Genetics (CMP00068). Funding for CARe genotyping was provided by NHLBI Contract N01-HC-65226.
Footnotes
Duality of Interests
There are no conflicts of interest relevant to this article.
Author Contributions
JMK wrote the manuscript and researched and analyzed the data. JNH, CG, MG, and NDP researched data, contributed to data analysis, and reviewed and edited the manuscript. JSP, MF, JGW, AC, LJRT, JIR, SSR, LEW, and BIF reviewed and edited the manuscript. BIF recruited and phenotyped WFSM participants. MCYN assisted with data analysis, designed the study, and reviewed and edited the manuscript. DWB contributed to manuscript writing and study design, contributed to the discussion, and reviewed and edited the manuscript. DWB is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1000 Genomes Project Consortium. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2014 Statistics Report | Data & Statistics | Diabetes | CDC. [Retrieved June 16, 2016]; (n.d.). from http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html.
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Cho YS, Chen C-H, Hu C, Long J, Ong RTH, Sim X, Seielstad M. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nature Genetics. 2012;44(1):67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastani Z, Hivert M-F, Timpson N, Perry JRB, Yuan X, Scott RA, Kathiresan S. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genetics. 2012;8(3):e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Gao C, Wang N, Guo X, Ziegler JT, Taylor KD, Xiang AH, Palmer ND. A Comprehensive Analysis of Common and Rare Variants to Identify Adiposity Loci in Hispanic Americans: The IRAS Family Study (IRASFS) PloS One. 2015;10(11):e0134649. doi: 10.1371/journal.pone.0134649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Lipids Genetics Consortium. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Abecasis GR. Discovery and refinement of loci associated with lipid levels. Nature Genetics. 2013;45(11):1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwege JN, Palmer ND, Dimitrov L, Keaton JM, Tabb KL, Sajuthi S, Bowden DW. Genome-wide linkage and association analysis of cardiometabolic phenotypes in Hispanic Americans. Journal of Human Genetics. 2017;62(2):175–184. doi: 10.1038/jhg.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwege JN, Palmer ND, Raffield LM, Ng MCY, Hawkins GA, Long J, Bowden DW. Genome-wide family-based linkage analysis of exome chip variants and cardiometabolic risk. Genetic Epidemiology. 2014;38(4):345–352. doi: 10.1002/gepi.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin L, Bergman RN, Bowden DW, Ellsworth DL, Haffner SM, Langefeld CD, Rich SS. Genetic epidemiology of insulin resistance and visceral adiposity. The IRAS Family Study design and methods. Annals of Epidemiology. 2003;13(4):211–217. doi: 10.1016/s1047-2797(02)00412-x. [DOI] [PubMed] [Google Scholar]
- Hester JM, Wing MR, Li J, Palmer ND, Xu J, Hicks PJ, Ng MCY. Implication of European-derived adiposity loci in African Americans. International Journal of Obesity (2005) 2012;36(3):465–473. doi: 10.1038/ijo.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genetics. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Palmer JP. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- Keaton JM, Hellwege JN, Ng MCY, Palmer ND, Pankow JS, Fornage M, Bowden DW. Genome-Wide Interaction with Insulin Secretion Loci Reveals Novel Loci for Type 2 Diabetes in African Americans. PloS One. 2016;11(7):e0159977. doi: 10.1371/journal.pone.0159977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft P, Yen Y-C, Stram DO, Morrison J, Gauderman WJ. Exploiting gene-environment interaction to detect genetic associations. Human Heredity. 2007;63(2):111–119. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- Lettre G, Palmer CD, Young T, Ejebe KG, Allayee H, Benjamin EJ, Boerwinkle E. Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: the NHLBI CARe Project. PLoS Genetics. 2011;7(2):e1001300. doi: 10.1371/journal.pgen.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JP, Palmer ND, Hicks PJ, Sale MM, Langefeld CD, Freedman BI, Bowden DW. Association Analysis in African Americans of European-Derived Type 2 Diabetes Single Nucleotide Polymorphisms From Whole-Genome Association Studies. Diabetes. 2008;57(8):2220–2225. doi: 10.2337/db07-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. The New England Journal of Medicine. 1993;329(27):1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- Liu CT, Raghavan S, Maruthur N, Kabagambe EK, Hong J, Ng MC, Hivert MF, Lu Y, An P, Bentley AR, Drolet AM, Gaulton KJ, Guo X, Armstrong LL, Irvin MR, Li M, Lipovich L, Rybin DV, Taylor KD, Agyemang C, Palmer ND, Cade BE, Chen WM, Dauriz M, Delaney JA, Edwards TL, Evans DS, Evans MK, Lange LA, Leong A, Liu J, Liu Y, Nayak U, Patel SR, Porneala BC, Rasmussen-Torvik LJ, Snijder MB, Stallings SC, Tanaka T, Yanek LR, Zhao W, Becker DM, Bielak LF, Biggs ML, Bottinger EP, Bowden DW, Chen G, Correa A, Couper DJ, Crawford DC, Cushman M, Eicher JD, Fornage M, Franceschini N, Fu YP, Goodarzi MO, Gottesman O, Hara K, Harris TB, Jensen RA, Johnson AD, Jhun MA, Karter AJ, Keller MF, Kho AN, Kizer JR, Krauss RM, Langefeld CD, Li X, Liang J, Liu S, Lowe WL, Jr, Mosley TH, North KE, Pacheco JA, Peyser PA, Patrick AL, Rice KM, Selvin E, Sims M, Smith JA, Tajuddin SM, Vaidya D, Wren MP, Yao J, Zhu X, Ziegler JT, Zmuda JM, Zonderman AB, Zwinderman AH, AAAG Consortium; CARe Consortium; COGENT-BP Consortium; eMERGE Consortium; MEDIA Consortium. Adeyemo A, Boerwinkle E, Ferrucci L, Hayes MG, Kardia SL, Miljkovic I, Pankow JS, Rotimi CN, Sale MM, Wagenknecht LE, Arnett DK, Chen YD, Nalls MA, MAGIC Consortium, Province MA. Kao WH, Siscovick DS, Psaty BM, Wilson JG, Loos RJ, Dupuis J, Rich SS, Florez JC, Rotter JI, Morris AP, Meigs JB. Trans-ethnic Meta-analysis and Functional Annotation Illuminates the Genetic Architecture of Fasting Glucose and Insulin. Am J Hum Genet. 2016;99(1):56–75. doi: 10.1016/j.ajhg.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Edwards T, Signorello LB, Cai Q, Zheng W, Shu X-O, Blot WJ. Evaluation of Genome-wide Association Study-identified Type 2 Diabetes Loci in African Americans. American Journal of Epidemiology. 2012;176(11):995–1001. doi: 10.1093/aje/kws176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AK, LaValley M, Liu CT, Rice K, An P, Liu Y, Miljkovic I, Rasmussen-Torvik L, Harris TB, Province MA, Borecki IB, Florez JC, Meigs JB, Cupples LA, Dupuis J. Meta-analysis of gene-environment interaction: joint estimation of SNP and SNP × environment regression coefficients. Genet Epidemiol. 2011;35(1):11–8. doi: 10.1002/gepi.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough CW, Palmer ND, Hicks PJ, Roh BH, An SS, Cooke JN, Bowden DW. A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney International. 2011;79(5):563–572. doi: 10.1038/ki.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AF, Jablonski KA, McAteer JB, Saxena R, Pollin TI, Franks PW, Florez JC. Extension of Type 2 Diabetes Genome-Wide Association Scan Results in the Diabetes Prevention Program. Diabetes. 2008;57(9):2503–2510. doi: 10.2337/db08-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MCY, Saxena R, Li J, Palmer ND, Dimitrov L, Xu J, Bowden DW. Transferability and fine mapping of type 2 diabetes loci in African Americans: the Candidate Gene Association Resource Plus Study. Diabetes. 2013;62(3):965–976. doi: 10.2337/db12-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell J, Gurdasani D, Delaneau O, Pirastu N, Ulivi S, Cocca M, Marchini J. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genetics. 2014;10(4):e1004234. doi: 10.1371/journal.pgen.1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ND, Hester JM, An SS, Adeyemo A, Rotimi C, Langefeld CD, Bowden DW. Resequencing and analysis of variation in the TCF7L2 gene in African Americans suggests that SNP rs7903146 is the causal diabetes susceptibility variant. Diabetes. 2011;60(2):662–668. doi: 10.2337/db10-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ND, McDonough CW, Hicks PJ, Roh BH, Wing MR, An SS, Sladek R. A genome-wide association search for type 2 diabetes genes in African Americans. PloS One. 2012;7(1):e29202. doi: 10.1371/journal.pone.0029202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad RB, Groop L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes. 2015;6(1):87–123. doi: 10.3390/genes6010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Retrieved from https://www.R-project.org/ [Google Scholar]
- Sakai K, Imamura M, Tanaka Y, Iwata M, Hirose H, Kaku K, Maeda S. Replication study for the association of 9 East Asian GWAS-derived loci with susceptibility to type 2 diabetes in a Japanese population. PloS One. 2013;8(9):e76317. doi: 10.1371/journal.pone.0076317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Elbers CC, Guo Y, Peter I, Gaunt TR, Mega JL, Keating BJ. Large-Scale Gene-Centric Meta-Analysis across 39 Studies Identifies Type 2 Diabetes Loci. American Journal of Human Genetics. 2012;90(3):410–425. doi: 10.1016/j.ajhg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Mohlke KL. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YJ, Schwander K, Arnett DK, Kardia SLR, Rankinen T, Bouchard C, Rao DC. An empirical comparison of meta-analysis and mega-analysis of individual participant data for identifying gene-environment interactions. Genetic Epidemiology. 2014;38(4):369–378. doi: 10.1002/gepi.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surakka I, Horikoshi M, Mägi R, Sarin A-P, Mahajan A, Lagou V ENGAGE Consortium. The impact of low-frequency and rare variants on lipid levels. Nature Genetics. 2015;47(6):589–597. doi: 10.1038/ng.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HA, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethnicity & Disease. 2005;15(4 Suppl 6) S6-4–17. [PubMed] [Google Scholar]
- The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American Journal of Epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- Waters KM, Stram DO, Hassanein MT, Le Marchand L, Wilkens LR, Maskarinec G, Haiman CA. Consistent Association of Type 2 Diabetes Risk Variants Found in Europeans in Diverse Racial and Ethnic Groups. PLoS Genetics. 2010;6(8) doi: 10.1371/journal.pgen.1001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AR, Perry JRB, Tanaka T, Hernandez DG, Zheng H-F, Melzer D, Frayling TM. Imputation of variants from the 1000 Genomes Project modestly improves known associations and can identify low-frequency variant-phenotype associations undetected by HapMap based imputation. PloS One. 2013;8(5):e64343. doi: 10.1371/journal.pone.0064343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Gao H, Li H, Tabara Y, Nakatochi M, Chiu Y-F, Tai ES. A meta-analysis of genome-wide association studies for adiponectin levels in East Asians identifies a novel locus near WDR11-FGFR2. Human Molecular Genetics. 2014;23(4):1108–1119. doi: 10.1093/hmg/ddt488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Lewinger JP, Conti D, Morrison JL, Gauderman WJ. Detecting Gene–Environment Interactions for a Quantitative Trait in a Genome-Wide Association Study. Genetic Epidemiology. 2016;40(5):394–403. doi: 10.1002/gepi.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.