Abstract
The aim of the present study was to establish T1 variation (T1v) thresholds for duplicated measurements of regional T1 values in left ventricle (LV) using magnetic resonance imaging (MRI). Eighteen healthy volunteers were recruited to undergo 2 consecutive cardiac MRI scans using modified Look-Locker Inversion recovery (MOLLI) with two spatial resolutions on different days to repeat T1 measurements on LV. The absolute differences (d) and standard deviations (SDs) of regional T1 values were acquired with the two scans and two readers. T1v threshold (mean difference + 2SD), intra-class correlation coefficient (ICC) and coefficient of variation (CoV) were calculated. T1 mapping using the MOLLI sequence (with multiple spatial resolutions) was successfully performed in all 18 volunteers twice. On a per-slice basis, ICCs for intra-observer, inter-observer, inter-resolution, inter-study T1v were 0.988, 0.899, 0.763 and 0.6. CoVs were 0.72%, 2.39%, 3.90% and 4.28%. T1v thresholds were 22 ms, 66 ms, 118 ms and 120 ms. On a per-segment basis, ICCs for T1v were 0.974, 0.859, 0.711 and 0.594. CoVs were 1.09%, 3.36%, 4.69% and 5.01%. T1v thresholds were 33 ms, 94 ms, 140 ms and 144 ms. Those thresholds may be useful for discriminating disease-initiated T1v from random errors of T1 measurements.
Keywords: Native T1 variation, MRI, Myocardium, MOLLI
Introduction
In magnetic resonance imaging (MRI), the longitudinal relaxation time (T1) is a time constant that represents the recovery of components of the nuclear spin magnetization that are parallel to the external magnetic field (B0). As a tissue-specific index under a given magnetic field, the T1 value may vary in the context of structural or functional variations in the myocardium [1–3]. For example, native T1 values have been found to be quantitatively related with the severity of structural abnormalities of the myocardial tissue, such as myocardial fibrosis [1]. Therefore, T1 variation (T1v) is expected to quantitatively reflect the progression of cardiovascular diseases.
Currently, a number of alternate MRI techniques have been developed to measure T1 values (pixel by pixel) in the beating heart, such as Modified Look-Locker Inversion recovery (MOLLI), Shortened Modified Look-Locker Inversion recovery (ShMOLLI), saturation pulse prepared heart rate independent inversion recovery (SAPPHIRE) and Saturation recovery Single Shot Acquisition (SASHA) [4]. To the best of our knowledge, systematic variations among those MRI methods for measuring regional myocardial T1 values have not been evaluated [5,6]. As a result, there is no clinical consensus on a unique threshold of T1v (increase or decrease) for discriminating “normal” and “abnormal” myocardial tissue. Such a knowledge gap may potentially affect clinical decisions when it is difficult to judge whether a local change in a T1 value is caused by errors of measurement or by improvement or deterioration of existing CVDs. To address this unmet clinical need, we tested intra-observer, inter-observer, inter-resolution and inter-study variability of regional T1 values, which were acquired using the MOLLI technique in healthy volunteers, on a per-slice and per-segment basis. The aim of the present study was to establish normal T1v thresholds for repeated measurements of regional T1 values using MRI. We expect that these reference values could be helpful for defining ranges of T1v that are associated with pathological changes in clinical studies.
Materials and Methods
Subject description
This HIPAA (Health Insurance Portability and Accountability Act of 1996) compliant study was approved by the institutional review board (IRB). Eighteen healthy volunteers (38.5 ± 15.4 years [ mean ± SD]; age range: 23 70 years; 12 male and 6 female) were recruited in this study. Each participant was invited to undergo 2 consecutive cardiac MRI scans at different days. All subjects were free of documented cardiovascular diseases or related cardiovascular risk factors, such as diabetes and hypertension (based on self-reporting and their medical histories). Exclusion criteria: 1) Subjects with significant cardiovascular risk factors, such as diabetes, hypertension (HTN) and renal dysfunction (defined as estimated Glomerular Filtration Rate [eGFR] < 60 mL/min/1.73 m2); and 2) Subjects with contradictions of clinical MRI scans. Written informed consents were provided by all participants. Table 1 presents general information of the study subjects.
Table 1.
Description of our study cohort
| Volunteers (N = 18) | ||
|---|---|---|
| Male (%) | 13 (72) | |
| Age (years old) | 48.4 ± 11.9 | |
| Weight (kg) | 82.5 ± 12.8 | |
| Height (cm) | 172.1 ± 9.2 | |
| Heart rate (beats/minute) | scan 1 | 68.3 ± 11.2 |
| scan 2 | 68.5 ± 12.2 | |
| Systolic blood pressure (mmHg) | scan 1 | 119.8 ± 29.1 |
| scan 2 | 121.5 ± 30.5 | |
| Diastolic blood pressure (mmHg) | scan 1 | 79.6 ± 20.1 |
| scan 2 | 80.1 ± 19.9 | |
MRI scans and parameters
All MRI scans were performed by 2 certified clinical technicians on a 1.5 T scanner (MAGNETOM Aera, Siemens AG, Erlangen, Germany) with an Eighteen-channel cardiac phased-array coil.
First, a 3-plane rapid localization sequence was run to provide anatomic orientation for the whole scan. A black-blood half-Fourier acquisition single-shot turbo spin-echo sequence was then performed to identify the 2-chamber, 4-chamber and short-axis views of the heart. T1 values data were acquired at “base”, “mid-ventricular” and “apex” of the LV with short-axis orientation using an ECG gated MOLLI sequence within a single breath hold. The acquisition strategy for T1 maps consisted of 2 inversion preparations, separated by 4 recovery heartbeats to ensure complete T1 recovery. Single shot balanced steady-state free precession (bSSFP) images were acquired in late diastole during 5 heartbeats after the first inversion and during 3 heartbeats after the second inversion. MOLLI sequence was run twice at the same LV planes during a single scan. For the first acquisition, imaging parameters included: field of view (FOV) = 380 x 380 mm2, base resolution = 256; slice thickness = 8 mm; repetition time (TR)/echo time (TE) = 277.6/1.1 ms; echo spacing = 2.7 ms; flip angle = 35 degrees; bandwidth = 1085 Hz/Pixel; TI start = 105 ms; TI increment = 80 ms; iPAT factor (GRAPPA) = 2. The second acquisition was run immediately after the first one and kept almost all parameters except an increased resolution (base resolution = 384). For each participant, a baseline scan (#1) and a repeat rescan (#2) was performed at different days, separated by a time interval (around 2 weeks) using identical imaging protocols.
Image processing workflow for T1 measurements
All images were transferred to an imaging workstation (Dell, Studio XPS 435T) for analysis by using a dedicated software package (Circle, CVI 42, Calgary, Canada). T1 maps were generated online using a motion correction algorithm, which is based on solving a variational energy minimization problem to assess synthetic images that present contrast changes similar to the acquired images [7]. T1 maps with poor image quality (defined as those having severe signal loss or artifacts) were excluded from quantitative analysis. Subsequently, the T1 estimate was computed on a per-pixel basis by performing non-linear curve fitting using the three-parameter signal model. All T1 maps were reviewed by reader #1 (KL, with 9 years of experience in cardiovascular imaging) to determine whether they were eligible for quantitative analysis. For all images, both epicardialand endocardialcontours of the LV were manually drawn (regularly excluding papillary muscles) by reader #1. T1 values were measured and mapped on a standard 16-segment LV model. For all volunteers, reviewer #1 re-analyzed images with a 1-month interval. A second reviewer (KS, with 10 years of experience of clinical cardiology) used the same method to independently measure the regional T1 values of those images to test the inter-observer and inter-study variability of the measurements. See figure 1 for an example of data processing.
Figure 1.
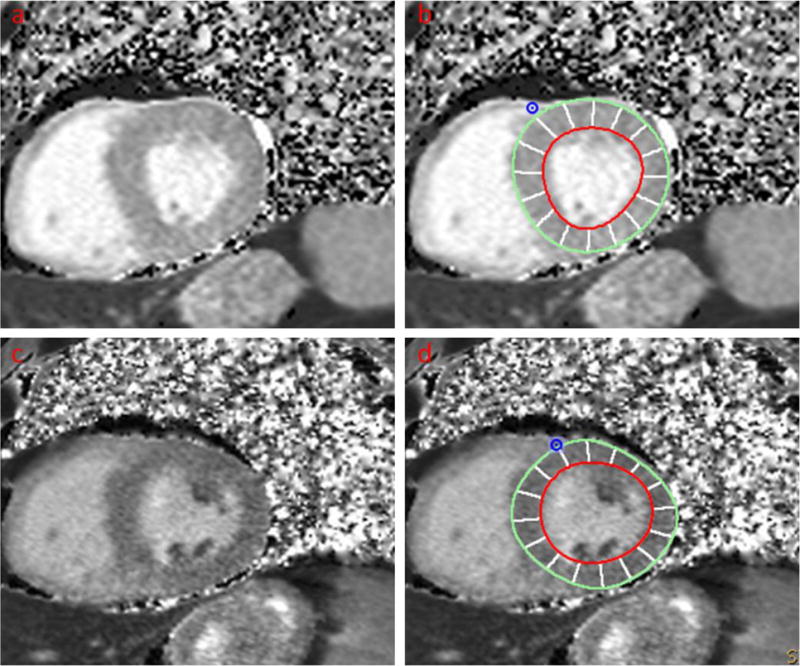
A healthy volunteer. Two consecutive scans were performed with interval of 14 days.
a Short-axis view of LV without contours (scan #1)
b Short-axis view of LV with contours (scan #1)
c Short-axis view of LV without contours (scan #2)
d Short-axis view of LV with contours (scan #2)
Statistical analysis
All quantitative indices are expressed as the means ± standard deviation (SD). Bland-Altman plots will be used to demonstrate T1v between two sets of measurements. On a per-slice and per-segment basis, the intra-class correlation coefficient (ICC) and coefficient of variation (CoV) were used to describe the concurrence of measurements acquired from the two analyses (by reader #1 with 1-month interval), two observers (reader #1 vs. reader #2), two resolutions (low vs. high) and two sequential scans (scan #1 vs. scan #2). An ICC value > 0.75 or a CoV < 20% between duplicated measurements was considered as good reproducibility.
The differences (d) (set as positive) between the measurements (defined as |T1scan1−T1scan2|) were calculated for each myocardial slice and segment. The average differences and SD were calculated. The T1v threshold was defined as .
Statistical analysis was performed by using SPSS software (Version 13.0, SPSS, Inc, Chicago, IL) and MATLAB (Version 2013a, Mathworks, Inc., Natick, MA). A p-value <0.05 was considered as statistically significant.
Results
T1 mapping using the MOLLI sequence (with multiple spatial resolutions) was successfully performed in all 18 volunteers twice. The interval between the two scans was 14 ± 5 days. For all participants, there was no significant difference of heart rates and blood pressure between two scans (Paired t test, p > 0.05) (table 1). All T1 maps were eligible for quantitative analysis and resulted in 54 groups of LV slices and 288 groups of myocardial segments for the comparison of regional T1 values. Figure 2 show side-by-side comparisons of the segmental distribution of T1 values in the American Heart Association (AHA) 16-segment LV model between both observers, both resolutions and both scans, indicating small differences in mean T1 between repeated measurements.
Figure 2.
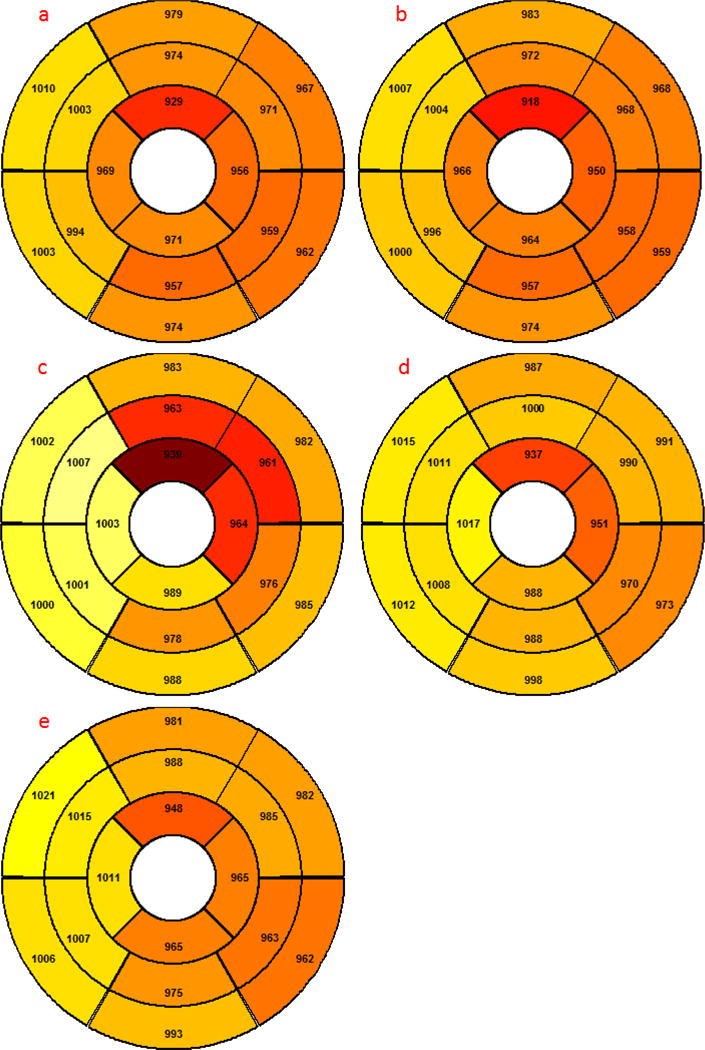
Segmental distributions of T1 values (averaged over 18 subjects) on the LV.
a Scan #1 (low resolution) analyzed with reader #1
b Scan #1 (low resolution) analyzed with reader #1 (repeated)
c Scan #1 (high resolution) analyzed with reader #1 (high resolution)
d Scan #2 (low resolution) analyzed with reader #1
e Scan #2 (low resolution) analyzed with reader #2
T1v on a per-slice basis
The results for intra-observer, inter-observer, inter-resolution and inter-study variability on a per-slice basis based on mean T1v for basal, mid-ventricular and apical short axis locations are summarized in table 2a and by Bland Altman plots shown in figure 3a. ICCs for intra-observer, inter-observer, inter-resolution and inter-study reproducibility were 0.988, 0.899, 0.766 and 0.6; CoVs were 0.72%, 2.39%, 3.90% and 4.28%, respectively. The intra-observer, inter-observer, inter-resolution and inter-study for T1 measurement on a per-slice basis were 8 ms, 18 ms, 36 ms and 36 ms and the SDs were 7 ms, 24 ms 41ms and 42 ms, respectively. As a result, the intra-observer, inter-observer, inter-resolution and inter-study thresholds for T1v were 22 ms, 66 ms, 118 ms and 120 ms.
Table 2a.
T1v calculated on a per-slice basis (low resolution). Intra-observer variability was based on results of scan #1 analyzed by reader #1. Inter-observer variability was based on results of scan #1 analyzed by reader #1 and reader #2. Inter-resolution variability was based on results of scan #1 analyzed by reader #1. Inter-study variability was based on results of scan #1 analyzed by reader #1 and scan #2 analyzed by reader #2
| Intra-observer | Inter-observer | Inter-resolution | Inter-study | |
|---|---|---|---|---|
| ICC | 0.988 | 0.899 | 0.766 | 0.6 |
| CoV (%) | 0.72 | 2.39 | 3.90 | 4.28 |
| (ms) | 8 | 18 | 36 | 36 |
| SD | 7 | 24 | 41 | 42 |
| T1v threshold (ms) | 22 | 66 | 118 | 120 |
Figure 3.
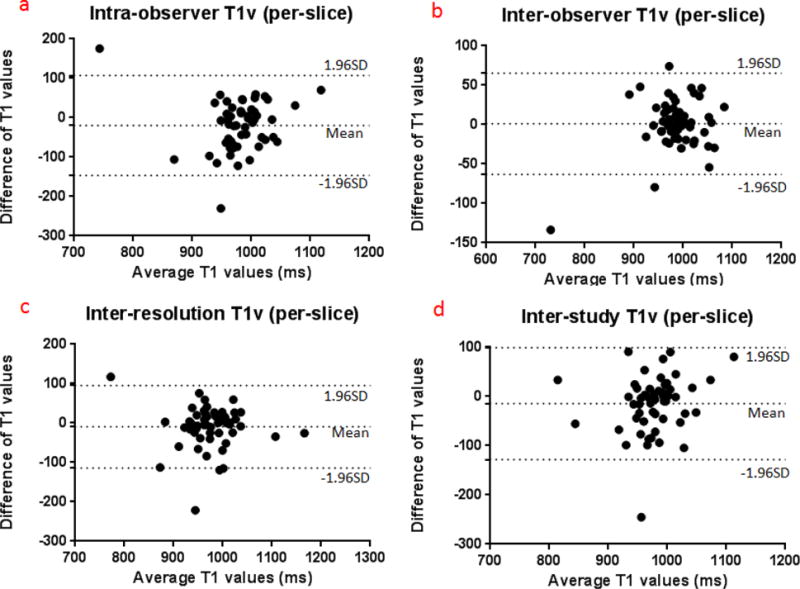
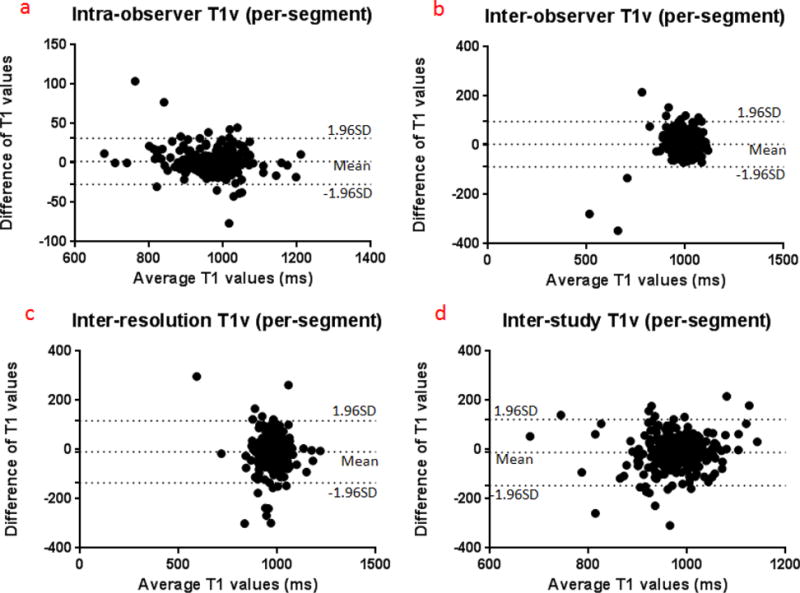
Bland-Altman plots presenting the differences between duplicated measurements (based on T1 values acquired with low resolution MOLLI).
a T1v on a per-slice basis
b T1v on a per-segment basis
T1v on a per-segment basis
On a per-segment basis, ICCs for intra-observer, inter-observer, inter-resolution and inter-study reproducibility were 0.974, 0.859, 0.711 and 0.594; CoVs were 1.09%, 3.36%, 4.69% and 5.01%, respectively. As shown in figure 3b, the for T1 measurement on a per-segment basis was higher than their counterparts on a per-slice basis. However, no statistically significance differences were found (Independent sample t test, p > 0.05). The intra-observer, inter-observer, inter-resolution and interstudy for T1 measurements were 9 ms, 20 ms, 40 ms and 52 ms, and the SDs were 12 ms, 37 ms, 50 ms and 46 ms, respectively. Therefore, the intra-observer, inter-observer and inter-study thresholds for T1v were 33 ms, 94 ms, 140 ms and 144 ms (table 2b). Figure 3b shows Bland-Altman plots for T1v at a per-segment basis.
Table 2b.
T1v calculated on a per-segment basis (low resolution). Intra-observer variability was based on results of scan #1 analyzed by reader #1. Inter-observer variability was based on results of scan #1 analyzed by reader #1 and reader #2. Inter-resolution variability was based on results of scan #1 analyzed by reader #1. Inter-study variability was based on results of scan #1 analyzed by reader #1 and scan #2 analyzed by reader #2
| Intra-observer | Inter-observer | Inter-resolution | Inter-study | |
|---|---|---|---|---|
| ICC | 0.974 | 0.859 | 0.711 | 0.594 |
| CoV (%) | 1.09 | 3.34 | 4.69 | 5.05 |
| (ms) | 9 | 20 | 40 | 52 |
| SD | 12 | 37 | 50 | 46 |
| T1v threshold (ms) | 33 | 94 | 140 | 144 |
Discussion
In the present study, we calculated concurrence and variability of regional T1 values that were acquired with two successive MRI scans of the same subjects with two resolutions and that were analyzed independently by two observers. Our data also suggest reasonable thresholds of T1v between measurements acquired with different circumstances. Such thresholds have the potential to help identify T1v that are caused by pathological changes, and they could be used in the design of a cardiovascular study using T1 mapping.
As a noninvasive imaging technique, T1 mapping has been applied to actively observe changes in native T1 values because of various cardiovascular diseases. A significant change in T1 values in the LV can represent the progression of certain myocardial lesions. Using the MOLLI technique, Hinojar et al. found that patients suffering from hypertrophy cardiomyopathy (HCM) had significantly higher T1 values than patients with primary HTN did (1169 ± 41 ms vs. 1059 ± 38 ms) [8]. Kato et al. found that patients with atrial fibrillation (AF) had a longer T1 time (global value) than healthy controls (1099 ± 52 ms vs. 1042 ± 20 ms) [9]. In addition, the increase of native T1 time has been shown to be related to the recurrence of AF after pulmonary vein isolation (PVI) [9]. Using shMOLLI technique, Ntusi et al. found that patients with rheumatoid arthritis (RA) had higher native T1 values (973 ± 27 ms vs. 961 ± 18 ms) than control subjects [10]. While a reduced myocardial T1 value could be found in Anderson-Fabry disease (882 ± 47 ms vs. 968 ± 32 ms) and high iron overload as compared with healthy controls [11,12].
However, variability of MRI-derived T1 measurements may come from many other sources than structural changes in the myocardium. First, the T1 mapping sequence was run at three predefined locations of the LV. Those imaging planes were manually defined by clinical MRI technicians during the scans. Therefore, slight myocardial slice mismatch between scans and rescans may exist because there are no significant anatomic landmarks for accurate slice selection. Second, the borders of the myocardium were manually drawn by analyzers. Variations of region of interest (ROI) may contribute to the variation in the measurements. Third, different physiological conditions, such as heart rate, may also lead to different MRI-derived T1 values at different time points [13]. As a result, it is important to assess the sensitivity of current T1 mapping techniques, such as MOLLI, for the detection of regional T1 changes. With a small sample size (n = 8), Lin et al. demonstrated good reproducibility of MOLLI in measuring native T1 values at a middle LV plane in healthy volunteers [14]. Roujol et al. studied inter-study variations of T1 measurements over the whole LV in 7 healthy volunteers using multiple MRI techniques [6]. Pica et al. found good agreement between duplicated measurements of T1 values in 21 patients with Fabry disease [15]. In the present study, our data (high ICC and low CoV) further demonstrated good intra-observer, inter-observer and inter-study reproducibility for regional native T1 measurements on per-slice and per-segment levels.
As absolute T1 values may vary due to many internal or external factors, T1v acquired with the same imaging protocol has become a more feasible method for the evaluation of the progression of cardiovascular diseases. Therefore, we focused on “normal” reference ranges of regional T1v in repeated measurements using MOLLI. The thresholds for T1v between repeated T1 measurements were calculated on a per-slice and per-segment basis. That means that more than 95% of the random variations of segmental T1v should be located within this range. These T1v thresholds may represent the potential of MRI techniques to distinguish regional T1 changes and may provide the minimal difference necessary to calculate sample sizes needed to identify patients with cardiovascular diseases in large scale clinical studies or trials. Compared to previous reproducibility studies for T1 measurements on a per-patient and per-slice basis, our study showed a higher inter-study T1v on a per-segment basis (including all 16 LV segments) in clinical settings. Our finding suggested that it is necessary to conservatively treat T1v (either positive or negative) as an indicator of the progression of cardiovascular diseases. Predictive values of these T1v thresholds need to be verified in future clinical studies.
This study has limitations. First, we did not calculate variations of regional extracellular volume (ECV) of our participants in this non-contrast MRI study. Non-contrast T1v is not the only factor that can affect ECV. We were unable to exclude other factors that affect ECV calibrations. T1 values of the myocardium and blood pool may vary greatly after the administration of different contrast agents [14]. Second, we were unable to compare T1v acquired with other MOLLI schemes, such as using 5(3)3 and 5(3s)3 or other MRI techniques [16]. A previous study has demonstrated that different T1 mapping sequences had similar in vivo reproducibility for native T1 measures (errors range from 25 ms to 50 ms) [6]. Therefore, we estimated that MOLLI with a 5(4)3 scheme could be an appropriate representative of existing MRI techniques for the investigation of T1v. Therefore, further studies are warranted for assessing T1v acquired with other MRI sequences or analysis tools. Third, we only tested distributions of regional T1v in healthy volunteers. Further studies are warranted to investigate T1v in subjects with special cardiovascular diseases who have characteristic native T1 values.
Conclusions
The regional T1v thresholds from repeated measurements found in our study demonstrate the underlying variability of the MOLLI techniques which is commonly used for myocardial native T1 quantification. These T1v thresholds are an important prerequisite for the clinical application of T1 mapping and should be taken into consideration when assess MRI-derived regional T1 changes associated with LV tissue abnormalities.
Acknowledgments
Funding source: This work was supported by grants from the national institute of Health R01HL117888 and K01HL121162.
Abbreviation
- MRI
Magnetic resonance imaging
- CVD
Cardiovascular diseases
- T1v
T1 variation
- MOLLI
Modified Look-Locker Inversion recovery
- ShMOLLI
Shortened Modified Look-Locker Inversion recovery
- SAPPHIRE
Saturation pulse prepared heart rate independent inversion recovery
- SASHA
Saturation Recovery Single Shot Acquisition
- TR
Repetition time
- TE
Echo time
- ECG
Electrocardiography
- bSSFP
balanced steady-state free precession
- HCM
hypertrophy cardiomyopathy
- ECV
extracellular volume
- AF
atrial fibrillation
- PVI
pulmonary vein isolation
- RA
rheumatoid arthritis
- HTN
hypertension
- ICC
intra-class correlation coefficient
- CoV
coefficient of variation
- SD
Standard deviation
Footnotes
Financial disclosure and competing interests: N/A
Compliance with Ethical Standards
Disclosure of potential conflicts of interest:
We have no competing interest to disclose.
Research involving human participants:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the IRB of Northwestern University.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
References
- 1.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, Neubauer S, Moon JC, Myerson SG. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99(13):932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, Hinojar R, Doltra A, Varma N, Child N, Rogers T, Suna G, Arroyo Ucar E, Goodman B, Khan S, Dabir D, Herrmann E, Zeiher AM, Nagel E, International TMCMROS T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovascular imaging. 2016;9(1):40–50. doi: 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Kali A, Choi EY, Sharif B, Kim YJ, Bi X, Spottiswoode B, Cokic I, Yang HJ, Tighiouart M, Conte AH, Li D, Berman DS, Choi BW, Chang HJ, Dharmakumar R. Native T1 Mapping by 3-T CMR Imaging for Characterization of Chronic Myocardial Infarctions. JACC Cardiovascular imaging. 2015;8(9):1019–1030. doi: 10.1016/j.jcmg.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovascular imaging. 2016;9(1):67–81. doi: 10.1016/j.jcmg.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Larmour S, Chow K, Kellman P, Thompson RB. Characterization of T bias in skeletal muscle from fat in MOLLI and SASHA pulse sequences: Quantitative fat-fraction imaging with T mapping. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2016 doi: 10.1002/mrm.26113. [DOI] [PubMed] [Google Scholar]
- 6.Roujol S, Weingartner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272(3):683–689. doi: 10.1148/radiol.14140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J, Kellman P. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012;67(6):1644–1655. doi: 10.1002/mrm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinojar R, Varma N, Child N, Goodman B, Jabbour A, Yu CY, Gebker R, Doltra A, Kelle S, Khan S, Rogers T, Arroyo Ucar E, Cummins C, Carr-White G, Nagel E, Puntmann VO. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy: Findings From the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circulation Cardiovascular imaging. 2015;8(12) doi: 10.1161/CIRCIMAGING.115.003285. [DOI] [PubMed] [Google Scholar]
- 9.Kato S, Foppa M, Roujol S, Basha T, Berg S, Kissinger KV, Goddu B, Manning WJ, Nezafat R. Left ventricular native T1 time and the risk of atrial fibrillation recurrence after pulmonary vein isolation in patients with paroxysmal atrial fibrillation. International journal of cardiology. 2016;203:848–854. doi: 10.1016/j.ijcard.2015.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Matthews PM, Robson MD, Wordsworth PB, Neubauer S, Karamitsos TD. Diffuse Myocardial Fibrosis and Inflammation in Rheumatoid Arthritis: Insights From CMR T1 Mapping. JACC Cardiovascular imaging. 2015;8(5):526–536. doi: 10.1016/j.jcmg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G, Fontana M, Maestrini V, Flett AS, Robson MD, Lachmann RH, Murphy E, Mehta A, Hughes D, Neubauer S, Elliott PM, Moon JC. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circulation Cardiovascular imaging. 2013;6(3):392–398. doi: 10.1161/CIRCIMAGING.112.000070. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, He T, Carpenter JP, Jabbour A, Alam MH, Gatehouse PD, Greiser A, Messroghli D, Firmin DN, Pennell DJ. In vivo comparison of myocardial T1 with T2 and T2* in thalassaemia major. Journal of magnetic resonance imaging: JMRI. 2013;38(3):588–593. doi: 10.1002/jmri.24010. [DOI] [PubMed] [Google Scholar]
- 13.Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, Sivananthan MU. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution–reproducibility study. Radiology. 2006;238(3):1004–1012. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 14.Lin K, Lloyd-Jones DM, Spottiswoode B, Bi X, Liu Y, Lu B, Xue H, Wang Y, Li D, Carr JC. T1 contrast in the myocardium and blood pool: a quantitative assessment of gadopentetate dimeglumine and gadofosveset trisodium at 1.5 and 3 T. Investigative radiology. 2014;49(4):243–248. doi: 10.1097/RLI.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 15.Pica S, Sado DM, Maestrini V, Fontana M, White SK, Treibel T, Captur G, Anderson S, Piechnik SK, Robson MD, Lachmann RH, Murphy E, Mehta A, Hughes D, Kellman P, Elliott PM, Herrey AS, Moon JC. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:99. doi: 10.1186/s12968-014-0099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:2. doi: 10.1186/1532-429X-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


